Effect of ε-Polylysine Addition on Pullulan Biodegradable Films for Blueberry Surface Coating
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of Blend Films
2.3. Physico-Chemical Characterization of the Film
2.3.1. Mechanical Properties
2.3.2. Water Vapor Permeability (WVP)
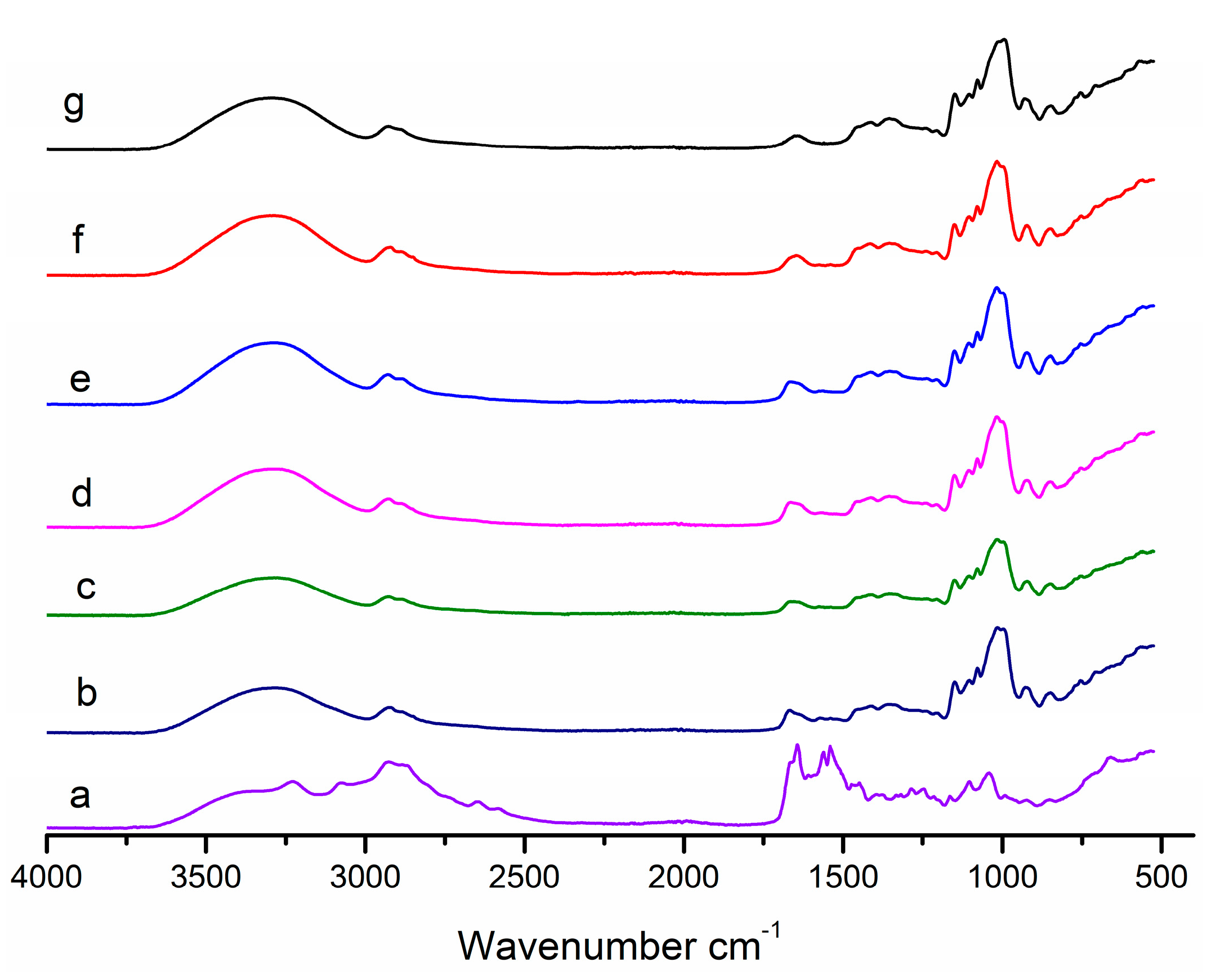
2.3.3. Fourier Transform Infrared (FTIR) Analysis
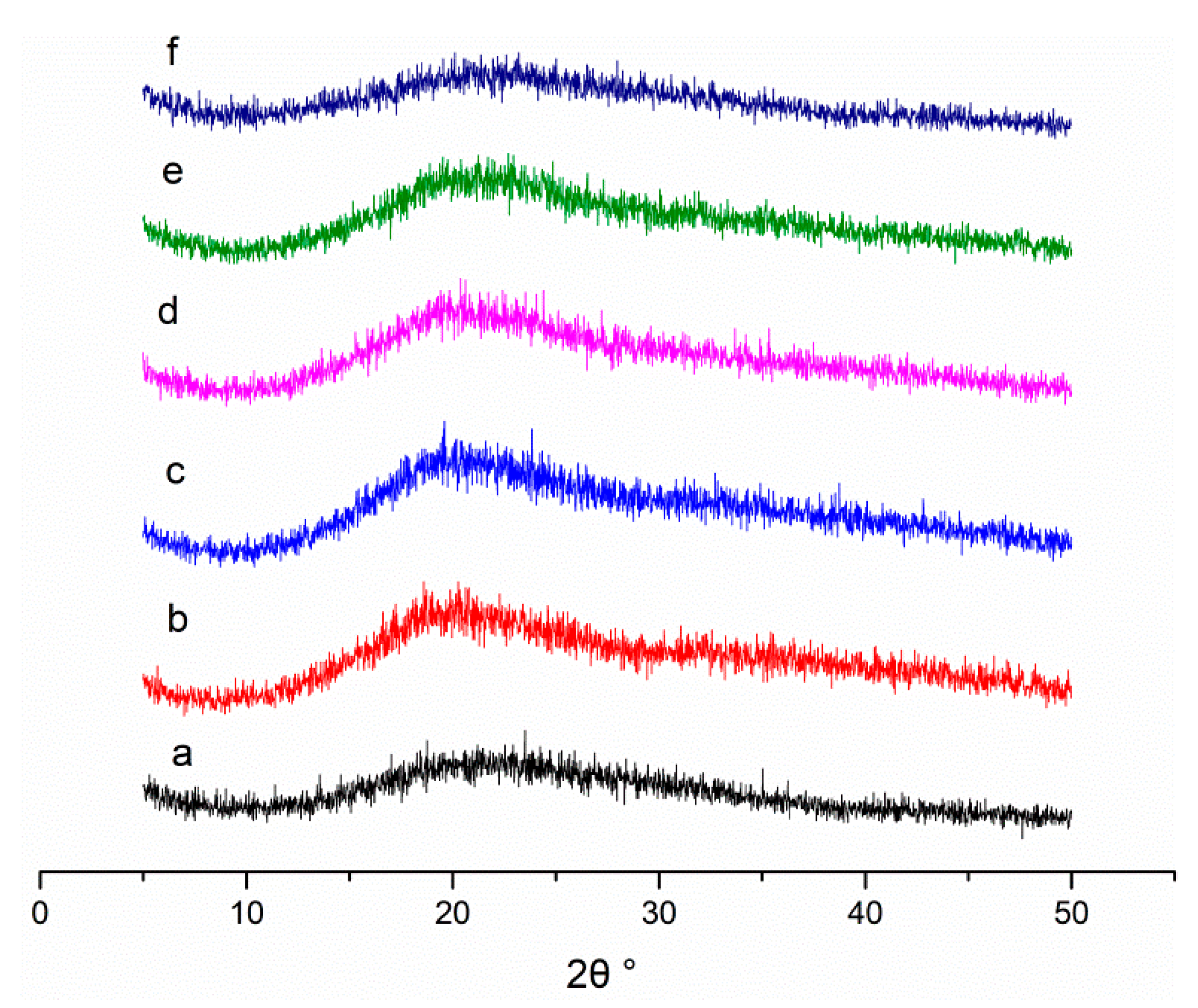
2.3.4. X-ray Diffraction (XRD)
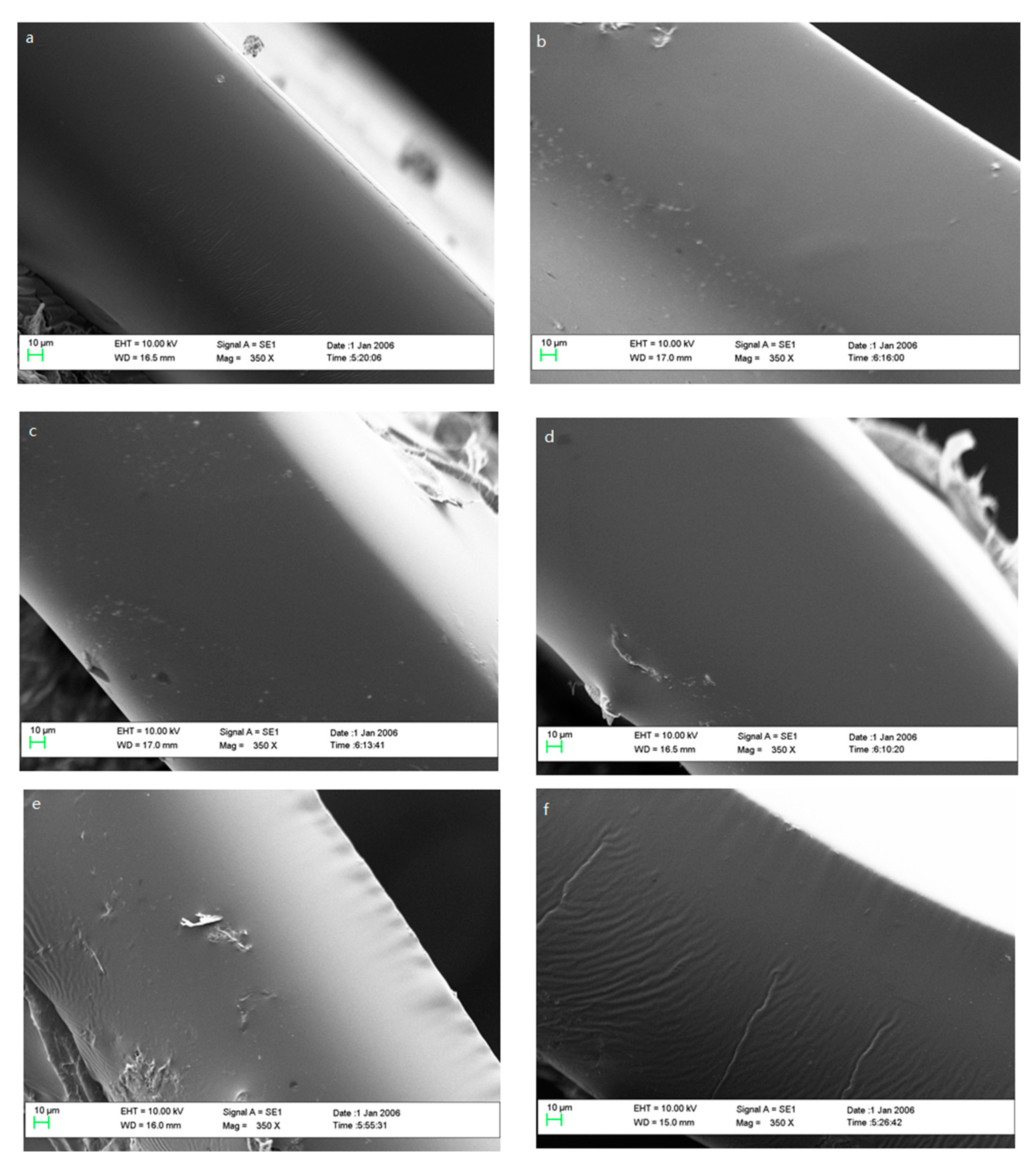
2.3.5. Scanning Electron Microscopy (SEM)
2.4. Preservation Experiment of Blueberries
2.5. Determination of Blueberry Properties
2.5.1. Mass Loss (ML)
2.5.2. Texture
2.5.3. Measurement of Blueberry Properties
2.6. Statistical Analysis
3. Results and Discussion
3.1. Characterization of Films
3.1.1. Mechanical Properties
3.1.2. WVP
3.1.3. FTIR
3.1.4. XRD
3.1.5. SEM
3.2. Preservation Experiment of Blueberries
3.2.1. Photographs of Blueberries in Storage
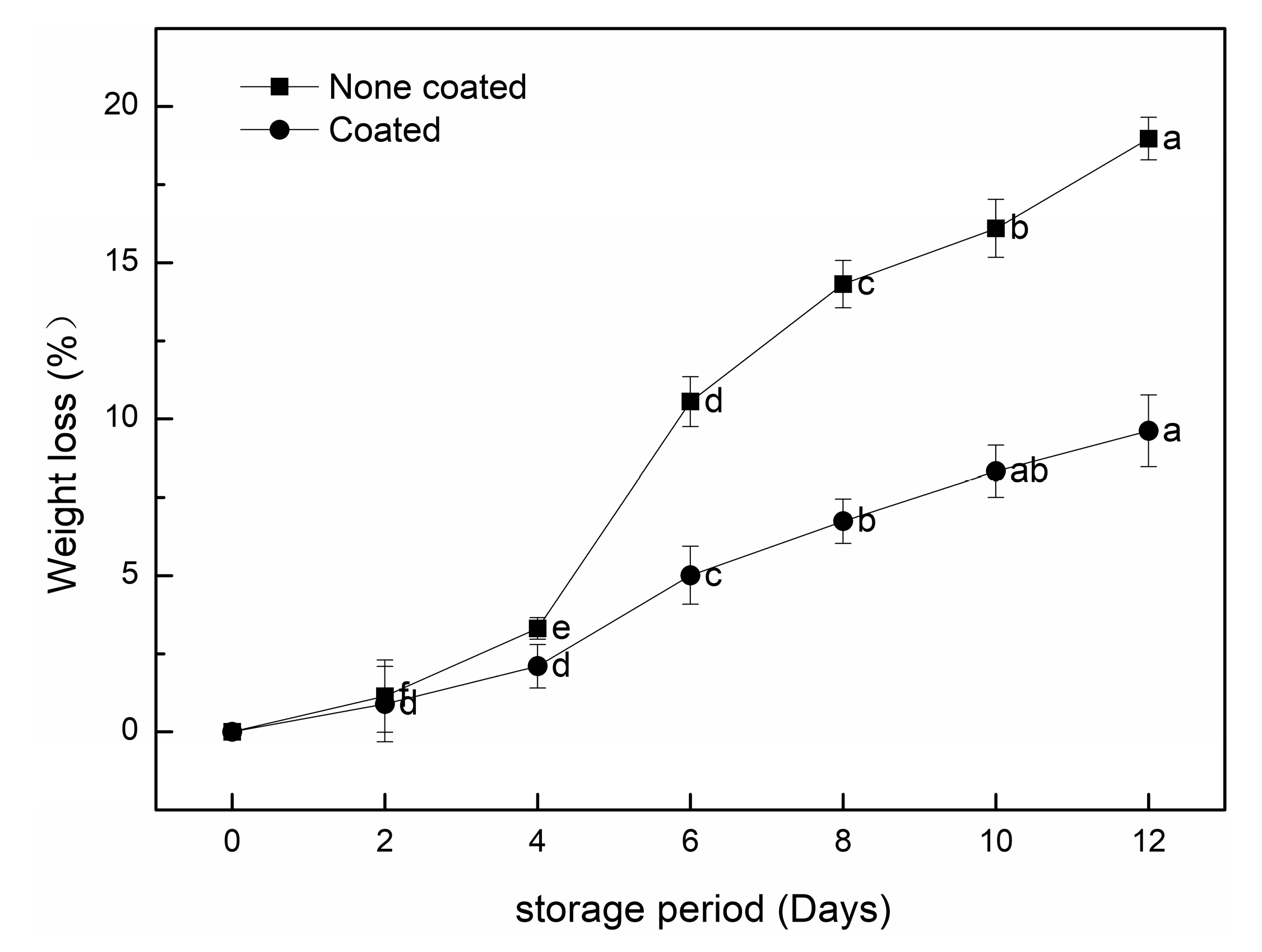
3.2.2. Mass Loss
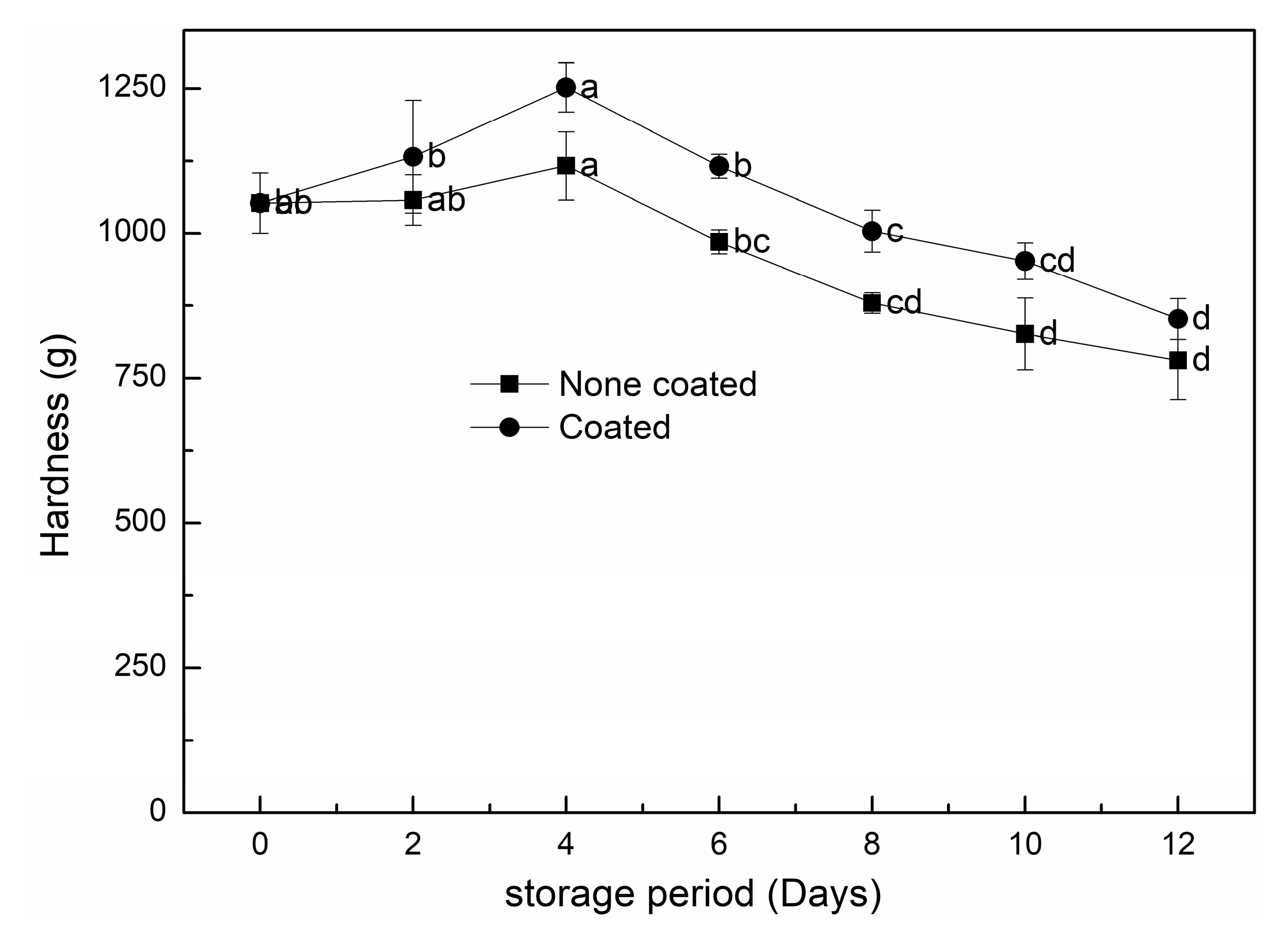
3.2.3. Texture
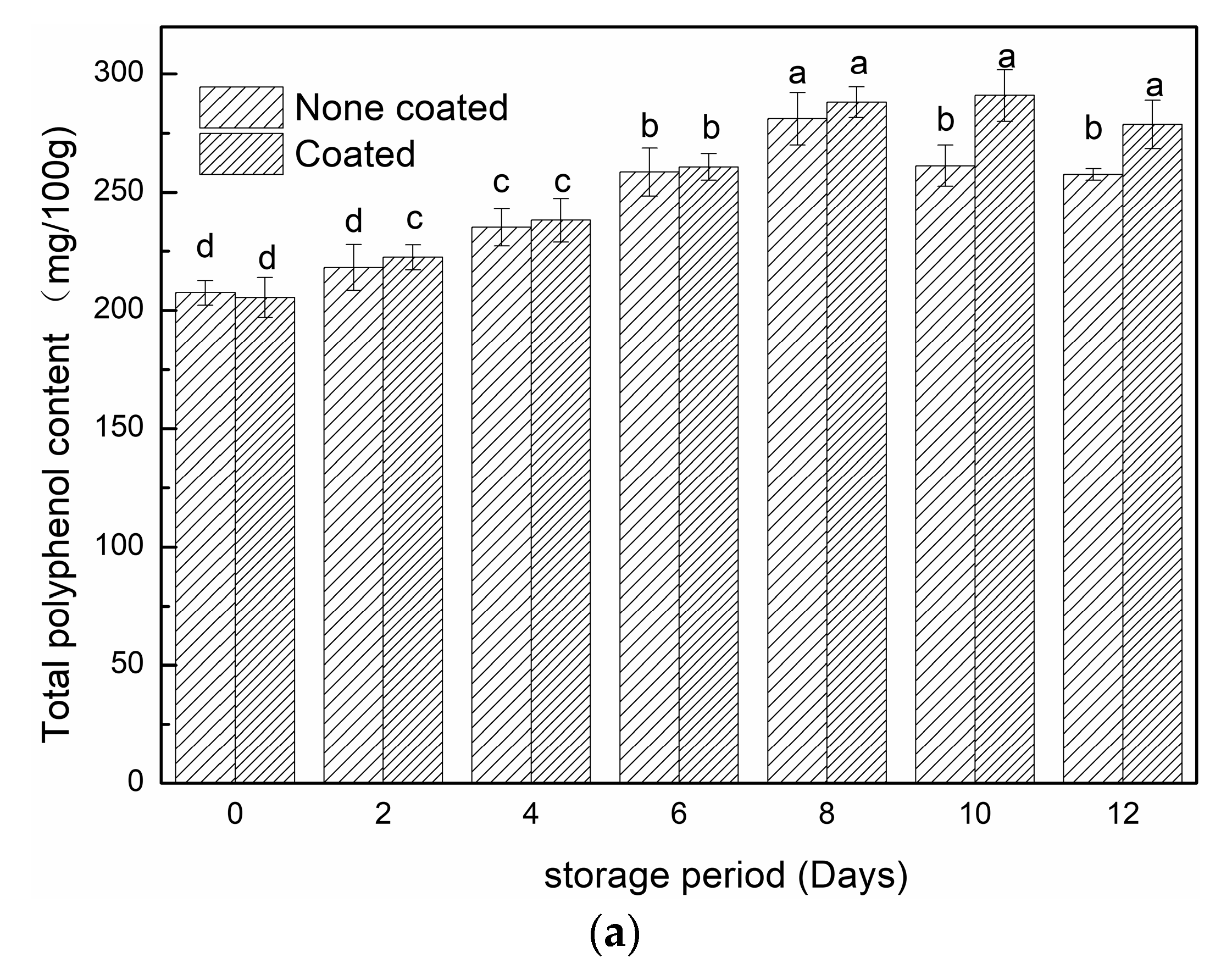
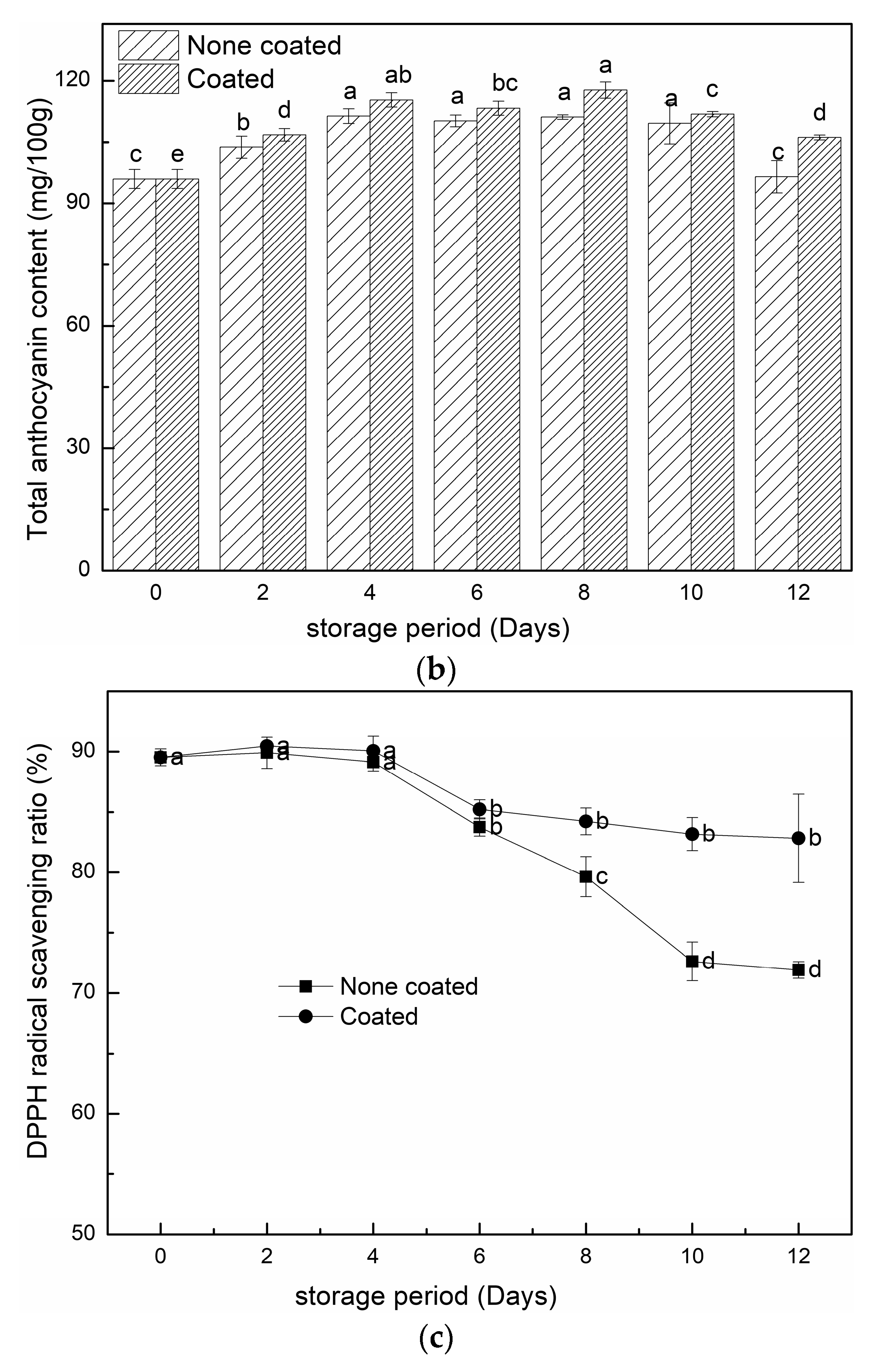
3.2.4. TPC, TAC and DPPH
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Tobar-Bolaos, G.; Casas-Forero, N.; Orellana-Palma, P.; Petzold, G. Blueberry juice: Bioactive compounds, health impact, and concentration technologies—A review. J. Food Sci. 2021, 86, 5062–5077. [Google Scholar] [CrossRef]
- Nilushni, S.; Nilanivetha, N.; Vasantha, H.R. Potential health benefits of fermented blueberry: A review of current scientific evidence. Trends Food Sci. Technol. 2023, 132, 103–120. [Google Scholar]
- Yang, W.; Guo, Y.; Liu, M.; Chen, X.; Xiao, X.; Wang, S.; Chen, F. Structure and function of blueberry anthocyanins: A review of recent advances. J. Funct. Foods 2022, 88, 104864. [Google Scholar] [CrossRef]
- Mannozzi, C.; Cecchini, J.P.; Tylewicz, U.; Romani, S. Study on the efficacy of edible coatings on quality of blueberry fruits during shelf-life. LWT Food Sci. Technol. 2017, 85, 440–444. [Google Scholar] [CrossRef]
- Li, C.; Luo, J.; MacLean, D. A novel instrument to delineate varietal and harvest effects on blueberry fruit texture during storage. J. Sci. Food Agric. 2011, 91, 1653–1658. [Google Scholar] [CrossRef]
- Huan, C.; An, X.; Yu, M.; Jiang, L.; Ma, R.; Tu, M.; Yu, Z. Effect of combined heat and 1-MCP treatment on the quality and antioxidant level of peach fruit during storage. Postharvest Biol. Technol. 2018, 145, 193–202. [Google Scholar] [CrossRef]
- Wang, C.; Gao, Y.; Tao, Y.; Wu, X.; Cui, Z. Influence of γ-irradiation on the reactive-oxygen metabolism of blueberry fruit during cold storage. Innov. Food Sci. Emerg. Technol. 2017, 41, 397–403. [Google Scholar] [CrossRef]
- Chu, W.; Gao, H.; Chen, H.; Fang, X.; Zheng, Y. Effects of cuticular wax on the postharvest quality of blueberry fruit. Food Chem. 2018, 239, 68–74. [Google Scholar] [CrossRef]
- Zhu, L.; Ling, J. Development of preservation technology of blueberry in the world. Food Ferment. Ind. 2011, 37, 173–176. [Google Scholar]
- Kraśniewska, K.; Ścibisz, I.; Gniewosz, M.; Mitek, M.; Pobiega, K.; Cendrowski, A. Effect of pullulan coating on postharvest quality and shelf-life of highbush blueberry (Vaccinium corymbosum L.). Materials 2017, 10, 965. [Google Scholar] [CrossRef]
- Pobiega, K.; Igielska, M.; Włodarczyk, P.; Gniewosz, M. The use of pullulan coatings with propolis extract to extend the shelf life of blueberry (Vaccinium corymbosum) fruit. Int. J. Food Sci. Technol. 2020, 56, 1013–1020. [Google Scholar] [CrossRef]
- Flores-López, M.L.; Cerqueira, M.A.; de Rodríguez, D.J.; Vicente, A.A. Perspectives on utilization of edible coatings and nano-laminate coatings for extension of postharvest storage of fruits and vegetables. Food Eng. Rev. 2016, 8, 292–305. [Google Scholar] [CrossRef]
- Mannozzi, C.; Tylewicz, U.; Chinnici, F.; Siroli, L.; Rocculi, P.; Dalla-Rosa, M.; Romani, S. Effects of chitosan based coatings enriched with procyanidin by-product on quality of fresh blueberries during storage. Food Chem. 2018, 251, 18–24. [Google Scholar] [CrossRef] [PubMed]
- Rojas-Graü, M.A.; Tapia, M.S.; Martín-Belloso, O. Using polysaccharide-based edible coatings to maintain quality of fresh-cut Fuji apples. LWT Food Sci. Technol. 2008, 41, 139–147. [Google Scholar] [CrossRef]
- Sima, P.; Mohammadreza, D.; Seyed Hadi, P.; Maral, S.; Beatriz, G.; Kazem, A.; Jose, M.L. Applications of carboxymethyl cellulose- and pectin-based active edible coatings in preservation of fruits and vegetables: A review. Trends Food Sci. Technol. 2021, 110, 663–673. [Google Scholar]
- Zhu, G.; Sheng, L.; Tong, Q. Preparation and characterization of carboxymethyl-gellan and pullulan blend films. Food Hydrocoll. 2014, 35, 341–347. [Google Scholar] [CrossRef]
- Priyadarshi, R.; Kim, S.; Rhim, J. Pectin/pullulan blend films for food packaging: Effect of blending ratio. Food Chem. 2021, 347, 129022. [Google Scholar] [CrossRef]
- Yan, Y.; Duan, S.; Zhang, H.; Wu, W. Preparation and characterization of Konjacglucomannan and pullulan composite films for strawberry preservation. Carbohydr. Polym. 2020, 243, 116446. [Google Scholar] [CrossRef]
- Dutta, P.K.; Tripathi, S.; Mehrotra, G.K.; Dutta, J. Perspectives for chitosan based antimicrobial films in food applications. Food Chem. 2009, 114, 1173–1182. [Google Scholar] [CrossRef]
- Takehara, M.; Hibino, A.; Saimura, M.; Hirohara, H. High-yield production of short chain length poly(ε-L-lysine) consisting of 5–20 residues by Streptomyces aureofaciens, and its antimicrobial activity. Biotechnol. Lett. 2010, 32, 1299–1303. [Google Scholar] [CrossRef]
- Zhou, C.; Li, P.; Qi, X.; Sharif, A.R.M.; Poon, Y.F.; Cao, Y.; Chang, M.W.; Jan Leong, S.S.; Chan-Park, M.B. A photopolymerized antimicrobial hydrogel coating derived from epsilon-poly-L-lysine. Biomaterials 2011, 32, 2704–2712. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.N.; Ye, Q.Q.; Hou, W.F.; Zhang, G.Q. Development of antibacterial ε-polylysine/chitosan hybrid films and the effect on citrus. Int. J. Biol. Macromol. 2018, 11, 2051–2056. [Google Scholar] [CrossRef] [PubMed]
- Jiao, W.; Liu, X.; Chen, Q.; Du, Y.; Li, Y.; Yue, F.; Dong, X.; Fu, M. Epsilon-poly-L-lysine (ε-PL) exhibits antifungal activity in vivo and in vitro against Botrytis cinerea and mechanism involved. Postharvest Biol. Technol. 2020, 168, 111270. [Google Scholar] [CrossRef]
- Tong, Q.; Xiao, Q.; Lim, L.T. Preparation and Properties of Pullulan- Alginate-Carboxymethylcellulose Blend Films. Food Res. Int. 2008, 41, 1007–1014. [Google Scholar] [CrossRef]
- Guo, N.; Zhu, G.; Chen, D.; Wang, D.; Zhang, F.; Zhang, Z. Preparation and characterization of gellan gum-guar gum blend films incorporated with nisin. J. Food Sci. 2020, 85, 1799–1804. [Google Scholar] [CrossRef] [PubMed]
- Bejaei, M.; Stanich, K.; Cliff, M.A. Modelling and Classification of Apple Textural Attributes Using Sensory, Instrumental and Compositional Analyses. Foods 2021, 10, 384. [Google Scholar]
- Tahir, H.E.; Zou, X.B.; Li, Z.H.; Zhu, Y. Comprehensive evaluation of antioxidant properties and volatile compounds of Sudanese honeys. J. Food Biochem. 2015, 39, 349–359. [Google Scholar] [CrossRef]
- Rodriguez, J.; Zoffoli, J.P. Effect of sulfur dioxide and modified atmosphere packaging on blueberry postharvest quality. Postharvest Biol. Technol. 2016, 117, 230–238. [Google Scholar] [CrossRef]
- Yang, Z.; Zou, X.; Li, Z.; Huang, X.; Zhai, X.; Zhang, W.; Shi, J.; Tahir, H.E. Improved postharvest quality of cold stored blueberry by edible coating based on composite gum arabic/roselle extract. Food Bioprocess Technol. 2019, 12, 1537–1547. [Google Scholar] [CrossRef]
- Hassan, B.; Chatha, S.A.S.; Hussain, A.I.; Zia, K.M.; Akhtar, N. Recent advances on polysaccharides, lipids and protein based edible films and coatings: A review. Int. J. Biol. Macromol. 2018, 109, 1095–1107. [Google Scholar] [CrossRef]
- Yu, Z.; Rao, G.; Wei, Y.; Yu, J.; Wu, S.; Fang, Y. Preparation, characterization, and antibacterial properties of biofilms comprising chitosan and ε-polylysine. Int. J. Biol. Macromol. 2019, 141, 545–552. [Google Scholar] [CrossRef]
- Hu, D.; Wang, H.; Wang, L. Physical properties and antibacterial activity of quaternized chitosan/carboxymethyl cellulose blend films. LWT Food Sci. Technol. 2016, 65, 398–405. [Google Scholar] [CrossRef]
- Ghanbarzadeh, B.; Almasi, H.; Entezami, A.A. Improving the barrier and mechanical properties of corn starch-based edible films: Effect of citric acid and carboxymethyl cellulose. Ind. Crops Prod. 2011, 33, 229–235. [Google Scholar] [CrossRef]
- Alirezalu, K.; Pirouzi, S.; Yaghoubi, M.; Karimi-Dehkord, M.; Jafarzadeh, S.; Khaneghah, A.M. Packaging of beef fillet with active chitosan film incorporated with ε-polylysine: An assessment of quality indices and shelf life. Meat Sci. 2021, 176, 108475. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Li, R.; Dong, F.; Tian, A.; Li, Z.; Dai, Y. Physical, mechanical and antimicrobial properties of starch films incorporated with ε-poly-L-lysine. Food Chem. 2015, 166, 107–114. [Google Scholar] [CrossRef] [PubMed]
- Hong, Y.J.; Lee, M.S.; Kim, J.C. pH-dependent release of alginate beadscoated with polylysine. J. Ind. Eng. Chem. 2011, 17, 410–414. [Google Scholar] [CrossRef]
- Wu, J.; Zhong, F.; Li, Y.; Shoemaker, C.F.; Xia, W. Preparation and characterization of pullulan-chitosan and pullulan-carboxymethyl chitosan blended films. Food Hydrocoll. 2013, 30, 82–91. [Google Scholar] [CrossRef]
- Lin, L.; Xue, L.; Duraiarasan, S.; Haiying, C. Preparation of ε-polylysine/chitosan nanofibers for food packaging against Salmonella on chicken. Food Packag. Shelf Life 2018, 17, 134–141. [Google Scholar] [CrossRef]
- Yin, Y.J.; Yao, K.D.; Cheng, G.X.; Ma, J.B. Properties of polyelectrolytecomplex films of chitosan and gelatin. Polym. Int. 1999, 48, 429–433. [Google Scholar] [CrossRef]
- Song, Z.; Li, F.; Guan, H.; Xu, Y.; Fu, Q.; Li, D. Combination of nisin and ε-polylysine with chitosan coating inhibits the white blush of fresh-cut carrots. Food Control 2017, 74, 34–44. [Google Scholar] [CrossRef]
- Tang, Q.; Pan, D.; Sun, Y.; Cao, J.; Zeng, X.; Wu, Z. Preparation and performance of antimicrobial films based on sodium alginateand ε-poly-L-lysine. J. Chin. Inst. Food Sci. Technol. 2016, 16, 101–107. [Google Scholar]
- Bonilla, J.; Sobral, P. Investigation of the physicochemical, antimicrobial and antioxidant properties of gelatin-chitosan edible film mixed with plant ethanolic extracts. Food Biosci. 2016, 16, 17–25. [Google Scholar] [CrossRef]
- Wang, C.Y.; Chen, C.T.; Wang, S.Y. Changes of flavonoid content and antioxidant capacity in blueberries after illumination with UV-C. Food Chem. 2009, 117, 426–431. [Google Scholar] [CrossRef]
- Cantín, C.M.; Minas, I.S.; Goulas, V.; Jiménez, M.; Manganaris, G.A.; Michailides, T.J.; Crisosto, C.H. Sulfur dioxide fumigation alone or in combination with CO2-enriched atmosphere extends the market life of highbush blueberry fruit. Postharvest Biol. Technol. 2012, 67, 84–91. [Google Scholar] [CrossRef]
- Zou, X.; Yang, Z.; Shi, J.; Huang, X.; Zhang, W.; Tahir, H.E. Preservation effect of gum Arabic edible coating incorporated with white roselle extract (Hibiscus sabdariffa L.) on cold-stored blueberries. Food Sci. 2019, 40, 204–211. [Google Scholar]
- Murmu, S.B.; Mishra, H.N. The effect of edible coating based on Arabic gum, sodium caseinate and essential oil of cinnamon and lemon grass on guava. Food Chem. 2018, 245, 820–828. [Google Scholar] [CrossRef]
- Reque, P.M.; Steffens, R.S.; Jablonski, A.; Flôres, S.H.; Rios, A.D.O.; De Jong, E.V. Cold storage of blueberry (Vaccinium spp.) fruits and juice: Anthocyanin stability and antioxidant activity. J. Food Compos. Anal. 2014, 33, 111–116. [Google Scholar] [CrossRef]
- Faria, A.; Oliveira, J.; Neves, P.; Gameiro, P.; Santos-Buelga, C.; de Freitas, V.; Mateus, N. Antioxidant properties of prepared blueberry (Vacciniummyrtillus) extracts. J. Agric. Food Chem. 2011, 53, 6896–6902. [Google Scholar] [CrossRef] [PubMed]
- Connor, A.M.; Luby, J.J.; Hancock, J.F.; Berkheimer, S.; Hanson, E.J. Changes in fruit antioxidant activity among blueberry cultivars during cold-temperature storage. J. Agric. Food Chem. 2002, 50, 893–898. [Google Scholar] [CrossRef]

| Content of ε-PL (g/100 mL) | TS (MPa) | EAB (%) | WVP × 10−6 (g m/Pa h m2) |
|---|---|---|---|
| 0 | 3.95 ± 0.26 b | 12.2 ± 1.20 e | 0.20 ± 0.05 c |
| 0.1 | 4.02 ± 0.24 b | 13.0 ± 0.95 e | 0.21 ± 0.01 c |
| 0.3 | 4.79 ± 0.11 ab | 17.5 ± 1.02 d | 0.24 ± 0.02 bc |
| 0.5 | 5.63 ± 0.57 a | 21.5 ± 1.23 c | 0.27 ± 0.01 b |
| 0.7 | 4.80 ± 0.52 ab | 25.0 ± 0.81 b | 0.40 ± 0.03 a |
| 0.9 | 4.10 ± 0.66 b | 30.0 ± 1.50 a | 0.41 ± 0.01 a |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhu, G.; Guo, N.; Yan, X.; Dong, J.; Chen, X.; Lu, H. Effect of ε-Polylysine Addition on Pullulan Biodegradable Films for Blueberry Surface Coating. Coatings 2023, 13, 1832. https://doi.org/10.3390/coatings13111832
Zhu G, Guo N, Yan X, Dong J, Chen X, Lu H. Effect of ε-Polylysine Addition on Pullulan Biodegradable Films for Blueberry Surface Coating. Coatings. 2023; 13(11):1832. https://doi.org/10.3390/coatings13111832
Chicago/Turabian StyleZhu, Guilan, Na Guo, Xingmei Yan, Jianyu Dong, Xiaozhong Chen, and Hongxia Lu. 2023. "Effect of ε-Polylysine Addition on Pullulan Biodegradable Films for Blueberry Surface Coating" Coatings 13, no. 11: 1832. https://doi.org/10.3390/coatings13111832
APA StyleZhu, G., Guo, N., Yan, X., Dong, J., Chen, X., & Lu, H. (2023). Effect of ε-Polylysine Addition on Pullulan Biodegradable Films for Blueberry Surface Coating. Coatings, 13(11), 1832. https://doi.org/10.3390/coatings13111832







