PVD Black Coating for Decorative Applications
Abstract
1. Introduction
2. Experimental Conditions
2.1. Coating Deposition
2.2. Coating Characterization
2.3. Simulation of Optical Properties
3. Results
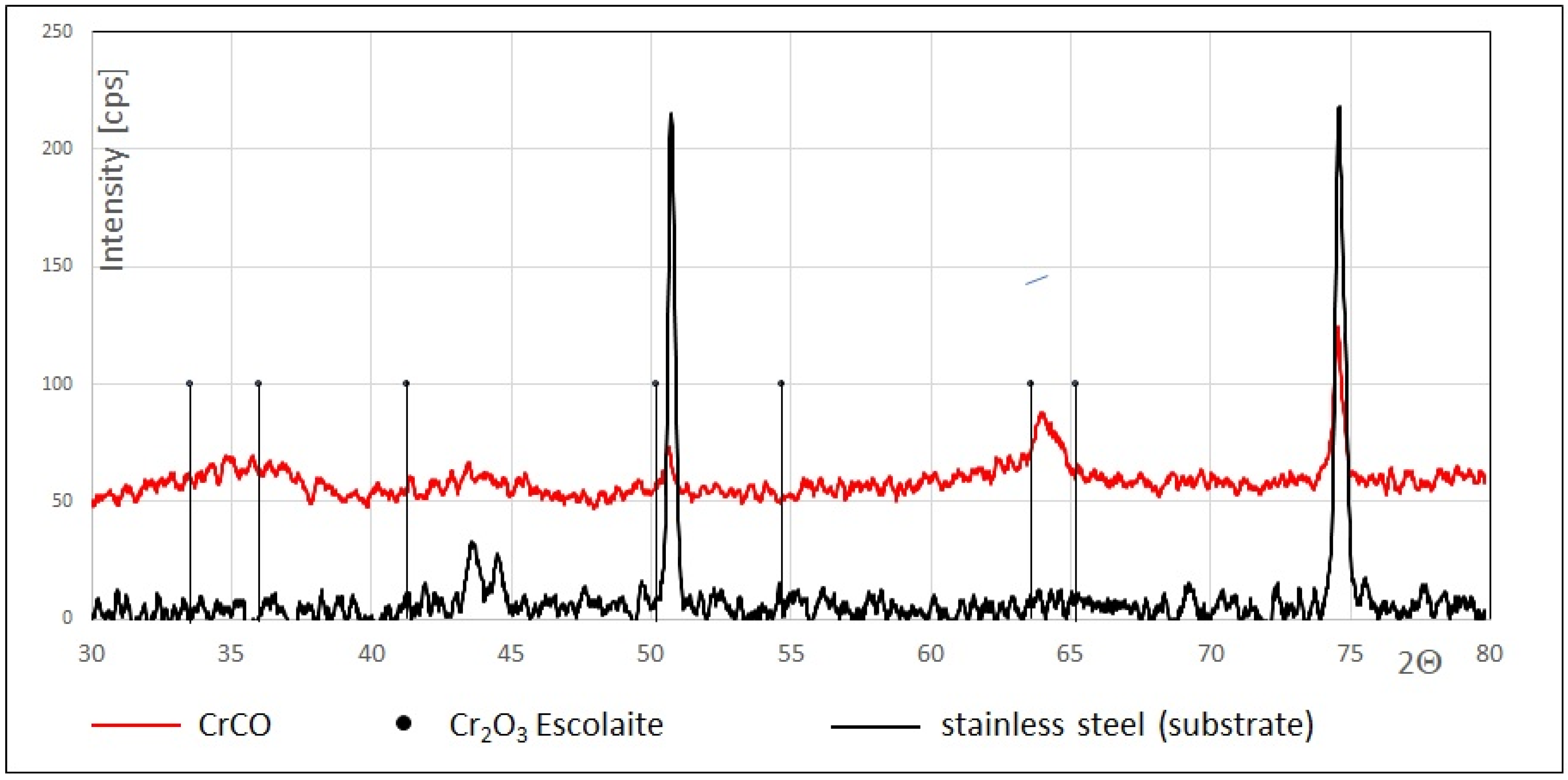
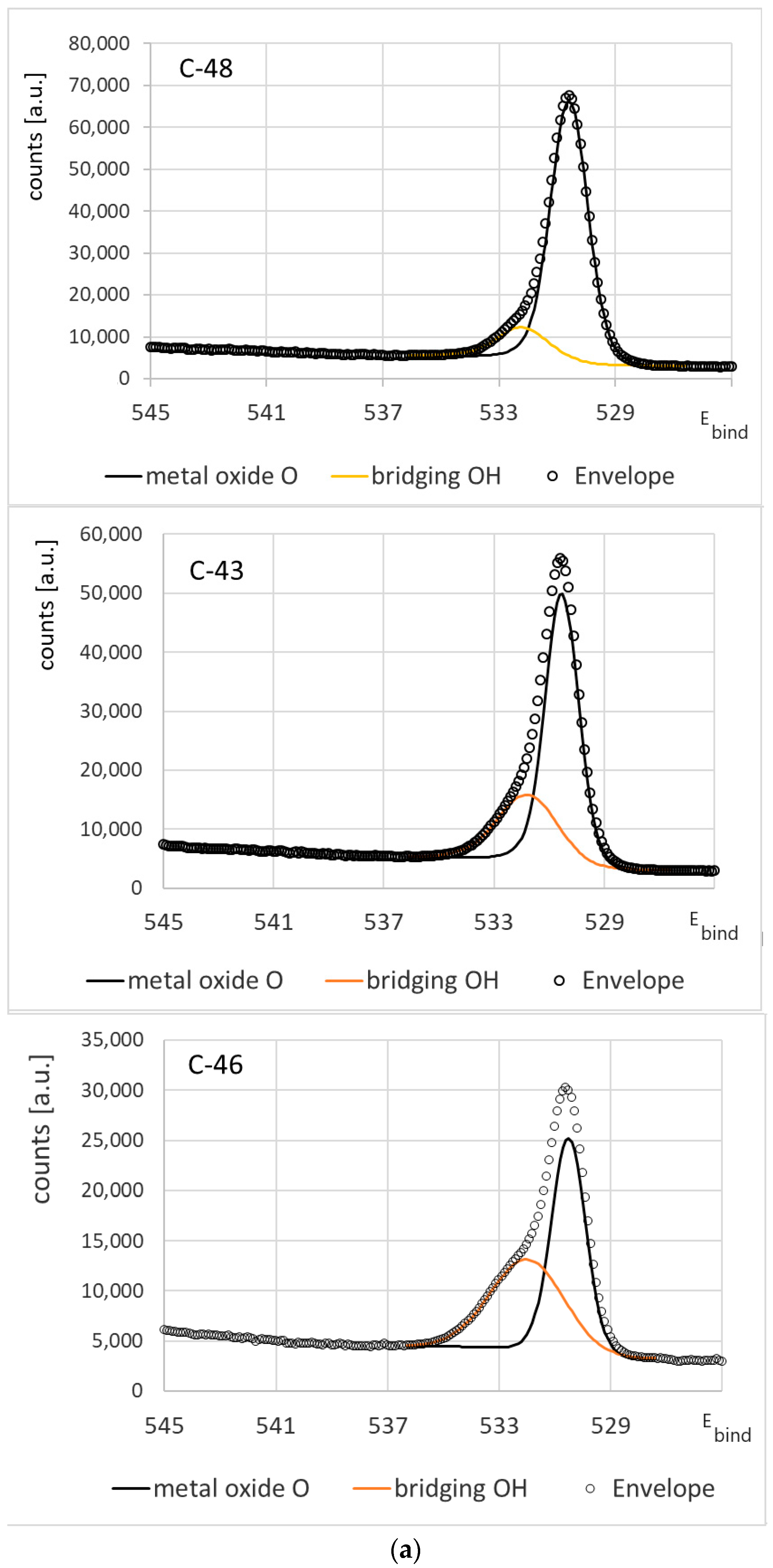
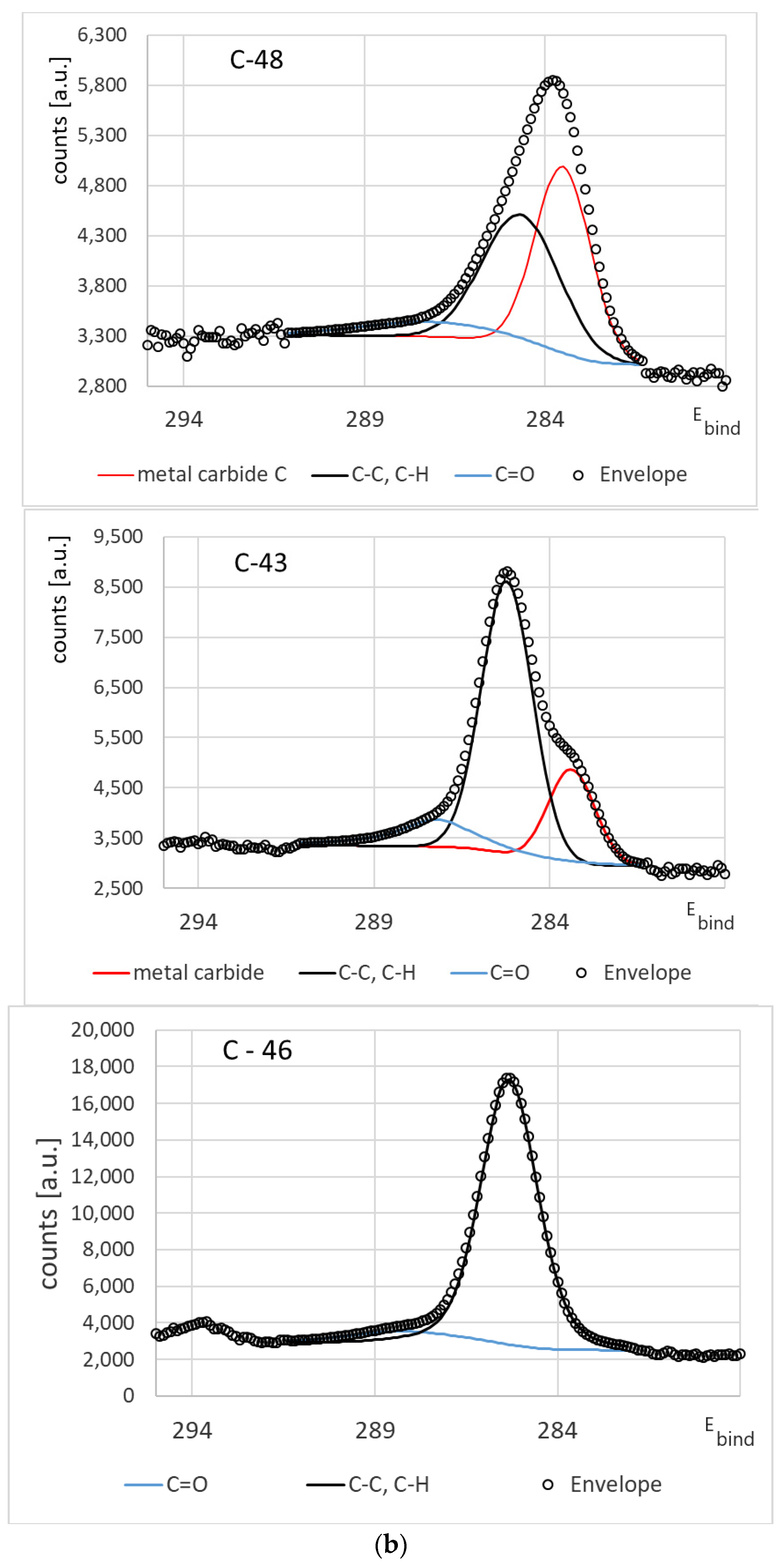
3.1. Chemical Composition
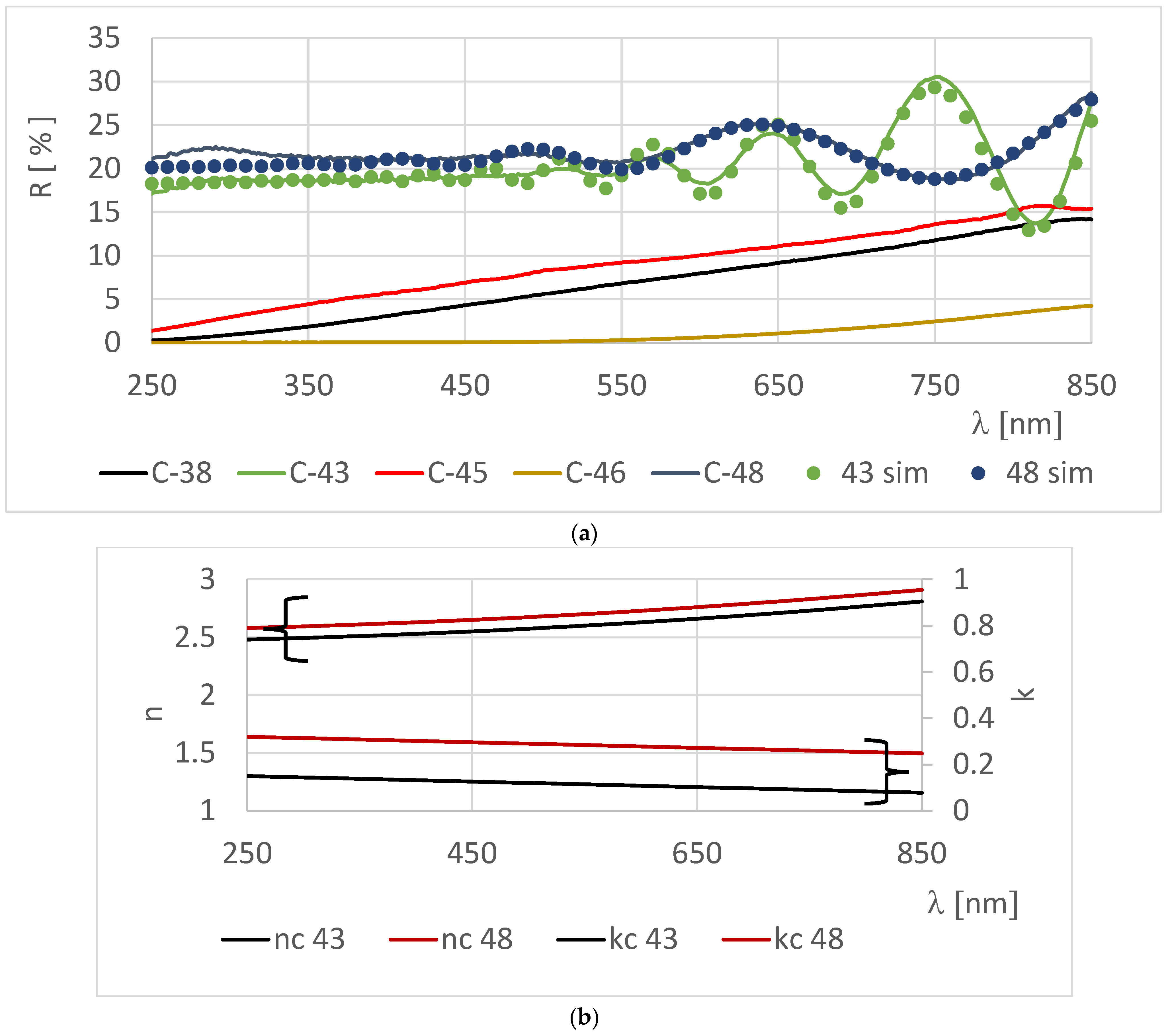
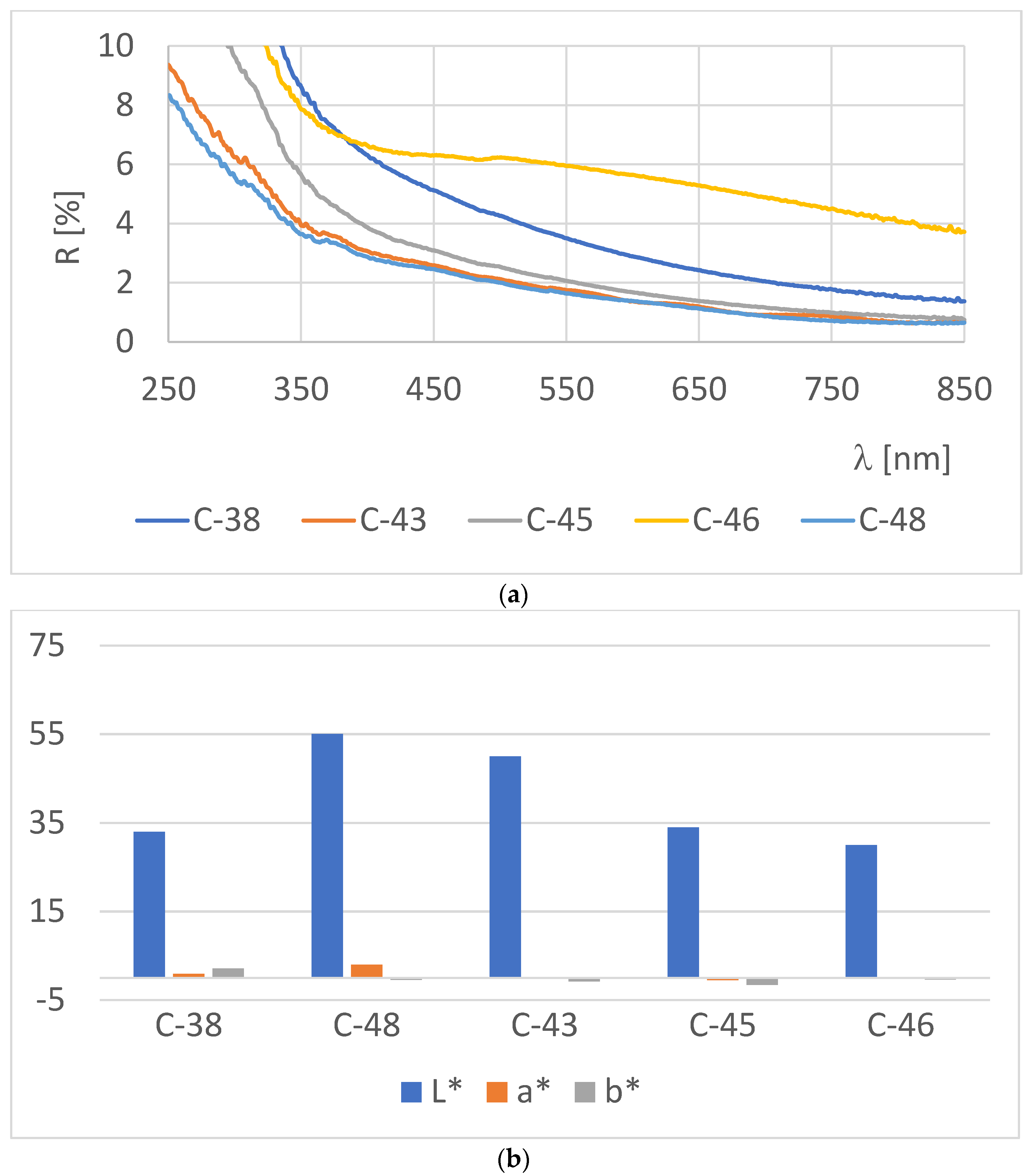
3.2. Optical Properties
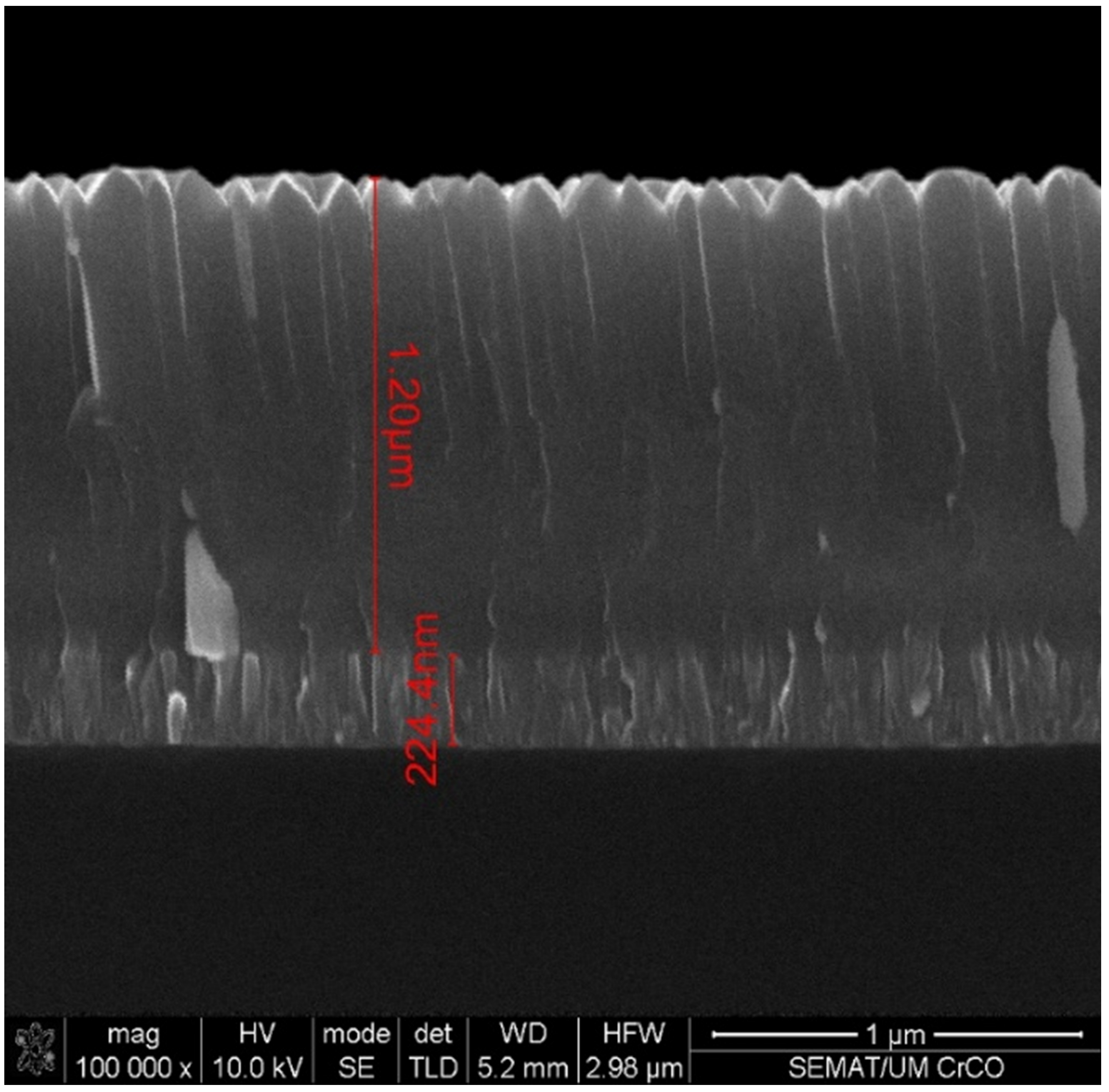
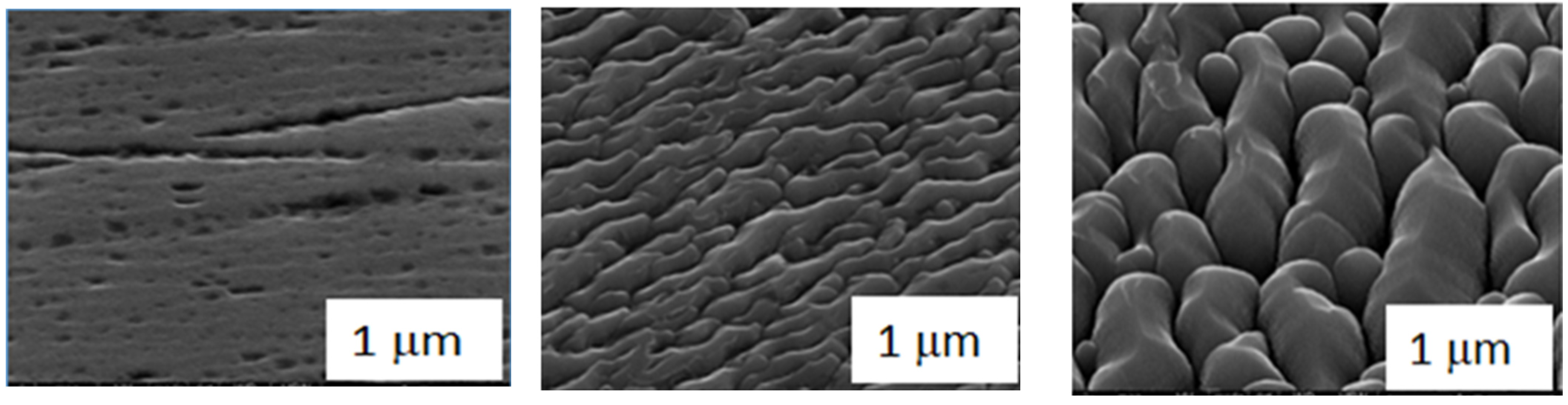
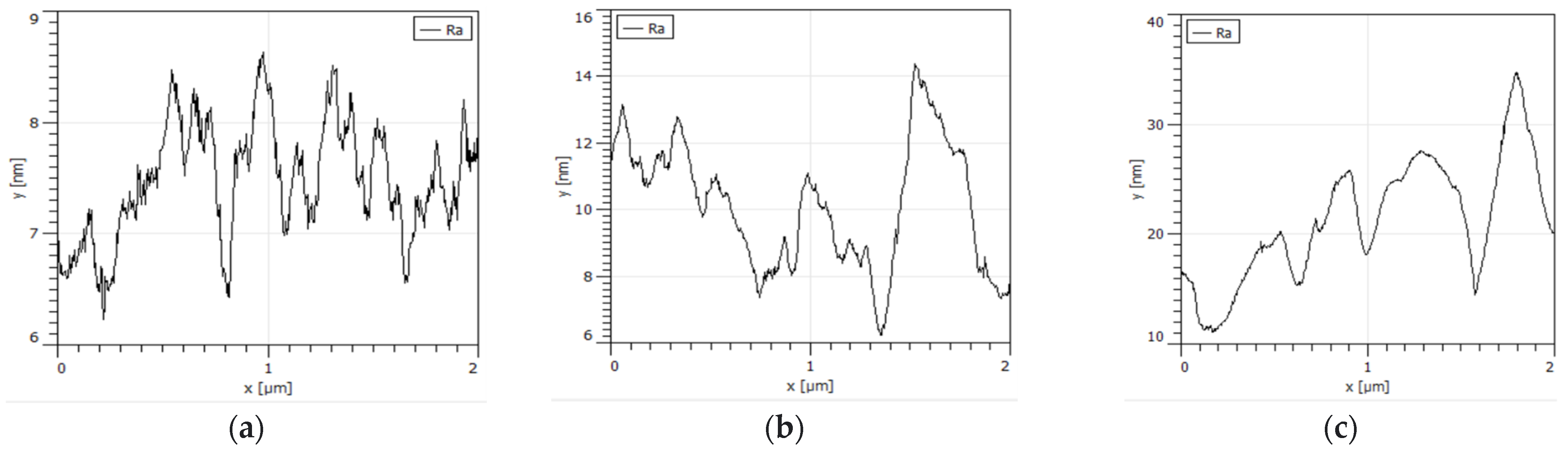
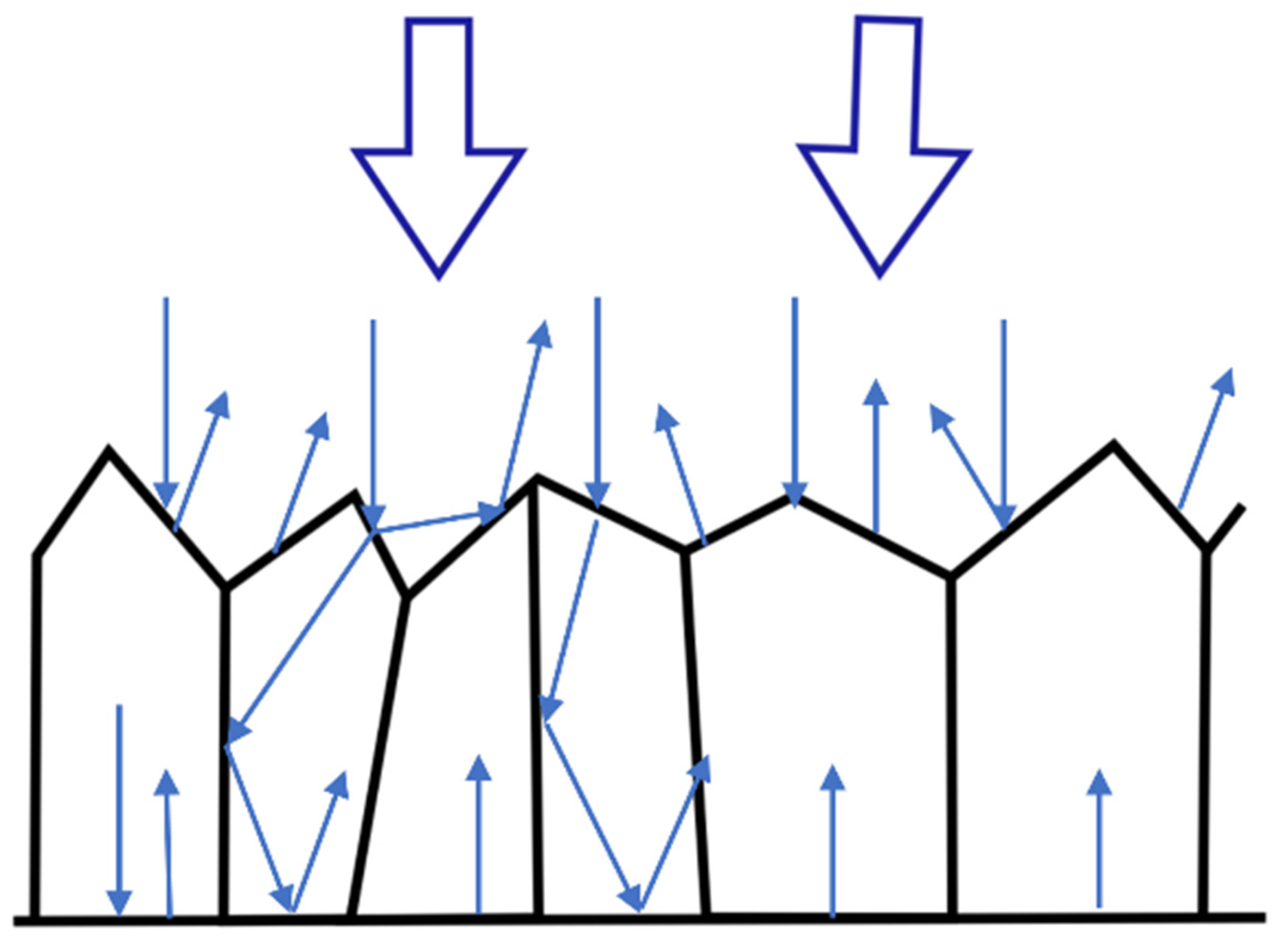
3.3. Microscopic Surface Analysis
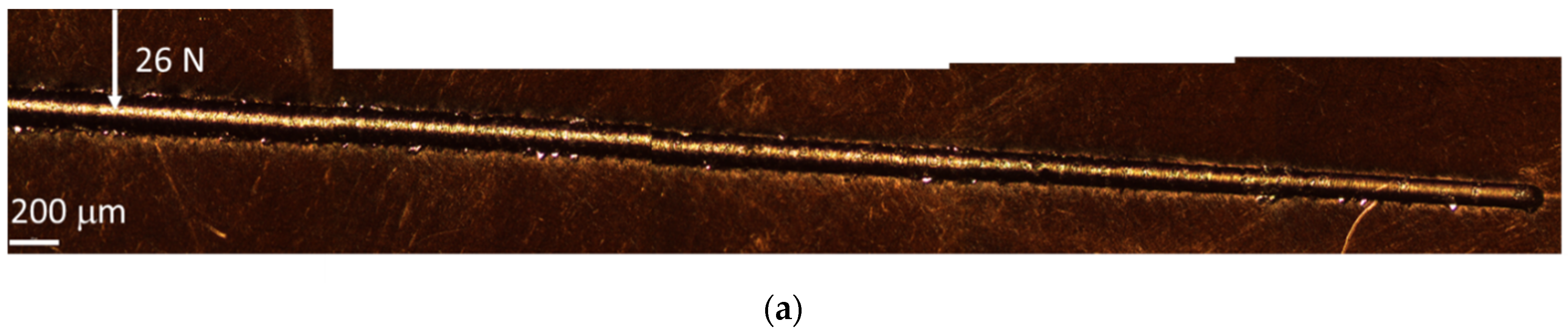
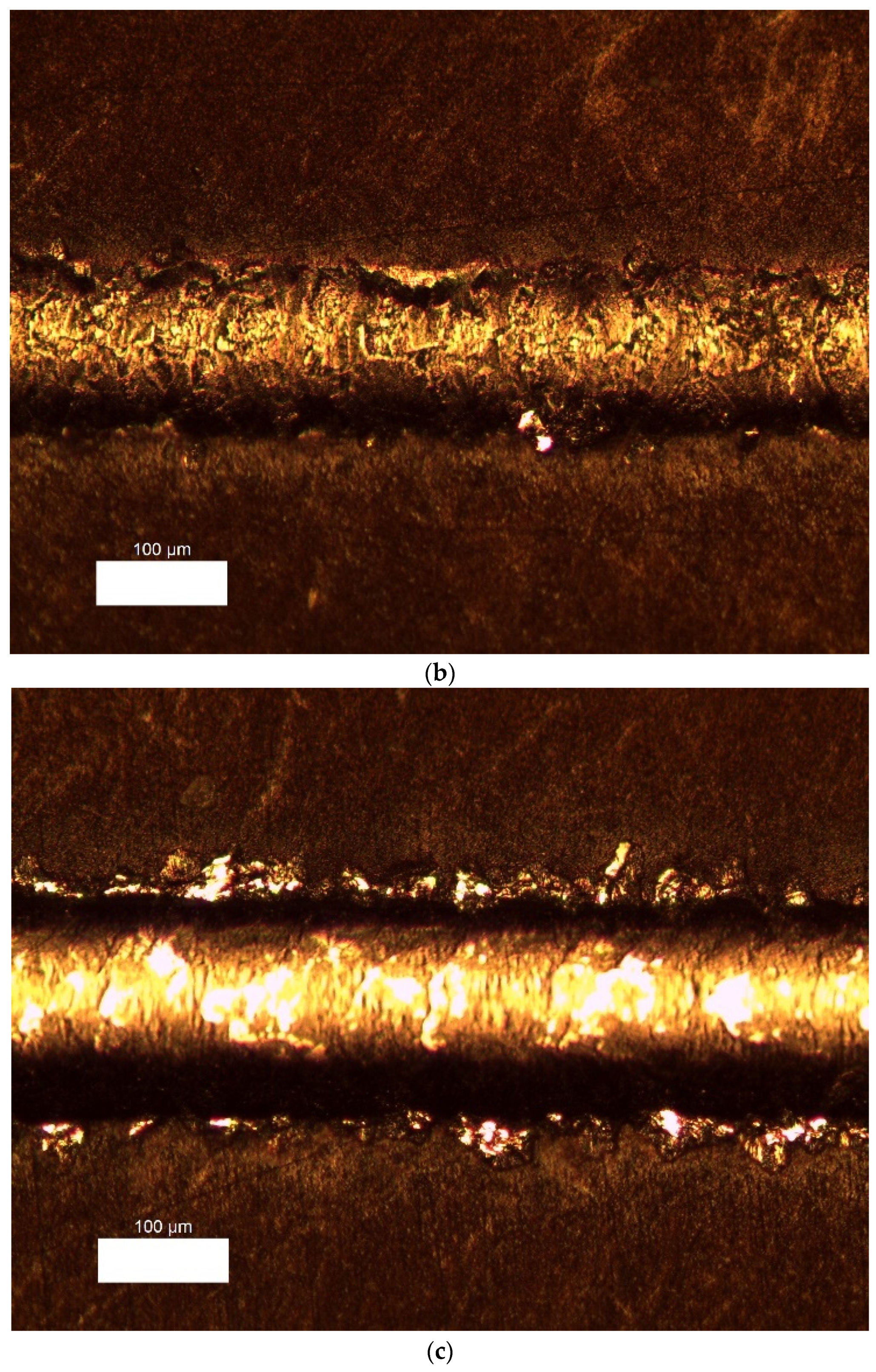
3.4. Mechanical Coating Properties
3.5. Surface Energy
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bewilogua, K.; Brauer, G.; Dietz, A.; Gabler, J.; Goch, G.; Karpuschewski, B.; Szyszka, B. Surface technology for automotive engineering. CIRP Ann. Manuf. Technol. 2009, 58, 608–627. [Google Scholar] [CrossRef]
- Carneiro, E.; Parreira, N.M.; Vuchkov, T.; Cavaleiro, A.; Ferreira, J.; Andritschky, M.; Carvalho, S. Cr-based sputtered decorative coatings for automotive industry. Materials 2021, 14, 5527. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, A.A.; Silva, F.J.G.; Pinto, A.G.; Sousa, V.F.C. Characterization of thin chromium coatings produced by PVD sputtering for optical applications. Coatings 2021, 11, 215. [Google Scholar] [CrossRef]
- Andritschky, M.; Atfeh, M.; Pischow, K. Multilayered decorative a-C:H/CrC coating on stainless steel. Surf. Coat. Technol. 2009, 203, 952–956. [Google Scholar] [CrossRef]
- Schossig, M.; Norkus, V.; Gerlach, G. Infrared Responsivity of Pyroelectric Detectors with Nanostructured NiCr Thin-Film Absorber. IEEE Sens. J. 2010, 10, 1564–1565. [Google Scholar] [CrossRef]
- Lee, J.; Kim, H.J.; Ko, Y.J.; Baek, J.Y.; Shin, G.; Jeon, J.G.; Lee, J.H.; Kim, J.H.; Jung, J.H.; Kang, T.J. Enhanced pyroelectric conversion of thermal radiation energy: Energy harvesting and non-contact proximity sensor. Nano Energy 2022, 97, 107178. [Google Scholar] [CrossRef]
- Pfund, A.H. The optical properties of metallic and crystalline powders. J. Opt. Soc. Am. 1933, 23, 375–378. [Google Scholar] [CrossRef]
- Nelms, N.; Dowson, J. Goldblack coating for thermal infrared detectors. Sens. Actuators A 2005, 120, 403–407. [Google Scholar] [CrossRef]
- Hao, Y.; Ye, Z.; Ye, M.; Dong, H.; Wang, L.; Du, Y. Construction and growth of black PEO coatings on aluminum alloys for enhanced wear and impact resistance. Ceram. Int. 2023, 49, 30782–30793. [Google Scholar] [CrossRef]
- Lin, Z.; Ma, C.; Ma, Z.; Gao, L.; Chen, W.; Chen, G. Laser etching ultra-black coating with novel anti-icing performance. Chem. Eng. J. 2023, 466, 143067. [Google Scholar] [CrossRef]
- Bello, M.; Shanmugan, S. Achievements in mid and high-temperature selective absorber coatings by physical vapor deposition (PVD) for solar thermal Application-A review Review. J. Alloys Compd. 2020, 839, 155510. [Google Scholar] [CrossRef]
- Rebouta, L.; Pitães, A.; Andritschky, M.; Capela, P.; Cerqueira, M.F.; Matilainen, A.; Pischow, K. Optical characterization of TiAlN/TiAlON/SiO2 absorber for solar selective applications Optical characterization of TiAlN/TiAlON/SiO2 absorber for solar selective applications. Surf. Coat. Technol. 2012, 211, 41–44. [Google Scholar] [CrossRef]
- Kam, D.; Wen, W.; Sadiq, T.O.; Idris, J. Deposition of black chromium coating for solar thermal application. J. Eng. Res. 2023, 11, 100023. [Google Scholar]
- Surviliene, S.; Cesuniene, A.; Juskenas, R.; Selskiene, A.; Bucinskiene, D.; Kalinauskas, P.; Juskevicius, K.; Jureviciute, I. The use of trivalent chromium bath to obtain a solar selective blackchromium coatings. Appl. Surf. Sci. 2014, 305, 492–497. [Google Scholar] [CrossRef]
- Saxena, V.; Rani, R.U.; Sharma, A.K. Studies on ultra high solar absorber black electroless nickel coatings on aluminum alloys for space application. Surf. Coat. Technol. 2006, 201, 855–862. [Google Scholar] [CrossRef]
- Ilic, B.; Czaplewski, D.; Neuzil, P.; Stancz, T.; Blough, J.; Maclay, G.J. Preparation and characterization of platinum black electrodes. J. Mater. Sci. 2000, 35, 3447–3457. [Google Scholar] [CrossRef]
- Pokorný, P.; Novotný, M.; More-Chevalier, J.; Dekhtyar, Y.; Romanova, M.; Davídkova, M.; Chertopalov, S.; Fitl, P.; Hruska, M.; Kawamura, M.; et al. Surface processes on thin layers of black aluminum in ultra-high vacuum. Vacuum 2022, 205, 111377. [Google Scholar] [CrossRef]
- Born, M.; Wolf, E. Principles of Optics; Pergamon: Oxford, UK, 1970. [Google Scholar]
- Wang, F.; Wu, J. Chapter 14—Applications in decorative films. In Modern Ion Plating Technology: Fundamentals and Applications; Elsevier: Amsterdam, The Netherlands, 2023; pp. 365–380. [Google Scholar] [CrossRef]
- Fernandes, A.C.; Cunha, L.; Moura, C.; Vaz, F.; Carvalho, P.; Le Bourhis, E.; Goudeau, P.; Rivière, J.P.; Parreira, N.M. The effect of bombarding conditions on the properties of multifunctional Ti-C-O thin films grown by magnetron sputtering. Surf. Coat. Technol. 2007, 202, 946–951. [Google Scholar] [CrossRef]
- Gupta, P.; Fang, F.; Rubanov, S.; Loho, T.; Koo, A.; Swift, N.; Fiedler, H.; Murmu, J.L.P.P.; Markwitz, A.; Kennedy, J. Decorative black coatings on titanium surfaces based on hard bi-layered carbon coatings synthesized by carbon implantation. Surf. Coat. Technol. 2019, 358, 386–393. [Google Scholar] [CrossRef]
- Xiao, W.; Jiang, X. Optical and mechanical properties of nanocrystalline aluminum oxynitride films prepared by electron cyclotron resonance plasma enhanced chemical vapor deposition. J. Cryst. Growth 2004, 264, 165–171. [Google Scholar] [CrossRef]
- Cerny, F.; Jech, V.; Stepanek, I.; Mackova, A.; Konvickova, S. Decorative a-C:H coatings. Appl. Surf. Sci. 2009, 256, S77–S81. [Google Scholar] [CrossRef]
- Chen, B.; Yang, D.; Charpentier, P.A.; Nikum, S. Optical and structural properties of pulsed laser deposited Ti:Al2O3 thin films. Sol. Energy Mater. Sol. Cells 2008, 92, 1025–1029. [Google Scholar] [CrossRef]
- Harish, C.; Barshilia, N.; Selvakumar, K.; Rajam, S.; Biswas, A. Structure and optical properties of pulsed sputter deposited CrxOy/Cr/Cr2O3 solar selective coatings. J. Appl. Phys. 2008, 103, 023507. [Google Scholar] [CrossRef]
- He, J.; Lan, X.; Liu, Z.; Jiao, D.; Zhong, X.; Cheng, Y.; Tang, C.; Qiu, W. Modification of Cr/CrN composite structure by Fe addition and its effect on decorative performance and corrosion resistance. Ceram. Int. 2021, 47, 23888–23894. [Google Scholar] [CrossRef]
- Cooke, K.E.; Bamber, M.; Bassas, J.; Boscarino, D.; Derby, B.; Figueras, A.; Inkson, B.J.; Rigato, V.; Steer, T.; Tee, D.G. Multilayer nitride coatings by closed field unbalanced magnetron sputter ion plating. Surf. Coat. Technol. 2003, 162, 276–287. [Google Scholar] [CrossRef]
- Numata, Y.; Nair, S.V.; Nakagawa, K.; Ishino, H.; Kobayashi, T.; Tokunaga, E. Optical size effect of organic nanocrystals studied by absorption spectroscopy within an integrating sphere. Chem. Phys. Lett. 2014, 601, 128–133. [Google Scholar] [CrossRef]
- Wang, Q.; Lai, F.; Shi, W.; Li, X.; Chen, R.; Liu, H.; Zhang, X.; Chang, Q.; Wang, Y. Synthesis and color properties of MnTiO3 black ceramic pigment. Mater. Chem. Phys. 2023, 296, 127310. [Google Scholar] [CrossRef]
- Carvalho, S.; Parreira, N.M.G.; Silva, M.Z.; Cavaleiro, A.; Rebouta, L. In-service behaviour of (Ti,Si,Al)Nx nanocomposite films. Wear 2012, 274–275, 68–74. [Google Scholar] [CrossRef]
- Tiss, B.; Bouguila, N.; Kraini, M.; Khirouni, K.; Vazquez–Vazquez, C.; Cunha, L.; Moura, C.; Alaya, S. Electrical transport of sprayed In2S3:Ag thin films. Mater. Sci. Semicond. Process. 2020, 114, 10508. [Google Scholar] [CrossRef]
- ISO 19403-2:2017; Paints and Varnishes—Wettability—Part 2: Determination of the Surface Free Energy of Solid Surfaces by Measuring the Contact Angle. ISO: Geneva, Switzerland, 2017.
- Johnson, B.; Christy, R.W. Optical constants of transition metals: Ti, V, Cr, Mn, Fe, Co, Ni, and PdP. Phys. Rev. B 1974, 9, 5056. [Google Scholar] [CrossRef]
- Abdullah, M.M.; Rajab, F.M.; Al-Abbas, S.M. Structural and optical characterizationof Cr2O3 nanostructures: Evaluation of its dielectric properties. AIP Adv. 2014, 4, 027121. [Google Scholar] [CrossRef]
- Fuentes, G.G.; Rodriguez, R.; Avelar-Batista, J.C.; Housden, J.; Montalá, F.; Carreras, L.J.; Cristóbal, A.B.; Damborenea, J.J.; Tate, T.J. Recent advances in the chromium nitride PVD process for forming and machining surface protection. J. Mater. Process. Technol. 2005, 167, 415–421. [Google Scholar] [CrossRef]
- Douard, A.; Maury, F. Chromium-based coatings by atmospheric chemical vapor deposition at low temperature from Cr(CO)6. Surf. Coat. Technol. 2005, 200, 1407–1412. [Google Scholar] [CrossRef][Green Version]
- Gomez, O.; Perales, E.; Chorro, E.; Burgos, F.J.; Viqueira, V.; Vilaseca, M.; Martınez-Verdu, F.M.; Pujol, J. Visual and instrumental assessments of color differences in automotive coatings. Color Res. Appl. 2016, 41, 384–391. [Google Scholar] [CrossRef]
- Chappé, J.M.; Vaz, F.; Cunha, L.; Moura, C.; de Lucas, M.M.; Imhoff, L.; Bourgeois, S.; Pierson, J.F. Development of dark Ti(C,O,N) coatings prepared by reactive sputtering. Surf. Coat. Technol. 2008, 203, 804–807. [Google Scholar] [CrossRef]
- Ono, K.; Wakabayashi, M.; Tsukakoshi, Y.; Abe, Y. Decorative black TiCxOy film fabricated by DC magnetron sputtering without importing oxygen reactive gas. Appl. Surf. Sci. 2016, 364, 69–74. [Google Scholar] [CrossRef]
- Nedfors, N.; Tengstrand, O.; Lewin, E.; Furlan, A.; Eklund, P.; Hultman, L.; Jansson, U. Structural, mechanical and electrical-contact properties of nanocrystalline-NbC/amorphous-C coatings deposited by magnetron sputtering. Surf. Coat. Technol. 2011, 206, 354–359. [Google Scholar] [CrossRef]
- Andersson, M.; Högström, J.; Urbonaite, S.; Furlan, A.; Nyholm, L.; Jansson, U. Deposition and characterization of magnetron sputtered amorphous Cr-C films. Vacuum 2012, 86, 148–1416. [Google Scholar] [CrossRef]
| Samples | Base Pressure [mbar] | Power Cr [W] | Power C [W] | Total Pressure [mbar] | Flow Ar [sccm] | Flow O2 [sccm] | Bias [A] | Bias [V] | Deposition Time [min] |
|---|---|---|---|---|---|---|---|---|---|
| C-38 | 1.2 × 10−6 | 600 | 1000 | 1.0 × 10−3 | 21.2 | 8 | 0.51 | −65 | 450 |
| C-43 | 1.2 × 10−6 | 600 | 1000 | 1.0 × 10−3 | 21.2 | 8 | 0.53 | −85 | 240 |
| C-45 | 4.0 × 10−6 | 600 | 1000 | 1.0 × 10−3 | 21.2 | 8 | 0.55 | −85 | 450 |
| C-46 | 4.4 × 10−6 | 600 | 1000 | 1.0 × 10−3 | 21.2 | 8 | 0.54 | −85 | 840 |
| C-48 | 1.2 × 10−6 | 600 | 1000 | 1.0 × 10−3 | 21.2 | 8 | 0.54 | −85 | 90 |
| Sample | EDX Composition [at %] | Thickness [μm] | XPS Composition [at %] | Critical Load LC2 [N] | Surface Energy σ [mN/m] | ||||
|---|---|---|---|---|---|---|---|---|---|
| Cr | O | C | Cr | O | C | ||||
| C-38 | 35.6 | 51.9 | 12.5 | 2.4 | 8 | 26.6 | |||
| C-43 | 35.2 | 53.4 | 11.4 | 1.2 | 28.2 | 52.0 | 19.8 | 12 | 30.8 |
| C-45 | 33.4 | 52.1 | 14.5 | 2.4 | 12 | 30.7 | |||
| C-46 | 36.0 | 52.4 | 11.6 | 4.8 | 15.4 | 37.8 | 46.8 | 44 | 26.3 |
| C-48 | - | - | - | 0.45 | 36.0 | 54.7 | 9.3 | - | 27.0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Arrousse, N.; Ferreira, J.; Carvalho, S.; Andritschky, M. PVD Black Coating for Decorative Applications. Coatings 2023, 13, 1838. https://doi.org/10.3390/coatings13111838
Arrousse N, Ferreira J, Carvalho S, Andritschky M. PVD Black Coating for Decorative Applications. Coatings. 2023; 13(11):1838. https://doi.org/10.3390/coatings13111838
Chicago/Turabian StyleArrousse, Nadia, Jorge Ferreira, Sandra Carvalho, and Martin Andritschky. 2023. "PVD Black Coating for Decorative Applications" Coatings 13, no. 11: 1838. https://doi.org/10.3390/coatings13111838
APA StyleArrousse, N., Ferreira, J., Carvalho, S., & Andritschky, M. (2023). PVD Black Coating for Decorative Applications. Coatings, 13(11), 1838. https://doi.org/10.3390/coatings13111838








