Influence of AC-DC-AC Cycling with Hydrostatic Pressure on Accelerated Protective Performance Test of Glass Flake Epoxy Coating
Abstract
:1. Introduction
2. Experimental
2.1. Specimen Preparation
2.2. Immersion Tests
2.3. EIS Measurement
2.4. AC-DC-AC Tests at Atmospheric and Hydrostatic Pressures
2.5. Surface Characterization
3. Results and Discussion
3.1. Immersion Test under Atmospheric Pressure
3.2. AC-DC-AC Test under Atmospheric Pressure
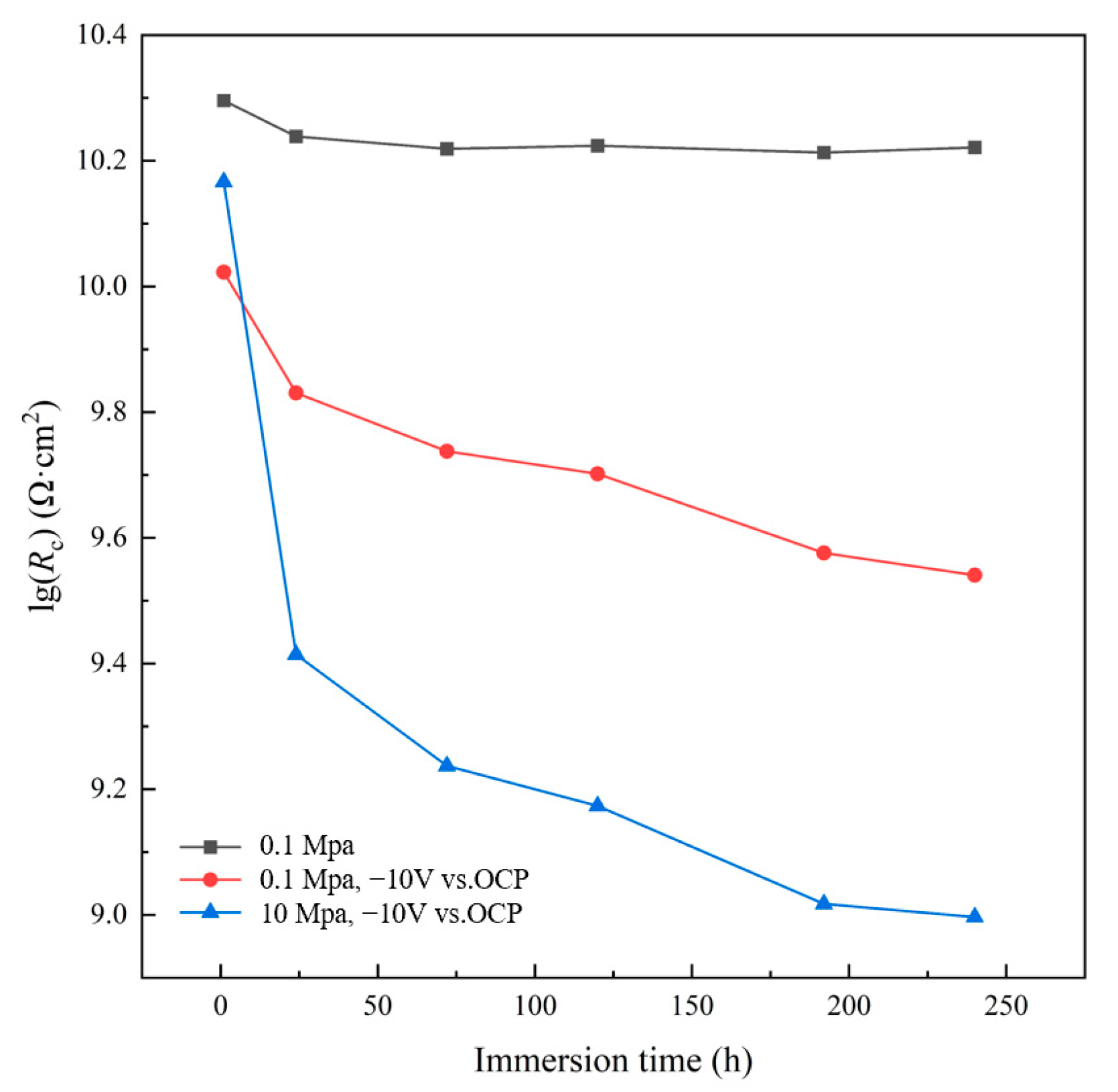
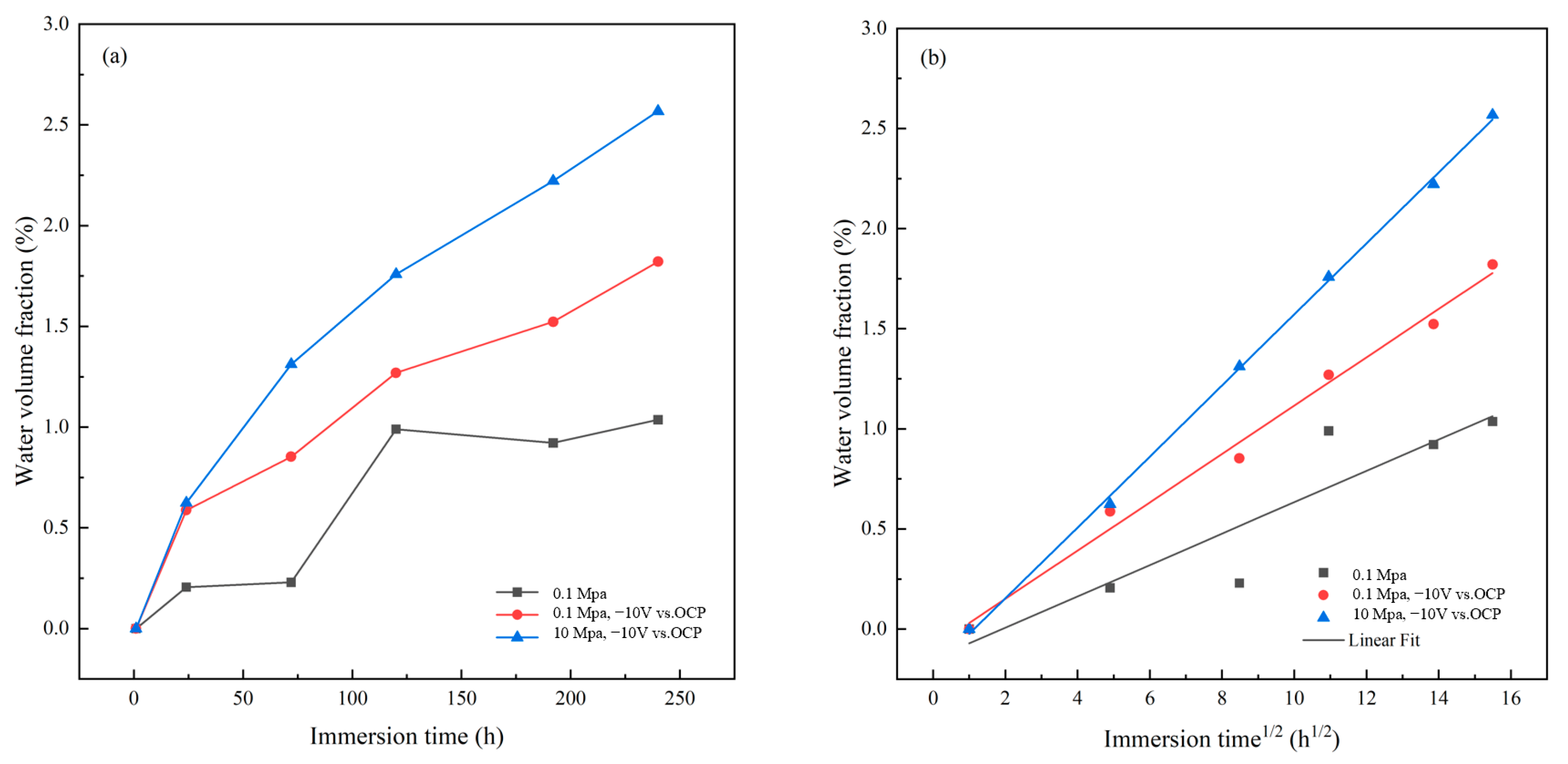
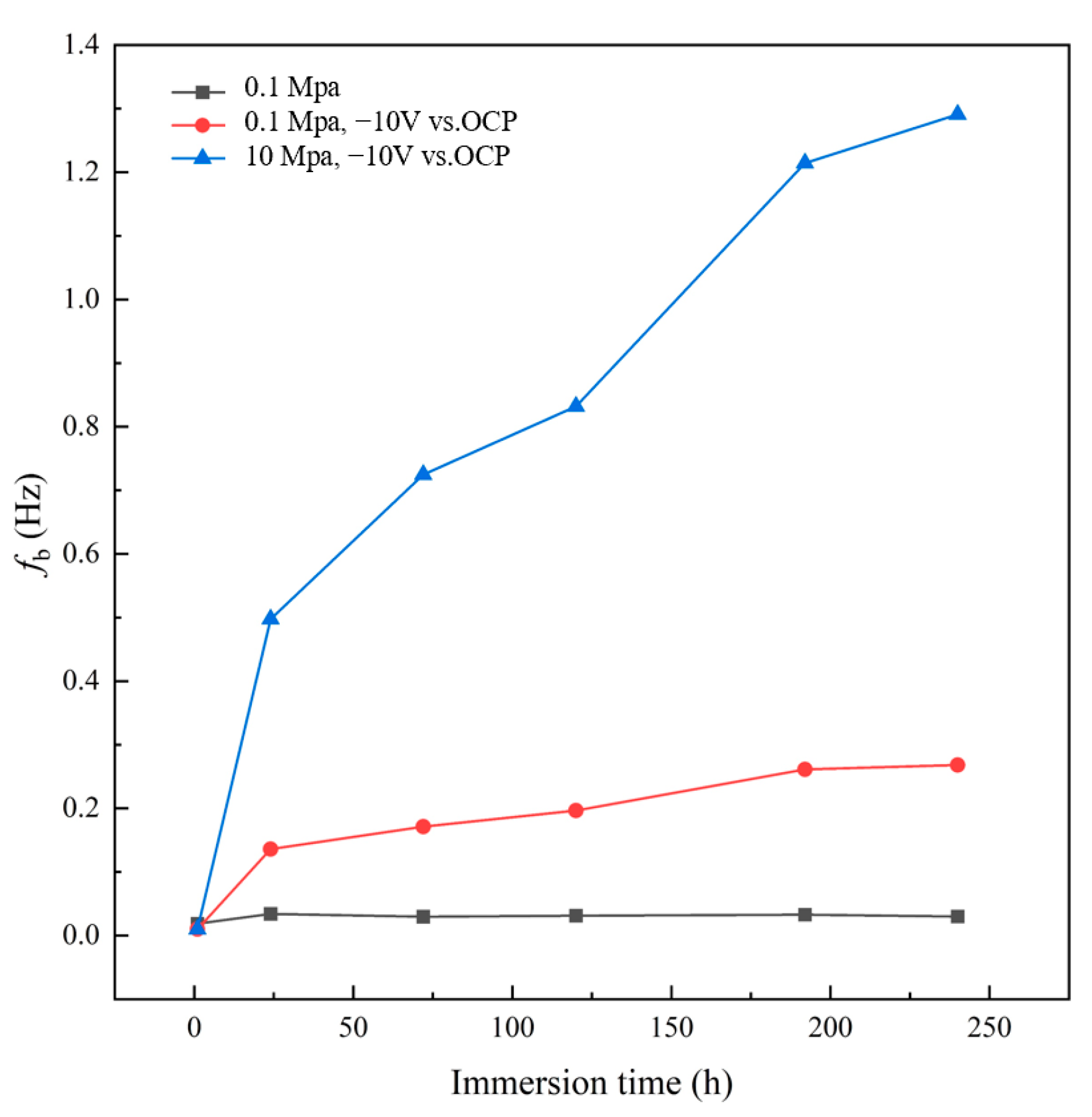
3.3. AC-DC-AC Test under Hydrostatic Pressure
4. Conclusions
- (1)
- The immersion test of the coating in seawater at atmospheric pressure shows that the EIS spectrum of the coating retains the feature of one time constant after immersion for 145 d. The impedance analysis indicates that the coating is still in good condition with high protective performance.
- (2)
- The AC-DC-AC test at atmospheric pressure, and the coupled test of AC-DC-AC with high hydrostatic pressure of 10 MPa show that the periodic cathodic polarization at −10 V vs. Ag/AgCl/seawater and high hydrostatic pressure can promote the degradation of the coating significantly. The coupled test has the greatest acceleration due to the synergetic effect. A faster testing method to evaluate the barrier properties of organic coatings can be provided by applying high hydrostatic pressure during the AC-DC-AC test. The results are helpful for the development of a new, modified accelerated testing method for marine coatings.
- (3)
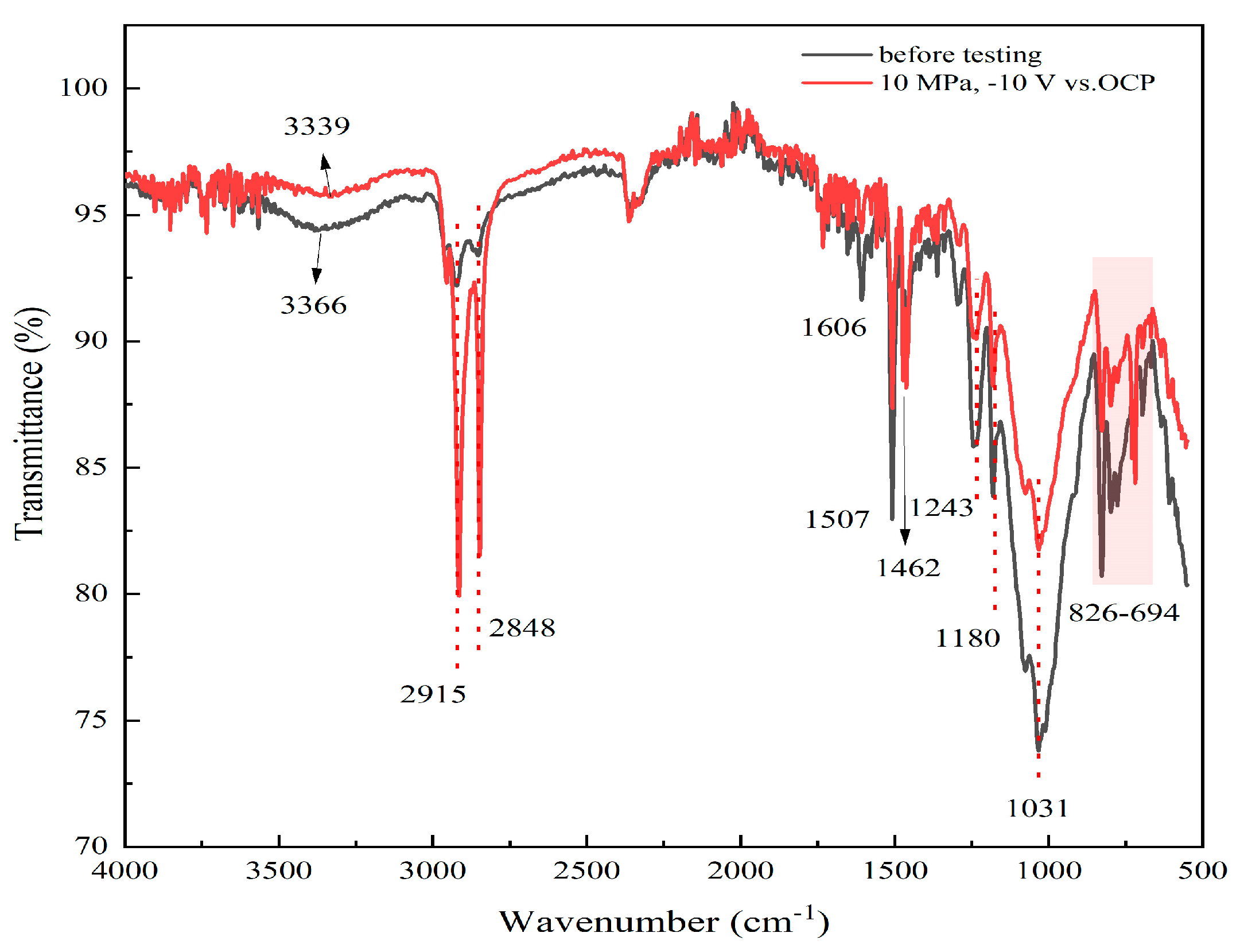
- The high hydrostatic pressure and periodic cathodic polarization are effective accelerators which facilitate the diffusion and uptake of water and other corrosive species in the micropores of the coating. This increases the delaminated area rate at the interface between coating and substrate, leading to relatively rapid degradation of the coating. The physical microstructure of the coating is changed under the stresses provided by cathodic polarization and hydrostatic pressure, while the chemical structure of the coating is not affected.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Xu, L.; Xin, Y.; Ma, L.; Zhang, H.; Lin, Z.; Li, X. Challenges and Solutions of Cathodic Protection for Marine Ships. Corros. Commun. 2021, 2, 33–40. [Google Scholar] [CrossRef]
- Woloszyk, K.; Garbatov, Y.; Kowalski, J. Experimental Ultimate Strength Assessment of Stiffened Plates Subjected to Marine Immersed Corrosion. Appl. Ocean Res. 2023, 138, 103679. [Google Scholar] [CrossRef]
- Bhandari, J.; Khan, F.; Abbassi, R.; Garaniya, V.; Ojeda, R. Modelling of Pitting Corrosion in Marine and Offshore Steel Structures—A Technical Review. J. Loss Prev. Process Ind. 2015, 37, 39–62. [Google Scholar] [CrossRef]
- Olajire, A.A. Recent Advances on Organic Coating System Technologies for Corrosion Protection of Offshore Metallic Structures. J. Mol. Liq. 2018, 269, 572–606. [Google Scholar] [CrossRef]
- Lyon, S.B.; Bingham, R.; Mills, D.J. Advances in Corrosion Protection by Organic Coatings: What We Know and What We Would like to Know. Prog. Org. Coat. 2017, 102, 2–7. [Google Scholar] [CrossRef]
- Buchheit, R.G. Chapter 18—Corrosion Resistant Coatings and Paints. In Handbook of Environmental Degradation of Materials; Kutz, M., Ed.; William Andrew Publishing: Norwich, NY, USA, 2005; pp. 367–385. ISBN 978-0-8155-1500-5. [Google Scholar]
- Zhang, F.; Ju, P.; Pan, M.; Zhang, D.; Huang, Y.; Li, G.; Li, X. Self-Healing Mechanisms in Smart Protective Coatings: A Review. Corros. Sci. 2018, 144, 74–88. [Google Scholar] [CrossRef]
- MSC.215(82); Performance Standard for Protective Coatings for Dedicated Seawater Ballast Tanks in All Types of Ships and Double-Side Skin Spaces of Bulk Carriers. International Maritime Organization: London, UK, 2006.
- Wei, C.; Wang, G.; Cridland, M.; Olson, D.L.; Liu, S. Chapter 25—Corrosion Protection of Ships. In Handbook of Environmental Degradation of Materials, 3rd ed.; Kutz, M., Ed.; William Andrew Publishing: Norwich, NY, USA, 2018; pp. 533–557. ISBN 978-0-323-52472-8. [Google Scholar]
- Iannarelli, P.; Beaumont, D.; Liu, Y.; Zhou, X.; Burnett, T.L.; Curioni, M.; Lyon, S.B.; Gibbon, S.R.; Morsh, S.; Emad, S.; et al. The Degradation Mechanism of a Marine Coating under Service Conditions of Water Ballast Tank. Prog. Org. Coat. 2022, 162, 106588. [Google Scholar] [CrossRef]
- Oriaifo, E.; Perera, N.; Guy, A.; Leung, P.S.; Tan, K. A Review of Test Protocols for Assessing Coating Performance of Water Ballast Tank Coatings; World Academy of Science, Engineering and Technology: Istanbul, Turkey, 2014; pp. 1610–1615. [Google Scholar]
- ISO 20340:2009; Paints and Varnishes—Performance Requirements for Protective Paint Systems for Offshore and Related Structures. ISO: Geneva, Switzerland, 2009.
- ISO 12944-9:2018; Paints and Varnishes—Corrosion Protection of Steel Structures by Protective Paint Systems—Part 9: Protective Paint Systems and Laboratory Performance Test Methods for Offshore and Related Structures. ISO: Geneva, Switzerland, 2018.
- Lajevardi Esfahani, S.; Ranjbar, Z.; Rastegar, S. Evaluation of Anticorrosion Behavior of Automotive Electrocoating Primers by the AC-DC-AC Accelerated Test Method. Prog. Color Color. Coat. 2013, 7, 187–199. [Google Scholar] [CrossRef]
- de Vooys, A.C.A.; Boelen, B.; van der Weijde, D.H. Screening of Coated Metal Packaging Cans Using EIS. Prog. Org. Coat. 2012, 73, 202–210. [Google Scholar] [CrossRef]
- Sørensen, P.A.; Kiil, S.; Dam-Johansen, K.; Weinell, C.E. Anticorrosive Coatings: A Review. J. Coat. Technol. Res. 2009, 6, 135–176. [Google Scholar] [CrossRef]
- Kotnarowska, D. Influence of Ultraviolet Radiation and Aggressive Media on Epoxy Coating Degradation. Prog. Org. Coat. 1999, 37, 149–159. [Google Scholar] [CrossRef]
- García, S.J.; Suay, J. Optimization of Deposition Voltage of Cataphoretic Automotive Primers Assessed by EIS and AC/DC/AC. Prog. Org. Coat. 2009, 66, 306–313. [Google Scholar] [CrossRef]
- Deflorian, F.; Rossi, S.; Fedel, M. Organic Coatings Degradation: Comparison between Natural and Artificial Weathering. Corros. Sci. 2008, 50, 2360–2366. [Google Scholar] [CrossRef]
- Yu, M.; Fan, C.; Ge, F.; Lu, Q.; Wang, X.; Cui, Z. Anticorrosion Behavior of Organic Offshore Coating Systems in UV, Salt Spray and Low Temperature Alternation Simulated Arctic Offshore Environment. Prog. Org. Coat. 2021, 28, 102545. [Google Scholar] [CrossRef]
- ISO 17463:2022; Paints and Varnishes—Guidelines for the Determination of Anticorrosive Properties of Organic Coatings by Accelerated Cyclic Electrochemical Technique. ISO: Geneva, Switzerland, 2022.
- Hollaender, J. Rapid Assessment of Food/Package Interactions by Electrochemical Impedance Spectroscopy (EIS). Food Addit. Contam. 1997, 14, 617–626. [Google Scholar] [CrossRef]
- Bierwagen, G.; Tallman, D.; Li, J.; He, L.; Jeffcoate, C. EIS Studies of Coated Metals in Accelerated Exposure. Prog. Org. Coat. 2003, 46, 149–158. [Google Scholar] [CrossRef]
- Liu, X.; Xiong, J.; Lv, Y.; Zuo, Y. Study on Corrosion Electrochemical Behavior of Several Different Coating Systems by EIS. Prog. Org. Coat. 2009, 64, 497–503. [Google Scholar] [CrossRef]
- García, S.J.; Suay, J. A Comparative Study between the Results of Different Electrochemical Techniques (EIS and AC/DC/AC). Prog. Org. Coat. 2007, 59, 251–258. [Google Scholar] [CrossRef]
- Usman, B.J.; Scenini, F.; Curioni, M. Exploring the Use of an AC-DC-AC Technique for the Accelerated Evaluation of Anticorrosion Performance of Anodic Films on Aluminium Alloys. Prog. Org. Coat. 2020, 144, 105648. [Google Scholar] [CrossRef]
- Gimeno, M.J.; Chamorro, S.; March, R.; Oró, E.; Pérez, P.; Gracenea, J.; Suay, J. Anticorrosive Properties Enhancement by Means of Phosphate Pigments in an Epoxy 2k Coating. Assessment by NSS and ACET. Prog. Org. Coat. 2014, 77, 2024–2030. [Google Scholar] [CrossRef]
- Puig, M.; Gimeno, M.J.; Gracenea, J.J.; Suay, J.J. Anticorrosive Properties Enhancement in Powder Coating Duplex Systems by Means of ZMP Anticorrosive Pigment. Assessment by Electrochemical Techniques. Prog. Org. Coat. 2014, 77, 1993–1999. [Google Scholar] [CrossRef]
- Abdolah Zadeh, M.; van der Zwaag, S.; García, S.J. Assessment of Healed Scratches in Intrinsic Healing Coatings by AC/DC/AC Accelerated Electrochemical Procedure. Surf. Coat. Technol. 2016, 303, 396–405. [Google Scholar] [CrossRef]
- Gimeno, M.J.; Puig, M.; Chamorro, S.; Molina, J.; March, R.; Oró, E.; Pérez, P.; Gracenea, J.J.; Suay, J.J. Improvement of the Anticorrosive Properties of an Alkyd Coating with Zinc Phosphate Pigments Assessed by NSS and ACET. Prog. Org. Coat. 2016, 95, 46–53. [Google Scholar] [CrossRef]
- Bethencourt, M.; Botana, F.J.; Cano, M.J.; Osuna, R.M.; Marcos, M. Lifetime Prediction of Waterborne Acrylic Paints with the AC–DC–AC Method. Prog. Org. Coat. 2004, 49, 275–281. [Google Scholar] [CrossRef]
- da Silva Lopes, T.; Lopes, T.; Martins, D.; Carneiro, C.; Machado, J.; Mendes, A. Accelerated Aging of Anticorrosive Coatings: Two-Stage Approach to the AC/DC/AC Electrochemical Method. Prog. Org. Coat. 2020, 138, 105365. [Google Scholar] [CrossRef]
- Zheng, D.; Gui, Q.; Xu, Y.; Song, G.-L. Modified AC-DC-AC Method for Evaluation of Corrosion Damage of Acrylic Varnish Paint Coating/Q215 Steel System. Prog. Org. Coat. 2021, 159, 106401. [Google Scholar] [CrossRef]
- Xu, Y.; Song, G.-L.; Zheng, D.; Feng, Z. The Corrosion Damage of an Organic Coating Accelerated by Different AC-DC-AC Tests. Eng. Fail. Anal. 2021, 126, 105461. [Google Scholar] [CrossRef]
- Esfahani, S.L.; Ranjbar, Z.; Rastegar, S. An Electrochemical and Mechanical Approach to the Corrosion Resistance of Cathodic Electrocoatings under Combined Cyclic and DC Polarization Conditions. Prog. Org. Coat. 2014, 77, 1264–1270. [Google Scholar] [CrossRef]
- Allahar, K.N.; Bierwagen, G.P.; Gelling, V.J. Understanding Ac–Dc–Ac Accelerated Test Results. Corros. Sci. 2010, 52, 1106–1114. [Google Scholar] [CrossRef]
- Kan, B.; Wu, W.; Yang, Z.; Zhang, X.; Li, J. Effects of Hydrostatic Pressure and pH on the Corrosion Behavior of 2205 Duplex Stainless Steel. J. Electroanal. Chem. 2021, 886, 115134. [Google Scholar] [CrossRef]
- Yang, Z.X.; Kan, B.; Li, J.X.; Su, Y.J.; Qiao, L.J. Hydrostatic Pressure Effects on Stress Corrosion Cracking of X70 Pipeline Steel in a Simulated Deep-Sea Environment. Int. J. Hydrogen Energy 2017, 42, 27446–27457. [Google Scholar] [CrossRef]
- Liu, R.; Cui, Y.; Liu, L.; Zhang, B.; Wang, F. A Primary Study of the Effect of Hydrostatic Pressure on Stress Corrosion Cracking of Ti-6Al-4V Alloy in 3.5% NaCl Solution. Corros. Sci. 2020, 165, 108402. [Google Scholar] [CrossRef]
- Liu, R.; Liu, L.; Wang, F. The Role of Hydrostatic Pressure on the Metal Corrosion in Simulated Deep-Sea Environments—A Review. J. Mater. Sci. Technol. 2022, 112, 230–238. [Google Scholar] [CrossRef]
- Meng, F.; Liu, L.; Liu, E.; Zheng, H.; Liu, R.; Cui, Y.; Wang, F. Synergistic Effects of Fluid Flow and Hydrostatic Pressure on the Degradation of Epoxy Coating in the Simulated Deep-Sea Environment. Prog. Org. Coat. 2021, 159, 106449. [Google Scholar] [CrossRef]
- Peng, W.; Duan, T.; Hou, J.; Guo, X.; Zhang, Y.; Ma, L.; Yu, M.; Xin, Y.; Xing, S.; Zhang, H. Electrochemical Corrosion Behavior of High Strength Steel in Simulated Deep-Sea Environment under Different Hydrostatic Pressure. J. Mater. Res. Technol. 2023, 23, 2301–2316. [Google Scholar] [CrossRef]
- Meng, F.; Zhang, T.; Liu, L.; Cui, Y.; Wang, F. Failure Behaviour of an Epoxy Coating with Polyaniline Modified Graphene Oxide under Marine Alternating Hydrostatic Pressure. Surf. Coat. Technol. 2019, 361, 188–195. [Google Scholar] [CrossRef]
- Shao, Z.; Ren, P.; Jia, T.; Lei, B.; Feng, Z.; Guo, H.; Chen, S.; Zhang, P.; Meng, G. High-Pressure Induced Acceleration Pathways for Water Diffusion in Heavy Duty Anticorrosion Coatings under Deep Ocean Environment: (I) The Samples Subjected to High-Pressure Pre-Processing. Prog. Org. Coat. 2022, 170, 106948. [Google Scholar] [CrossRef]
- Liu, Y.; Wang, J.; Liu, L.; Li, Y.; Wang, F. Study of the Failure Mechanism of an Epoxy Coating System under High Hydrostatic Pressure. Corros. Sci. 2013, 74, 59–70. [Google Scholar] [CrossRef]
- Dong, J.-J.; Fan, L.; Zhang, H.-B.; Xu, L.-K.; Xue, L.-L. Electrochemical Performance of Passive Film Formed on Ti–Al–Nb–Zr Alloy in Simulated Deep Sea Environments. Acta Metall. Sin. Engl. Lett. 2020, 33, 595–604. [Google Scholar] [CrossRef]
- ISO-5668:2023; Corrosion of Metals and Alloys Guidelines and Requirements for Corrosion Testing in Simulated Environment of Deep-Sea Water. ISO: Geneva, Switzerland, 2023.
- Corfias, C.; Pébère, N.; Lacabanne, C. Characterization of Protective Coatings by Electrochemical Impedance Spectroscopy and a Thermostimulated Current Method: Influence of the Polymer Binder. Corros. Sci. 2000, 42, 1337–1350. [Google Scholar] [CrossRef]
- Liu, J.; Lu, Z.; Zhang, L.; Li, C.; Ding, R.; Zhao, X.; Zhang, P.; Wang, B.; Cui, H. Studies of Corrosion Behaviors of a Carbon Steel/Copper-Nickel Alloy Couple under Epoxy Coating with Artificial Defect in 3.5 wt.% NaCl Solution Using the WBE and EIS Techniques. Prog. Org. Coat. 2020, 148, 105909. [Google Scholar] [CrossRef]
- Trentin, A.; Pakseresht, A.; Duran, A.; Castro, Y.; Galusek, D. Electrochemical Characterization of Polymeric Coatings for Corrosion Protection: A Review of Advances and Perspectives. Polymers 2022, 14, 2306. [Google Scholar] [CrossRef] [PubMed]
- Shi, C.; Shao, Y.; Wang, Y.; Meng, G.; Liu, B. Influence of Submicro-Sheet Zinc Phosphate Modified by Urea-Formaldehyde on the Corrosion Protection of Epoxy Coating. Surf. Interfaces 2020, 18, 100403. [Google Scholar] [CrossRef]
- Zhang, Y.; Shao, Y.; Liu, X.; Shi, C.; Wang, Y.; Meng, G.; Zeng, X.; Yang, Y. A Study on Corrosion Protection of Different Polyaniline Coatings for Mild Steel. Prog. Org. Coat. 2017, 111, 240–247. [Google Scholar] [CrossRef]
- Ganborena, L.; Vega, J.M.; Özkaya, B.; Grande, H.-J.; García-Lecina, E. AN SKP and EIS Study of Microporous Nickel-Chromium Coatings in Copper Containing Electrolytes. Electrochimica Acta 2019, 318, 683–694. [Google Scholar] [CrossRef]
- Monetta, T.; Bellucci, F.; Nicodemo, L.; Nicolais, L. Protective Properties of Epoxy-Based Organic Coatings on Mild Steel. Prog. Org. Coat. 1993, 21, 353–369. [Google Scholar] [CrossRef]
- Del Grosso Destreri, M.; Vogelsang, J.; Fedrizzi, L.; Deflorian, F. Water Up-Take Evaluation of New Waterborne and High Solid Epoxy Coatings. Part II: Electrochemical Impedance Spectroscopy. Prog. Org. Coat. 1999, 37, 69–81. [Google Scholar] [CrossRef]
- Del Grosso Destreri, M.; Vogelsang, J.; Fedrizzi, L. Water Up-Take Evaluation of New Waterborne and High Solid Epoxy Coatings. Prog. Org. Coat. 1999, 37, 57–67. [Google Scholar] [CrossRef]
- Loveday, D.; Peterson, P.; Rodgers, B. Evaluation of Organic Coatings with Electrochemical Impedance Spectroscopy. JCT Coat. Tech. 2004, 8, 46–52. [Google Scholar]
- Lashgari, S.M.; Yari, H.; Mahdavian, M.; Ramezanzadeh, B.; Bahlakeh, G.; Ramezanzadeh, M. Application of Nanoporous Cobalt-Based ZIF-67 Metal-Organic Framework (MOF) for Construction of an Epoxy-Composite Coating with Superior Anti-Corrosion Properties. Corros. Sci. 2021, 178, 109099. [Google Scholar] [CrossRef]
- Brasher, D.M.; Kingsbury, A.H. Electrical Measurements in the Study of Immersed Paint Coatings on Metal. I. Comparison between Capacitance and Gravimetric Methods of Estimating Water-Uptake. J. Appl. Chem. 1954, 4, 62–72. [Google Scholar] [CrossRef]
- Miszczyk, A.; Darowicki, K. Water Uptake in Protective Organic Coatings and Its Reflection in Measured Coating Impedance. Prog. Org. Coat. 2018, 124, 296–302. [Google Scholar] [CrossRef]
- Fan, C.; Shi, J.; Dilger, K. Water Uptake and Interfacial Delamination of an Epoxy-Coated Galvanized Steel: An Electrochemical Impedance Spectroscopic Study. Prog. Org. Coat. 2019, 137, 105333. [Google Scholar] [CrossRef]
- Liu, R.; Liu, L.; Meng, F.; Tian, W.; Liu, Y.; Li, Y.; Wang, F. Finite Element Analysis of the Water Diffusion Behaviour in Pigmented Epoxy Coatings under Alternating Hydrostatic Pressure. Prog. Org. Coat. 2018, 123, 168–175. [Google Scholar] [CrossRef]
- Mansfeld, F. Use of Electrochemical Impedance Spectroscopy for the Study of Corrosion Protection by Polymer Coatings. J. Appl. Electrochem. 1995, 25, 187–202. [Google Scholar] [CrossRef]
- Liu, J.; Xiang-Bo, L.; Jia, W.; Tian-Yuan, L.; Xiao-Ming, W. Studies of Impedance Models and Water Transport Behaviours of Epoxy Coating at Hydrostatic Pressure of Seawater. Prog. Org. Coat. 2013, 76, 1075–1081. [Google Scholar] [CrossRef]
- Fadl, A.M.; Abdou, M.I.; Hamza, M.A.; Sadeek, S.A. Corrosion-Inhibiting, Self-Healing, Mechanical-Resistant, Chemically and UV Stable PDMAS/TiO2 Epoxy Hybrid Nanocomposite Coating for Steel Petroleum Tanker Trucks. Prog. Org. Coat. 2020, 146, 105715. [Google Scholar] [CrossRef]
- Contu, F.; Fenzy, L.; Taylor, S.R. An FT-IR Investigation of Epoxy Coatings as a Function of Electrolyte Composition. Prog. Org. Coat. 2012, 75, 92–96. [Google Scholar] [CrossRef]
- Bajat, J.B.; Milošev, I.; Jovanović, Ž.; Mišković-Stanković, V.B. Studies on Adhesion Characteristics and Corrosion Behaviour of Vinyltriethoxysilane/Epoxy Coating Protective System on Aluminium. Appl. Surf. Sci. 2010, 256, 3508–3517. [Google Scholar] [CrossRef]
- Jaisai, M.; Baruah, S.; Dutta, J. Paper Modified with ZnO Nanorods—Antimicrobial Studies. Beilstein J. Nanotechnol. 2012, 3, 684–691. [Google Scholar] [CrossRef]
- Meng, F.; Liu, L.; Tian, W.; Wu, H.; Li, Y.; Zhang, T.; Wang, F. The Influence of the Chemically Bonded Interface between Fillers and Binder on the Failure Behaviour of an Epoxy Coating under Marine Alternating Hydrostatic Pressure. Corros. Sci. 2015, 101, 139–154. [Google Scholar] [CrossRef]
- Tian, W.; Meng, F.; Liu, L.; Li, Y.; Wang, F. The Failure Behaviour of a Commercial Highly Pigmented Epoxy Coating under Marine Alternating Hydrostatic Pressure. Prog. Org. Coat. 2015, 82, 101–112. [Google Scholar] [CrossRef]
- Tian, W.; Liu, L.; Meng, F.; Liu, Y.; Li, Y.; Wang, F. The Failure Behaviour of an Epoxy Glass Flake Coating/Steel System under Marine Alternating Hydrostatic Pressure. Corros. Sci. 2014, 86, 81–92. [Google Scholar] [CrossRef]
- Hongyang GAO, W.W. Degradation Behavior of a Modified Epoxy Coating in Simulated Deep-Sea Environment. J. Chin. Soc. Corros. Prot. 2017, 37, 247–263. [Google Scholar] [CrossRef]
- Liu, L.; Cui, Y.; Li, Y.; Zhang, T.; Wang, F. Failure Behavior of Nano-SiO2 Fillers Epoxy Coating under Hydrostatic Pressure. Electrochimica Acta 2012, 62, 42–50. [Google Scholar] [CrossRef]
- Zhang, X.; Wu, W.; Li, Y.; Li, J.; Qiao, L. Corrosion Form Transition of Mooring Chain in Simulated Deep-Sea Environments: Remarkable Roles of Dissolved Oxygen and Hydrostatic Pressure. J. Mater. Sci. Technol. 2023, 162, 118–130. [Google Scholar] [CrossRef]
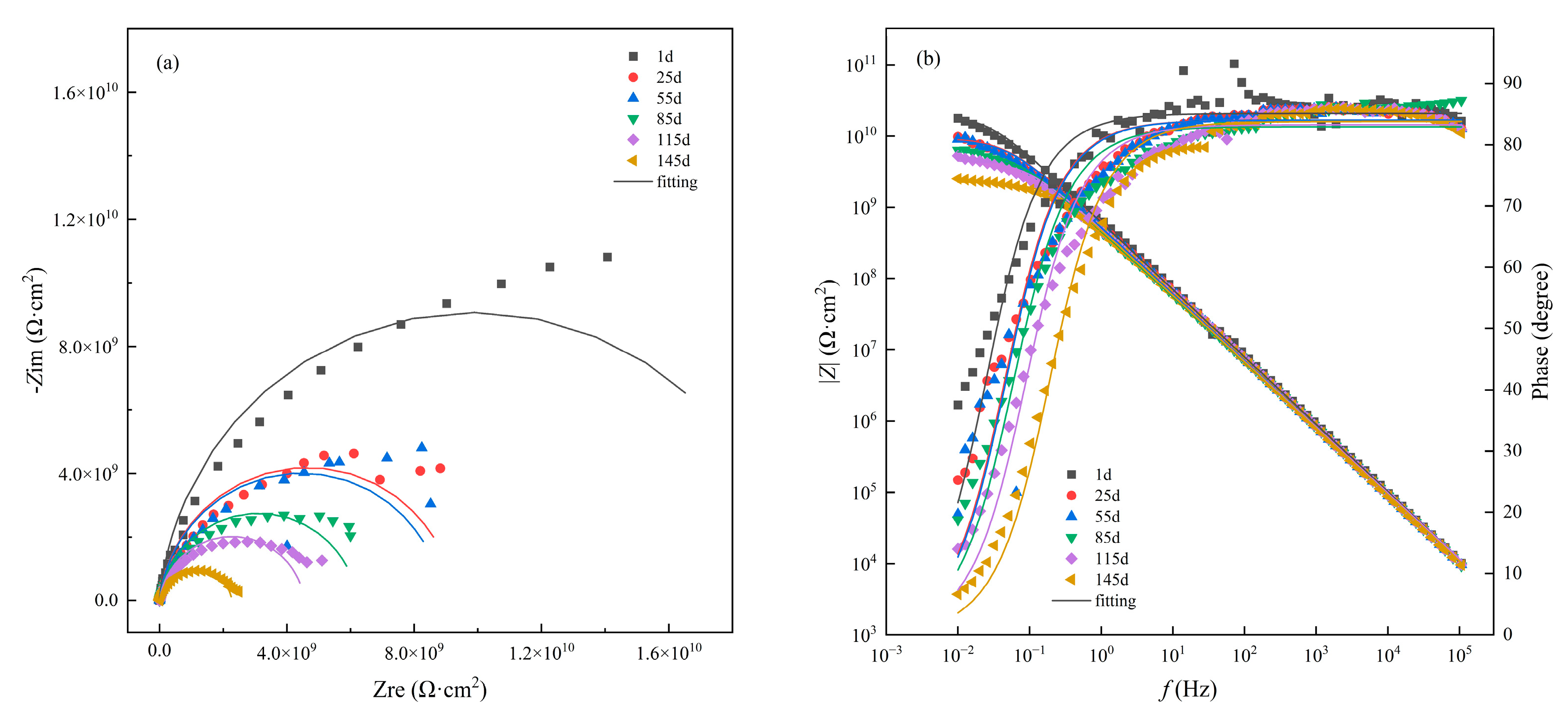
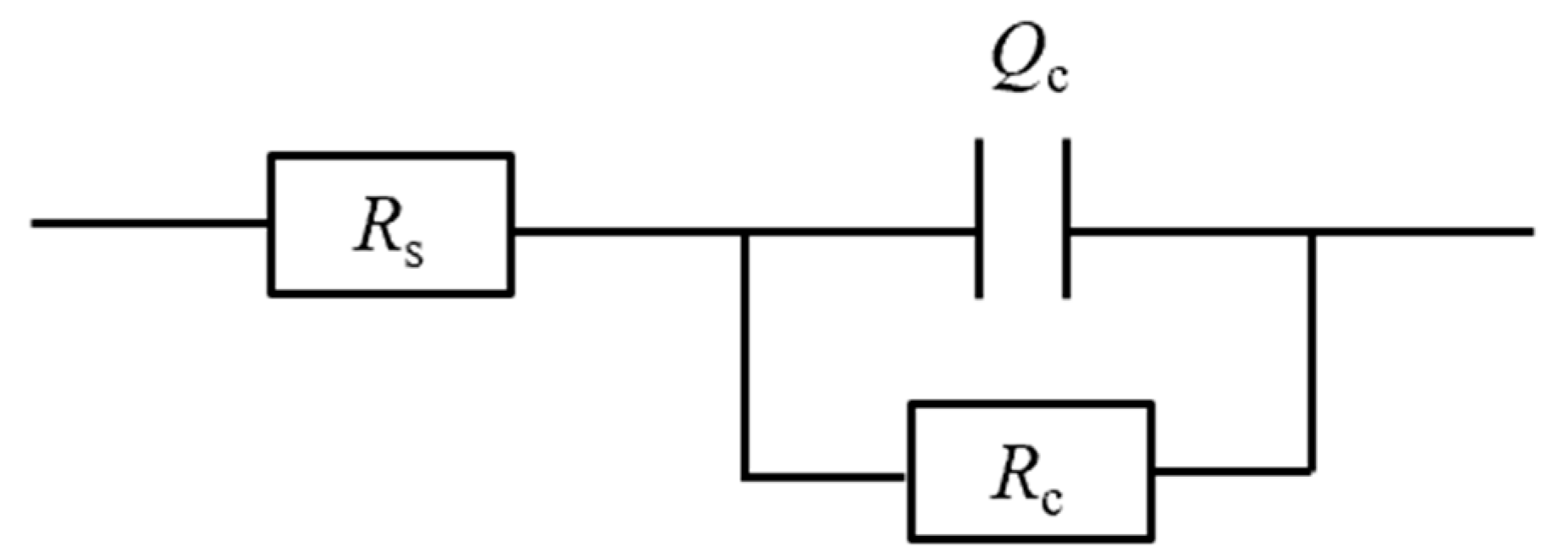
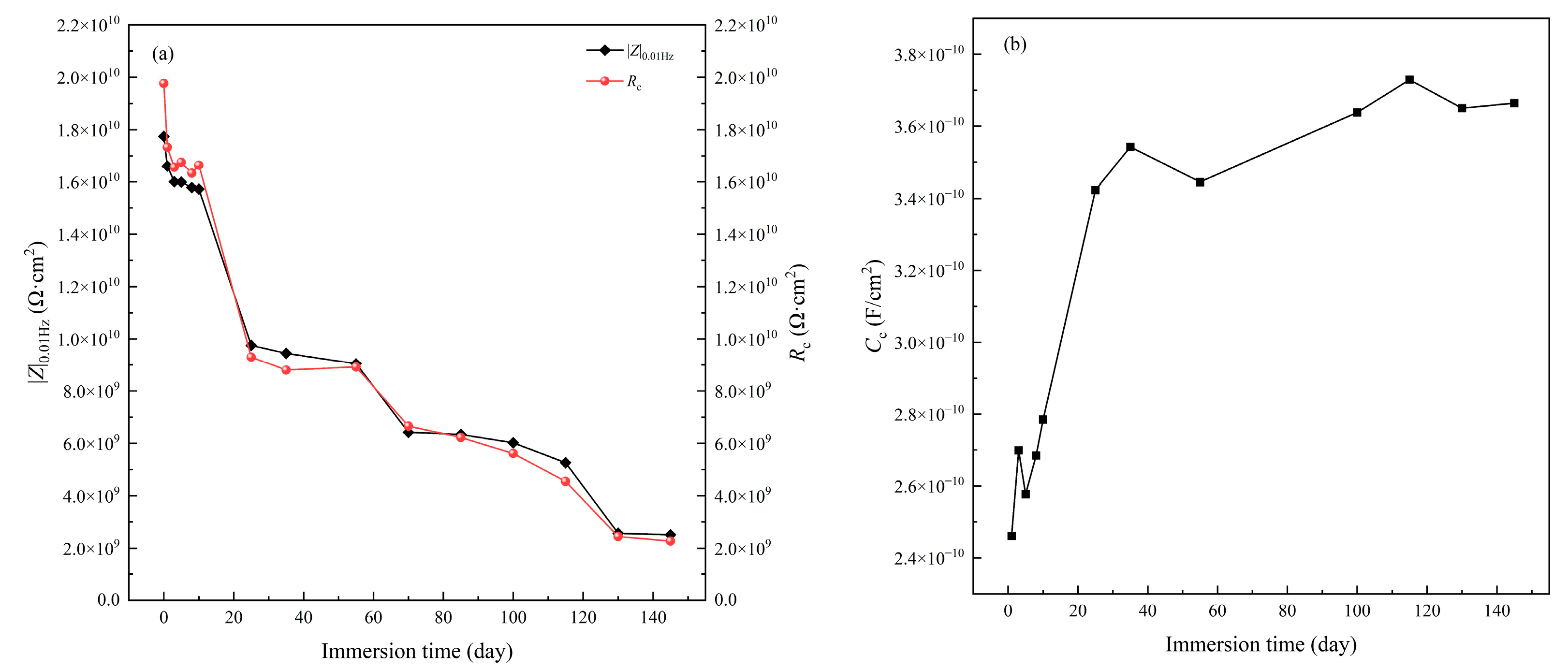




| Testing Conditions | Fitting Parameters | 1 h | 24 h | 72 h | 120 h | 192 h | 240 h |
|---|---|---|---|---|---|---|---|
| Immersion in seawater at atmospheric pressure | Rs (Ω·cm2) | 0.61 | 0.31 | 0.54 | 0.41 | 0.21 | 0.11 |
| Rc (Ω·cm2) | 1.98 ×1010 | 1.73 ×1010 | 1.67 ×1010 | 1.66 ×1010 | 1.65 ×1010 | 1.63 ×1010 | |
| Qc (F/cm2) | 2.46 ×10−10 | 2.58 ×10−10 | 2.69 ×10−10 | 2.70 ×10−10 | 2.79 ×10−10 | 2.84 ×10−10 | |
| n | 0.95 | 0.96 | 0.96 | 0.95 | 0.95 | 0.95 | |
| AC-DC-AC cycling at atmospheric pressure | Rs (Ω·cm2) | 1.72 | 1.90 | 1.84 | 1.65 | 1.55 | 1.32 |
| Rc (Ω·cm2) | 1.05 ×1010 | 6.77 ×109 | 5.47 ×109 | 5.03 ×109 | 3.77 ×109 | 3.47 ×109 | |
| Qc (F/cm2) | 1.76 ×10−10 | 1.88 ×10−10 | 1.89 ×10−10 | 1.91 ×10−10 | 1.97 ×10−10 | 2.04 ×10−10 | |
| n | 0.93 | 0.94 | 0.96 | 0.95 | 0.93 | 0.95 | |
| AC-DC-AC coupled with hydrostatic pressure | Rs (Ω·cm2) | 0.73 | 0.81 | 0.91 | 0.80 | 0.84 | 0.72 |
| Rc (Ω·cm2) | 1.47 ×1010 | 2.60 ×109 | 1.73 ×109 | 1.49 ×109 | 1.04 ×109 | 9.92 ×108 | |
| Qc (F/cm2) | 1.19 ×10−10 | 1.44 ×10−10 | 1.50 ×10−10 | 1.57 ×10−10 | 1.58 ×10−10 | 1.72 ×10−10 | |
| n | 0.96 | 0.95 | 0.94 | 0.95 | 0.95 | 0.94 |
| Testing Conditions | Ad (cm2) | α |
|---|---|---|
| Immersion in seawater at atmospheric pressure | 2.981 × 10−6 | 4.217 × 10−7 |
| AC-DC-AC cycling at atmospheric pressure | 2.642 × 10−5 | 3.738 × 10−6 |
| AC-DC-AC coupled with hydrostatic pressure | 1.272 × 10−4 | 1.800 × 10−5 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shen, Y.; Xu, L.; Liu, Y.; Lu, Y.; Xu, H.; Zhao, R.; Bai, S.; Xin, Y.; Hou, J.; Liu, X.; et al. Influence of AC-DC-AC Cycling with Hydrostatic Pressure on Accelerated Protective Performance Test of Glass Flake Epoxy Coating. Coatings 2023, 13, 1843. https://doi.org/10.3390/coatings13111843
Shen Y, Xu L, Liu Y, Lu Y, Xu H, Zhao R, Bai S, Xin Y, Hou J, Liu X, et al. Influence of AC-DC-AC Cycling with Hydrostatic Pressure on Accelerated Protective Performance Test of Glass Flake Epoxy Coating. Coatings. 2023; 13(11):1843. https://doi.org/10.3390/coatings13111843
Chicago/Turabian StyleShen, Yong, Likun Xu, Yilong Liu, Yonghong Lu, Haibo Xu, Rongrong Zhao, Shuangfeng Bai, Yonglei Xin, Jian Hou, Xuehui Liu, and et al. 2023. "Influence of AC-DC-AC Cycling with Hydrostatic Pressure on Accelerated Protective Performance Test of Glass Flake Epoxy Coating" Coatings 13, no. 11: 1843. https://doi.org/10.3390/coatings13111843
APA StyleShen, Y., Xu, L., Liu, Y., Lu, Y., Xu, H., Zhao, R., Bai, S., Xin, Y., Hou, J., Liu, X., & Liu, F. (2023). Influence of AC-DC-AC Cycling with Hydrostatic Pressure on Accelerated Protective Performance Test of Glass Flake Epoxy Coating. Coatings, 13(11), 1843. https://doi.org/10.3390/coatings13111843






