Influence of Electron Beam Treatment on Structure and Phase Composition of TiB2–Ag Coating Deposited by Electrical Explosion Spraying
Abstract
:1. Introduction
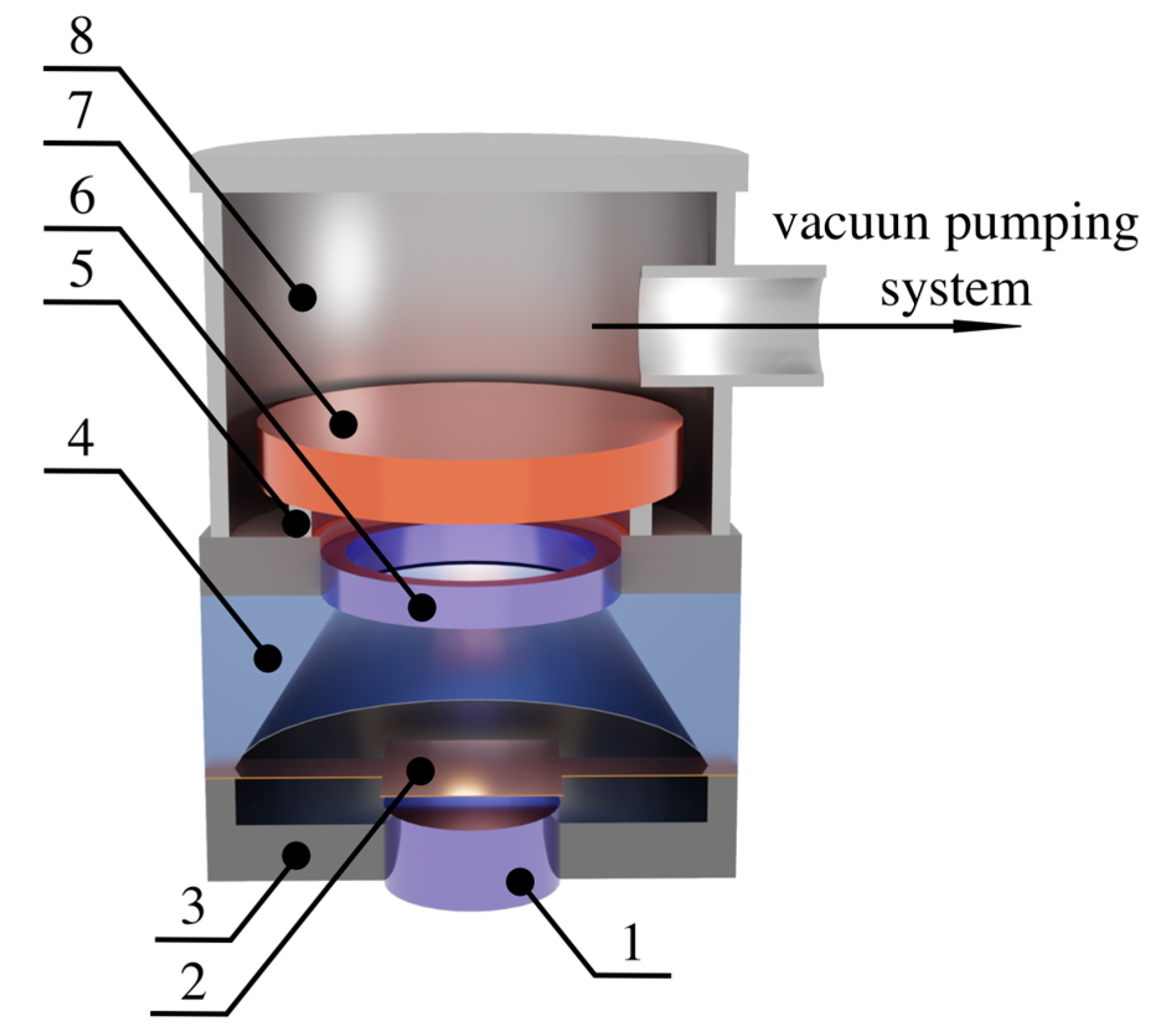
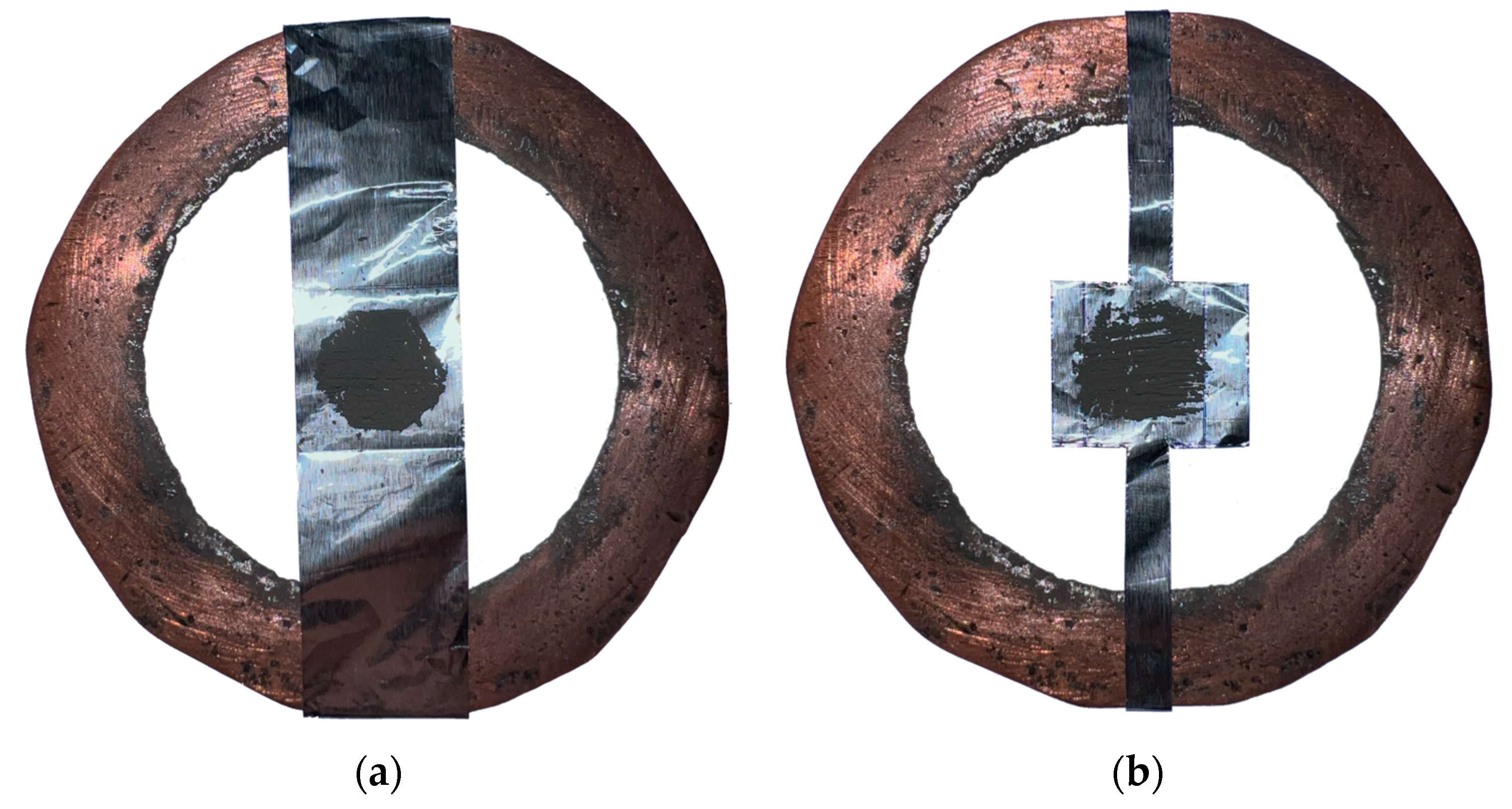
2. Materials and Methods
3. Results and Discussion
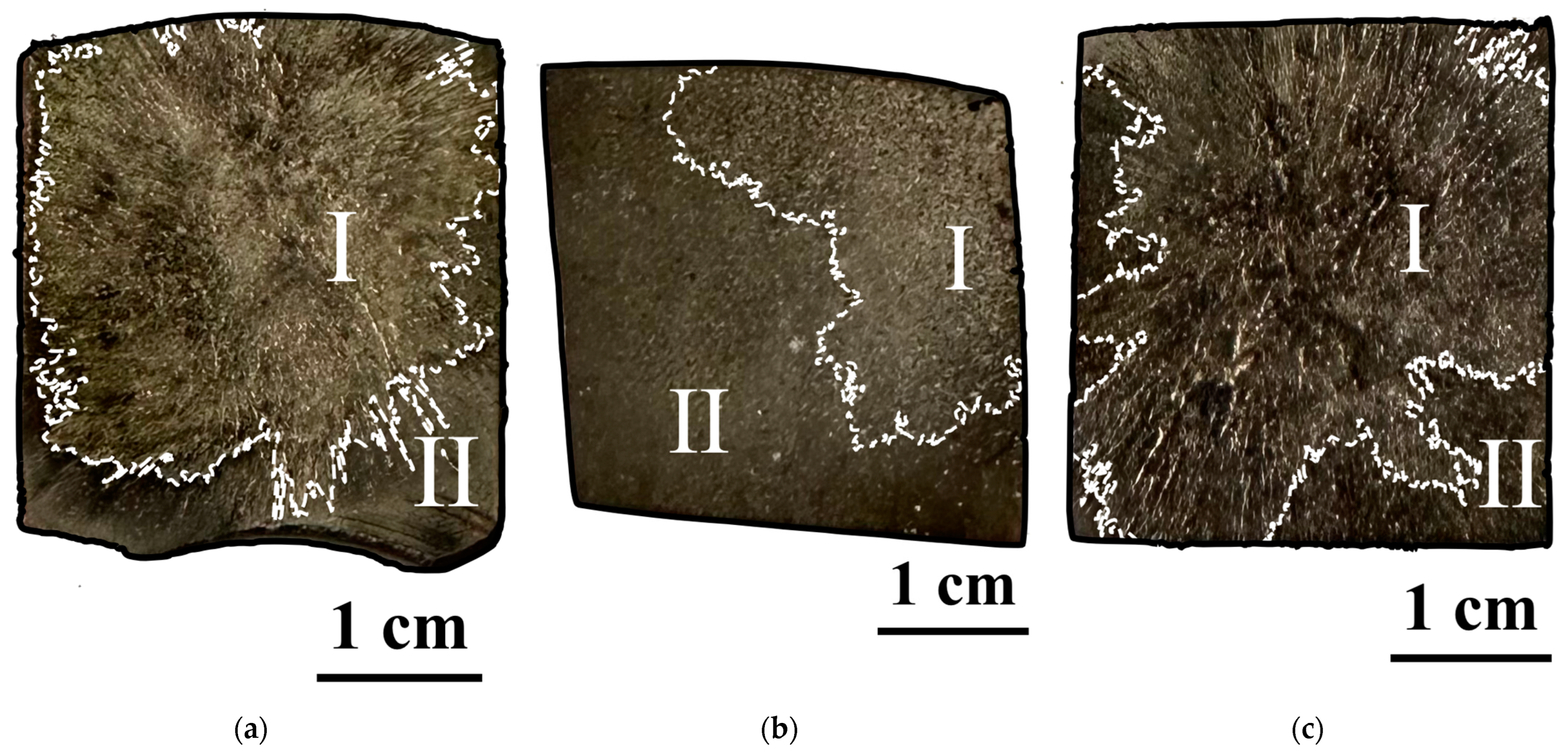
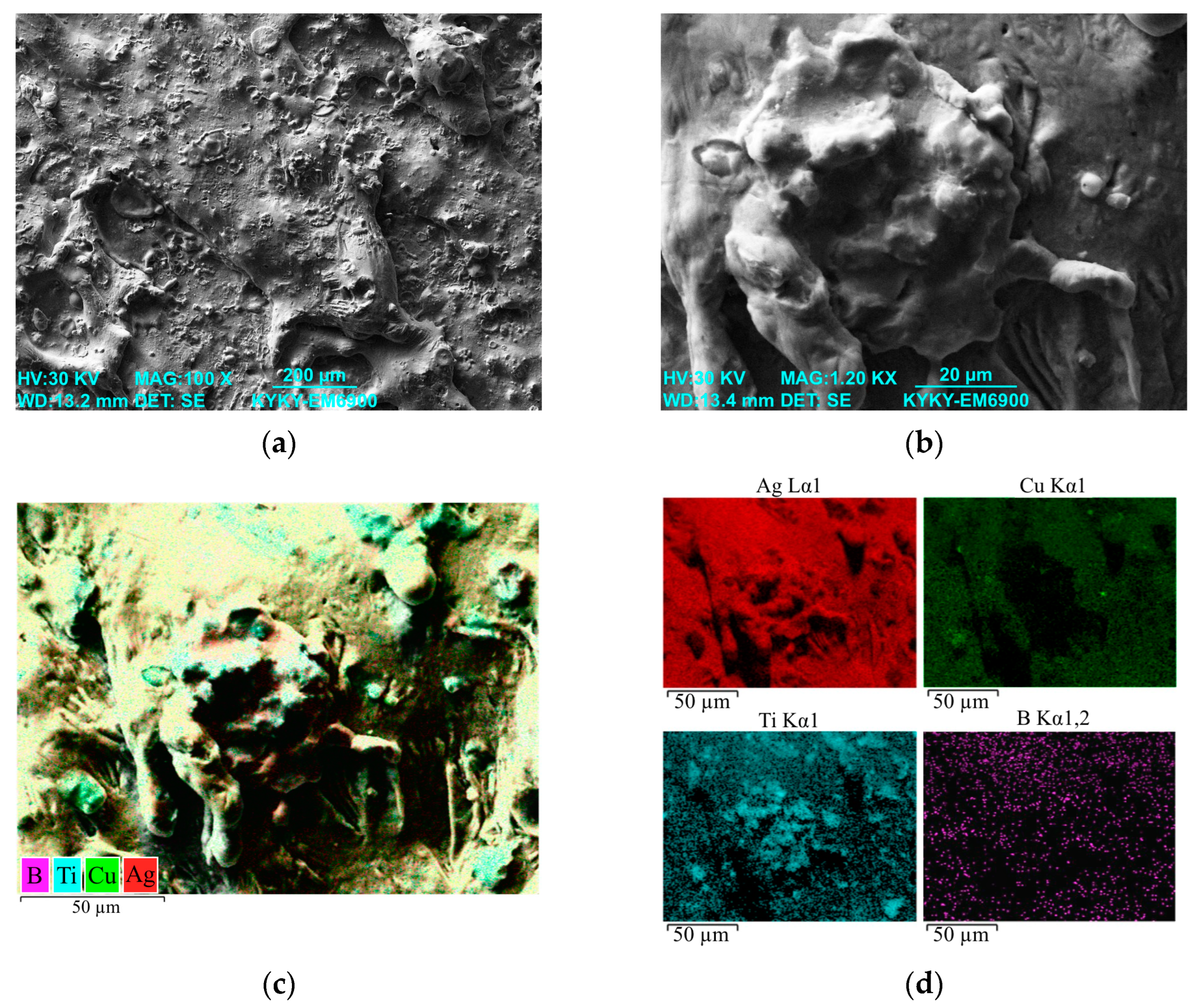
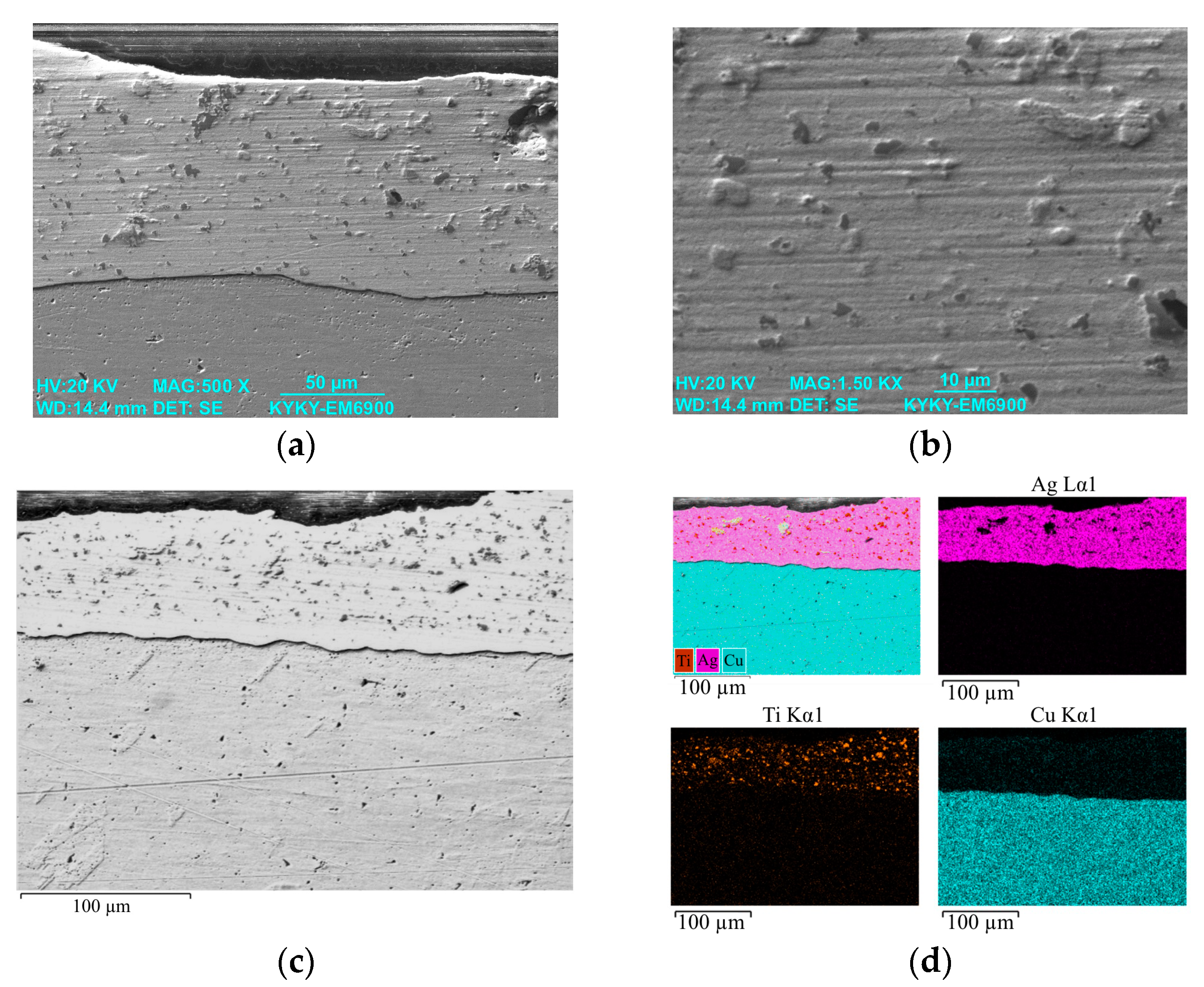
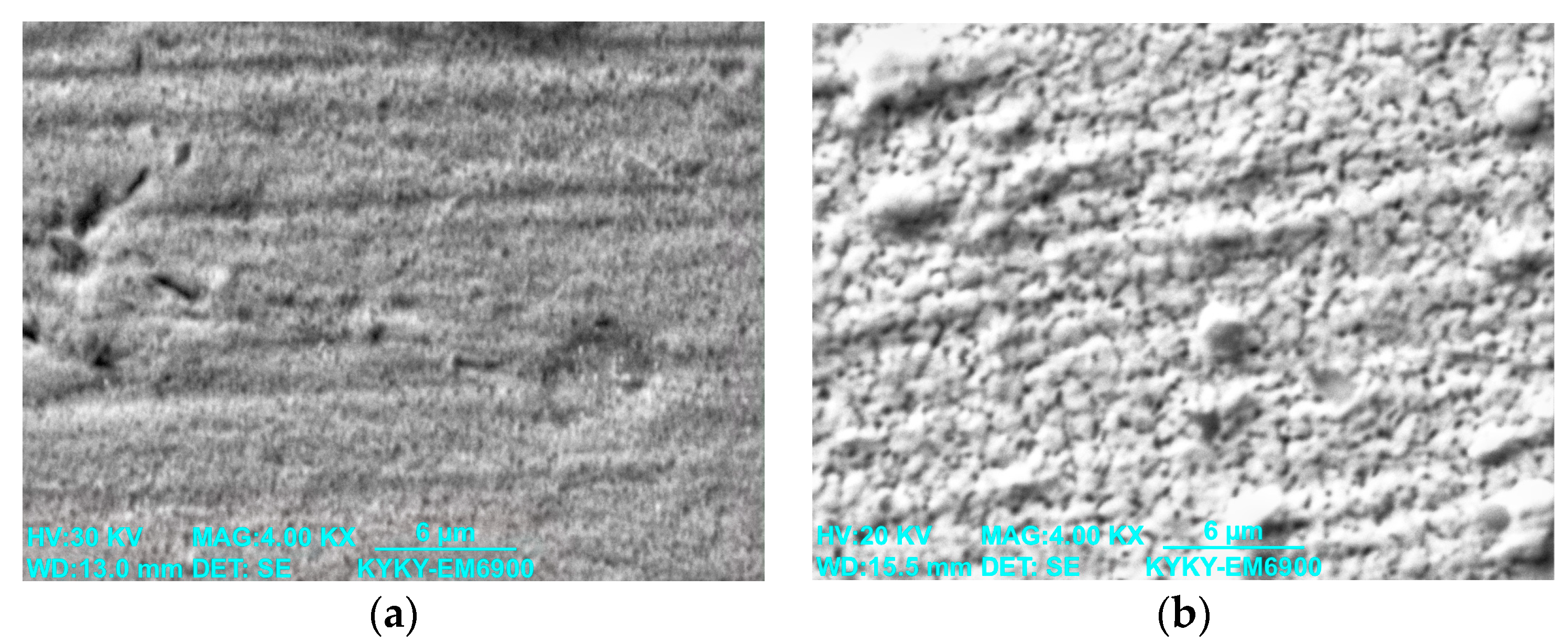
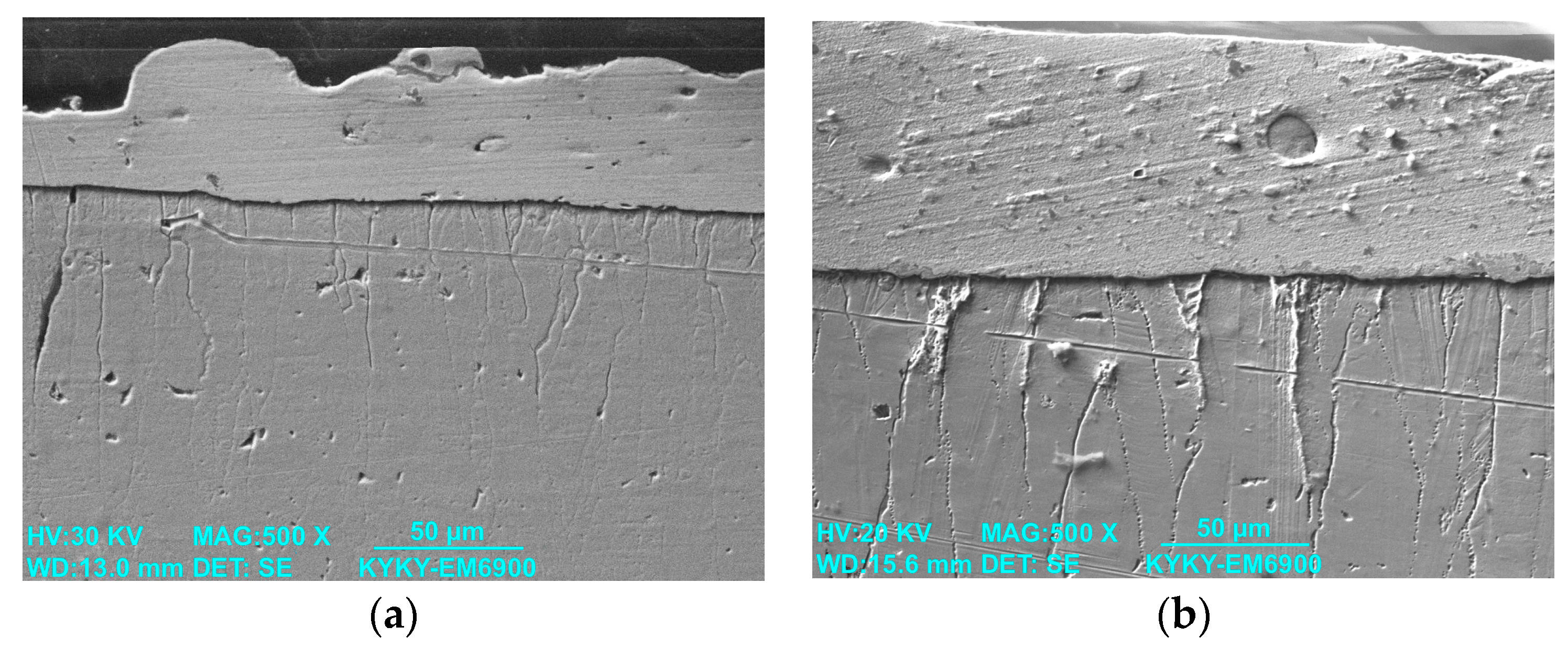
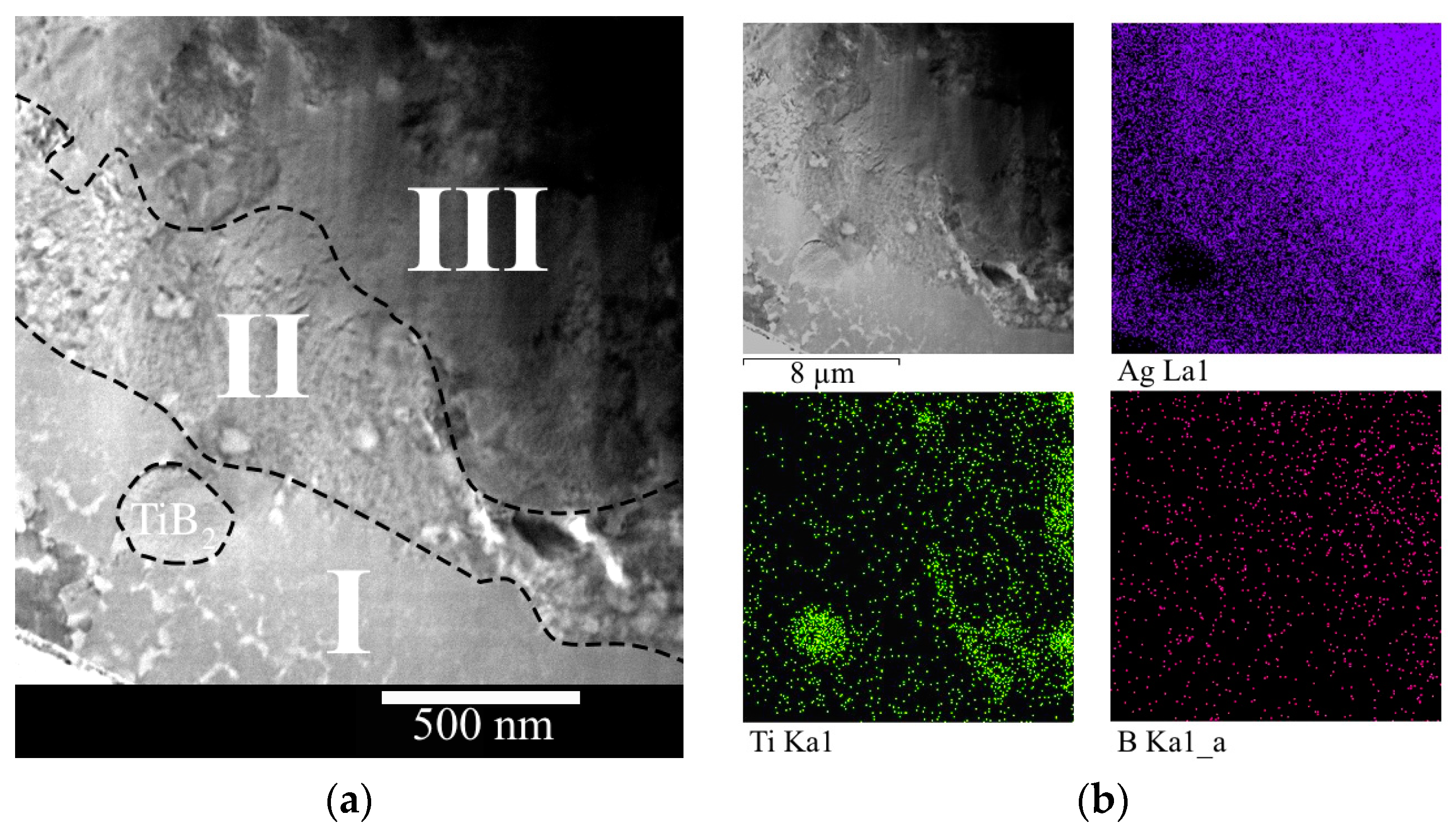
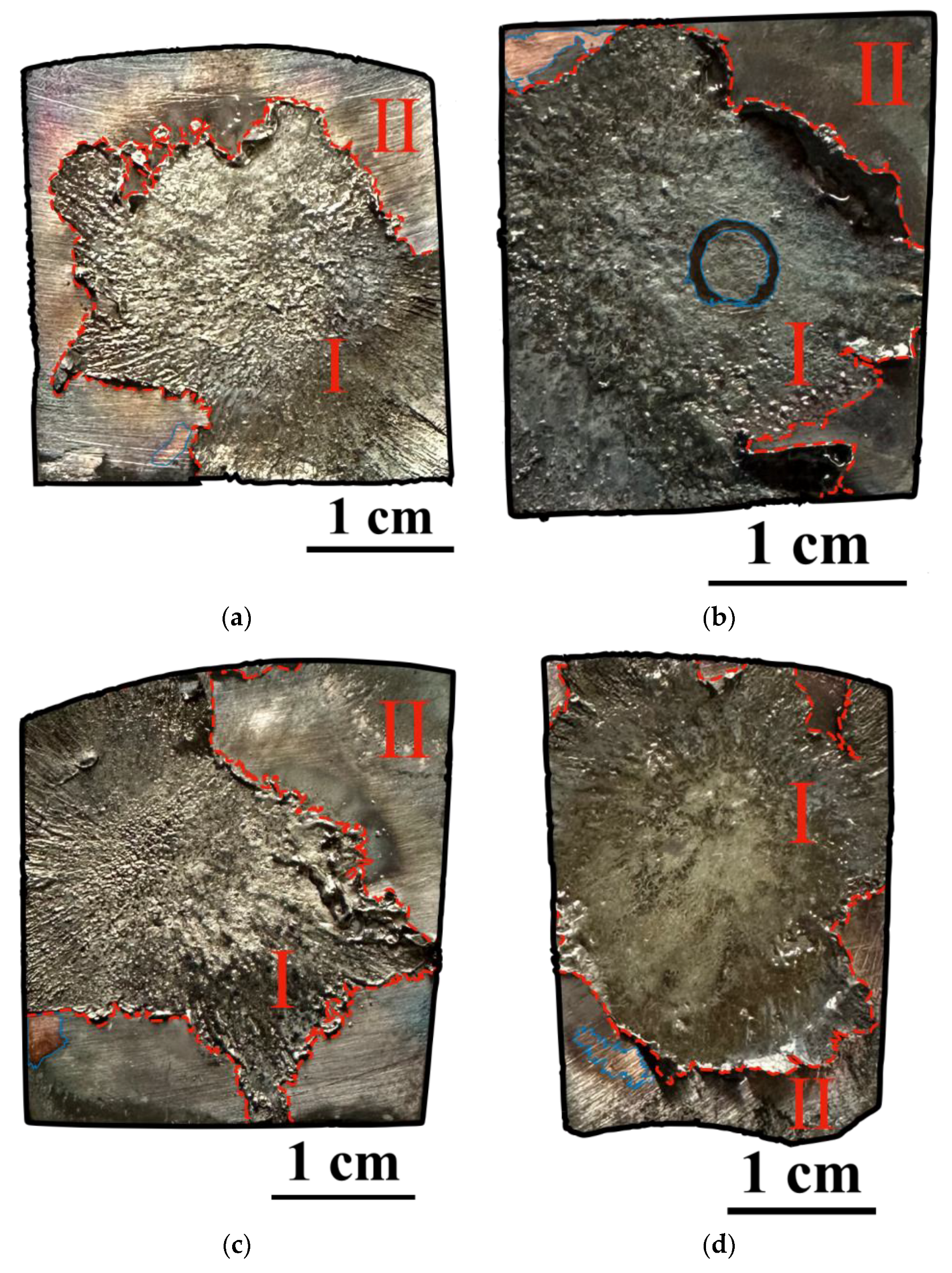
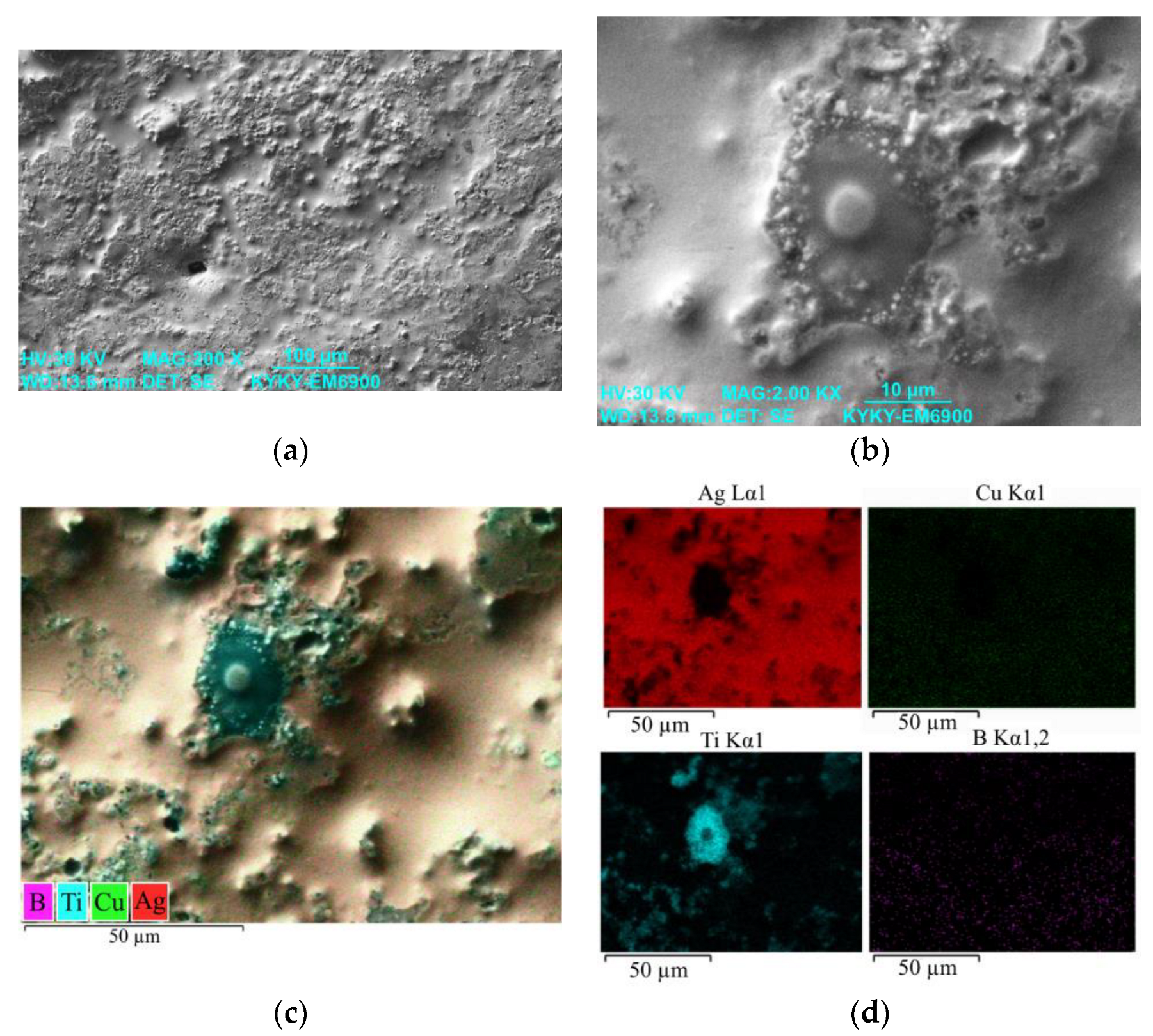
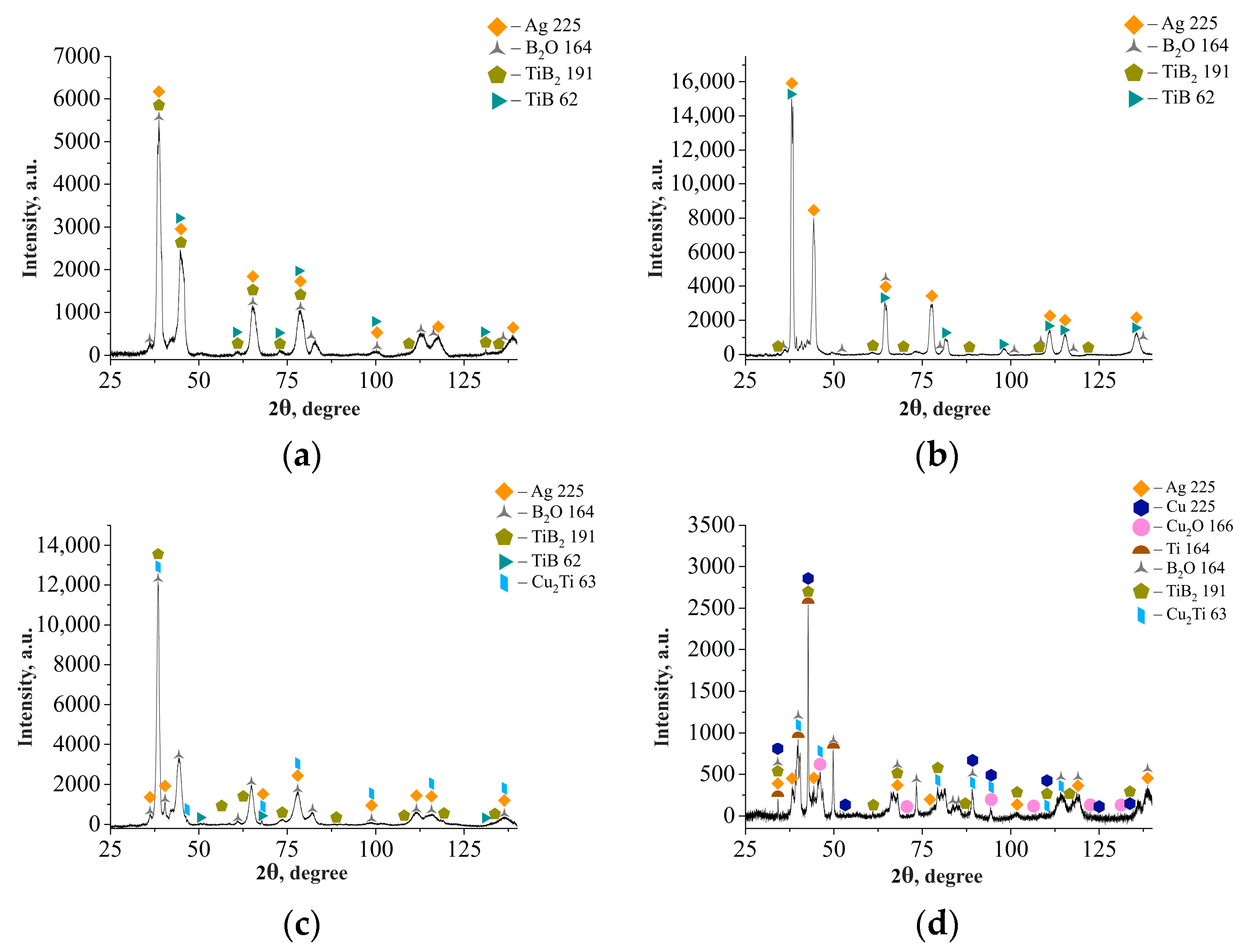
3.1. Study of the TiB2–Ag Coating Obtained by Electrical Explosion
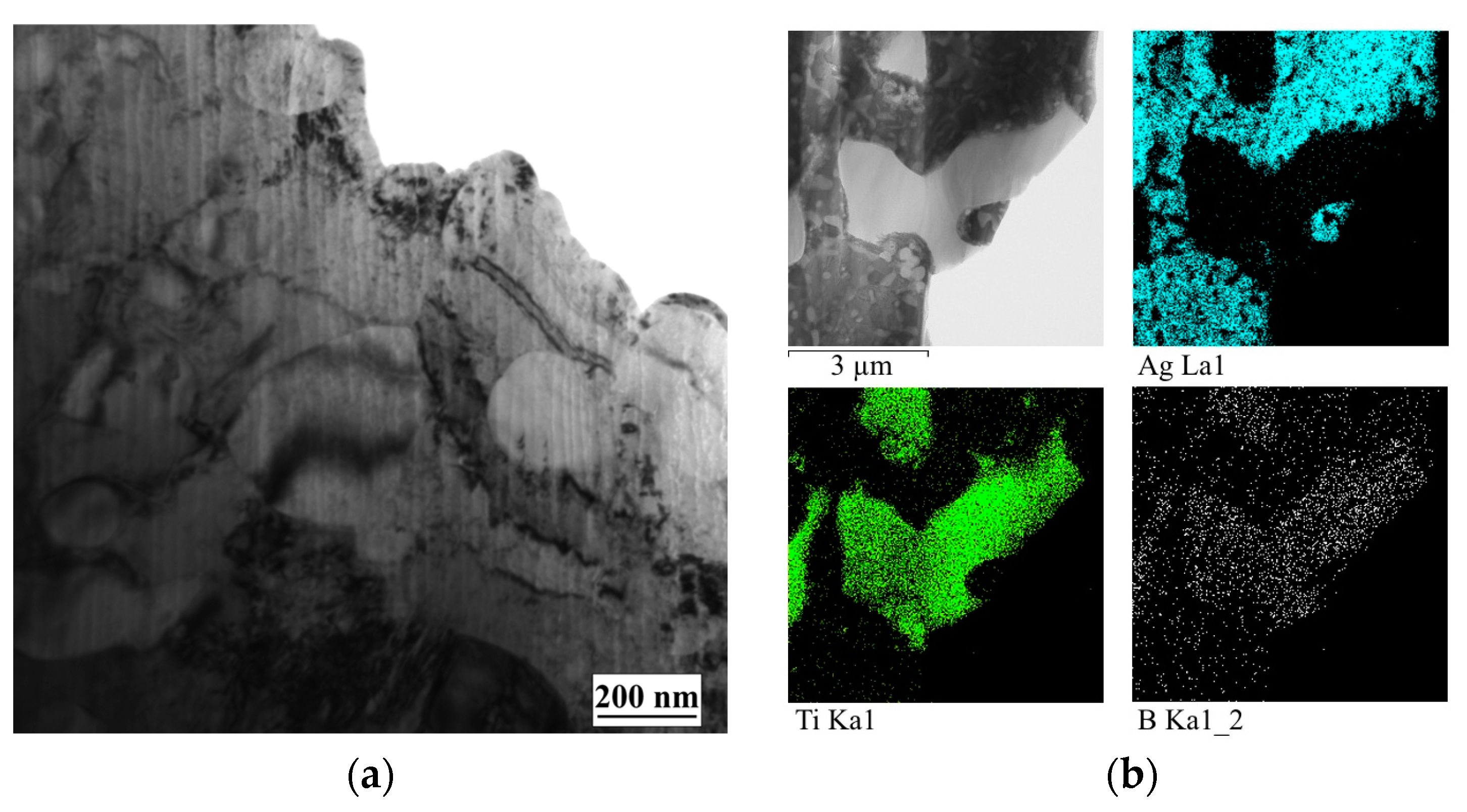
3.2. Study of the TiB2–Ag Coating Electron Beam Treatment Influence
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Grieseler, R.; Camargo, M.K.; Hopfeld, M.; Schmidt, U.; Bund, A.; Schaaf, P. Copper-MAX-Phase Composite Coatings Obtained by Electro-Co-Deposition: A Promising Material for Electrical Contacts. Surf. Coat. Technol. 2017, 321, 219–228. [Google Scholar] [CrossRef]
- Yang, F.; Wu, Y.; Rong, M.; Sun, H.; Murphy, A.B.; Ren, Z.; Niu, C. Low-Voltage Circuit Breaker Arcs—Simulation and Measurements. J. Phys. D Appl. Phys. 2013, 46, 273001. [Google Scholar] [CrossRef]
- Song, J.; Yuan, H.; Schinow, V. Fretting Corrosion Behavior of Electrical Contacts with Tin Coating in Atmosphere and Vacuum. Wear 2019, 426–427, 1439–1445. [Google Scholar] [CrossRef]
- Biyik, S.; Arslan, F.; Aydin, M. Arc-Erosion Behavior of Boric Oxide-Reinforced Silver-Based Electrical Contact Materials Produced by Mechanical Alloying. J. Electron. Mater. 2015, 44, 457–466. [Google Scholar] [CrossRef]
- Wu, C.; Zhao, Q.; Li, N.; Wang, H.; Yi, D.; Weng, W. Influence of Fabrication Technology on Arc Erosion of Ag/10SnO2 Electrical Contact Materials. J. Alloys Compd. 2018, 766, 161–177. [Google Scholar] [CrossRef]
- Zhou, Z.; Zhao, T.; Feng, Y.; Zhao, H.; Qian, G. Arc Erosion Characteristics Evolution of Ag/Ti3SiC2 Composites during Repetitive Arc Breakdowns in SF6 Gaseous Medium. Int. J. Appl. Ceram. Technol. 2021, 18, 1716–1724. [Google Scholar] [CrossRef]
- Ray, N.; Kempf, B.; Mützel, T.; Froyen, L.; Vanmeensel, K.; Vleugels, J. Effect of WC Particle Size and Ag Volume Fraction on Electrical Contact Resistance and Thermal Conductivity of Ag–WC Contact Materials. Mater. Des. 2015, 85, 412–422. [Google Scholar] [CrossRef]
- Kesim, M.T.; Yu, H.; Sun, Y.; Aindow, M.; Alpay, S.P. Corrosion, Oxidation, Erosion and Performance of Ag/W-Based Circuit Breaker Contacts: A Review. Corros. Sci. 2018, 135, 12–34. [Google Scholar] [CrossRef]
- Yangfang, C.; Xiaofang, Y.; Xiaoping, B.; Mingjiang, Z.; Jie, L.; Changlin, Y.; Xiufang, Z. Research on the Influence of Different Oxide Particles on Properties of AgCdO Contact Material. J. Phys. Conf. Ser. 2021, 1948, 012192. [Google Scholar] [CrossRef]
- Wang, X.; Yang, H.; Chen, M.; Zou, J.; Liang, S. Fabrication and Arc Erosion Behaviors of AgTiB2 Contact Materials. Powder Technol. 2014, 256, 20–24. [Google Scholar] [CrossRef]
- Nilsson, O.; Hauner, F.; Jeannot, D. Replacement of AgCdO by AgSnO/Sub2/ in DC Contactors. In Proceedings of the 50th IEEE Holm Conference on Electrical Contacts and the 22nd International Conference on Electrical Contacts Electrical Contacts, Seattle, WA, USA, 23–23 September 2004; pp. 70–74. [Google Scholar]
- Senthil, S.M.; Parameshwaran, R.; Rathanasamy, R.; Praveen Kumar, S. Fabrication of a Novel Silver-Based Electrical Contact Composites and Assessment of Its Mechanical and Electrical Properties. Arch. Metall. Mater. 2021, 66, 1087–1094. [Google Scholar]
- Ćosović, V.; Talijan, N.; Živković, D.; Minić, D.; Živković, Ž. Comparison of Properties of Silver-Metal Oxide Electrical Contact Materials. J. Min. Metall. Sect. B Metall. 2012, 48, 131–141. [Google Scholar] [CrossRef]
- Wang, X.; Li, G.; Zou, J.; Liang, S.; Fan, Z. Investigation on Preparation, Microstructure, and Properties of AgTiB2 Composite. J. Compos. Mater. 2011, 45, 1285–1293. [Google Scholar] [CrossRef]
- Li, H.; Wang, X.; Liu, Y.; Guo, X. Effect of Strengthening Phase on Material Transfer Behavior of Ag-Based Contact Materials under Different Voltages. Vacuum 2017, 135, 55–65. [Google Scholar] [CrossRef]
- Li, H.; Wang, X.; Guo, X.; Yang, X.; Liang, S. Material Transfer Behavior of AgTiB2 and AgSnO2 Electrical Contact Materials under Different Currents. Mater. Des. 2017, 114, 139–148. [Google Scholar] [CrossRef]
- Güler, O.; Varol, T.; Alver, Ü.; Biyik, S. The Wear and Arc Erosion Behavior of Novel Copper Based Functionally Graded Electrical Contact Materials Fabricated by Hot Pressing Assisted Electroless Plating. Adv. Powder Technol. 2021, 32, 2873–2890. [Google Scholar] [CrossRef]
- Güler, O.; Alver, Ü.; Varol, T. Fabrication and Characterization of Novel Layered Materials Produced by Electroless Plating and Hot Pressing. J. Alloys Compd. 2020, 835, 155278. [Google Scholar] [CrossRef]
- Güler, O.; Varol, T.; Alver, Ü.; Canakci, A. Effect of Al2O3 Content and Milling Time on the Properties of Silver Coated Cu Matrix Composites Fabricated by Electroless Plating and Hot Pressing. Mater. Today Commun. 2020, 24, 101153. [Google Scholar] [CrossRef]
- Wei, S.; Xu, B.; Wang, H.; Jin, G.; Lv, H. Comparison on Corrosion-Resistance Performance of Electro-Thermal Explosion Plasma Spraying FeAl-Based Coatings. Surf. Coat. Technol. 2007, 201, 5294–5297. [Google Scholar] [CrossRef]
- Tamura, H.; Konoue, M.; Sawaoka, A.B. Zirconium Boride and Tantalum Carbide Coatings Sprayed by Electrothermal Explosion of Powders. J. Therm. Spray Tech. 1997, 6, 463–468. [Google Scholar] [CrossRef]
- Koval’, N.N.; Ivanov, Y.F. Nanostructuring of Surfaces of Metalloceramic and Ceramic Materials by Electron-Beams. Russ. Phys. J. 2008, 51, 505–516. [Google Scholar] [CrossRef]
- Li, C.; Feng, J.; Yuan, W.; Cao, Y.; Han, R. Discharge Characteristics and Dynamic Process of Directional Spraying Binary and Ternary Alloy Coating via Electrical Explosion Method. In Proceedings of the 4th International Symposium on Plasma and Energy Conversion, Foshan, China, 14–16 October 2022; Dai, D., Zhang, C., Fang, Z., Lu, X., Eds.; Springer Nature: Singapore, 2023; pp. 302–311. [Google Scholar]
- Romanov, D.; Moskovskii, S.; Konovalov, S.; Sosnin, K.; Gromov, V.; Ivanov, Y. Improvement of Copper Alloy Properties in Electro-Explosive Spraying of ZnO-Ag Coatings Resistant to Electrical Erosion. J. Mater. Res. Technol. 2019, 8, 5515–5523. [Google Scholar] [CrossRef]
- Vlasova, M.; Kakazey, M.; Aguilar, P.A.M.; Tapia, R.G.; Reséndiz-González, M.C.; Hernandez, A.C.; Mel’nikov, I.V.; Fironov, Y. TiN–TiB2 Ceramics Degradation in the Region of a Steady-State Laser Heating. Surf. Coat. Technol. 2019, 378, 124738. [Google Scholar] [CrossRef]
- Ma, G.; Chen, S.; Wang, H. Impact Spread Behavior of Flying Droplets and Properties of Splats. In Micro Process and Quality Control of Plasma Spraying; Ma, G., Chen, S., Wang, H., Eds.; Springer Series in Advanced Manufacturing; Springer Nature: Singapore, 2022; pp. 87–202. ISBN 978-981-19274-2-3. [Google Scholar]
- Liu, Q.; Wang, Y.; Bai, Y.; Li, Z.D.; Tan, G.L.; Bao, M.Y.; Li, X.J.; Zhan, H.; Sun, Y.W.; Chong, N.J.; et al. Formation Mechanism of Gas Phase in Supersonic Atmospheric Plasma Sprayed NiCr-Cr3C2 Cermet Coatings. Surf. Coat. Technol. 2020, 397, 126052. [Google Scholar] [CrossRef]
- Romanov, D.A.; Moskovskii, S.V.; Glezer, A.M.; Gromov, V.E.; Sosnin, K.V. Phase Composition, Structure, and Wear Resistance of Electric-Explosive CuO–Ag System Coatings after Electron Beam Processing. Bull. Russ. Acad. Sci. Phys. 2019, 83, 1270–1274. [Google Scholar] [CrossRef]
- Wang, X.; Zhou, H.; Wei, Y.; Zhang, A.; Zhu, L. Electrical Explosion Spray of Ag/C Composite Coating and Its Deposition Behavior. Ceram. Int. 2022, 48, 4497–4504. [Google Scholar] [CrossRef]
- Sarychev, V.; Nevskii, S.; Konovalov, S.; Granovskii, A. Modeling of the Initial Stages of the Formation of Heterogeneous Plasma Flows in the Electric Explosion of Conductors. Curr. Appl. Phys. 2018, 18, 1101–1107. [Google Scholar] [CrossRef]
- Romanov, D.A.; Budovskikh, E.A.; Zhmakin, Y.D.; Gromov, V.E. Surface Modification by the EVU 60/10 Electroexplosive System. Steel Transl. 2011, 41, 464–468. [Google Scholar] [CrossRef]
- Nevskii, S.; Sarychev, V.; Konovalov, S.; Granovskii, A.; Gromov, V. Formation Mechanism of Micro- and Nanocrystalline Surface Layers in Titanium and Aluminum Alloys in Electron Beam Irradiation. Metals 2020, 10, 1399. [Google Scholar] [CrossRef]
- Sarychev, V.; Nevskii, S.; Konovalov, S.; Granovskii, A.; Ivanov, Y.; Gromov, V. Model of Nanostructure Formation in Al–Si Alloy at Electron Beam Treatment. Mater. Res. Express 2018, 6, 026540. [Google Scholar] [CrossRef]




| Regime, No | Pulse Energy Density, J/cm2 | Pulse Duration, µs | Number of Pulses | Pulse Frequency, s−1 |
|---|---|---|---|---|
| 1 | 45 | 50 | 30 | 0.3 |
| 2 | 50 | 200 | 30 | 0.3 |
| 3 | 60 | 200 | 30 | 0.3 |
| 4 | 70 | 200 | 30 | 0.3 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Filyakov, A.D.; Pochetukha, V.V.; Romanov, D.A.; Vashchuk, E.S. Influence of Electron Beam Treatment on Structure and Phase Composition of TiB2–Ag Coating Deposited by Electrical Explosion Spraying. Coatings 2023, 13, 1867. https://doi.org/10.3390/coatings13111867
Filyakov AD, Pochetukha VV, Romanov DA, Vashchuk ES. Influence of Electron Beam Treatment on Structure and Phase Composition of TiB2–Ag Coating Deposited by Electrical Explosion Spraying. Coatings. 2023; 13(11):1867. https://doi.org/10.3390/coatings13111867
Chicago/Turabian StyleFilyakov, Artem D., Vasilii V. Pochetukha, Denis A. Romanov, and Ekaterina S. Vashchuk. 2023. "Influence of Electron Beam Treatment on Structure and Phase Composition of TiB2–Ag Coating Deposited by Electrical Explosion Spraying" Coatings 13, no. 11: 1867. https://doi.org/10.3390/coatings13111867





