Electrodeposition of Iron Selenide: A Review
Abstract
:1. Introduction
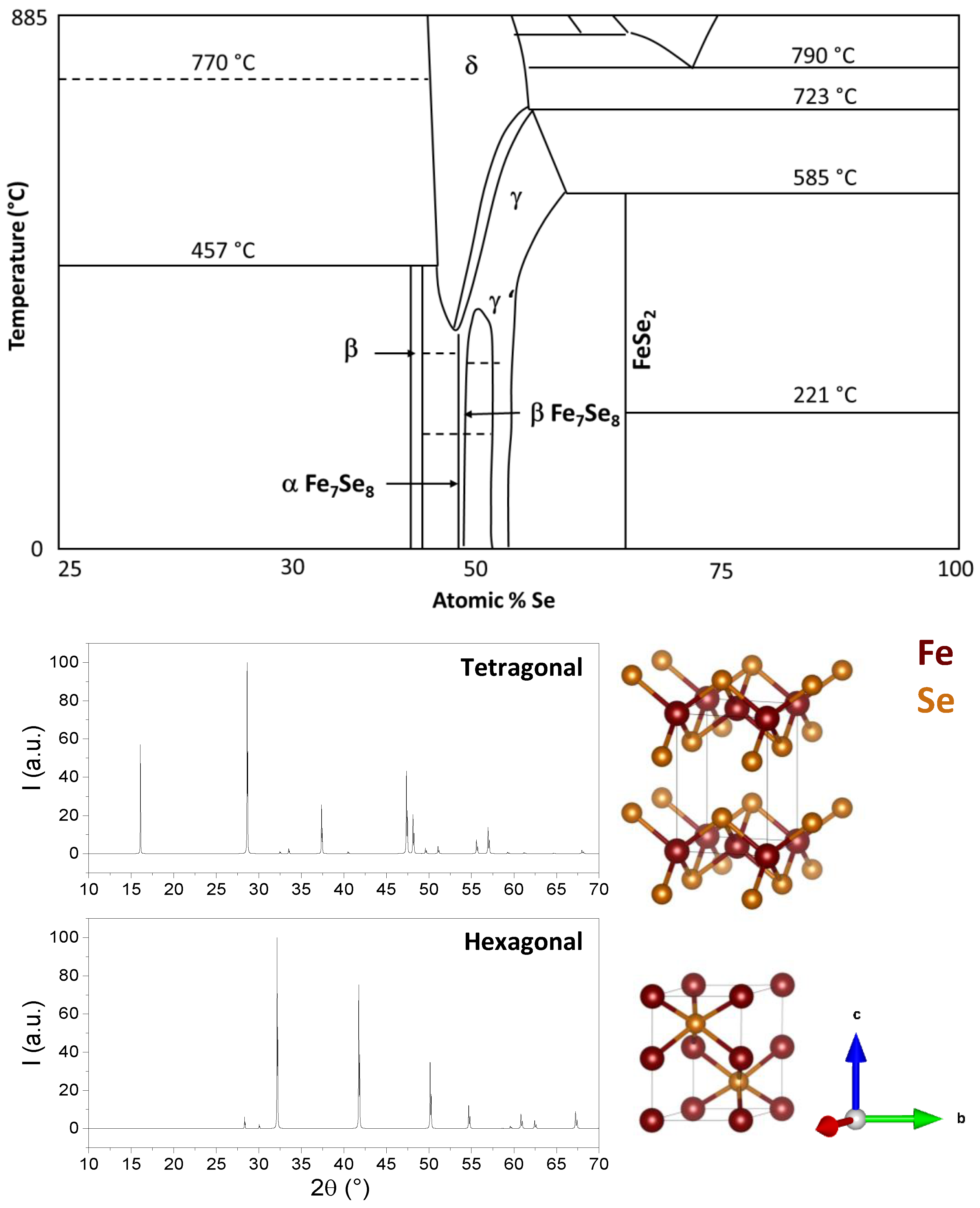
2. FeSe Structure
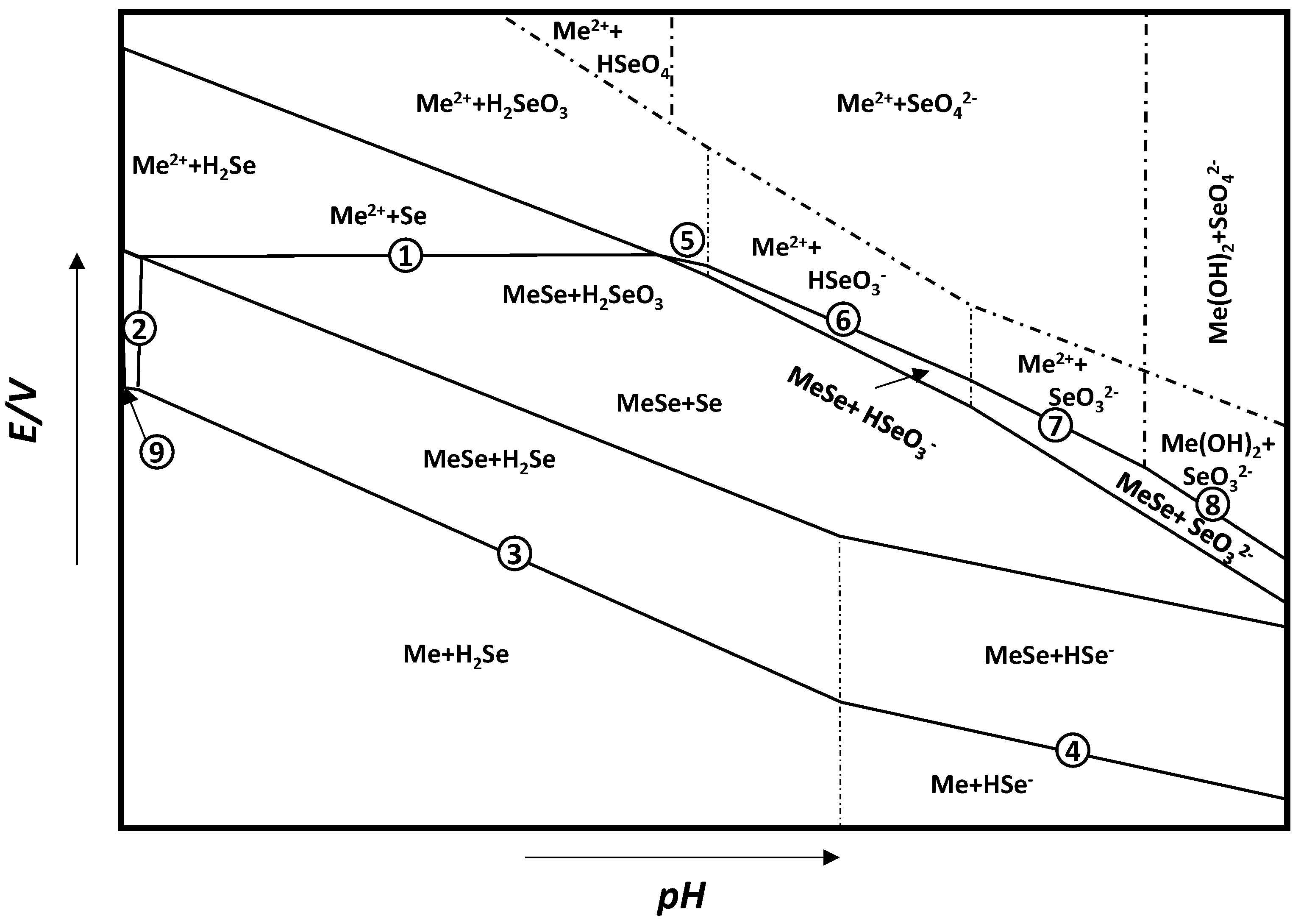
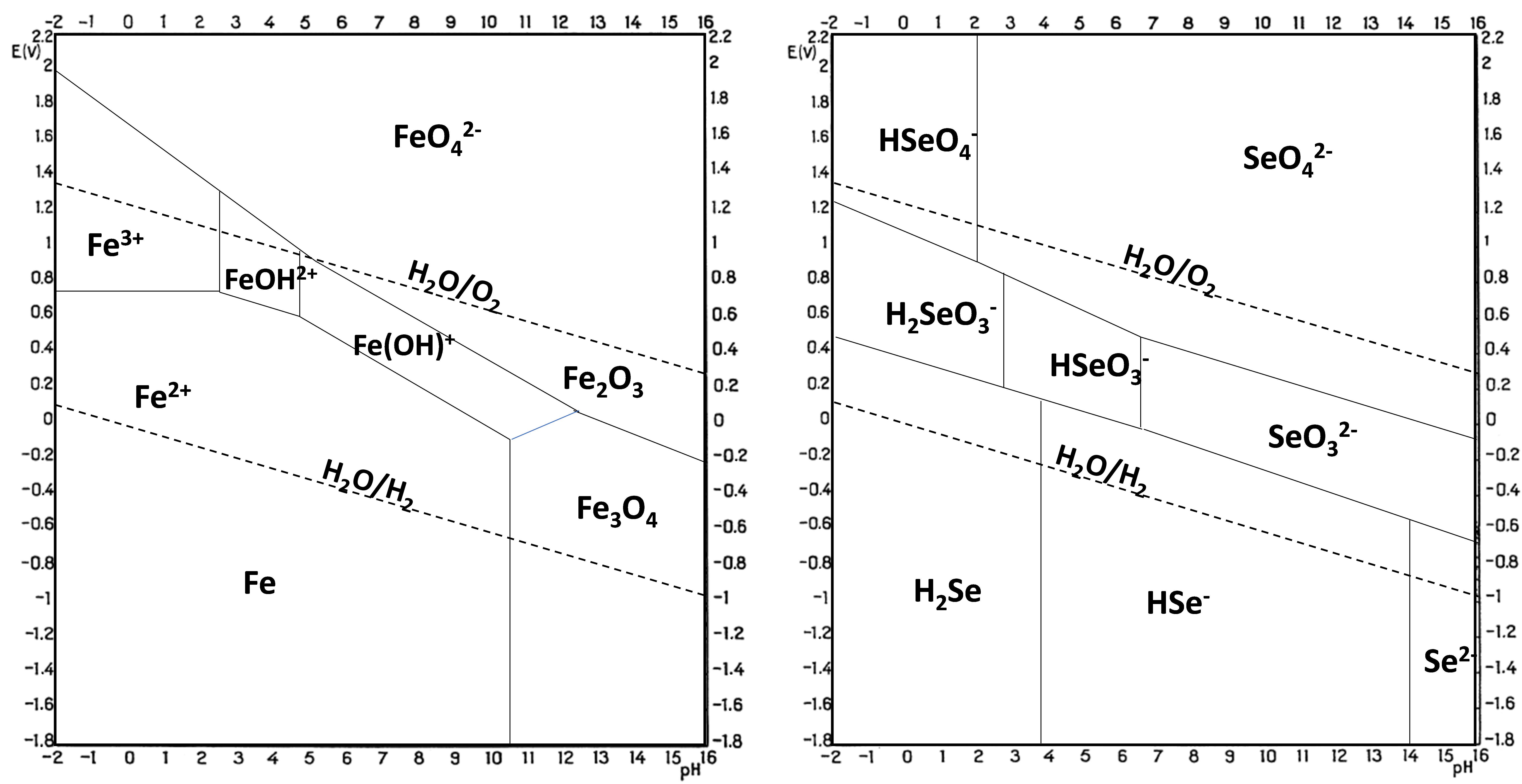
3. Thermodynamic Analysis
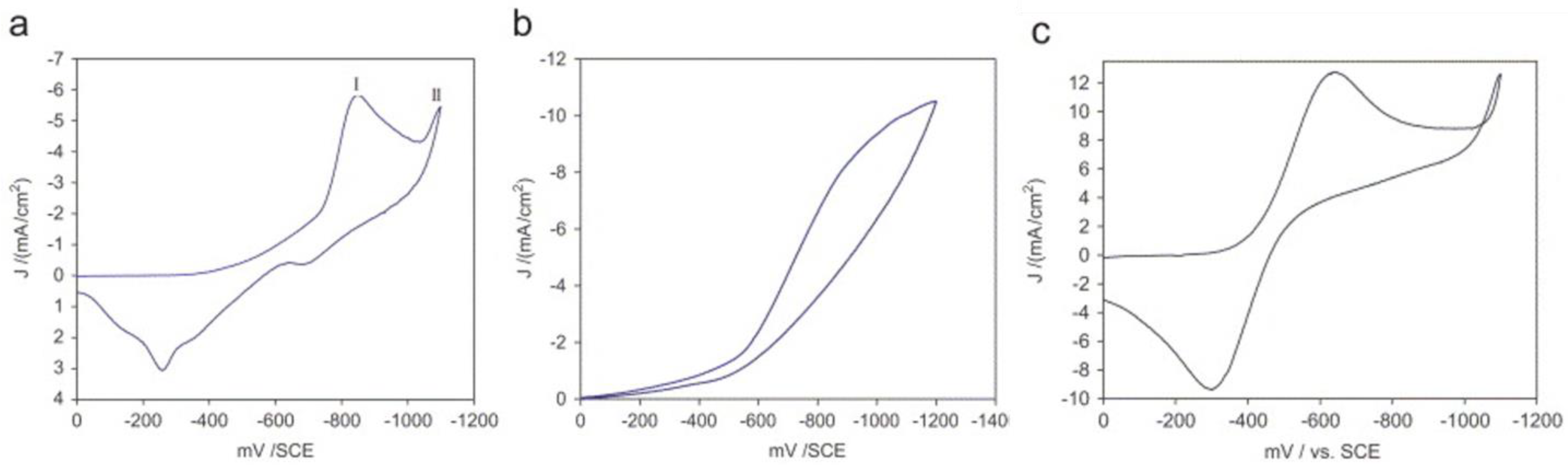
4. Electrodeposition of FeSe
- The electrolyte: i.e., the precursors and the solution. Different precursors give different results even if the element to be deposited is in the same oxidation state. Solubility, solvation effects, and other phenomena indirectly influence the reactions in the solution/on the electrode.
- Electrode materials, working electrode potential (with respect to the reference electrode); even if the working electrode (substrate) is non-reacting, different materials will give different working potentials and, therefore, different results.
- The pH of the solution influences the stability of the electrodes, the precursor salts, the conductivity of the solution, et cetera.
- Additives/complexing agents: used to increase the solubility of precursors, they influence the adsorption of metal ions at the substrate surface, film nucleation, and growth.
- The operation temperature (usually between room temperature and T < 100 °C)
5. Summary of the Literature
5.1. FeCl3 + SeO2 + TEA
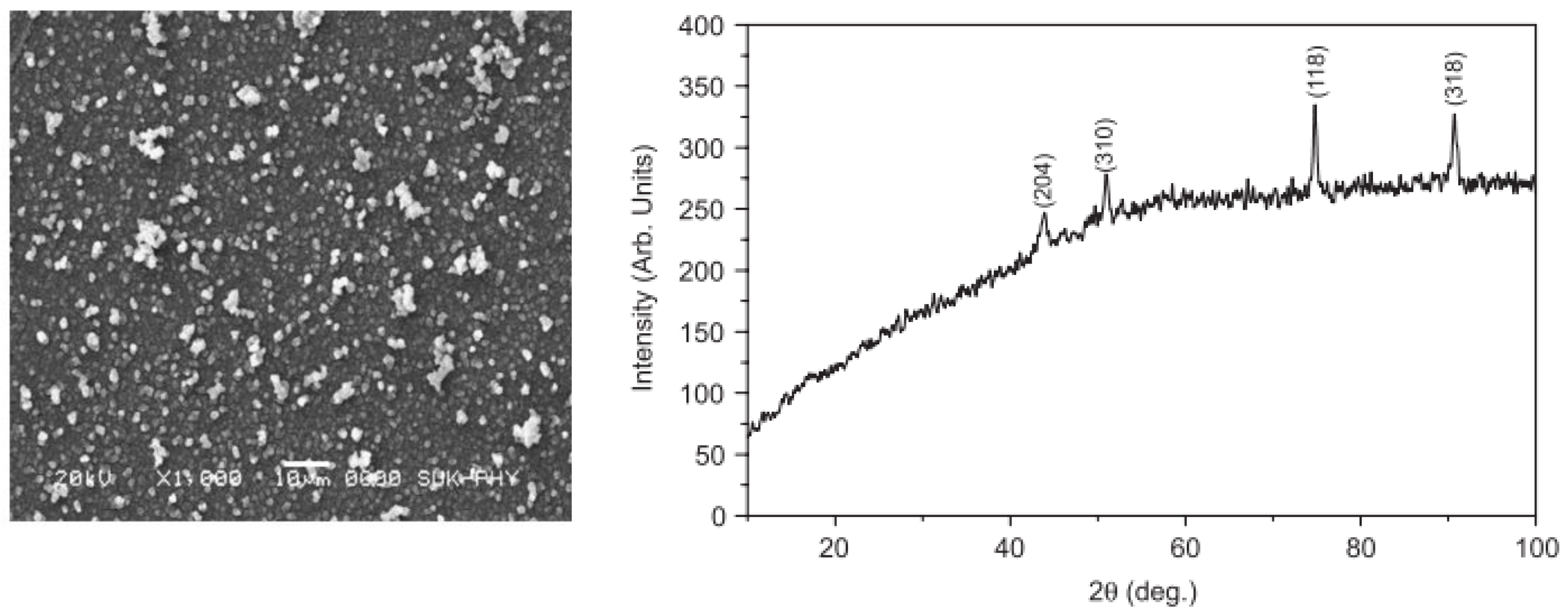
5.1.1. Characterization of Precursors
5.1.2. Characterization of the Samples
5.2. FeSO4 + SeO2
5.2.1. Characterization of Precursors
5.2.2. Effect of Bath Temperature
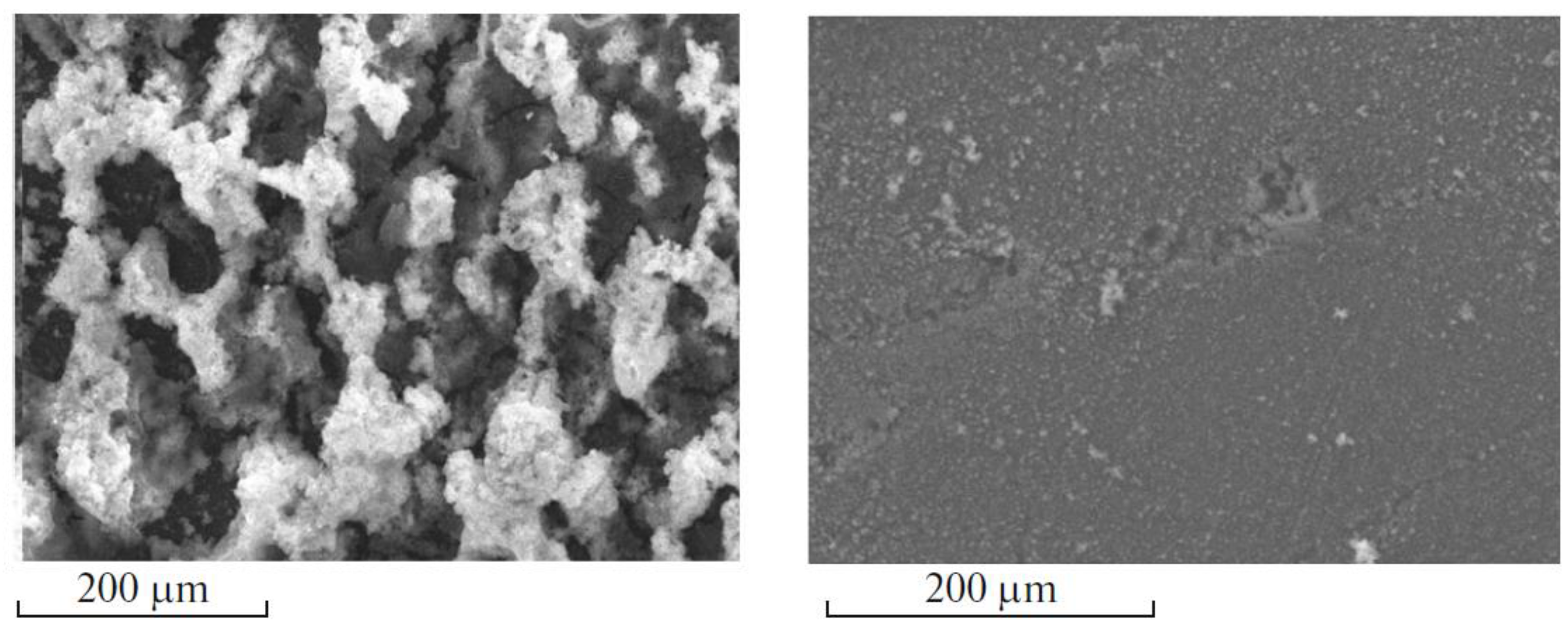
5.2.3. Characterization of the Samples
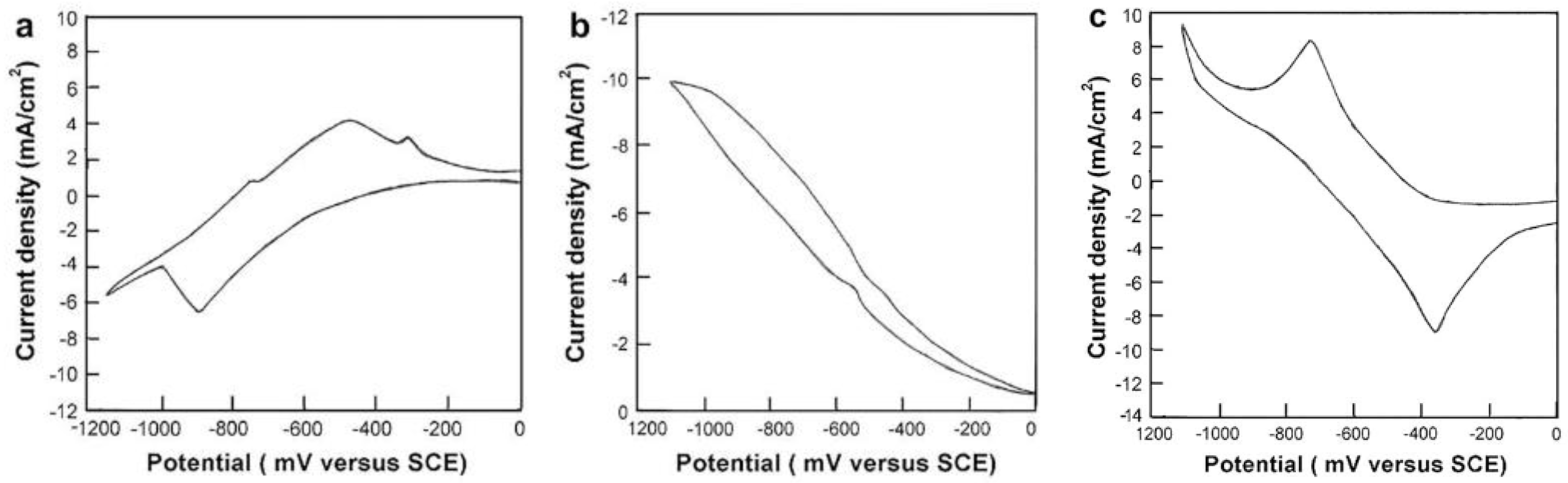
5.2.4. Effect of Deposition Potential
5.2.5. Effect of Solution pH
5.3. Na2SeO3 + (NH4)2Fe(SO4)2 in Na2SO4
5.3.1. Characterization of the Precursors
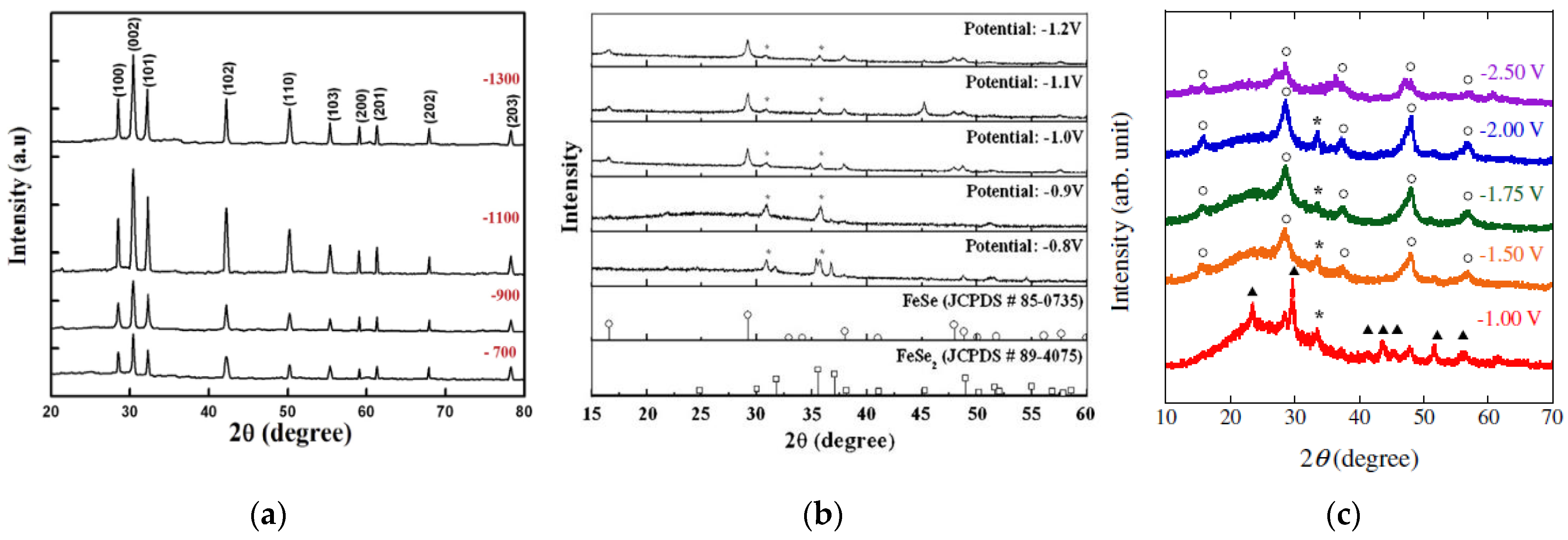
5.3.2. Effect of Bath Temperature
5.3.3. Effect of Deposition Mode
5.4. FeSO4 + H2SeO3 in Na2SO4
5.4.1. Characterization of the Precursors
5.4.2. Effect of Bath Temperature
5.4.3. Characterization of the Samples
5.5. Fe(NO3)3 + H2SeO3
5.5.1. Characterization of the Precursors
5.5.2. Effect of Bath Temperature
5.5.3. Effect of Precursor Concentration
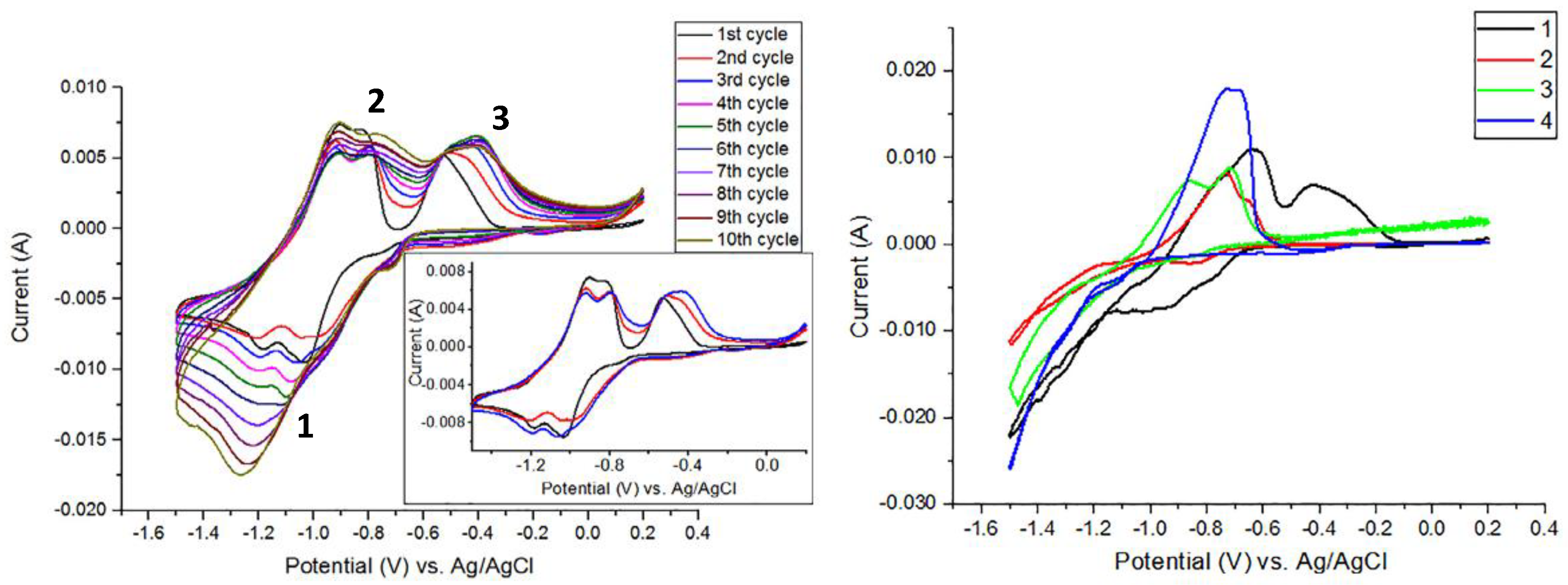
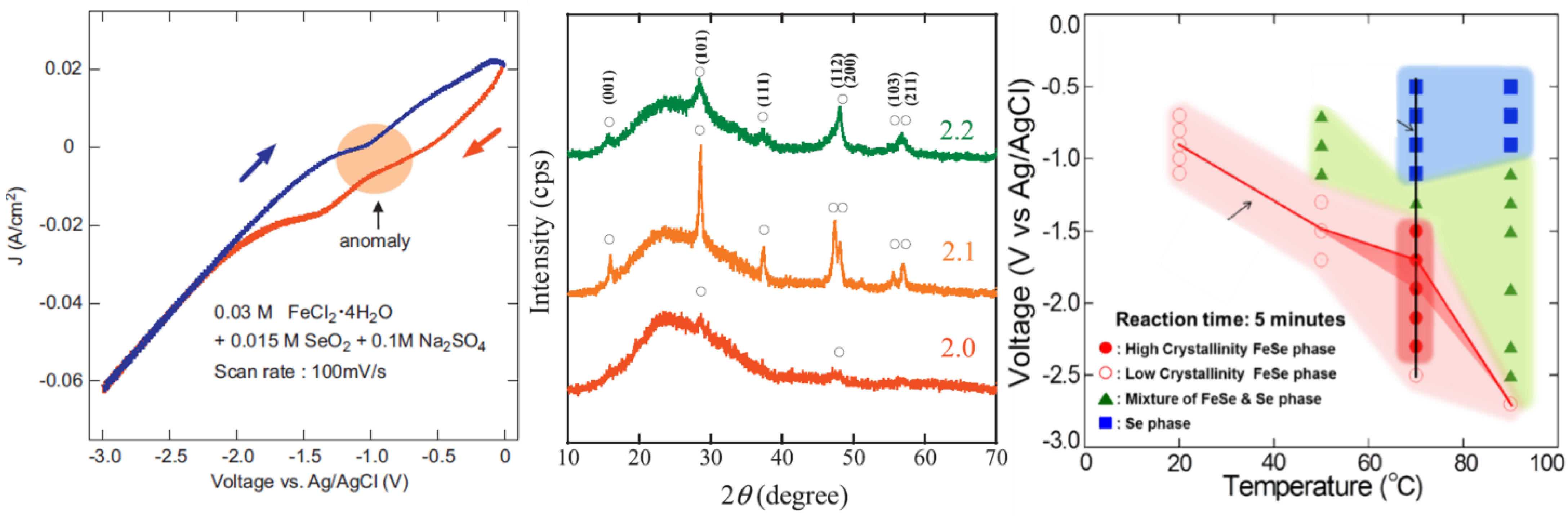
5.6. FeCl2 + SeO2 in Na2SO4
5.6.1. Effect of Deposition Potential/Temperature
5.6.2. Effect of Solution pH
6. Discussion
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zhang, K.; Hu, Z.; Liu, X.; Tao, Z.; Chen, J. FeSe2 microspheres as a high-performance anode material for Na-ion batteries. Adv. Mater. 2015, 27, 3305–3309. [Google Scholar] [CrossRef] [PubMed]
- Yoo, H.; Lee, G.H.; Kim, D.W. FeSe hollow spheroids as electrocatalysts for high-rate Li–O2 battery cathodes. J. Alloys Compd. 2021, 856, 158269. [Google Scholar] [CrossRef]
- Wang, W.; Pan, X.; Liu, W.; Zhang, B.; Chen, H.; Fang, X.; Yao, J.; Dai, S. FeSe2 films with controllable morphologies as efficient counter electrodes for dye-sensitized solar cells. Chem. Commun. 2014, 50, 2618–2620. [Google Scholar] [CrossRef] [PubMed]
- Kwon, J.; Jun, S.W.; Choi, S.I.; Mao, X.; Kim, J.; Koh, E.K.; Kim, Y.H.; Kim, S.K.; Hwang, D.Y.; Kim, C.S.; et al. FeSe quantum dots for in vivo multiphoton biomedical imaging. Sci. Adv. 2019, 5, eaay0044. [Google Scholar] [CrossRef] [PubMed]
- Chanda, D.; Tufa, R.A.; Birdja, Y.Y.; Basu, S.; Liu, S. Hydrothermally/electrochemically decorated FeSe on Ni-foam electrode: An efficient bifunctional electrocatalysts for overall water splitting in an alkaline medium. Int. J. Hydrogen Energy 2020, 45, 27182–27192. [Google Scholar] [CrossRef]
- Majhi, K.C.; Yadav, M. Transition metal chalcogenides based nanocomposites as efficient electrocatalyst for hydrogen evolution reaction over the entire pH range. Int. J. Hydrogen Energy 2020, 45, 24219–24231. [Google Scholar] [CrossRef]
- Cheng, Z.; Gao, M.; Sun, L.; Zheng, D.; Xu, H.; Kong, L.; Gao, C.; Yu, H.; Lin, J. FeSe/FeSe2 Heterostructure as a Low-Cost and High-Performance Electrocatalyst for Oxygen Evolution Reaction. ChemElectroChem 2022, 9, e202200399. [Google Scholar] [CrossRef]
- Hou, B.; Benito-alifonso, D.; Webster, R.F.; Cherns, D.; Galan, M.C.; Fermín, D.J. Synthetic mechanism studies of iron selenides: An emerging class of materials for electrocatalysis. Catalysts 2021, 11, 681. [Google Scholar] [CrossRef]
- Iida, K.; Hänisch, J.; Tarantini, C. Fe-based superconducting thin films on metallic substrates: Growth, characteristics, and relevant properties. Appl. Phys. Rev. 2018, 5, 031304. [Google Scholar] [CrossRef]
- Sakoda, M.; Iida, K.; Naito, M. Recent progress in thin-film growth of Fe-based superconductors: Superior superconductivity achieved by thin films. Supercond. Sci. Technol. 2018, 31, 093001. [Google Scholar] [CrossRef]
- Piperno, L.; Vannozzi, A.; Augieri, A.; Masi, A.; Mancini, A.; Rufoloni, A.; Celentano, G.; Braccini, V.; Cialone, M.; Iebole, M.; et al. High-performance Fe(Se,Te) films on chemical CeO2-based buffer layers. Sci. Rep. 2023, 13, 569. [Google Scholar] [CrossRef] [PubMed]
- Vannozzi, A.; Prili, S.; Sylva, G.; Masi, A.; Armenio, A.A.; Mancini, A.; Pinto, V.; Rufoloni, A.; Piperno, L.; Augieri, A.; et al. Epitaxial Zr-doped CeO2 films by chemical solution deposition as buffer layers for Fe(Se,Te) film growth. Supercond. Sci. Technol. 2020, 33, 9. [Google Scholar] [CrossRef]
- Piperno, L.; Vannozzi, A.; Augieri, A.; Pinto, V.; Armenio, A.A.; Rizzo, F.; Mancini, A.; Rufoloni, A.; Celentano, G.; Braccini, V.; et al. Chemical CeO2-based buffer layers for Fe(Se,Te) films. IEEE Trans. Appl. Supercond. 2022, 32, 1–5. [Google Scholar] [CrossRef]
- Alesini, D.; Braggio, C.; Carugno, G.; Crescini, N.; D’Agostino, D.; Di Gioacchino, D.; Di Vora, R.; Falferi, P.; Gallo, S.; Gambardella, U.; et al. Galactic axions search with a superconducting resonant cavity. Phys. Rev. D 2019, 99, 101101. [Google Scholar] [CrossRef]
- Hussain, R.A.; Badshah, A.; Lal, B. Fabrication, characterization and applications of iron selenide. J. Solid State Chem. 2016, 243, 179–189. [Google Scholar] [CrossRef]
- Pesko, E.; Zukowska, G.; Zero, E.; Krzton-Maziopa, A. Electrocrystallization of nanostructured iron-selenide films for potential application in dye sensitized solar cells. Thin Solid Film. 2020, 709, 138121. [Google Scholar] [CrossRef]
- Demura, S.; Ozaki, T.; Okazaki, H.; Mizuguchi, Y.; Kawasaki, Y.; Deguchi, K.; Watanabe, T.; Hara, H.; Yamaguchi, T.; Takeya, H.; et al. Electrochemical synthesis of iron-based superconductor FeSe films. J. Phys. Soc. Jpn. 2012, 81, 043702. [Google Scholar] [CrossRef]
- Demura, S.; Tanaka, M.; Yamashita, A.; Denholme, S.J.; Okazaki, H.; Fujioka, M.; Yamaguchi, T.; Takeya, H.; Iida, K.; Holzapfel, B.; et al. Electrochemical deposition of FeSe on RABiTS tapes. J. Phys. Soc. Jpn. 2016, 85, 015001. [Google Scholar] [CrossRef]
- Demura, S.; Okazaki, H.; Ozaki, T.; Hara, H.; Kawasaki, Y.; Deguchi, K.; Watanabe, T.; Denholme, S.J.; Mizuguchi, Y.; Yamaguchi, T.; et al. Electrodeposition as a new route to synthesize superconducting FeSe. Solid State Commun. 2013, 154, 40–42. [Google Scholar] [CrossRef]
- Okamoto, H. The fese (ironselenium) system. J. Phase Equilibria 1991, 12, 383–389. [Google Scholar] [CrossRef]
- Sines, I.T.; Schaak, R.E. Phase-selective chemical extraction of selenium and sulfur from nanoscale metal chalcogenides: A general strategy for synthesis, purification, and phase targeting. J. Am. Chem. Soc. 2011, 133, 1294–1297. [Google Scholar] [CrossRef] [PubMed]
- Amcoff; Ericsson, T.; Gismelseed, A. An X-ray, Mössbauer and magnetization investigation of hexagonal FeSe. Z. Krist. New Cryst. Struct. 1994, 209, 197–205. [Google Scholar] [CrossRef]
- Haindl, S. Iron-Based Superconducting Thin Films; Springer International Publishing: Cham, Switzerland, 2021. [Google Scholar] [CrossRef]
- Hsu, F.C.; Luo, J.Y.; Yeh, K.W.; Chen, T.K.; Huang, T.W.; Wu, P.M.; Lee, Y.C.; Huang, Y.L.; Chu, Y.Y.; Yan, D.C.; et al. Superconductivity in the PbO-type structure α-FeSe. Proc. Natl. Acad. Sci. USA 2008, 105, 14262–14264. [Google Scholar] [CrossRef] [PubMed]
- Margadonna, S.; Takabayashi, Y.; McDonald, M.T.; Kasperkiewicz, K.; Mizuguchi, Y.; Takano, Y.; Fitch, A.N.; Suard, E.; Prassides, K. Crystal structure of the new FeSe1−x superconductor. Chem. Commun. 2008, 43, 5607–5609. [Google Scholar] [CrossRef] [PubMed]
- Kumar, R.S.; Zhang, Y.; Sinogeikin, S.; Xiao, Y.; Kumar, S.; Chow, P.; Cornelius, A.L.; Chen, C. Crystal and electronic structure of FeSe at high pressure and low temperature. J. Phys. Chem. B 2010, 114, 12597–12606. [Google Scholar] [CrossRef] [PubMed]
- Ubale, A.U.; Sakhare, Y.S.; Bhute, M.V.; Belkhedkar, M.R.; Singh, A. Size-dependent structural, electrical and optical properties of nanostructured iron selenide thin films deposited by Chemical Bath Deposition Method. Solid State Sci. 2013, 16, 134–142. [Google Scholar] [CrossRef]
- Wang, X.; Li, H.; Huang, Y.; Dong, Z.; Zhong, C.; Liu, J. Tuning tetrahedral structure and electronic properties of FeSe films through strain engineering. J. Phys. Chem. Solids 2020, 145, 109541. [Google Scholar] [CrossRef]
- Hara, Y.; Takase, K.; Yamasaki, A.; Sato, H.; Miyakawa, N.; Umeyama, N.; Ikeda, S.I. Structural and physical properties of FeSe crystals fabricated by the chemical vapor transport method. Phys. C Supercond. Appl. 2010, 470, S313–S314. [Google Scholar] [CrossRef]
- Guterding, D.; Jeschke, H.O.; Valentí, R. Basic electronic properties of iron selenide under variation of structural parameters. Phys. Rev. B 2017, 96, 125107. [Google Scholar] [CrossRef]
- Minakshi, M.; Mitchell, D.R.G.; Munnangi, A.R.; Barlow, A.J.; Fichtner, M. New insights into the electrochemistry of magnesium molybdate hierarchical architectures for high performance sodium devices. Nanoscale 2018, 10, 13277–13288. [Google Scholar] [CrossRef]
- Minakshi, M.; Singh, P.; Issa, T.B.; Thurgate, S.; De Marco, R. Lithium insertion into manganese dioxide electrode in MnO2/Zn aqueous battery: Part III. Electrochemical behavior of γ-MnO2 in aqueous lithium hydroxide electrolyte. J. Power Sources 2006, 153, 165–169. [Google Scholar] [CrossRef]
- Lai, Y.; Han, C.; Yan, C.; Liu, F.; Li, J.; Liu, Y. Thermodynamic analysis on metal selenides electrodeposition. J. Alloys Compd. 2013, 557, 40–46. [Google Scholar] [CrossRef]
- Bouroushian, M. Electrochemistry of Metal Chalcogenides; Springer Science & Business Media: Berlin/Heidelberg, Germany, 2010. [Google Scholar] [CrossRef]
- Ray, A. Electrodeposition of Thin Films for Low-cost Solar Cells. In Electroplating of Nanostructures; IntechOpen: London, UK, 2015. [Google Scholar] [CrossRef]
- Saloniemi, H. Electrodeposition of PbS, PbSe and PbTe Thin Films; VTT Publications: Espoo, Finland, 2000; pp. 2–82. [Google Scholar]
- Gao, Z.; Qi, J.; Chen, M.; Zhang, W.; Cao, R. An Electrodeposited NiSe for Electrocatalytic Hydrogen and Oxygen Evolution Reactions in Alkaline Solution. Electrochim. Acta 2017, 224, 412–418. [Google Scholar] [CrossRef]
- Dhanasekaran, V.; Mahalingam, T.; Rhee, J.K.; Chu, J.P. Structural and optical properties of electrosynthesized ZnSe thin films. Optik 2013, 124, 255–260. [Google Scholar] [CrossRef]
- Kowalik, R.; Kazimierczak, H.; Zabiński, P. Electrodeposition of cadmium selenide. Mater. Sci. Semicond. Process 2016, 50, 43–48. [Google Scholar] [CrossRef]
- Kröger, F.A. Cathodic Deposition and Characterization of Metallic or Semiconducting Binary Alloys or Compounds. J. Electrochem. Soc. 1978, 125, 2028–2034. [Google Scholar] [CrossRef]
- Pourbaix, M. Atlas of Electrochemical Equilibria in Aqueous Solutions; American Association for the Advancement of Science (AAAS): Washington, DC, USA, 1966. [Google Scholar] [CrossRef]
- Cook, W.G.; Olive, R.P. Pourbaix diagrams for the iron-water system extended to high-subcritical and low-supercritical conditions. Corros. Sci. 2012, 55, 326–331. [Google Scholar] [CrossRef]
- Chen, P.Y.; Hu, S.F.; Liu, R.S.; Huang, C.Y. Electrodeposition of nano-dimensioned FeSe. Thin Solid Film. 2011, 519, 8397–8400. [Google Scholar] [CrossRef]
- Zeynalova, A.O.; Javadova, S.P.; Majidzade, V.A.; Aliyev, A.S. Electrochemical Synthesis of Iron Monoselenide Thin Films. Chem. Probl. 2021, 19, 262–271. [Google Scholar] [CrossRef]
- Aricò, A.S.; Antonucci, V.; Antonucci, P.L.; Cocke, D.L.; Giordano, N. A voltammetric study of the electrodeposition chemistry in the FeS system. Electrochim. Acta. 1991, 36, 581–590. [Google Scholar] [CrossRef]
- Asakai, T.; Hioki, A. Evaluation of the Stability of Iron(II) Solutions by Precise Coulometric Titration with Electrogenerated Cerium(IV). Anal. Sci. 2012, 28, 601–606. [Google Scholar] [CrossRef]
- Pawar, S.M.; Moholkar, A.V.; Suryavanshi, U.B.; Rajpure, K.Y.; Bhosale, C.H. Electrosynthesis and characterization of iron selenide thin films. Sol. Energy Mater. Sol. Cells 2007, 91, 560–565. [Google Scholar] [CrossRef]
- Saji, V.S.; Lee, C.W. Selenium electrochemistry. RSC Adv. 2013, 3, 10058–10077. [Google Scholar] [CrossRef]
- Thanikaikarasan, S.; Mahalingam, T.; Sundaram, K.; Kathalingam, A.; Kim, Y.D.; Kim, T. Growth and characterization of electrosynthesized iron selenide thin films. Vacuum 2009, 83, 1066–1072. [Google Scholar] [CrossRef]
- Thanikaikarasan, S.; Perumal, R.; Marjorie, S.R. Influence of potential on structural, compositional, optical and magnetic properties of electrochemically grown iron selenide thin films. J. Alloys Compd. 2020, 848, 156348. [Google Scholar] [CrossRef]
- Thanikaikarasan, S.; Mahalingam, T.; Raja, M.; Kim, T.; Kim, Y.D. Characterization of electroplated FeSe thin films. J. Mater. Sci. Mater. Electron. 2009, 20, 727–734. [Google Scholar] [CrossRef]
- Laurinavichyute, V.K.; Bakhtenkova, S.E.; Drozhzhin, O.A.; Kazakov, S.M.; Antipov, E.V. Electrodeposition of FexSey films from acidic solutions. Russ. J. Electrochem. 2016, 52, 1048–1056. [Google Scholar] [CrossRef]
- Yamashita, A.; Tanaka, M.; Takeya, H.; Takano, Y. Phase diagram of FeSe deposited by electrochemical technique with different temperature and voltage. J. Phys. Soc. Jpn. 2017, 86, 075001. [Google Scholar] [CrossRef]
- Yamashita, A.; Matsumoto, R.; Tanaka, M.; Hara, H.; Iida, K.; Holzapfel, B.; Takeya, H.; Takano, Y. Observation of zero resistance in as-electrodeposited FeSe. Solid State Commun. 2018, 270, 72–75. [Google Scholar] [CrossRef]
- Masi, A.; Frangini, S.; Pumiglia, D.; Della Seta, L.; Masci, A.; McPhail, S.J.; Carlini, M. LaFeO3 perovskite conversion coatings grown on a 13Cr ferritic stainless steel: A corrosion degradation study in simulated solid oxide fuel cell (SOFC) interconnect conditions at 700 °C. Mater. Corros. 2017, 68, 536–545. [Google Scholar] [CrossRef]
- Barkley, D.P. Structure and Pattern Formation in Electrodeposition. In Advances in Electrochemical Sciences and Engineering; Wiley-VCH Verlag GmbH & Co.: Weinheim Germany, 2001. [Google Scholar]
- Banik, S.J.; Akolkar, R. Suppressing Dendritic Growth during Alkaline Zinc Electrodeposition using Polyethylenimine Additive. Electrochim. Acta. 2015, 179, 475–481. [Google Scholar] [CrossRef]
- Shao, W.; Zangari, G. Dendritic growth and morphology selection in copper electrodeposition from acidic sulfate solutions containing chlorides. J. Phys. Chem. C 2009, 113, 10097–10102. [Google Scholar] [CrossRef]
- Stickney, J.L.; Wade, T.L.; Flowers, B.H.; Vaidyanathan, R.; Happek, U. Electrodeposition of Compound Semiconductors by Electrochemical Atomic Layer Epitaxy (EC-ALE). Encycl. Electrochem. 2002. [Google Scholar] [CrossRef]
- Wade, T.; Flowers, B.; Varazo, K.; Lay, M.; Happek, U.; Stickney, J.L. Morphology control in the formation of compound semiconductors using electrochemical atomic layer epitaxy (EC-ALE). In Morphological Evolution of Electrodeposits and Electrochemical Processing in ULSI Fabrication and Electrodeposition of and on Semiconductors IV: Proceedings of the International Symposia; The Electrochemical Society: Pennington, NJ, USA, 2001. [Google Scholar]




| a, b, c, n, m | Stoichiometric Number | |
| Standard Gibbs free energy at 298 K | kJ mol−1 | |
| Standard molar reactive Gibbs free energy | kJ mol−1 | |
| Molar reactive Gibbs free energy | kJ mol−1 | |
| Molar gas constant | 8.314 J K−1 mol−1 at 298 K | |
| Temperature | K | |
| Activity of substance X | ||
| F | Faraday’s constant | 96,485 C mol−1 |
| Standard electrode potential | V | |
| E | Electrode Potential | V |
| Substance | (kJ/mol) | Substance | (kJ/mol) |
|---|---|---|---|
| FeSe | −87.533 | Fe | −8.31 |
| Fe2+ | −35.585 | Fe3+ | 64.332 |
| Se | −12.592 | HSe− | −14.035 |
| H2Se (g) | −35.950 | SeO32− | −525.577 |
| H2SeO3 | −569.233 | HSeO3− | −560.894 |
| Potential–pH formulas for FeSe0.96 | |||
| Fe2+ + 2e− = Fe | E = −0.5985 | ||
| Fe2+ + 0.96Se + 2e− = FeSe0.96 | E = −0.249 | ||
| FeSe0.96 + 1.92H+ + 1.92e− = Fe + 0.96H2Se | E = −0.278 − 0.059pH | ||
| FeSe0.96 + 0.96H+ + 1.92e− = Fe + 0.96HSe− | E = −0.424 − 0.030pH | ||
| Fe2+ + 0.96SeO32− +5.76H+ + 5.84e− = FeSe0.96 + 2.88H2O | E = 0.430 − 0.058pH | ||
| Substrate | Precursors | pH | Fe:Se | Tdep (°C) | Potential (V) | Dep Time (min) | Thermal Treatment | Phase | |
|---|---|---|---|---|---|---|---|---|---|
| [43] | ITO/AAO | FeSo4 + SeO2 | N.D. | 10:1 | 25 | −1 vs. Ag/AgCl | 60 | No | FeSetetra |
| [47] | Stainless steel/FTO | FeCl3 + SeO2+ additives | N.D. | 1:1 | 25 | −1.05 vs. SCE | 15 | No | Fe3Se4 |
| [52] | Glassy carbon | Na2SeO3 + (NH4)2 Fe(SO4)2 | 2.1 | 2:1 to 6:1 | 25 | −1.1 vs. Ag/AgCl | 60 | No | FexSey |
| [44] | Ni | Fe(NO3)3 + H2SeO3 | N.D. | 14:1 | 25–35 | 0.67 vs. Ag/Agcl | 60 | 450 °C, 1 h | Fe42.2Se57.8 |
| [16] | Pt/Au | FeSO4 + H2SeO3 | 2 | 5:1 | 25 | −1.2 vs. Ag/AgCl | 60 | 400 °C, 96 h | FeSe + FeSe2 |
| [49,51] | ITO | FeSO4 + SeO2 | 3.0 | 10:1 | 30–90 | −1.1 vs. SCE | 10–60 | No | FeSetetra |
| [17] | Fe | FeSO4 + SeO2 | 2.3 | 2:1 | 20 | −1.75 vs. Ag/AgCl | 60 | No | FeSetetra |
| [19] | Fe | FeCl2 + SeO2 | 2.1 | 2:1 | 20 | −0.9 vs. Ag/AgCl | 60 | No | FeSetetra |
| [18] | Ni Rabits | FeCl2 + SeO2 | 2.1 | 2:1 | 20 | −0.8 to −1.0 vs. Ag/AgCl | 60 | No | FeSetetra |
| [53] | ITO | FeCl2 + SeO2 | 2.1 | 2:1 | 70 | −1.7 vs. Ag/AgCl | 5 | No | FeSetetra |
| [54] | Ni Rabits | FeCl2 + SeO2 | 2.1 | 2:1 | 70 | −0.9 to −1.1 vs. Ag/AgCl | 5 | No | FeSetetra |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Piperno, L.; Celentano, G.; Sotgiu, G. Electrodeposition of Iron Selenide: A Review. Coatings 2023, 13, 1905. https://doi.org/10.3390/coatings13111905
Piperno L, Celentano G, Sotgiu G. Electrodeposition of Iron Selenide: A Review. Coatings. 2023; 13(11):1905. https://doi.org/10.3390/coatings13111905
Chicago/Turabian StylePiperno, Laura, Giuseppe Celentano, and Giovanni Sotgiu. 2023. "Electrodeposition of Iron Selenide: A Review" Coatings 13, no. 11: 1905. https://doi.org/10.3390/coatings13111905
APA StylePiperno, L., Celentano, G., & Sotgiu, G. (2023). Electrodeposition of Iron Selenide: A Review. Coatings, 13(11), 1905. https://doi.org/10.3390/coatings13111905







