Analysis of Surface and Physicochemical Properties of Novel Hydrogel Materials Supported with Magnetic Nanoparticles
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
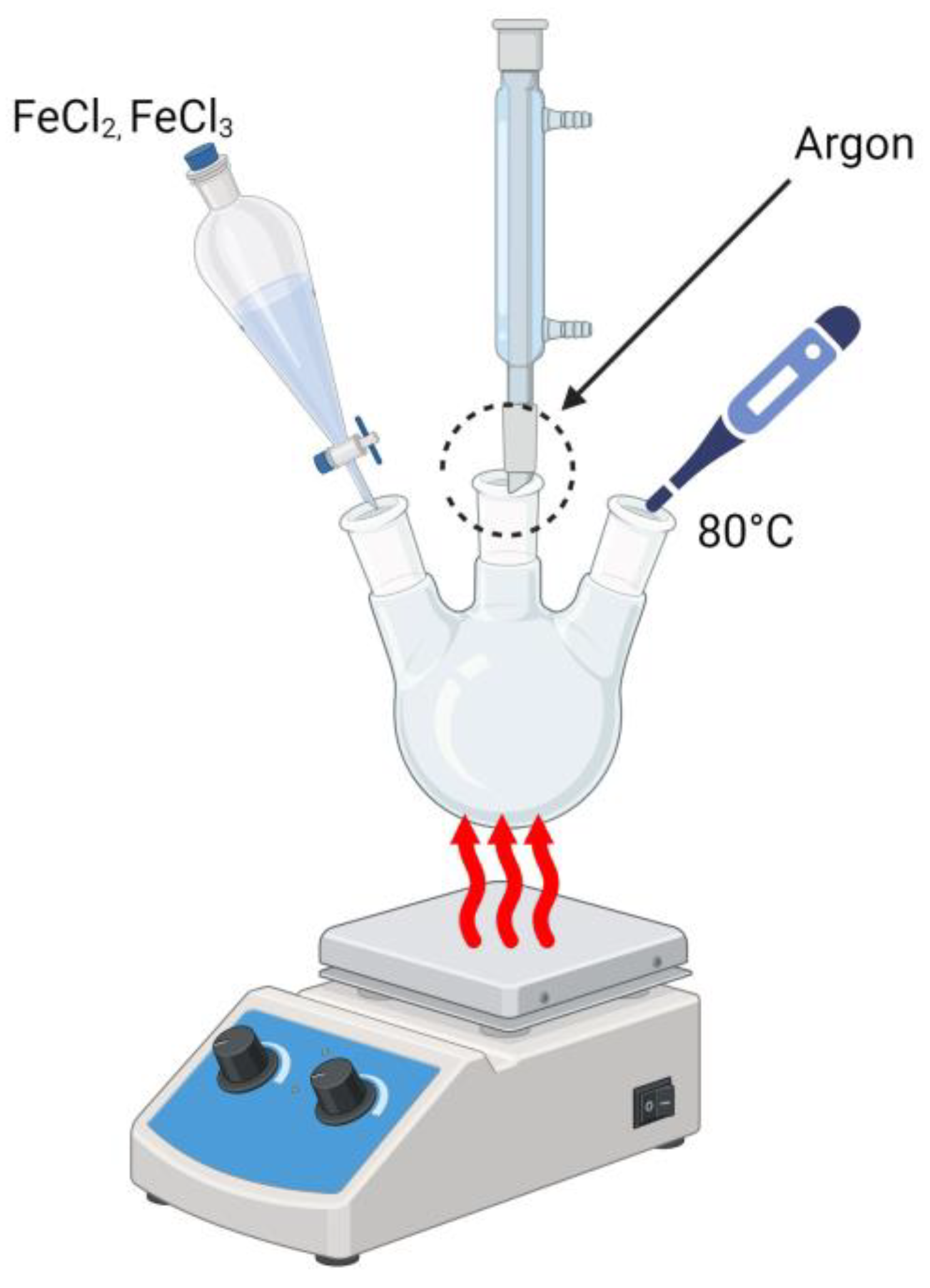
2.2. Synthesis of Magnetic Nanoparticles Modified with Silver
2.3. Synthesis of Hydrogel Materials
2.4. Characterization of the Obtained Nanoparticles
2.5. Infrared Spectroscopy Analysis
2.6. Testing the Sorption Capacity of Hydrogels
2.7. Incubation Analysis in Simulated Body Fluids
2.8. Microscopic Observations and Surface Roughness Profile Analysis
2.9. Statistical Analysis
3. Results
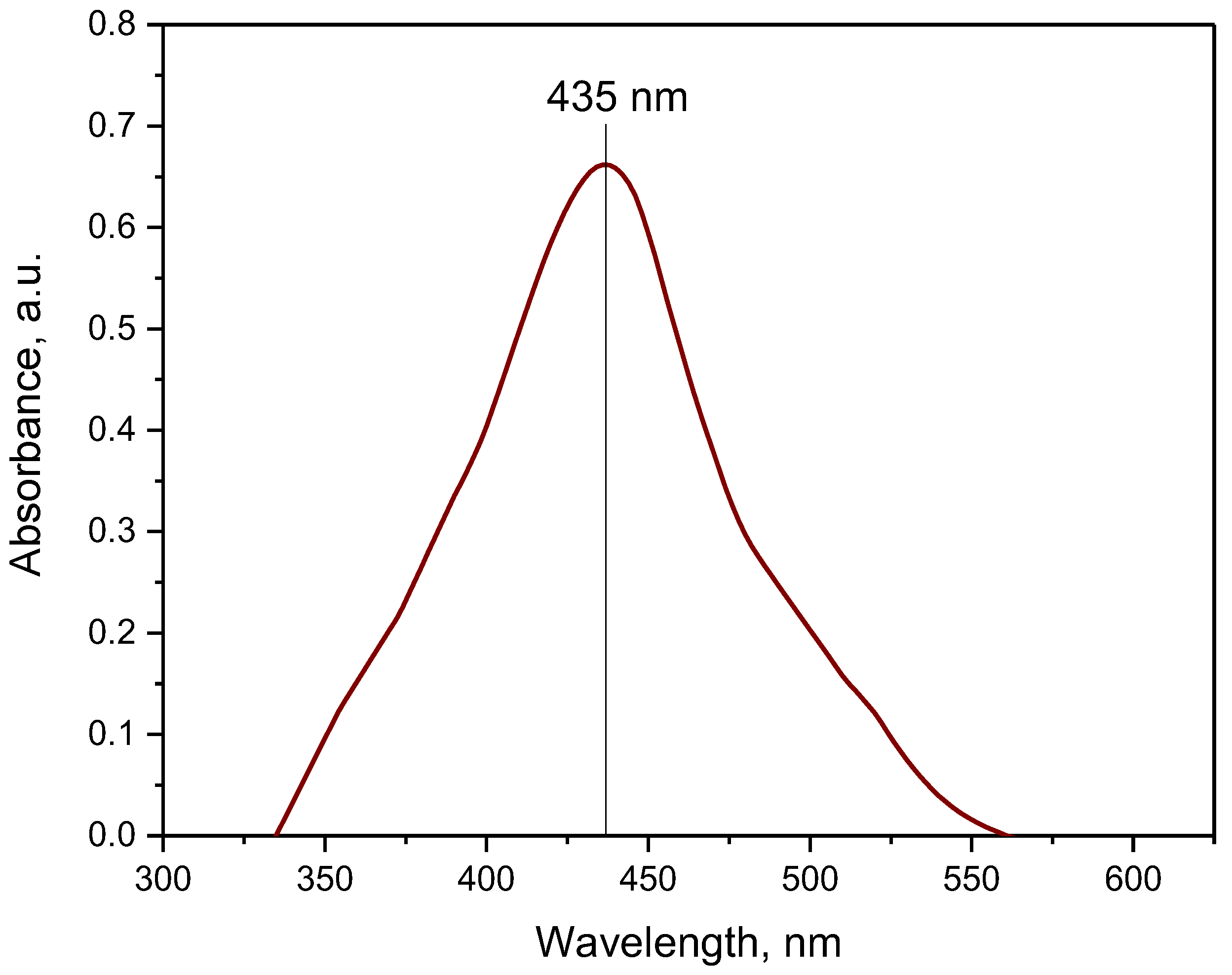
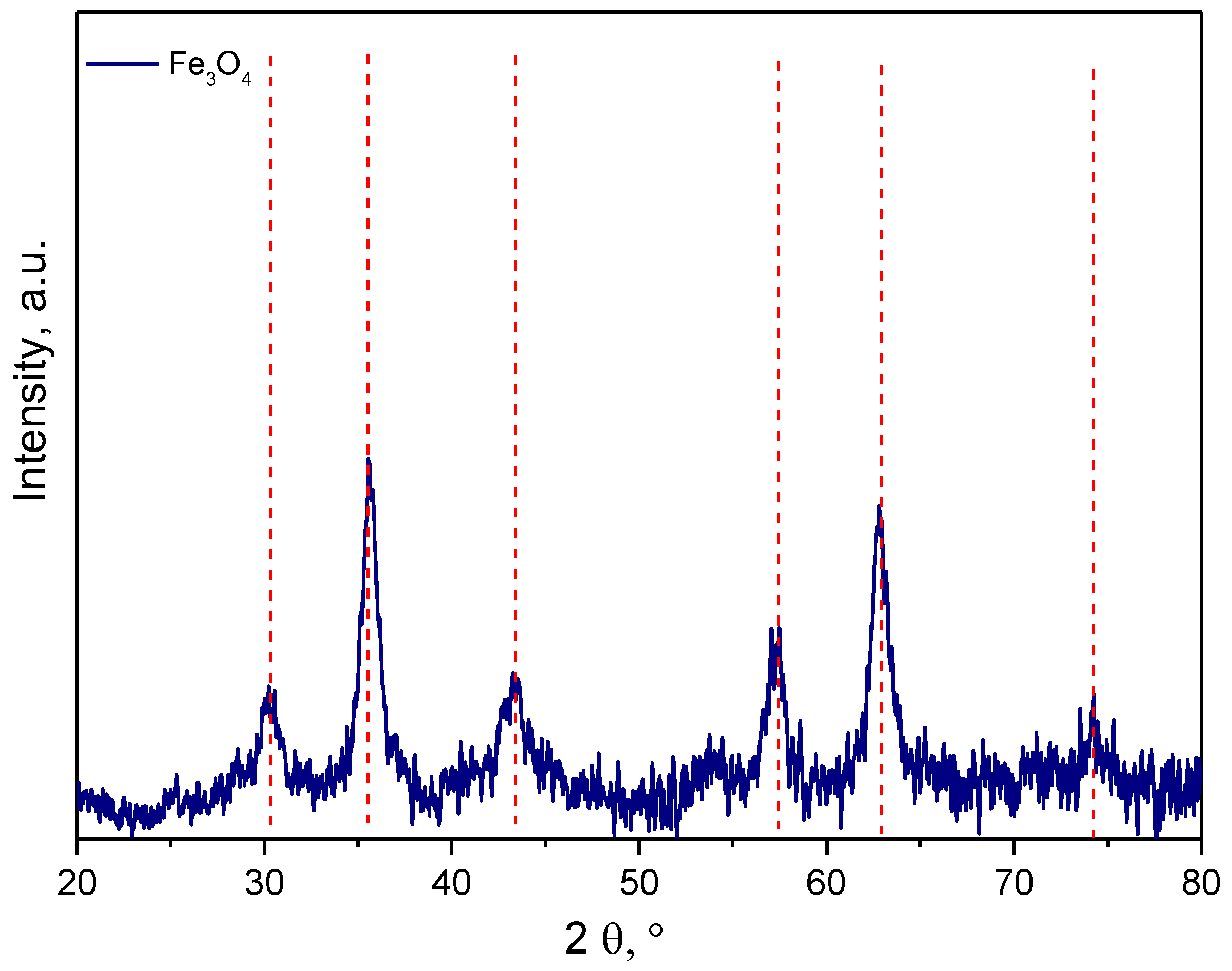
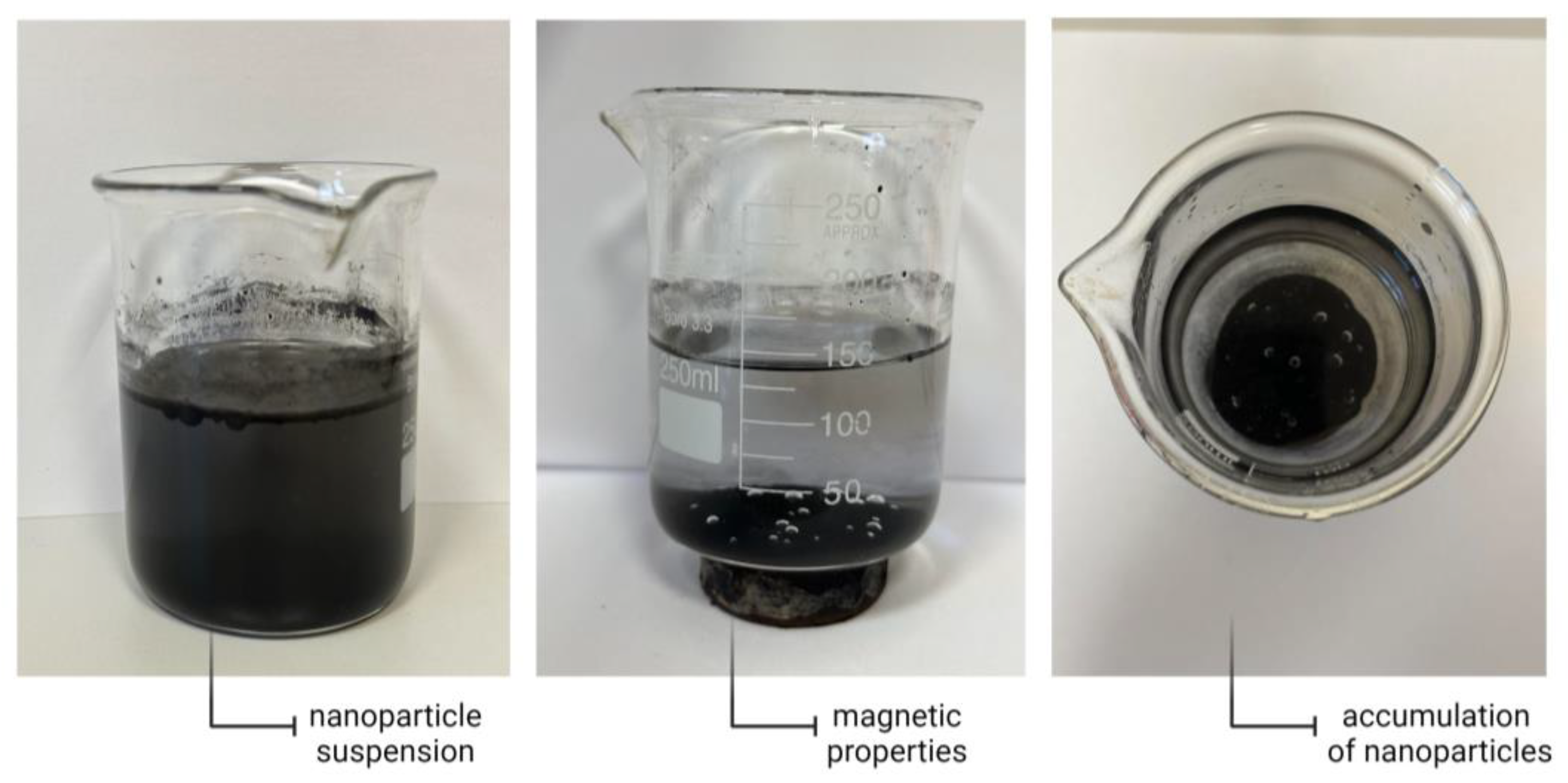
3.1. Characterization of the Obtained Nanoparticles Using FT-IR and UV-Vis Methods
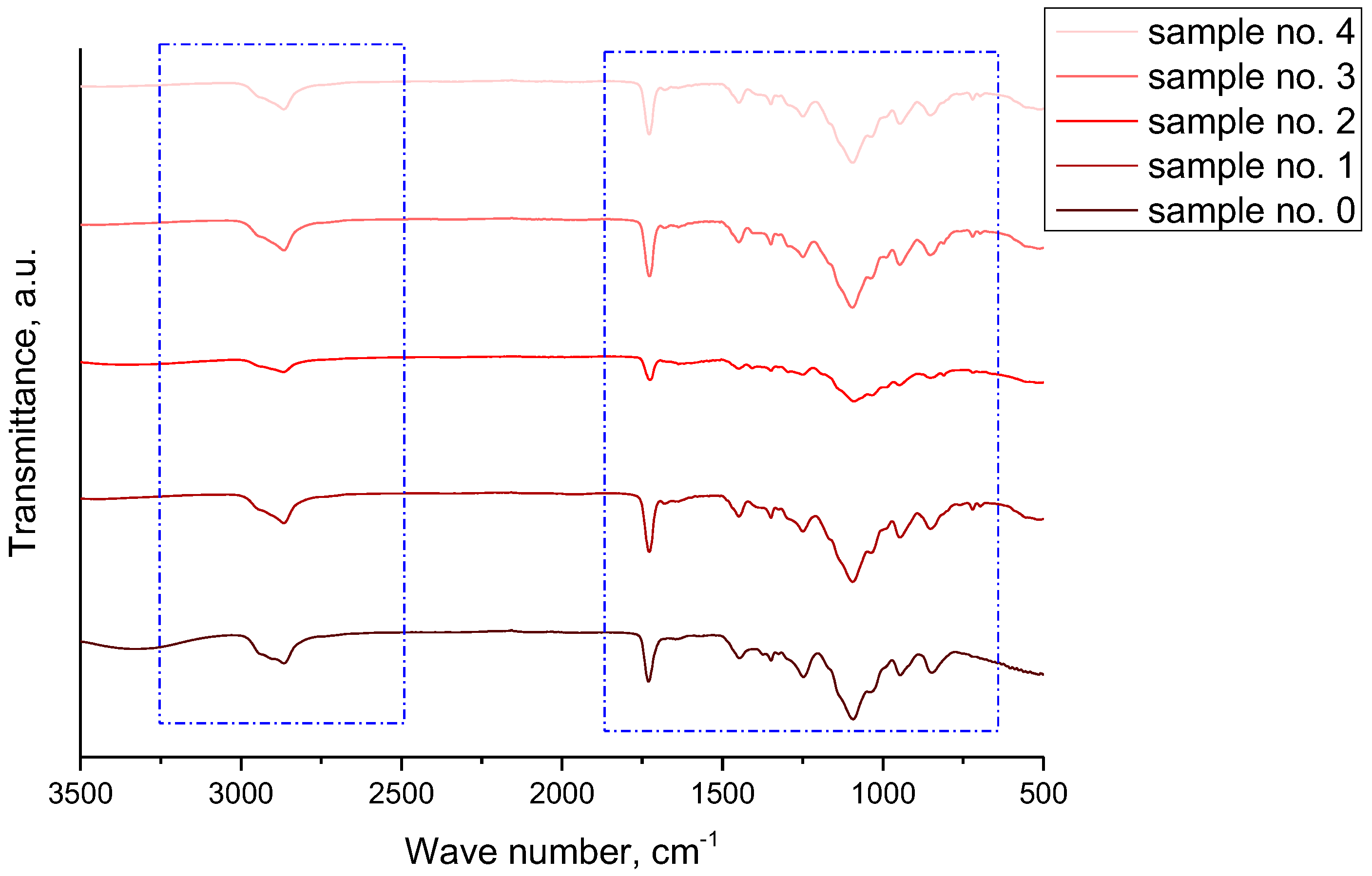
3.2. Infrared Spectroscopy Analysis of Hydrogel Materials
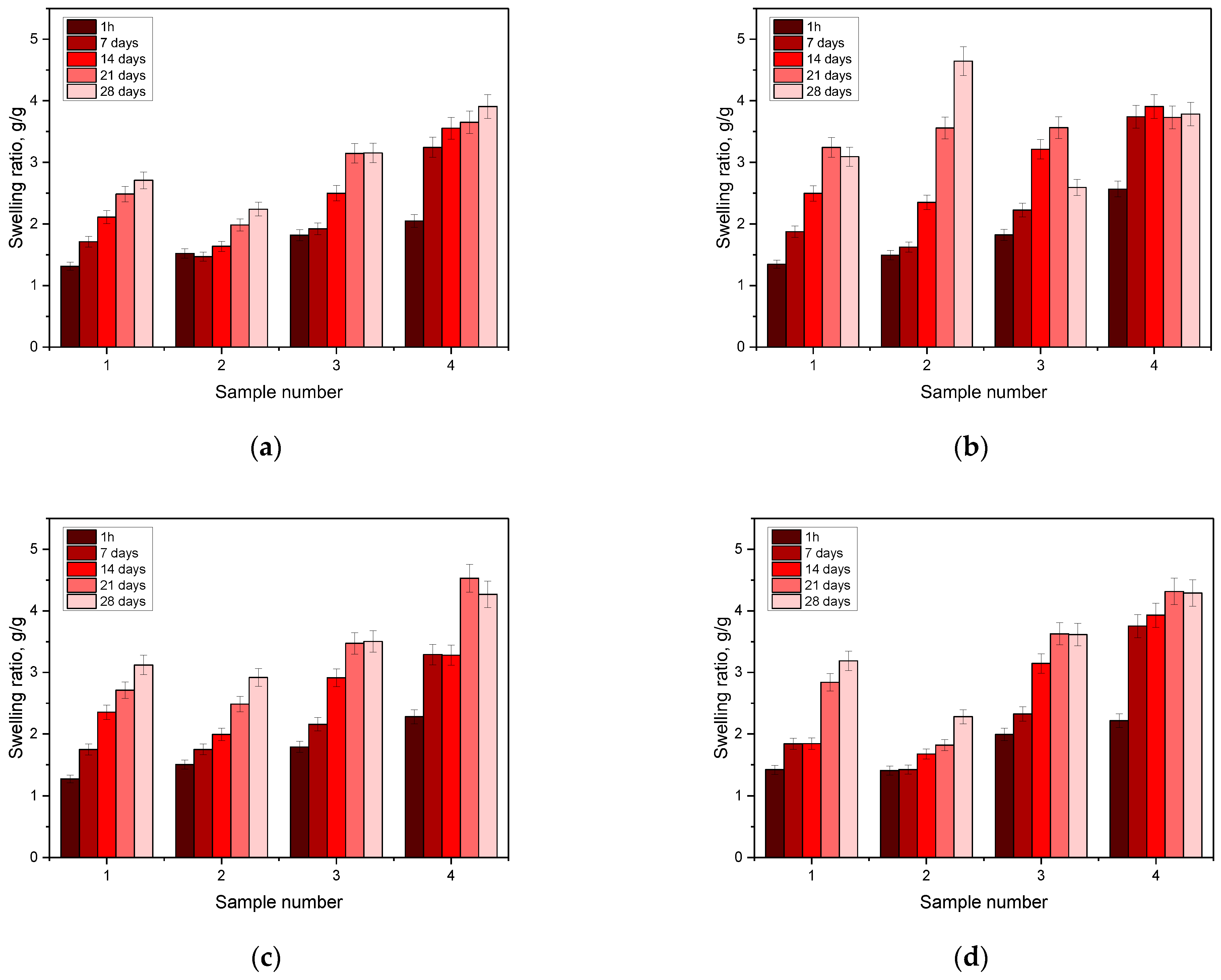
3.3. Testing the Sorption Capacity of Hydrogels
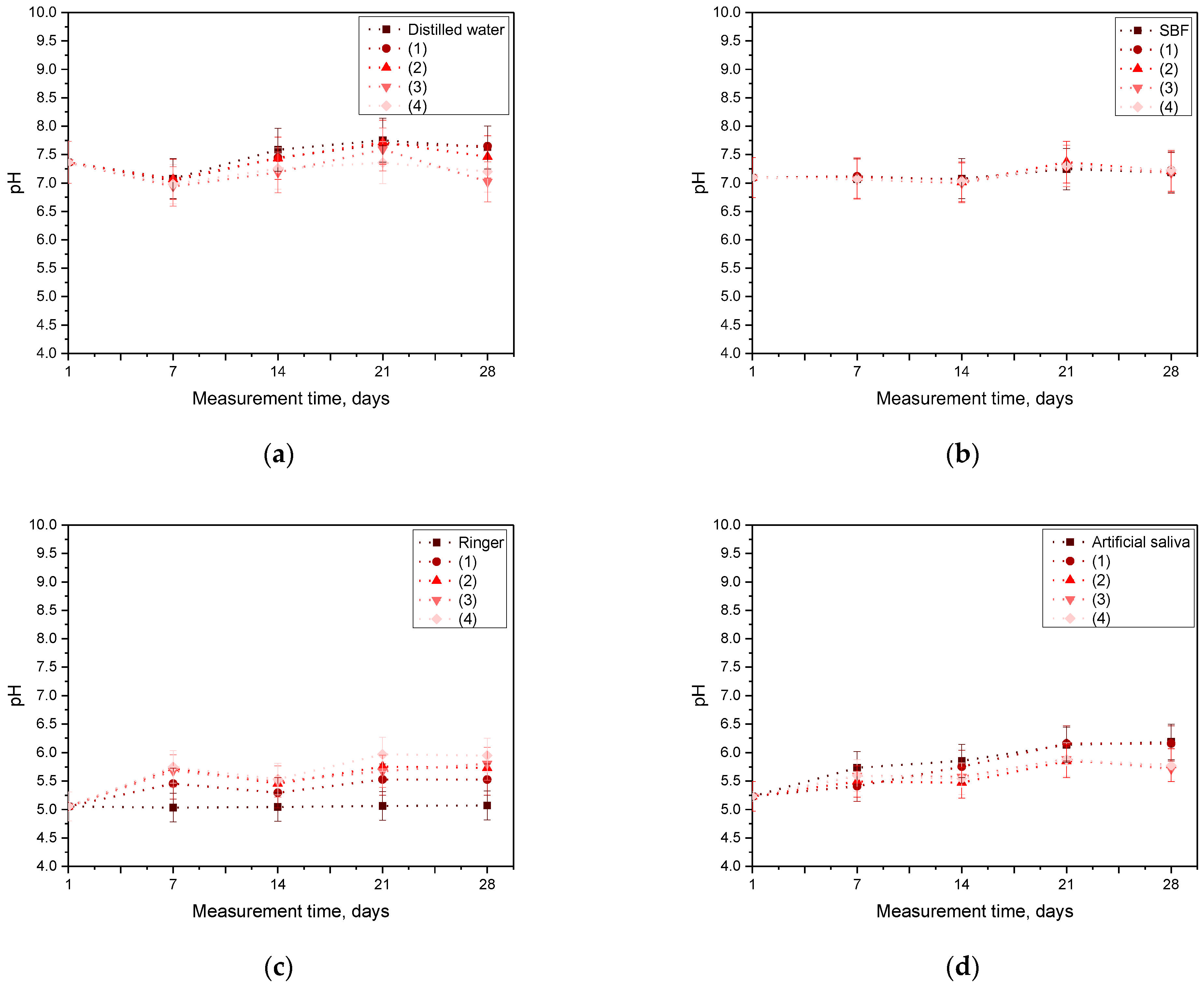
3.4. Incubation Analysis in Simulated Body Fluids
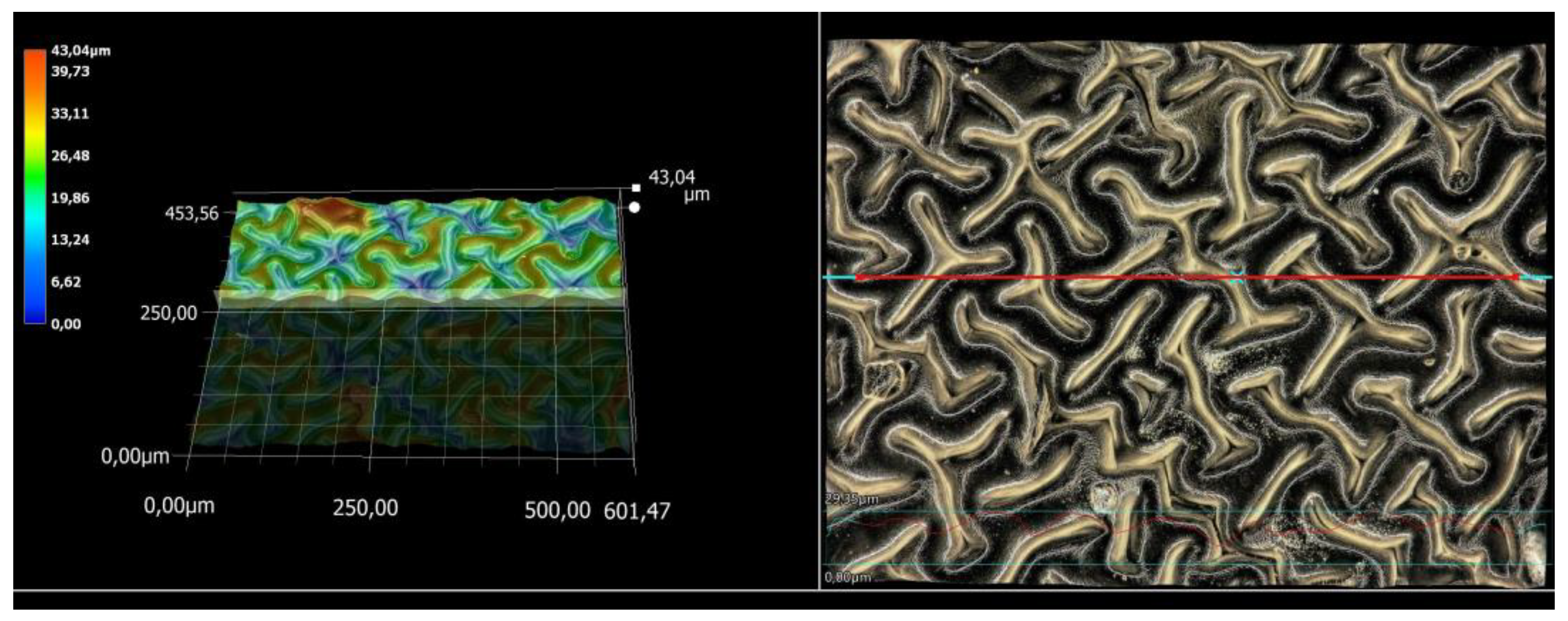
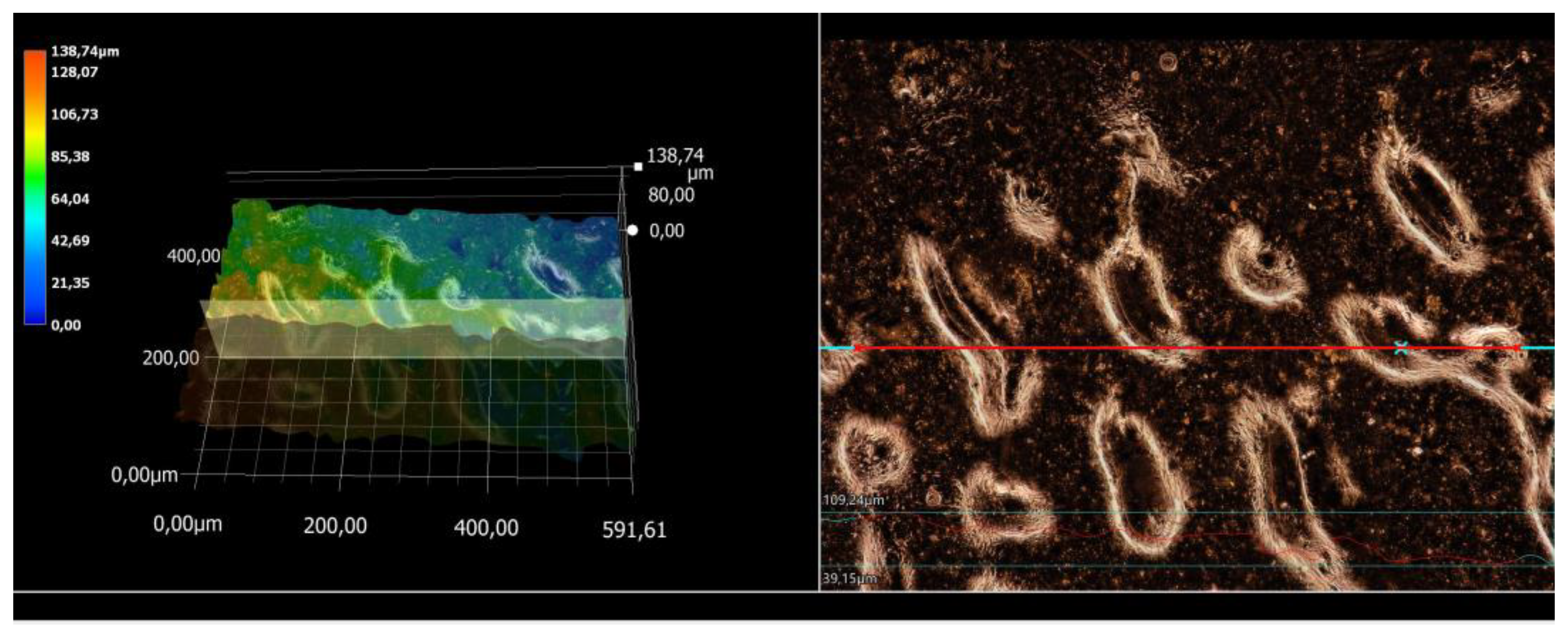
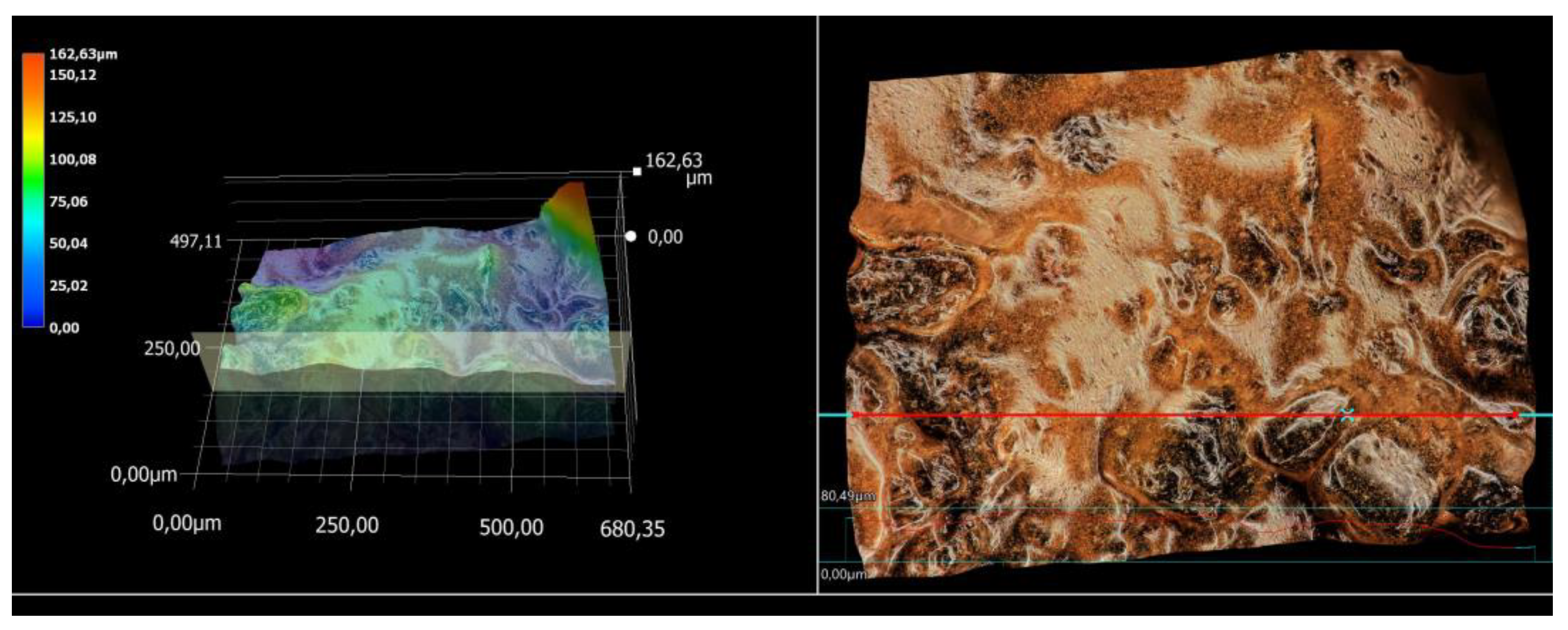
3.5. Microscopic Observations and Surface Roughness Profile Analysis
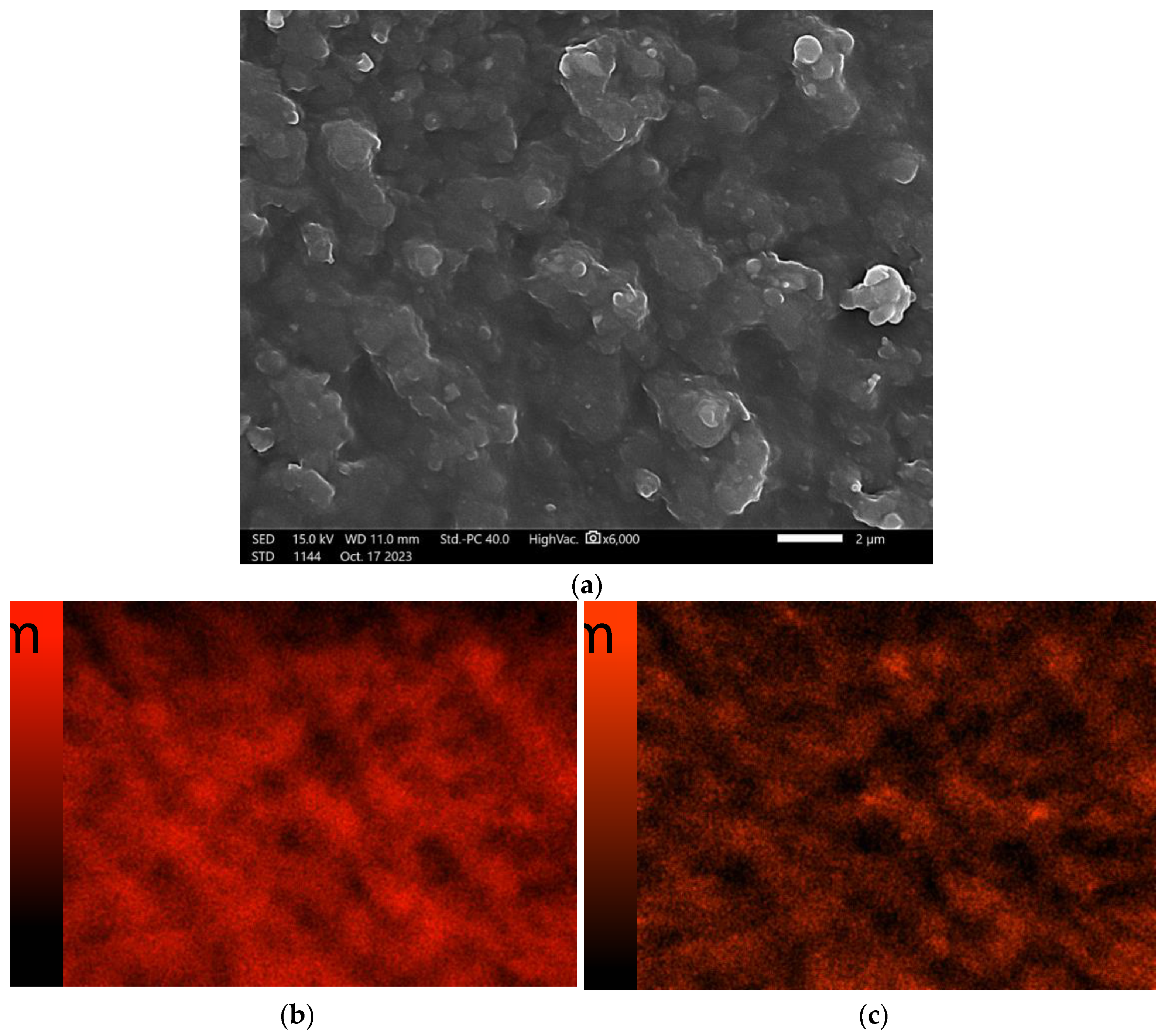
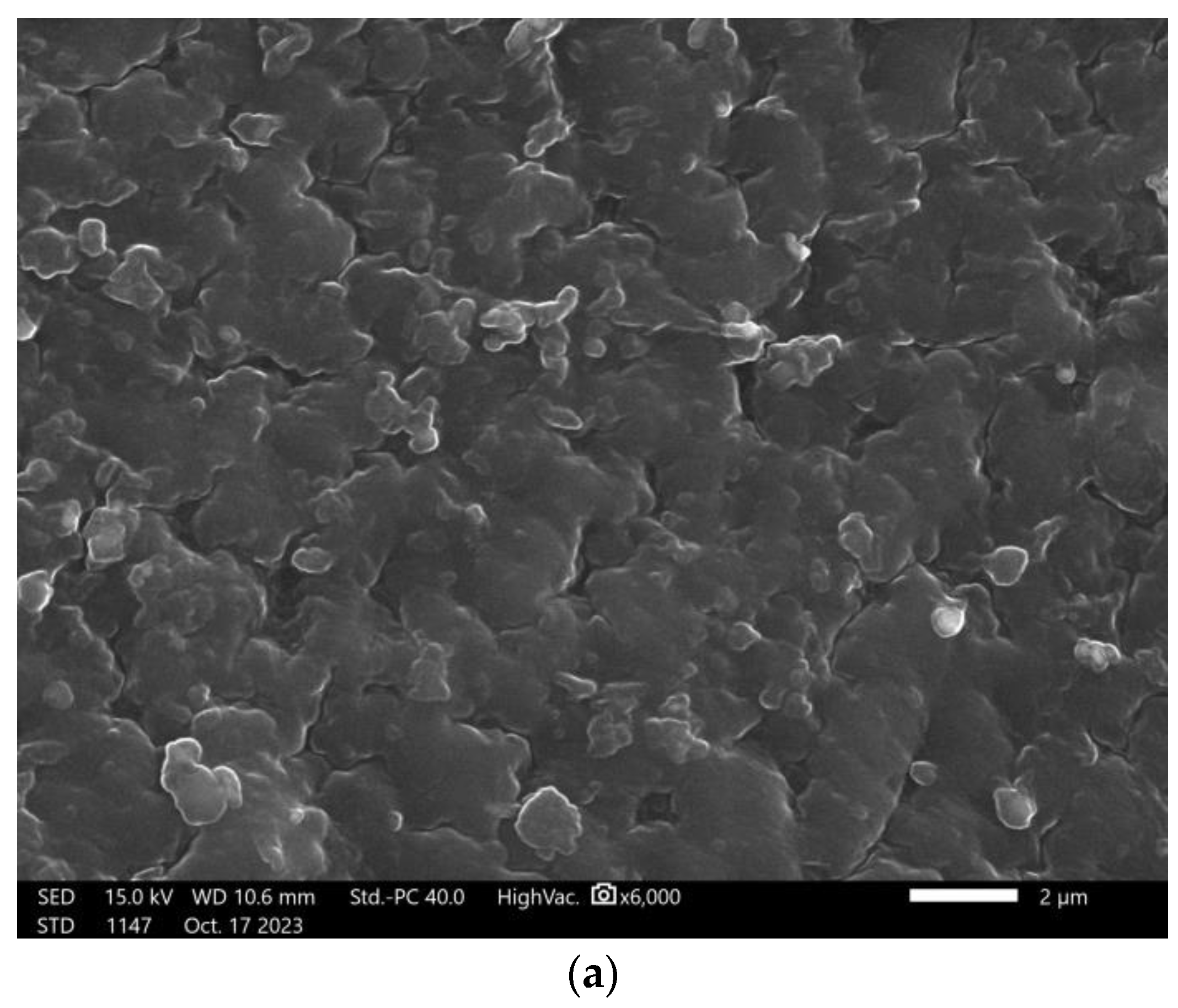
3.6. Scanning Electron Microscopy
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Akbarzadeh, A.; Samiei, M.; Davaran, S. Magnetic Nanoparticles: Preparation, Physical Properties, and Applications in Biomedicine. Nanoscale Res. Lett. 2012, 7, 144. [Google Scholar] [CrossRef] [PubMed]
- Katz, E. Synthesis, Properties and Applications of Magnetic Nanoparticles and Nanowires—A Brief Introduction. Magnetochemistry 2019, 5, 61. [Google Scholar] [CrossRef]
- Issa, B.; Obaidat, I.M.; Albiss, B.A.; Haik, Y. Magnetic Nanoparticles: Surface Effects and Properties Related to Biomedicine Applications. Int. J. Mol. Sci. 2013, 14, 21266–21305. [Google Scholar] [CrossRef] [PubMed]
- Astefanoaei, I.; Gimaev, R.; Zverev, V.; Tishin, A.; Stancu, A. Cubic and Sphere Magnetic Nanoparticles for Magnetic Hyperthermia Therapy: Computational Results. Nanomaterials 2023, 13, 2383. [Google Scholar] [CrossRef]
- Zhang, Y.; Xi, K.; Fu, X.; Sun, H.; Wang, H.; Yu, D.; Li, Z.; Ma, Y.; Liu, X.; Huang, B.; et al. Versatile Metal-Phenolic Network Nanoparticles for Multitargeted Combination Therapy and Magnetic Resonance Tracing in Glioblastoma. Biomaterials 2021, 278, 121163. [Google Scholar] [CrossRef]
- Wang, J.; Chen, P.; Dong, Y.; Xie, H.; Wang, Y.; Soto, F.; Ma, P.; Feng, X.; Du, W.; Liu, B.-F. Designer Exosomes Enabling Tumor Targeted Efficient Chemo/Gene/Photothermal Therapy. Biomaterials 2021, 276, 121056. [Google Scholar] [CrossRef] [PubMed]
- Kiru, L.; Zlitni, A.; Tousley, A.M.; Dalton, G.N.; Wu, W.; Lafortune, F.; Liu, A.; Cunanan, K.M.; Nejadnik, H.; Sulchek, T.; et al. In Vivo Imaging of Nanoparticle-Labeled CAR T Cells. Proc. Natl. Acad. Sci. USA 2022, 119, e2102363119. [Google Scholar] [CrossRef]
- Dorjsuren, B.; Chaurasiya, B.; Ye, Z.; Liu, Y.; Li, W.; Wang, C.; Shi, D.; Evans, C.E.; Webster, T.J.; Shen, Y. Cetuximab-Coated Thermo-Sensitive Liposomes Loaded with Magnetic Nanoparticles and Doxorubicin for Targeted EGFR-Expressing Breast Cancer Combined Therapy. Int. J. Nanomed. 2020, 15, 8201–8215. [Google Scholar] [CrossRef]
- Park, Y.; Demessie, A.A.; Luo, A.; Taratula, O.R.; Moses, A.S.; Do, P.; Campos, L.; Jahangiri, Y.; Wyatt, C.R.; Albarqi, H.A.; et al. Targeted Nanoparticles with High Heating Efficiency for the Treatment of Endometriosis with Systemically Delivered Magnetic Hyperthermia. Small 2022, 18, e2107808. [Google Scholar] [CrossRef]
- Zhang, F.; Lu, G.; Wen, X.; Li, F.; Ji, X.; Li, Q.; Wu, M.; Cheng, Q.; Yu, Y.; Tang, J.; et al. Magnetic Nanoparticles Coated with Polyphenols for Spatio-Temporally Controlled Cancer Photothermal/Immunotherapy. J. Control. Release 2020, 326, 131–139. [Google Scholar] [CrossRef]
- Daya, R.; Xu, C.; Nguyen, N.-Y.T.; Liu, H.H. Angiogenic Hyaluronic Acid Hydrogels with Curcumin-Coated Magnetic Nanoparticles for Tissue Repair. ACS Appl. Mater. Interfaces 2022, 14, 11051–11067. [Google Scholar] [CrossRef] [PubMed]
- Ho, T.-C.; Chang, C.-C.; Chan, H.-P.; Chung, T.-W.; Shu, C.-W.; Chuang, K.-P.; Duh, T.-H.; Yang, M.-H.; Tyan, Y.-C. Hydrogels: Properties and Applications in Biomedicine. Molecules 2022, 27, 2902. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Wu, Y.; Long, L.; Yang, L.; Fu, D.; Hu, C.; Kong, Q.; Wang, Y. Inflammation-Responsive Drug-Loaded Hydrogels with Sequential Hemostasis, Antibacterial, and Anti-Inflammatory Behavior for Chronically Infected Diabetic Wound Treatment. ACS Appl. Mater. Interfaces 2021, 13, 33584–33599. [Google Scholar] [CrossRef] [PubMed]
- Zeng, N.; He, L.; Jiang, L.; Shan, S.; Su, H. Synthesis of Magnetic/PH Dual Responsive Dextran Hydrogels as Stimuli-Sensitive Drug Carriers. Carbohydr. Res. 2022, 520, 108632. [Google Scholar] [CrossRef] [PubMed]
- Ailincai, D.; Mititelu-Tartau, L.; Marin, L. Citryl-Imine-PEG-Ylated Chitosan Hydrogels—Promising Materials for Drug Delivery Applications. Int. J. Biol. Macromol. 2020, 162, 1323–1337. [Google Scholar] [CrossRef] [PubMed]
- Ji, L.; Zhang, F.; Zhu, L.; Jiang, J. An In-Situ Fabrication of Bamboo Bacterial Cellulose/Sodium Alginate Nanocomposite Hydrogels as Carrier Materials for Controlled Protein Drug Delivery. Int. J. Biol. Macromol. 2021, 170, 459–468. [Google Scholar] [CrossRef] [PubMed]
- Cai, X.; He, Y.; Cai, L.; Zhan, J.; Li, Q.; Zhong, S.; Hou, H.; Wang, W.; Qiu, X. An Injectable Elastic Hydrogel Crosslinked with Curcumin-Gelatin Nanoparticles as a Multifunctional Dressing for the Rapid Repair of Bacterially Infected Wounds. Biomater. Sci. 2023, 11, 3227–3240. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.; Huang, R.; Zheng, B.; Guo, W.; Li, C.; He, W.; Wei, Y.; Du, Y.; Wang, H.; Wu, D.; et al. Highly Stretchable, Adhesive, Biocompatible, and Antibacterial Hydrogel Dressings for Wound Healing. Adv. Sci. 2021, 8, 2003627. [Google Scholar] [CrossRef]
- Lobato, N.; Mansur, M.; Ferreira, A. Characterization and Chemical Stability of Hydrophilic and Hydrophobic Magnetic Nanoparticles. Mater. Res. 2017, 20, 736–746. [Google Scholar] [CrossRef]
- Silva, C.; Silva, R.E.; De Figueiredo, A.; Alves, V. Magnetic Solid-Phase Microextraction for Lead Detection in Aqueous Samples Using Magnetite Nanoparticles. J. Braz. Chem. Soc. 2020, 31, 109–115. [Google Scholar] [CrossRef]
- Nalbandian, L.; Patrikiadou, E.; Zaspalis, V.; Patrikidou, A.; Hatzidaki, E.; Papandreou, C. Magnetic Nanoparticles in Medical Diagnostic Applications: Synthesis, Characterization and Proteins Conjugation. Curr. Nanosci. 2015, 12, 455–468. [Google Scholar] [CrossRef]
- Ibekwe, C.; Oyatogun, G.; Esan, T.; Oluwasegun, K. Synthesis and Characterization of Chitosan/Gum Arabic Nanoparticles for Bone Regeneration. Am. J. Mater. Sci. Eng. 2017, 5, 28–36. [Google Scholar] [CrossRef]
- Palumbo, G.; Górny, M.; Banaś, J. Corrosion Inhibition of Pipeline Carbon Steel (N80) in CO2-Saturated Chloride (0.5 M of KCl) Solution Using Gum Arabic as a Possible Environmentally Friendly Corrosion Inhibitor for Shale Gas Industry. J. Mater. Eng. Perform. 2019, 28, 6458–6470. [Google Scholar] [CrossRef]
- Park, S.B.; White, S.B.; Steadman, C.S.; Pechan, T.; Pechanova, O.; Clemente, H.J.; Thirumalai, R.V.K.G.; Willard, S.T.; Ryan, P.L.; Feugang, J.M. Silver-Coated Magnetic Nanocomposites Induce Growth Inhibition and Protein Changes in Foodborne Bacteria. Sci. Rep. 2019, 9, 17499. [Google Scholar] [CrossRef] [PubMed]
- Iglesias-Silva, E.; Rivas, J.; León Isidro, L.M.; López-Quintela, M.A. Synthesis of Silver-Coated Magnetite Nanoparticles. J. Non-Cryst. Solids 2007, 353, 829–831. [Google Scholar] [CrossRef]
- Mehrabi, F.; Shamspur, T.; Sheibani, H.; Mostafavi, A.; Mohamadi, M.; Hakimi, H.; Bahramabadi, R.; Salari, E. Silver-Coated Magnetic Nanoparticles as an Efficient Delivery System for the Antibiotics Trimethoprim and Sulfamethoxazole against E. Coli and S. Aureus: Release Kinetics and Antimicrobial Activity. BioMetals 2021, 34, 1237–1246. [Google Scholar] [CrossRef] [PubMed]
- Bertolucci, E.; Galletti, A.; Antonetti, C.; Marracci, M.; Tellini, B.; Piccinelli, F.; Visone, C. Chemical and Magnetic Properties Characterization of Magnetic Nanoparticles. In Proceedings of the 2015 IEEE International Instrumentation and Measurement Technology Conference (I2MTC) Proceedings, Pisa, Italy, 11–14 May 2015; Volume 2015, pp. 1492–1496. [Google Scholar] [CrossRef]
- Shagholani, H.; Ghoreishi, S.M.; Mousazadeh, M. Improvement of Interaction between PVA and Chitosan via Magnetite Nanoparticles for Drug Delivery Application. Int. J. Biol. Macromol. 2015, 78, 130–136. [Google Scholar] [CrossRef]
- Loh, K.-S.; Lee, Y.H.; Musa, A.; Salmah, A.A.; Zamri, I. Use of Fe3O4 Nanoparticles for Enhancement of Biosensor Response to the Herbicide 2,4-Dichlorophenoxyacetic Acid. Sensors 2008, 8, 5775–5791. [Google Scholar] [CrossRef]
- Bhosale, M.; Ummineni, D.; Sasaki, T.; Nishio-Hamane, D.; Bhanage, B. Magnetically Separable γ-Fe2O3 Nanoparticles: An Efficient Catalyst for Acylation of Alcohols, Phenols, and Amines Using Sonication Energy under Solvent Free Condition. J. Mol. Catal. A Chem. 2015, 404, 8–17. [Google Scholar] [CrossRef]
- Akakuru, O.; Isiuku, B. Chitosan Hydrogels and Their Glutaraldehyde-Crosslinked Counterparts as Potential Drug Release and Tissue Engineering Systems—Synthesis, Characterization, Swelling Kinetics and Mechanism. J. Phys. Chem. Biophys. 2017, 7, 1000256. [Google Scholar] [CrossRef]
- Arulmoorthy, M.P.; Anbarasi, G.; Srinivasan, M.; Vishnupriya, B. Biosynthesis and Characterization of Chitosan Based Hydrogel: A Potential in Vitro Wound Healing Agent. Mater. Today Proc. 2022, 48, 263–275. [Google Scholar] [CrossRef]
- Jamnongkan, T.; Kaewpirom, S. Potassium Release Kinetics and Water Retention of Controlled-Release Fertilizers Based on Chitosan Hydrogels. J. Polym. Environ. 2010, 18, 413–421. [Google Scholar] [CrossRef]
- Imani, M.; Sharifi, S.; Ziaee, F. Monitoring of Polyethylene Glycol-Diacrylate-Based Hydrogel Formation by Real Time NMR Spectroscopy. Iran. Polym. J. 2007, 16, 13–20. [Google Scholar]
- ALSamman, M.T.; Sánchez, J. Chitosan- and Alginate-Based Hydrogels for the Adsorption of Anionic and Cationic Dyes from Water. Polymers 2022, 14, 1498. [Google Scholar] [CrossRef] [PubMed]
- Indrani, D.J.; Lukitowati, F.; Yulizar, Y. Preparation of Chitosan/Collagen Blend Membranes for Wound Dressing: A Study on FTIR Spectroscopy and Mechanical Properties. IOP Conf. Ser. Mater. Sci. Eng. 2017, 202, 12020. [Google Scholar] [CrossRef]
- Vo, T.S.; Vo, T.T.B.C.; Tran, T.T.; Pham, N.D. Enhancement of Water Absorption Capacity and Compressibility of Hydrogel Sponges Prepared from Gelatin/Chitosan Matrix with Different Polyols. Prog. Nat. Sci. Mater. Int. 2022, 32, 54–62. [Google Scholar] [CrossRef]
- Li, Y.; Tan, Y.; Xu, K.; Lu, C.; Liang, X.; Wang, P. In Situ Crosslinkable Hydrogels Formed from Modified Starch and O-Carboxymethyl Chitosan. RSC Adv. 2015, 5, 30303–30309. [Google Scholar] [CrossRef]
- Lv, Q.; Wu, M.; Shen, Y. Enhanced Swelling Ratio and Water Retention Capacity for Novel Super-Absorbent Hydrogel. Colloids Surfaces A Physicochem. Eng. Asp. 2019, 583, 123972. [Google Scholar] [CrossRef]
- Majhy, B.; Priyadarshini, P.; Sen, A.K. Effect of Surface Energy and Roughness on Cell Adhesion and Growth—Facile Surface Modification for Enhanced Cell Culture. RSC Adv. 2021, 11, 15467–15476. [Google Scholar] [CrossRef]
- Gradilla-Orozco, J.L.; Hernández-Jiménez, J.Á.; Robles-Vásquez, O.; Cortes-Ortega, J.A.; Renteria-Urquiza, M.; Lomelí-Ramírez, M.G.; Rendón, J.G.T.; Jiménez-Amezcua, R.M.; García-Enriquez, S. Physicomechanical and Morphological Characterization of Multi-Structured Potassium-Acrylate-Based Hydrogels. Gels 2022, 8, 627. [Google Scholar] [CrossRef]
- Kaberova, Z.; Karpushkin, E.; Nevoralová, M.; Vetrík, M.; Šlouf, M.; Dušková-Smrčková, M. Microscopic Structure of Swollen Hydrogels by Scanning Electron and Light Microscopies: Artifacts and Reality. Polymers 2020, 12, 578. [Google Scholar] [CrossRef]



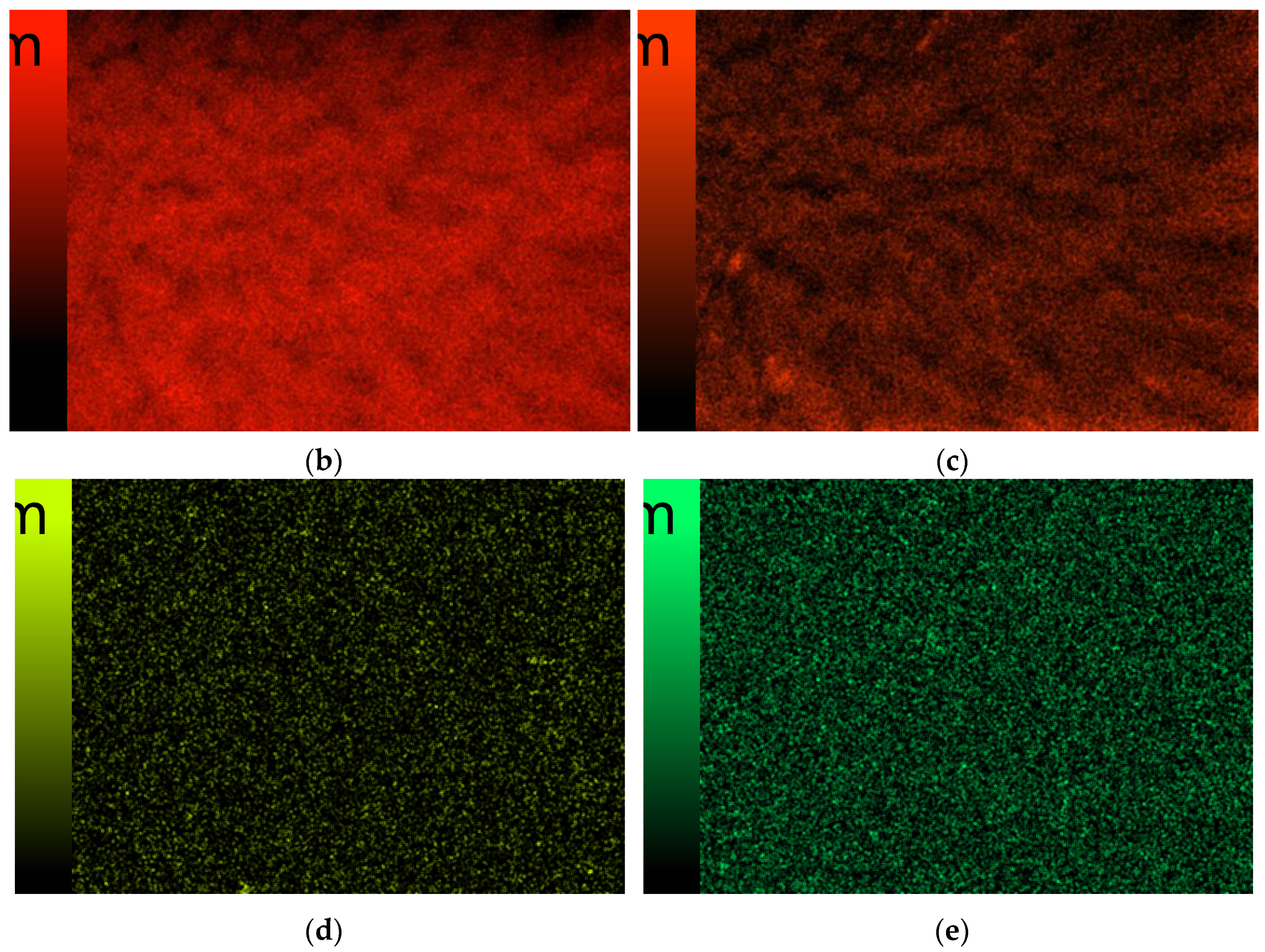
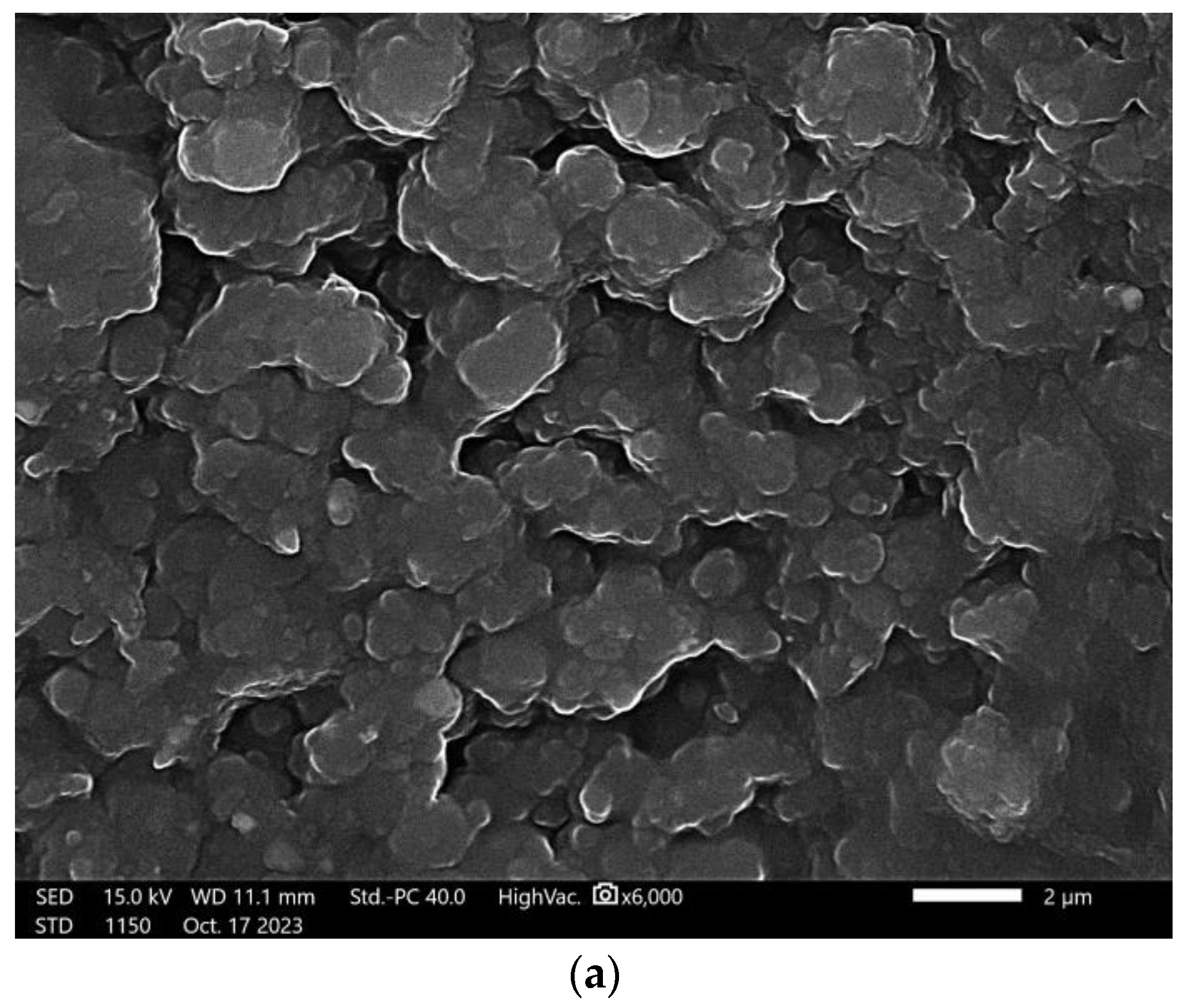
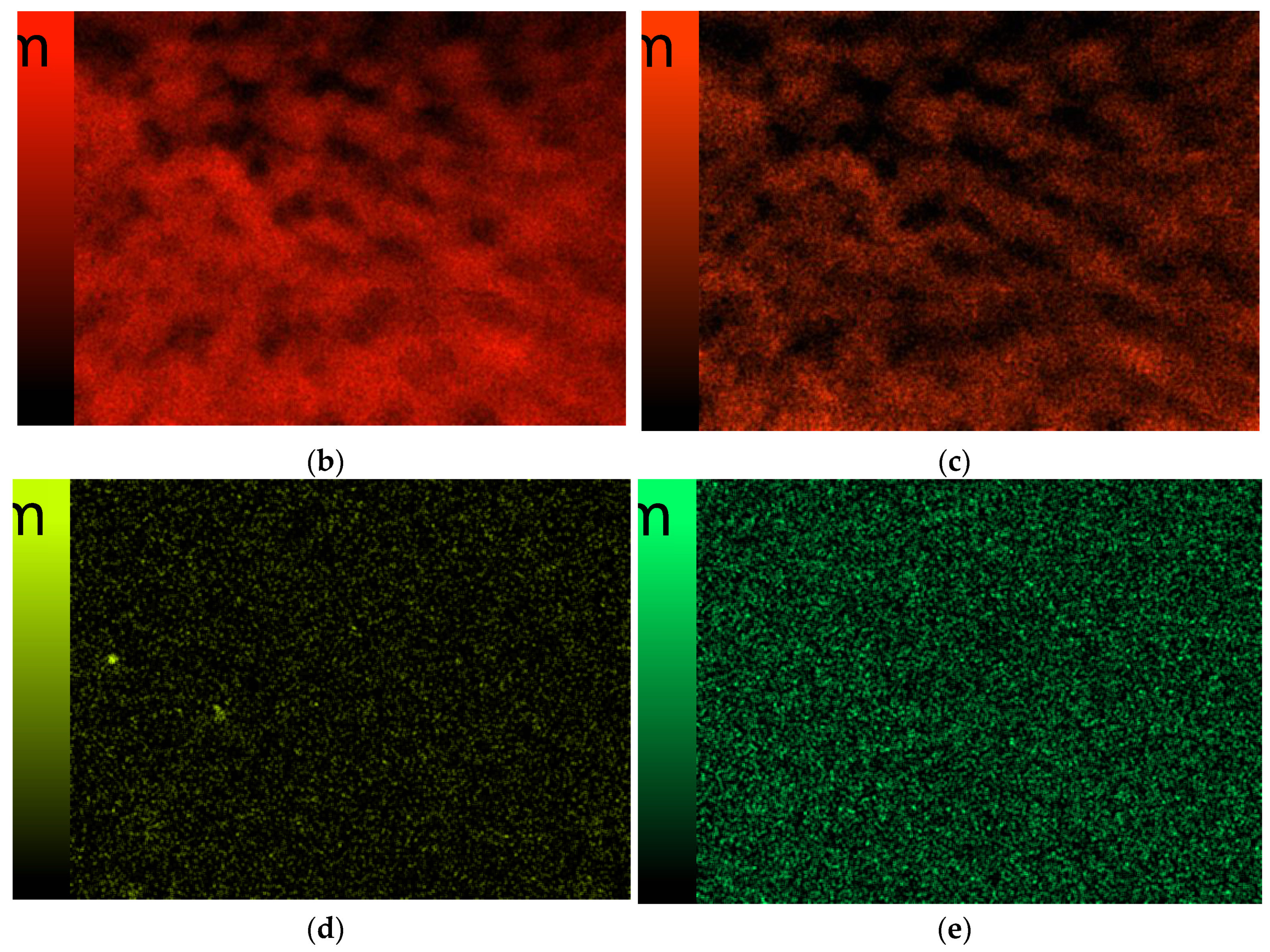

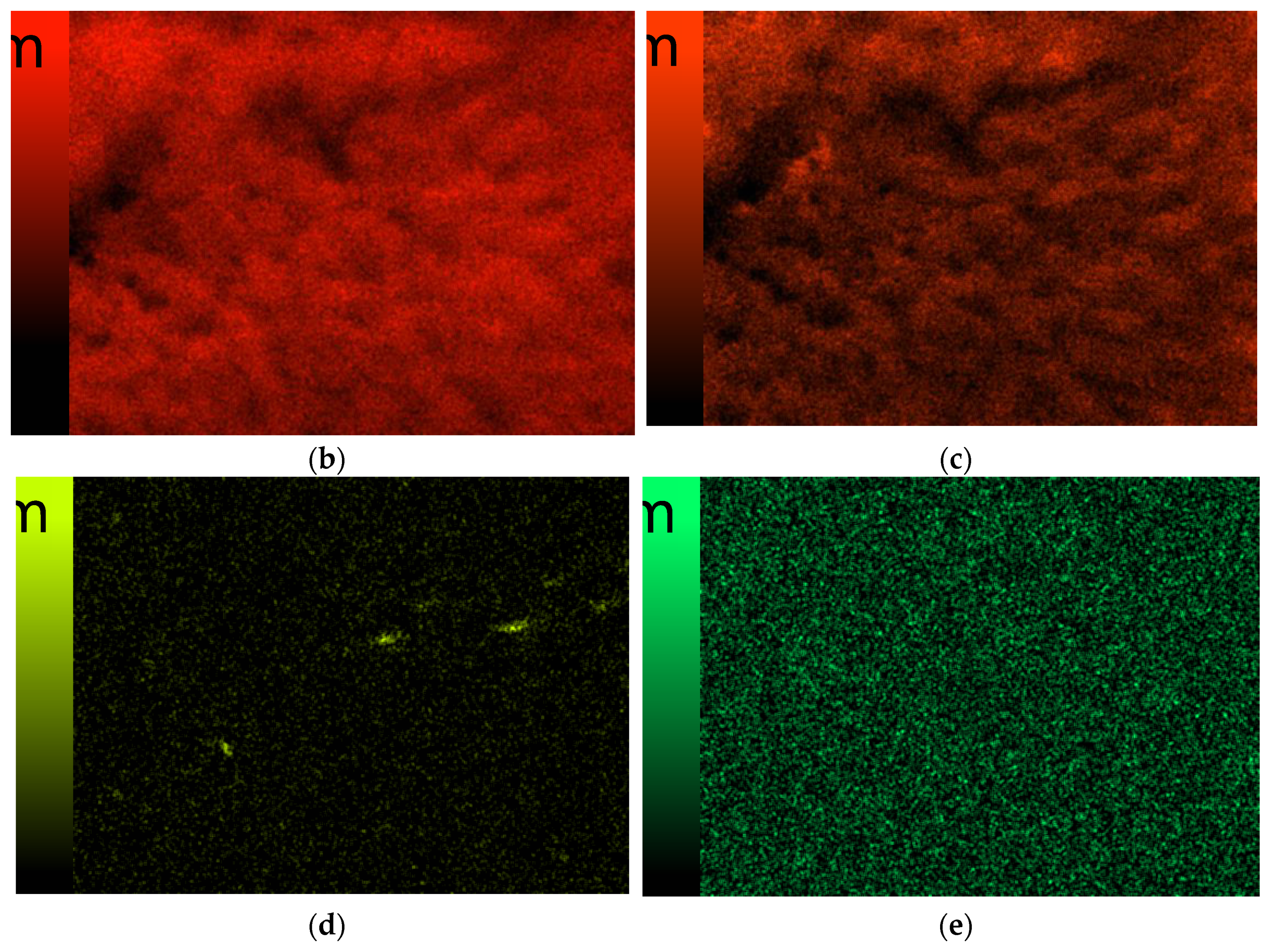
| Sample Number | 1% Solution of Chitosan in 0.05% Acetic Acid Solution (mL) | Ag-Coated Magnetic Nanoparticles (mL) | Photoinitiator (mL) | Crosslinking Agent Mn = 575 g/mol (mL) |
|---|---|---|---|---|
| 1. | 10 | 0 | 0.25 | 2.5 |
| 2. | 3 | |||
| 3. | 5 | |||
| 4. | 7 |
| Parameter | Value |
|---|---|
| Angular range | 20–80° 2θ |
| Measurement step | 0.0027166° 2θ |
| Components | Nickel filter on the lamp Mask 13 mm Slot 1° Knife in position LOW |
| Independent Variable | Sum of Squares | Mean Square | F-Value | p-Value |
|---|---|---|---|---|
| Type of incubation fluid | 0.09470 | 0.03156 | 41.59 | 3.759 × 10−11 |
| Nanoparticles content | 6.21679 | 2.07226 | 2730.60 | 1.273 × 10−38 |
| Interaction | 0.40238 | 0.04470 | 58.91 | 2.223 × 10−17 |
| Ag-Coated Magnetic Nanoparticles (mL) | Ra (μm) | Rz (μm) |
|---|---|---|
| 0 | 3.52 | 19.28 |
| 3 | 11.31 | 56.76 |
| 5 | 11.38 | 59.94 |
| 7 | 15.95 | 67.16 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sala, K.; Cholewa, K.; Bańkosz, M.; Tyliszczak, B. Analysis of Surface and Physicochemical Properties of Novel Hydrogel Materials Supported with Magnetic Nanoparticles. Coatings 2023, 13, 1907. https://doi.org/10.3390/coatings13111907
Sala K, Cholewa K, Bańkosz M, Tyliszczak B. Analysis of Surface and Physicochemical Properties of Novel Hydrogel Materials Supported with Magnetic Nanoparticles. Coatings. 2023; 13(11):1907. https://doi.org/10.3390/coatings13111907
Chicago/Turabian StyleSala, Katarzyna, Krzysztof Cholewa, Magdalena Bańkosz, and Bożena Tyliszczak. 2023. "Analysis of Surface and Physicochemical Properties of Novel Hydrogel Materials Supported with Magnetic Nanoparticles" Coatings 13, no. 11: 1907. https://doi.org/10.3390/coatings13111907






