Antifungal Properties of Ozone Treatment against P. citrinum and R. stolonifera in Fresh-Peeled Garlic
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Ozone Exposure
2.3. Sample Preparation and Ozone Treatment
2.4. Exosmosis Ratio (ER) and Protein Dissolution Rate (PDR) Determination
2.5. Mycelial Growth Inhibition Rate (MGIR) and Lethality Rate (LR) Determination
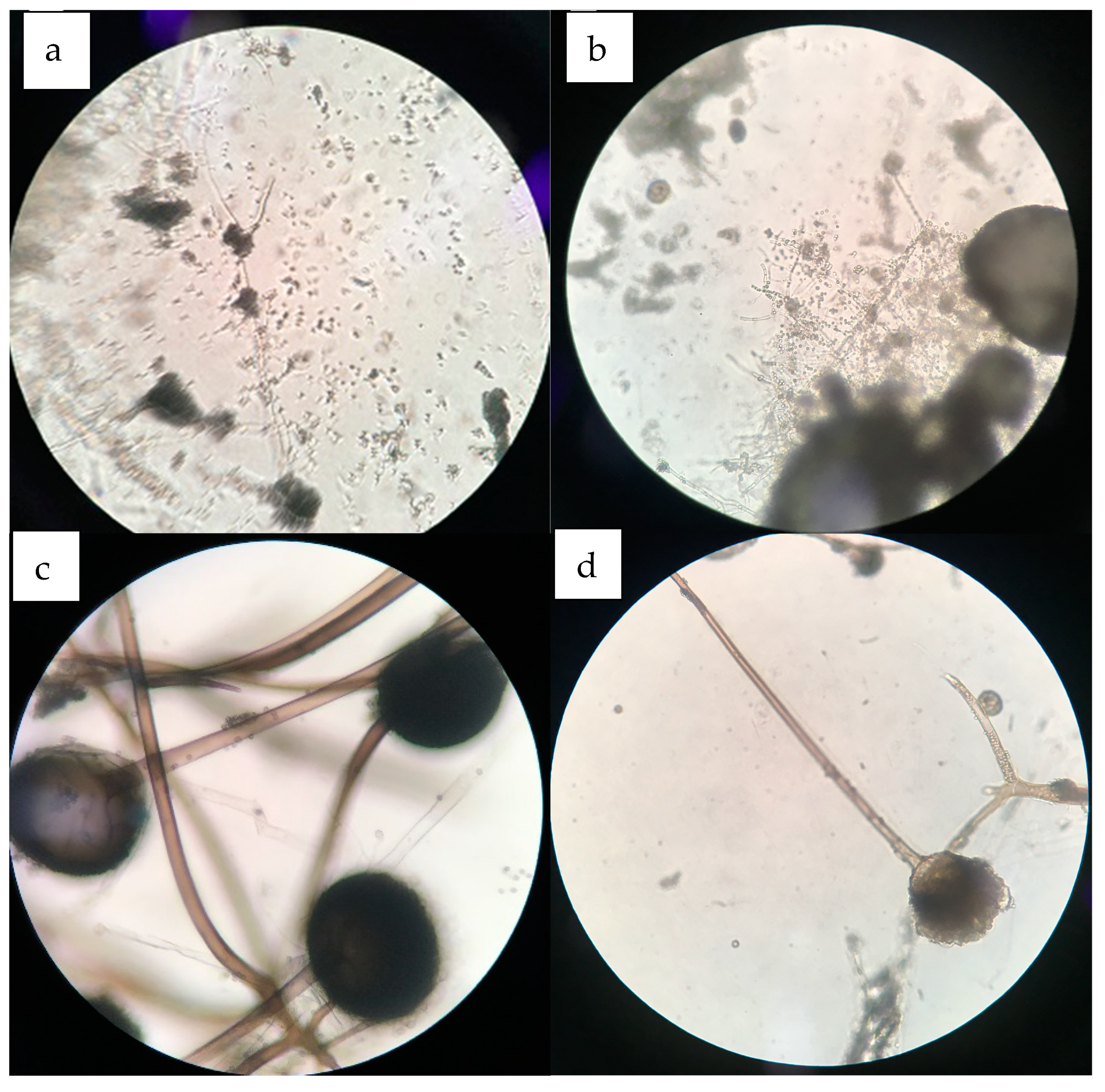
2.6. Mycelial Morphology
2.7. Microbiological Counts
2.8. Disease Incidence Rate (DIR) and Disease Spot Diameter and Depth
2.9. Statistical Analysis
3. Results and Discussion
3.1. Results
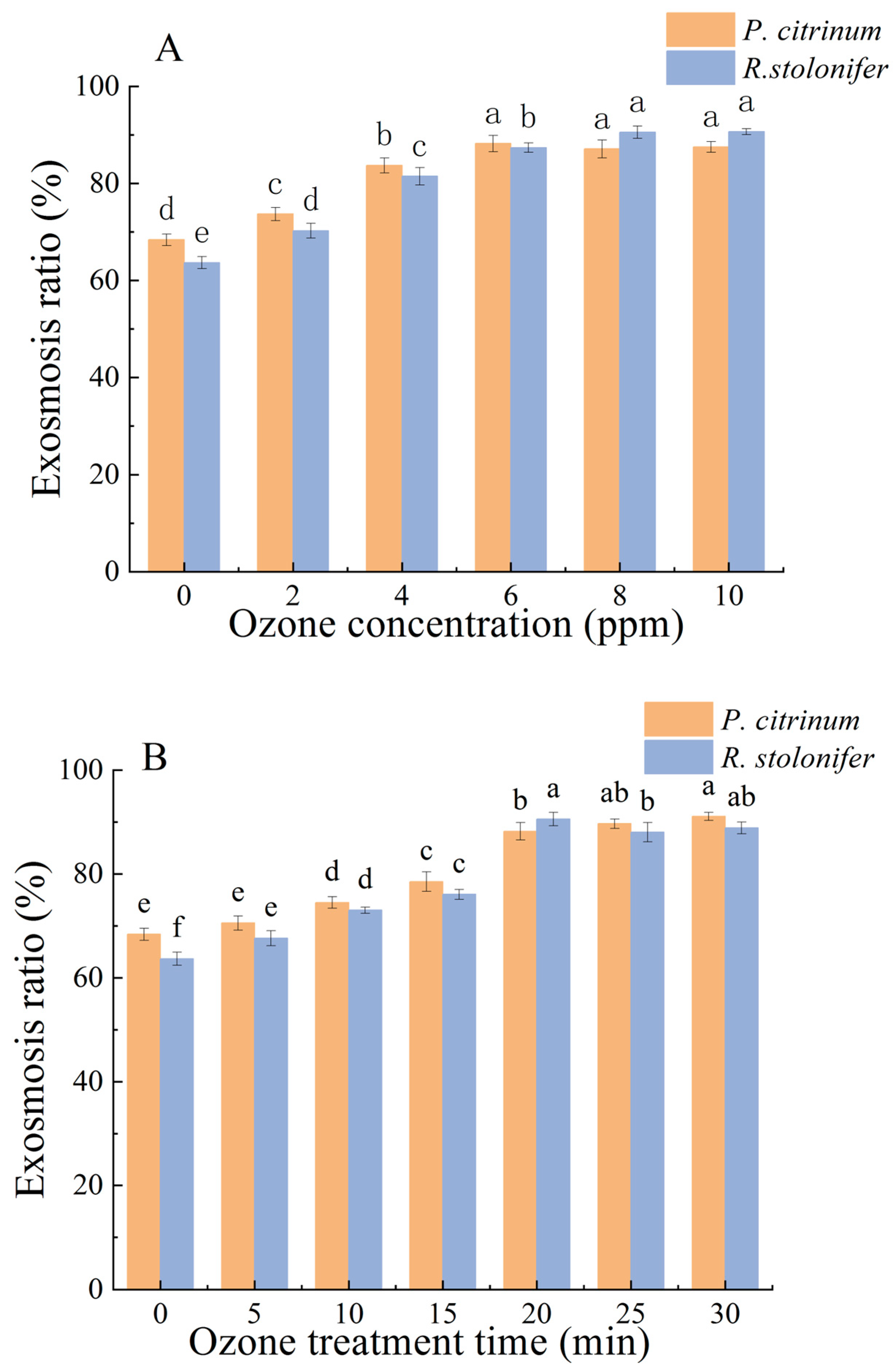
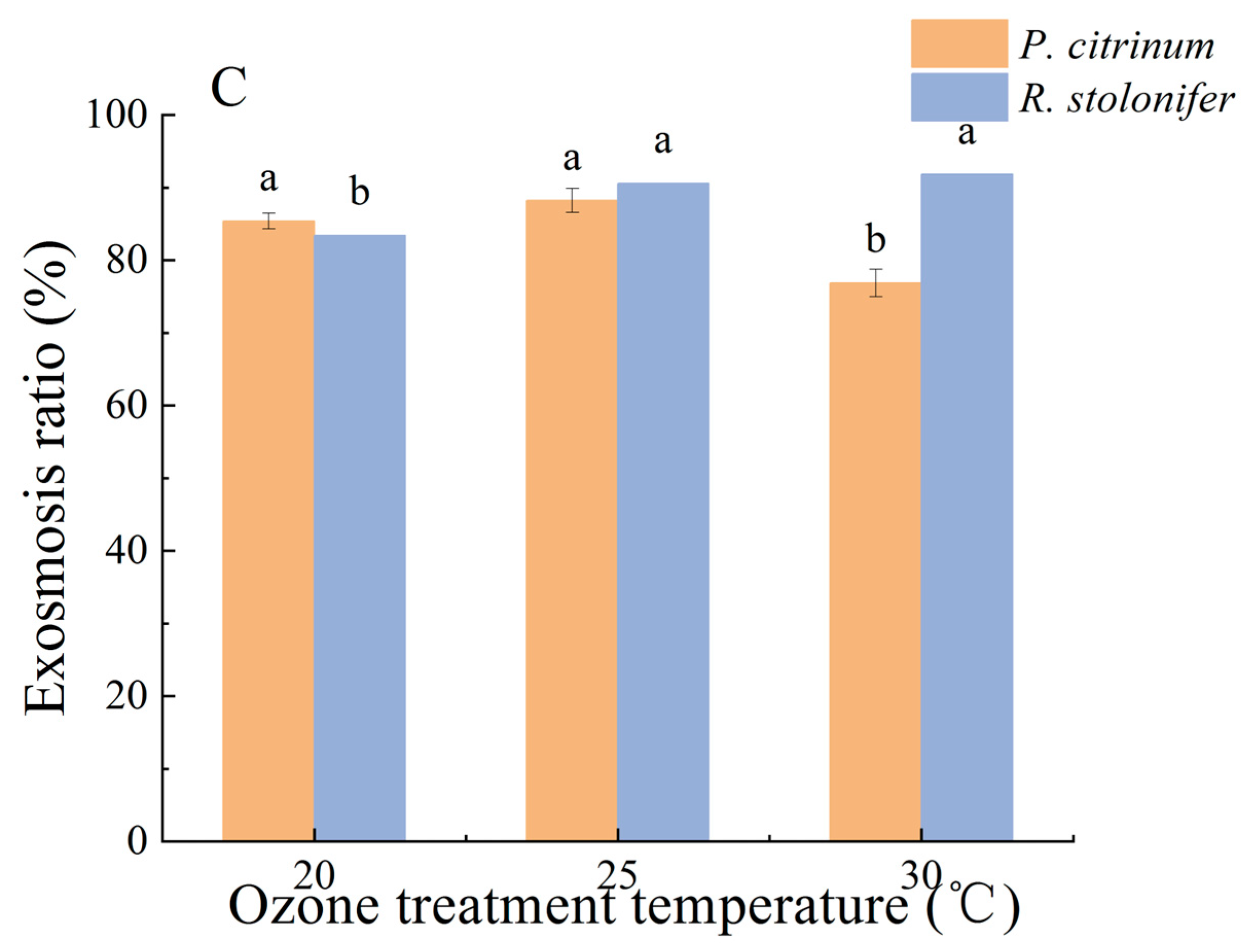
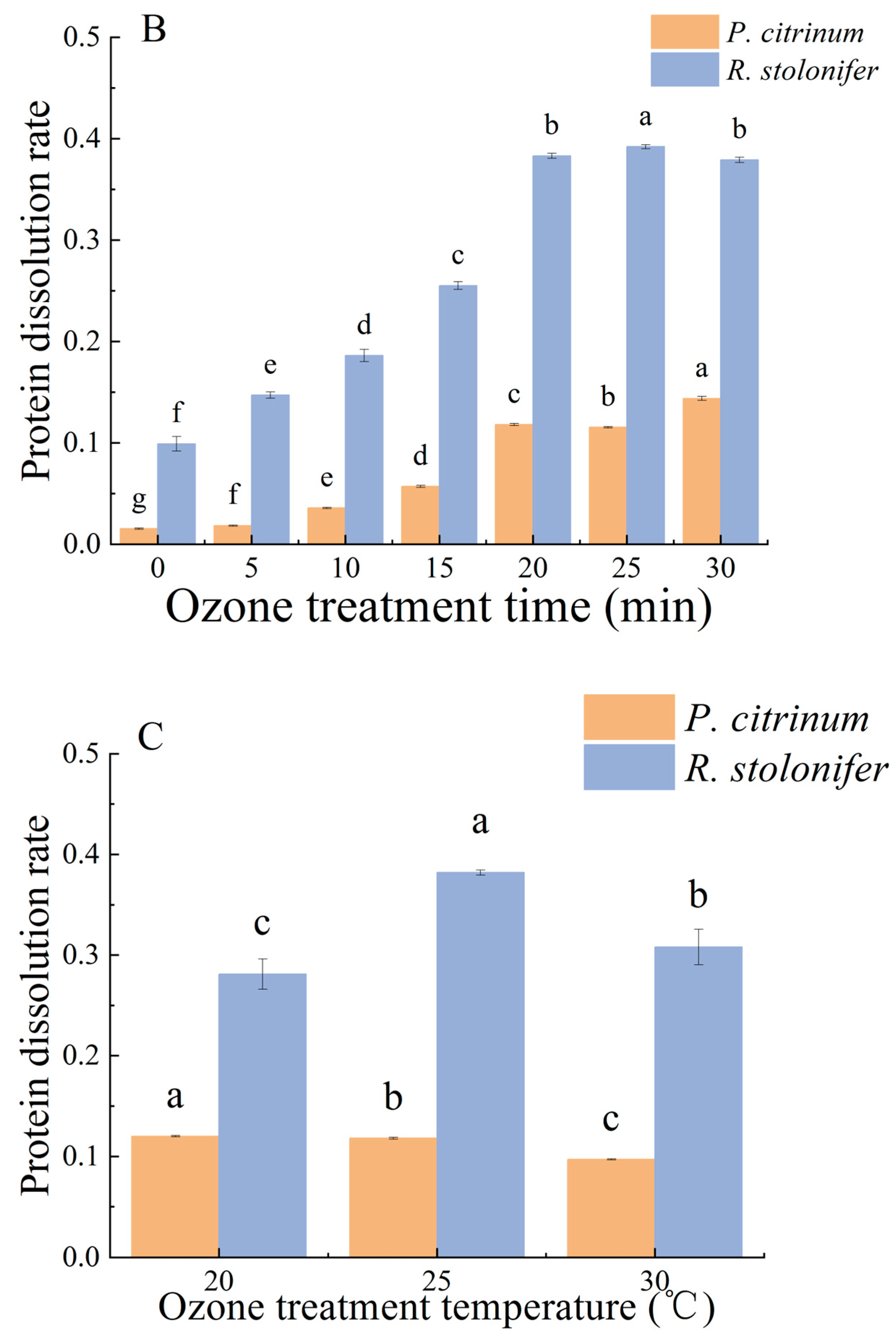
3.1.1. Changes in ER and PDR
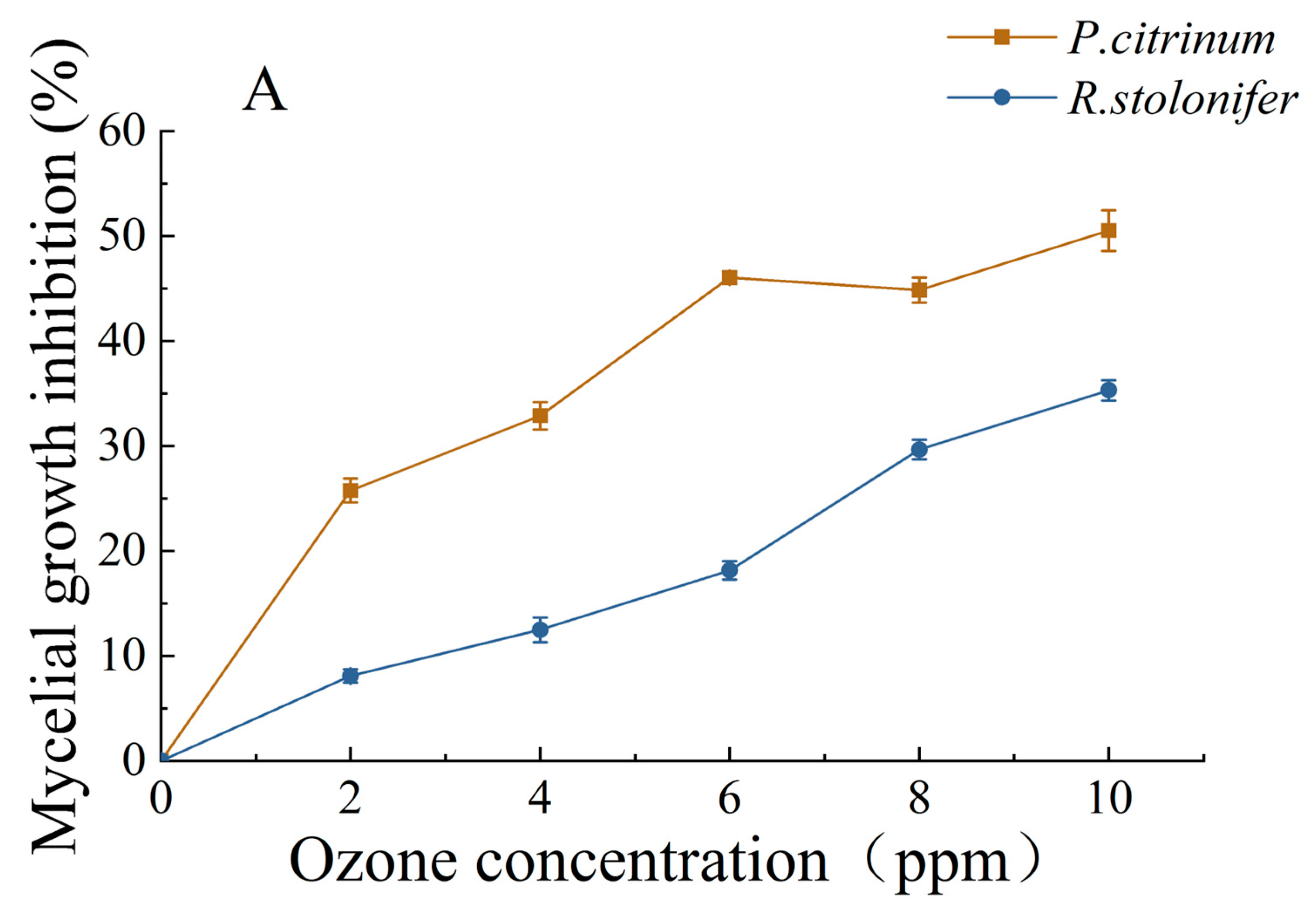
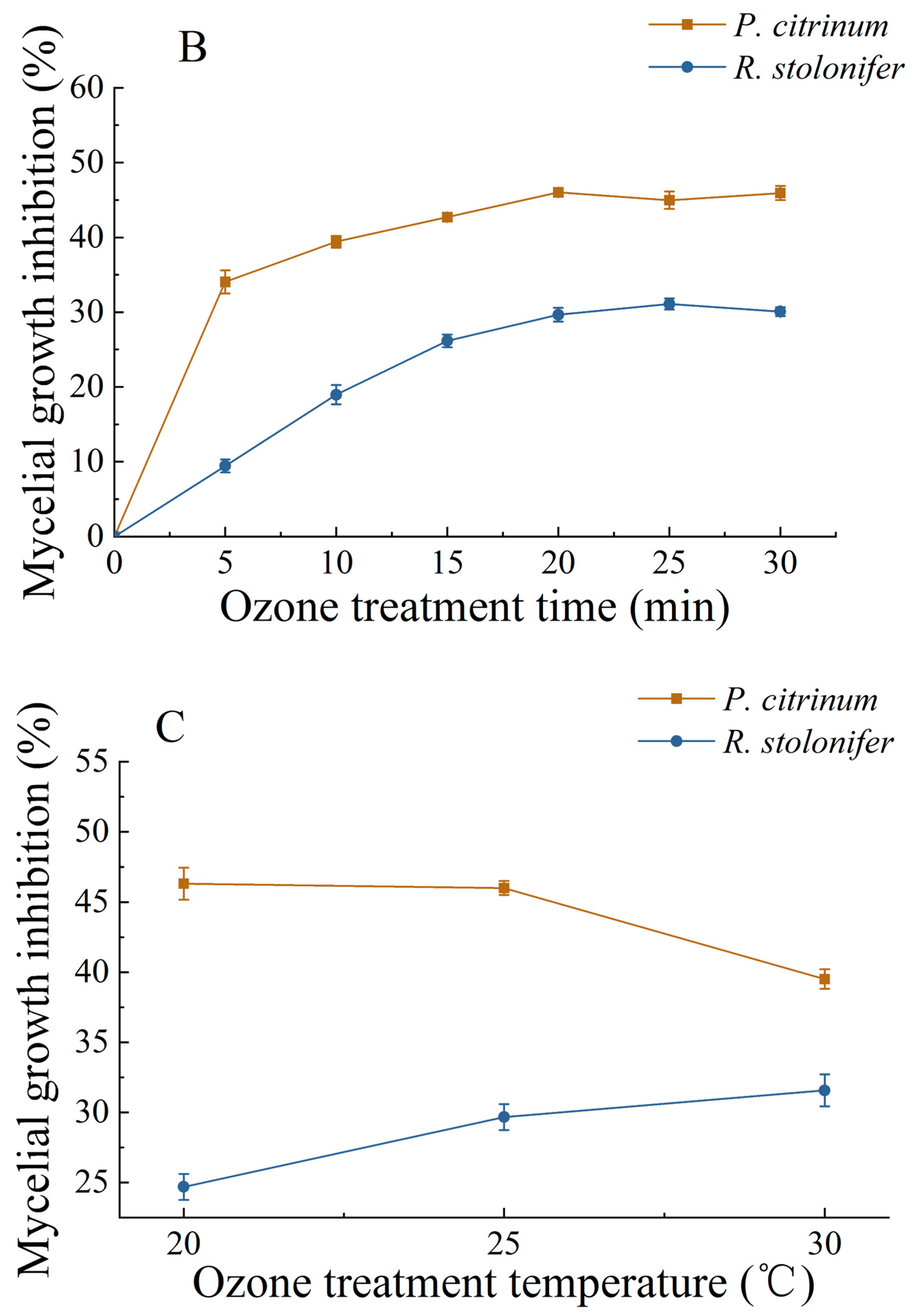
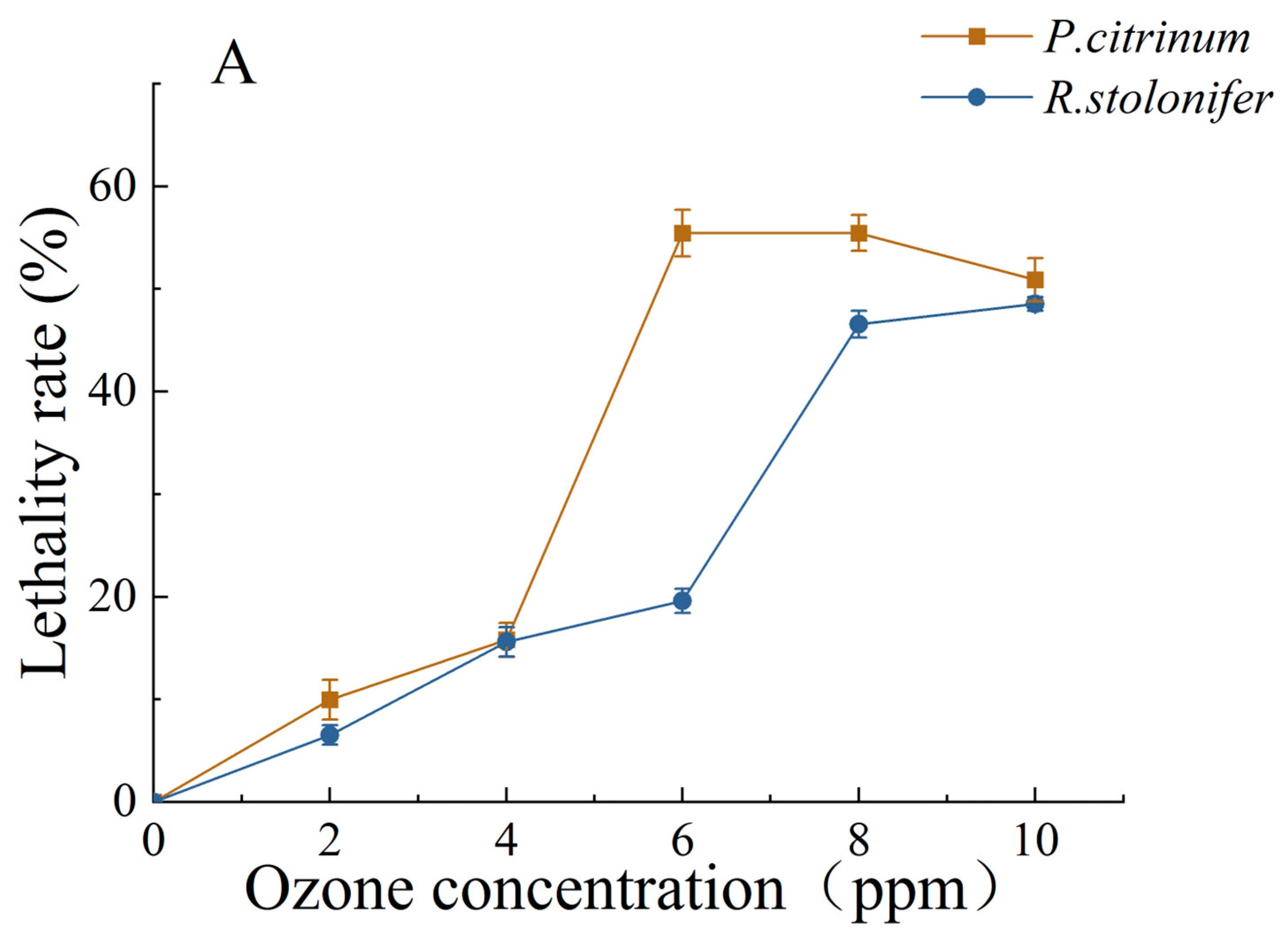
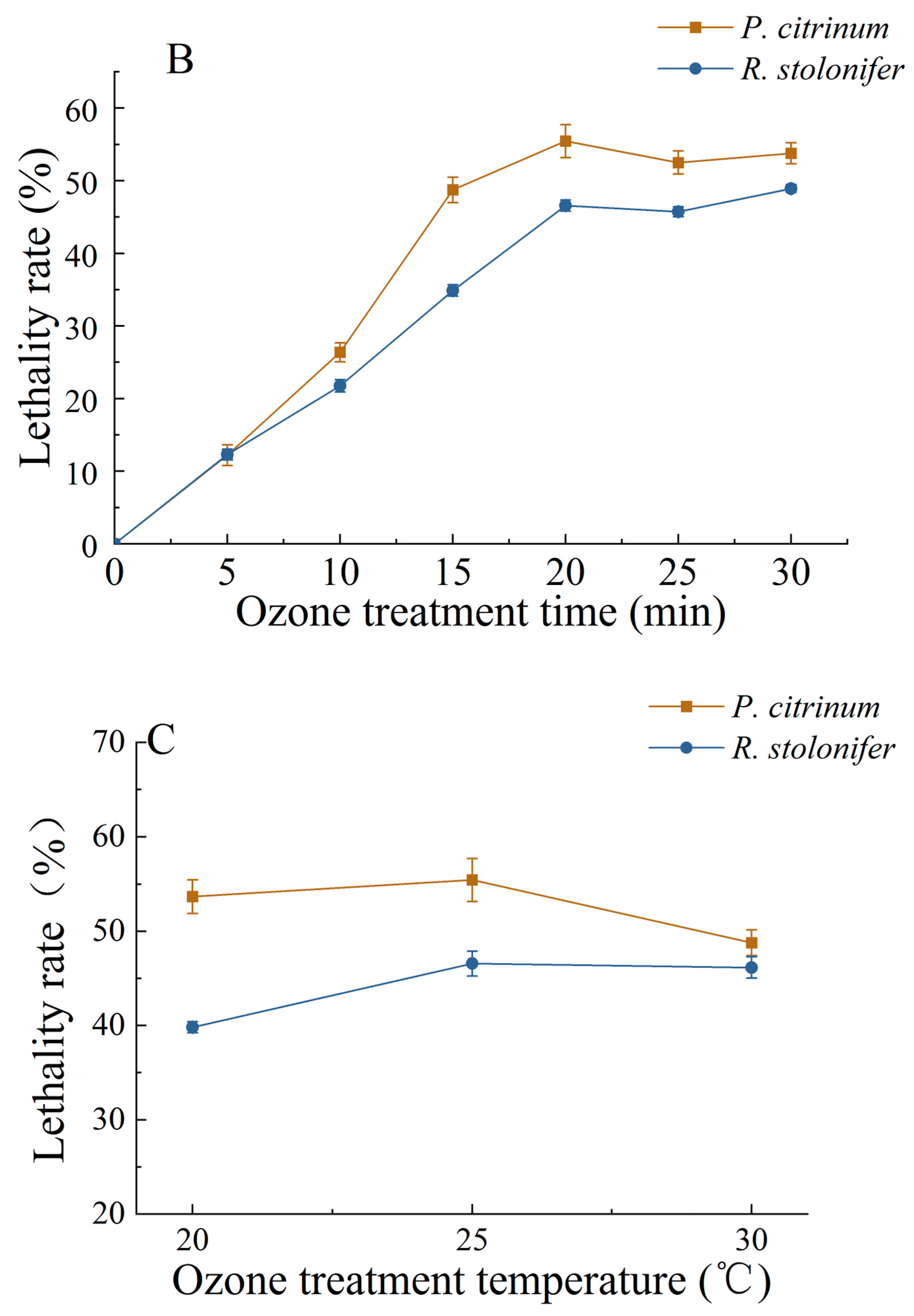
3.1.2. Changes in MGIR and LR
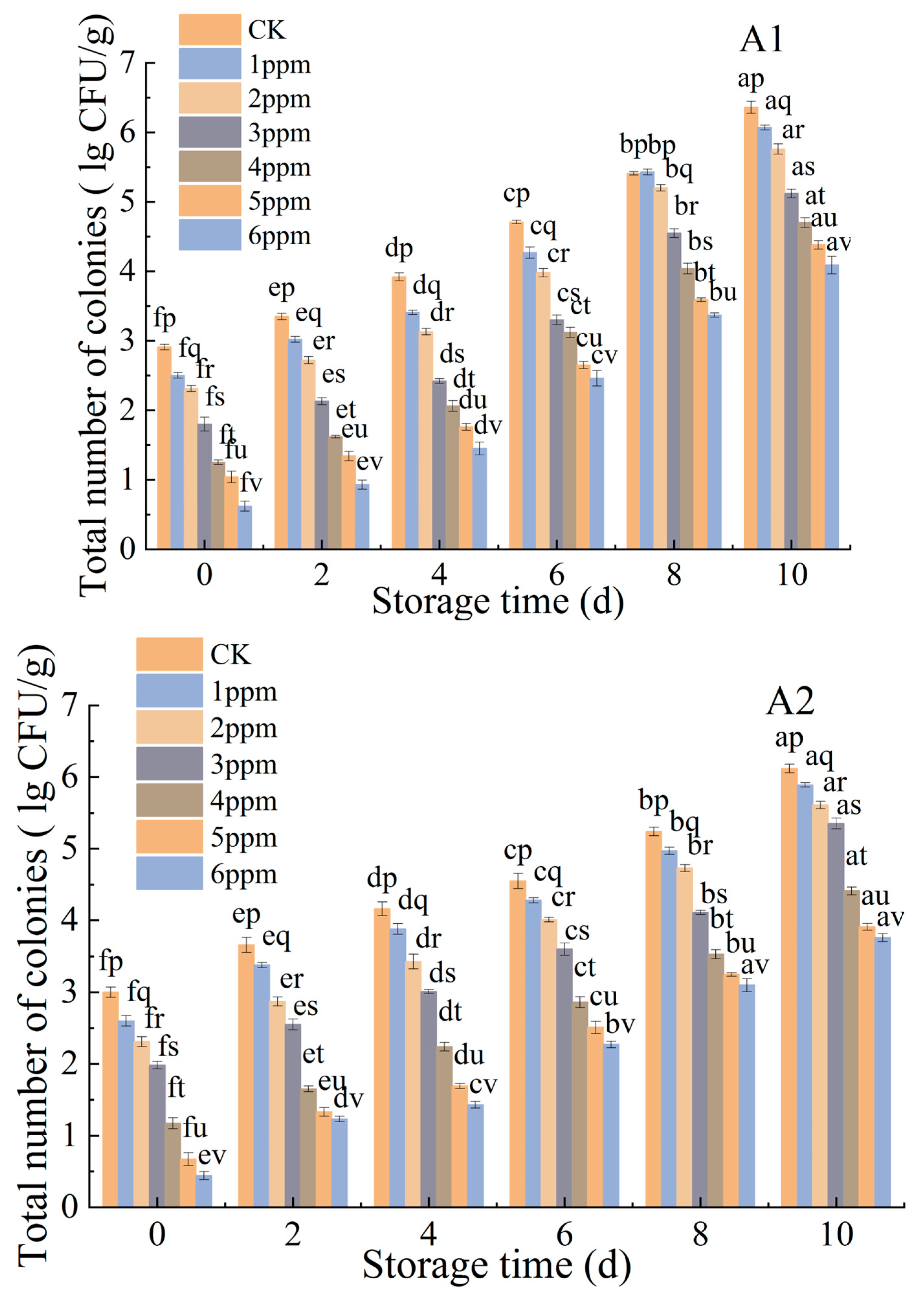
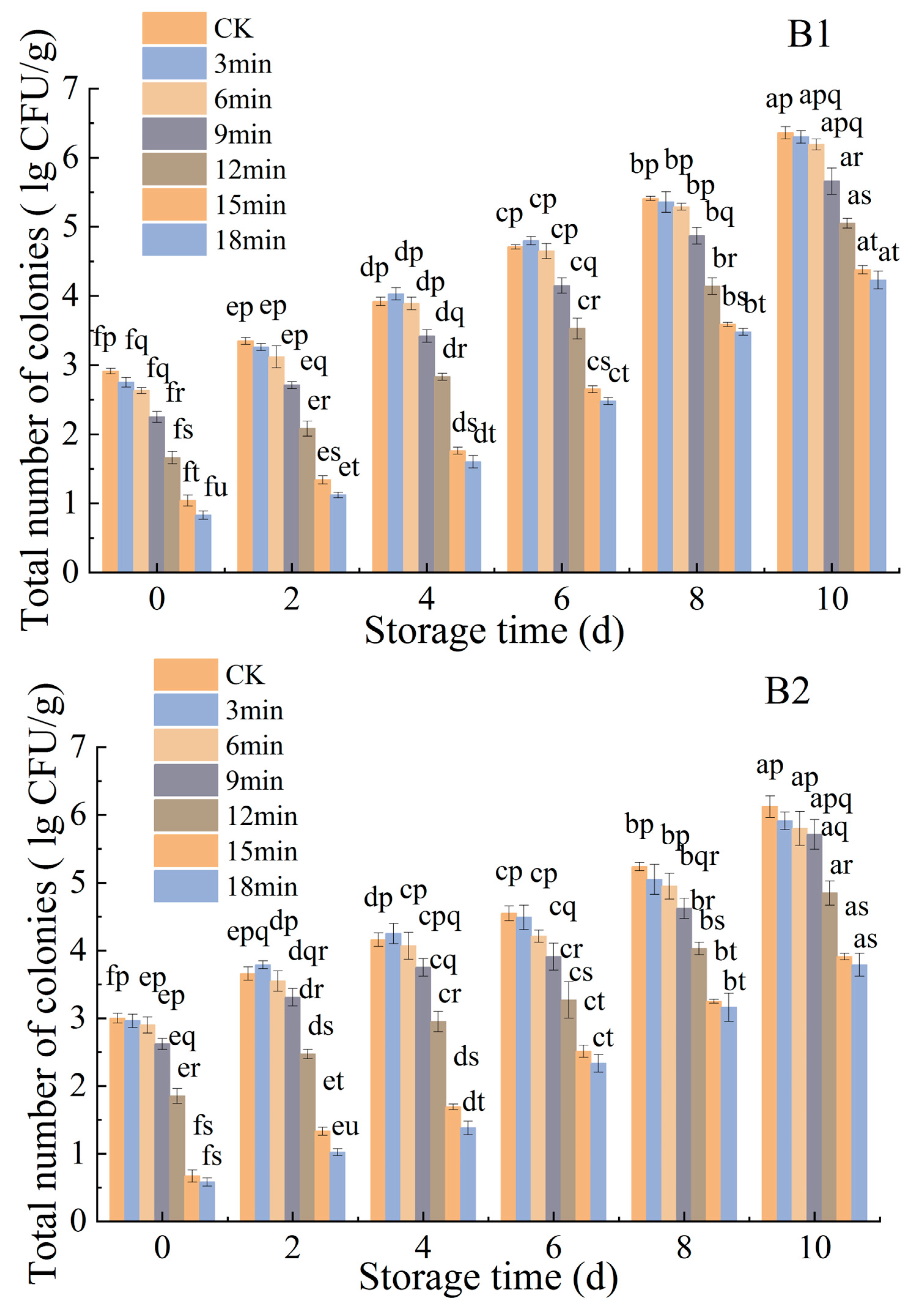
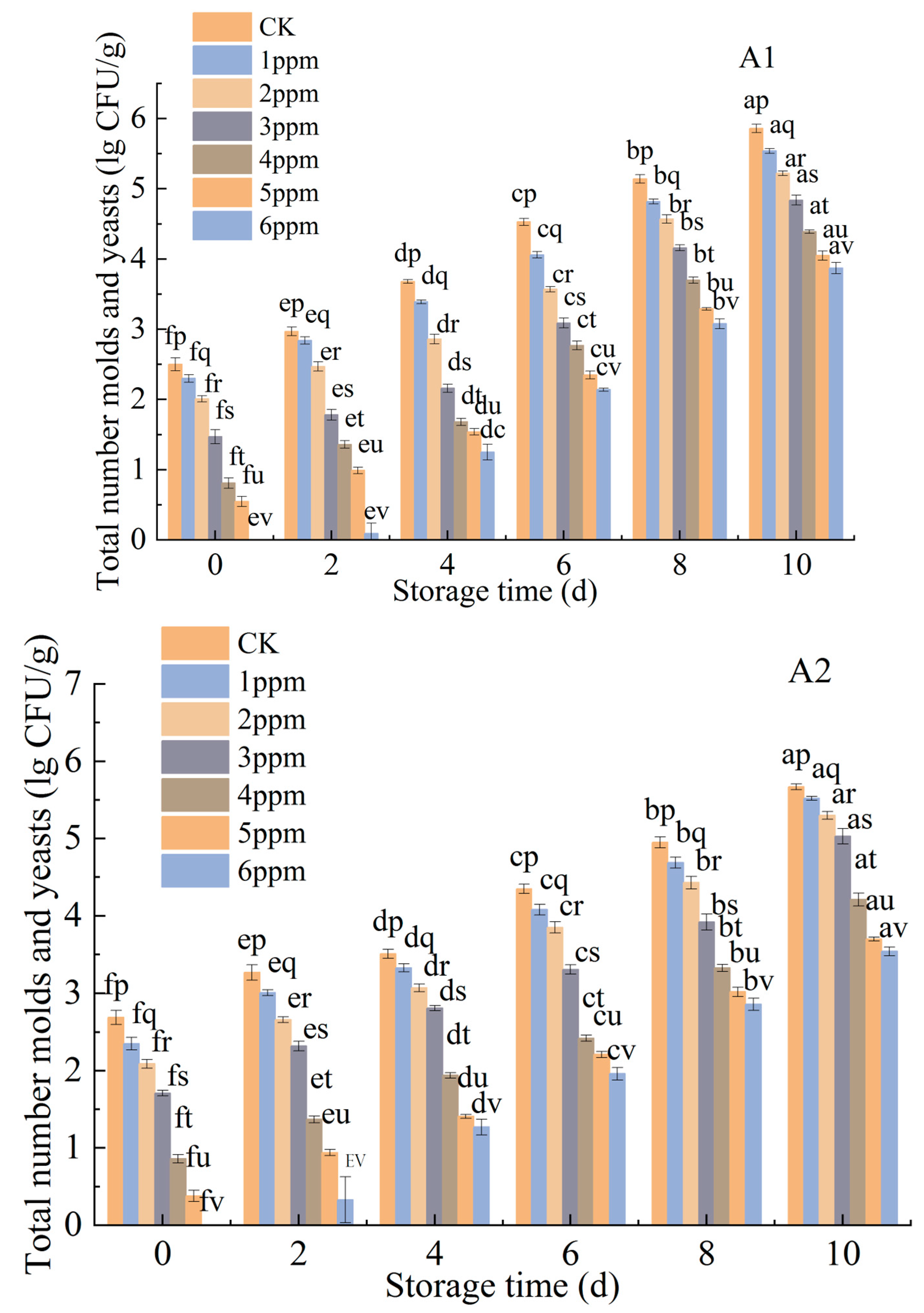
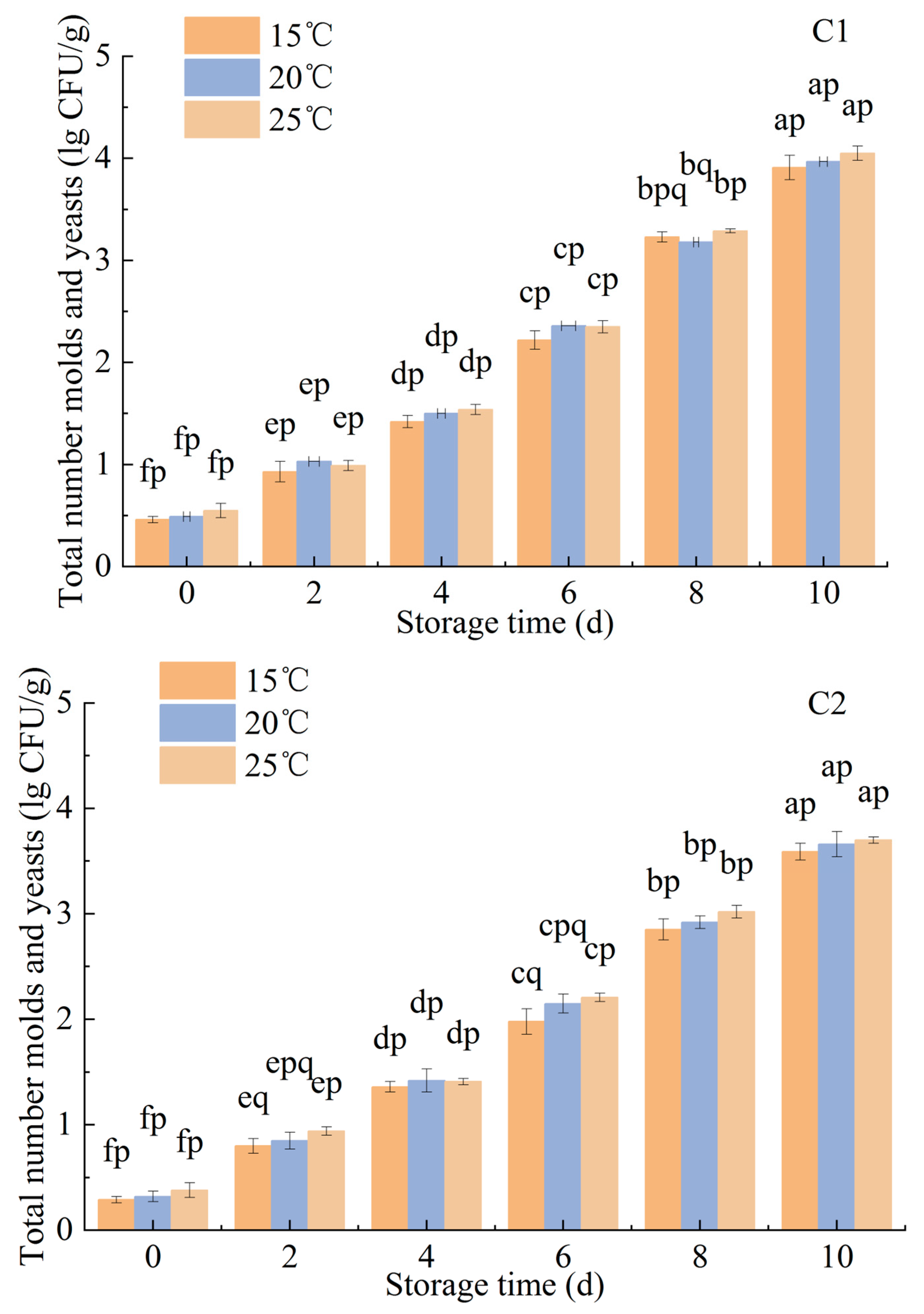
3.1.3. Effects of Ozone Treatment on the Microbiological Counts of P. citrinum and R. stolonifer
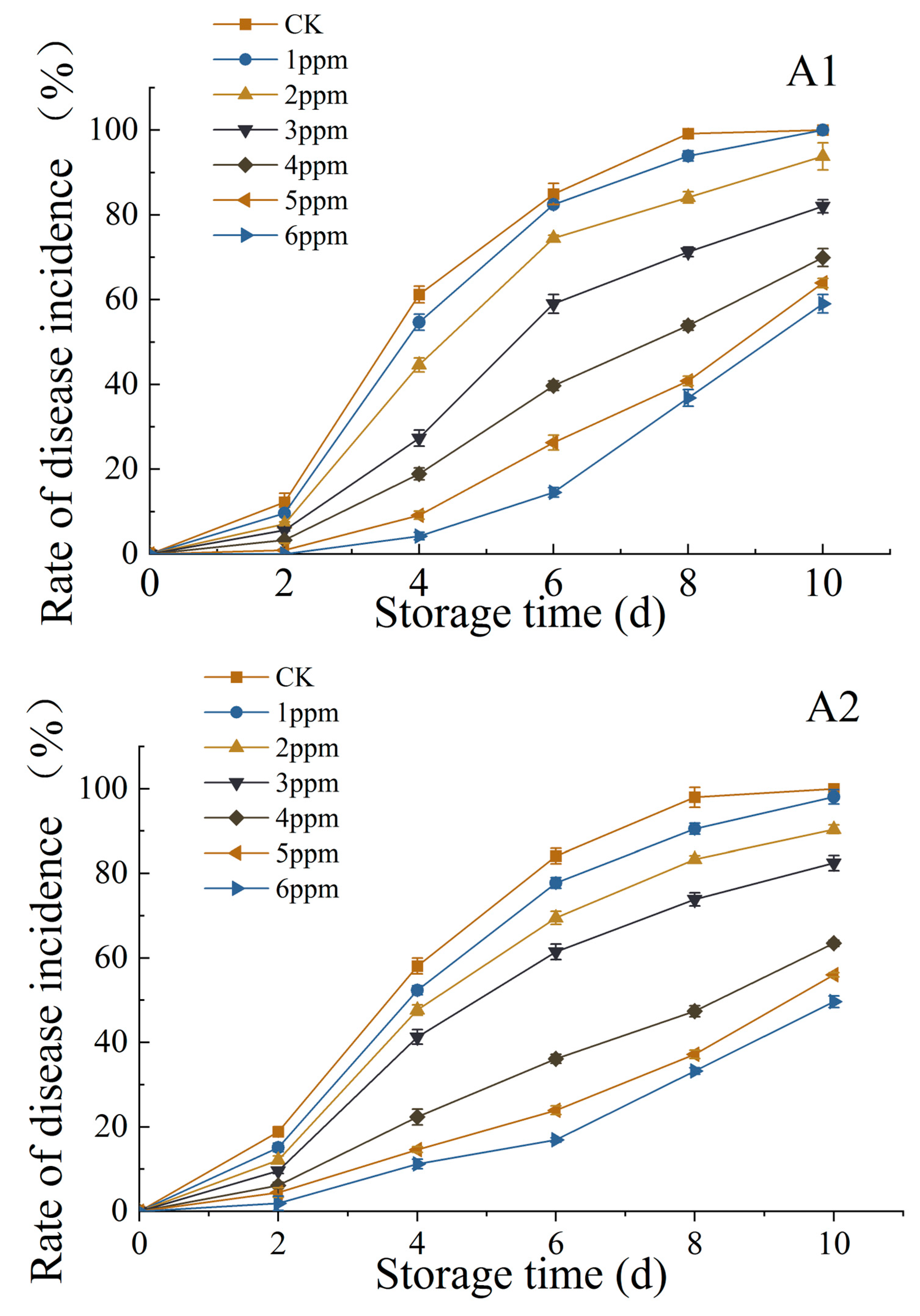
3.1.4. Effects of Ozone Treatment on DIR
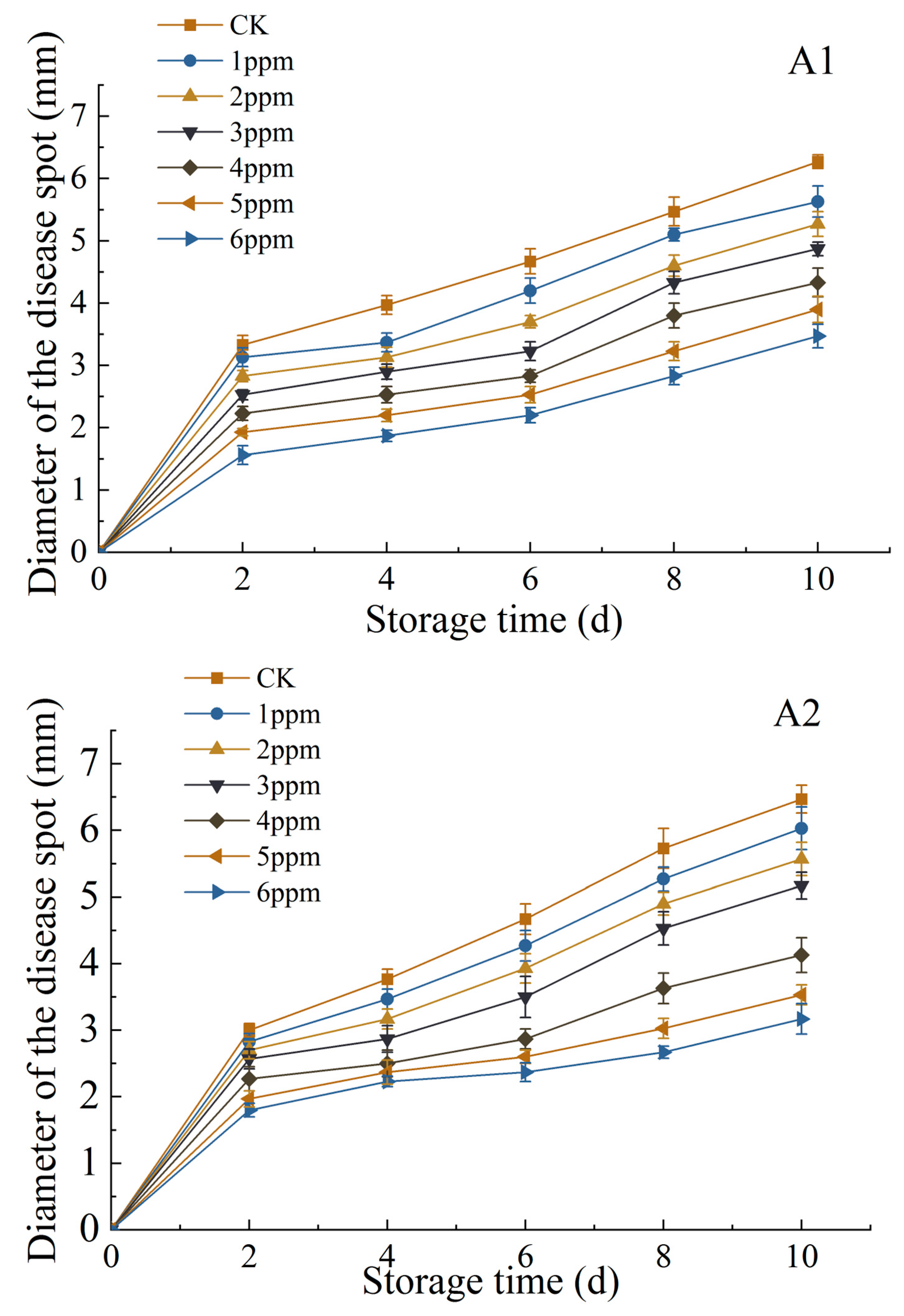
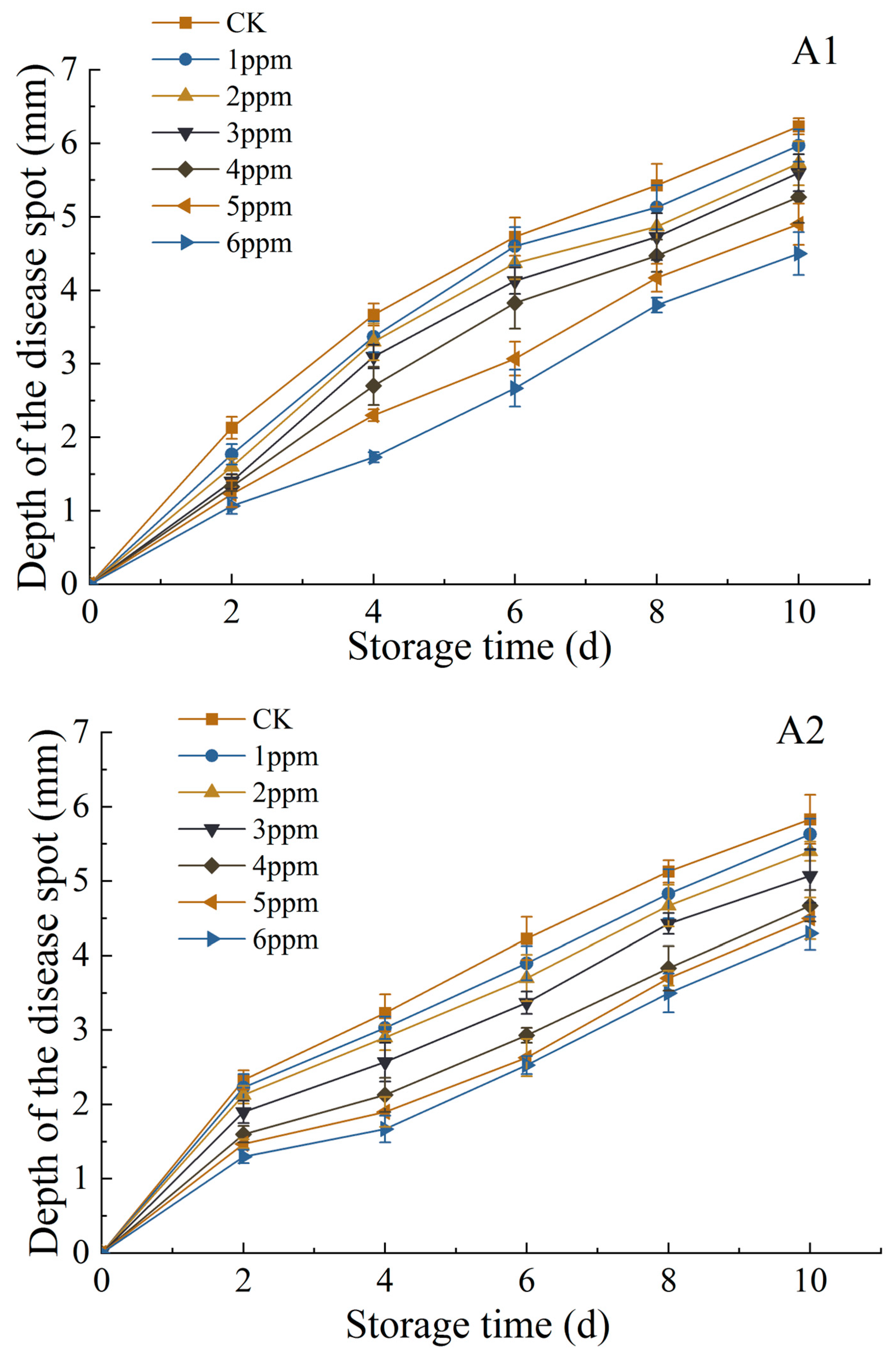
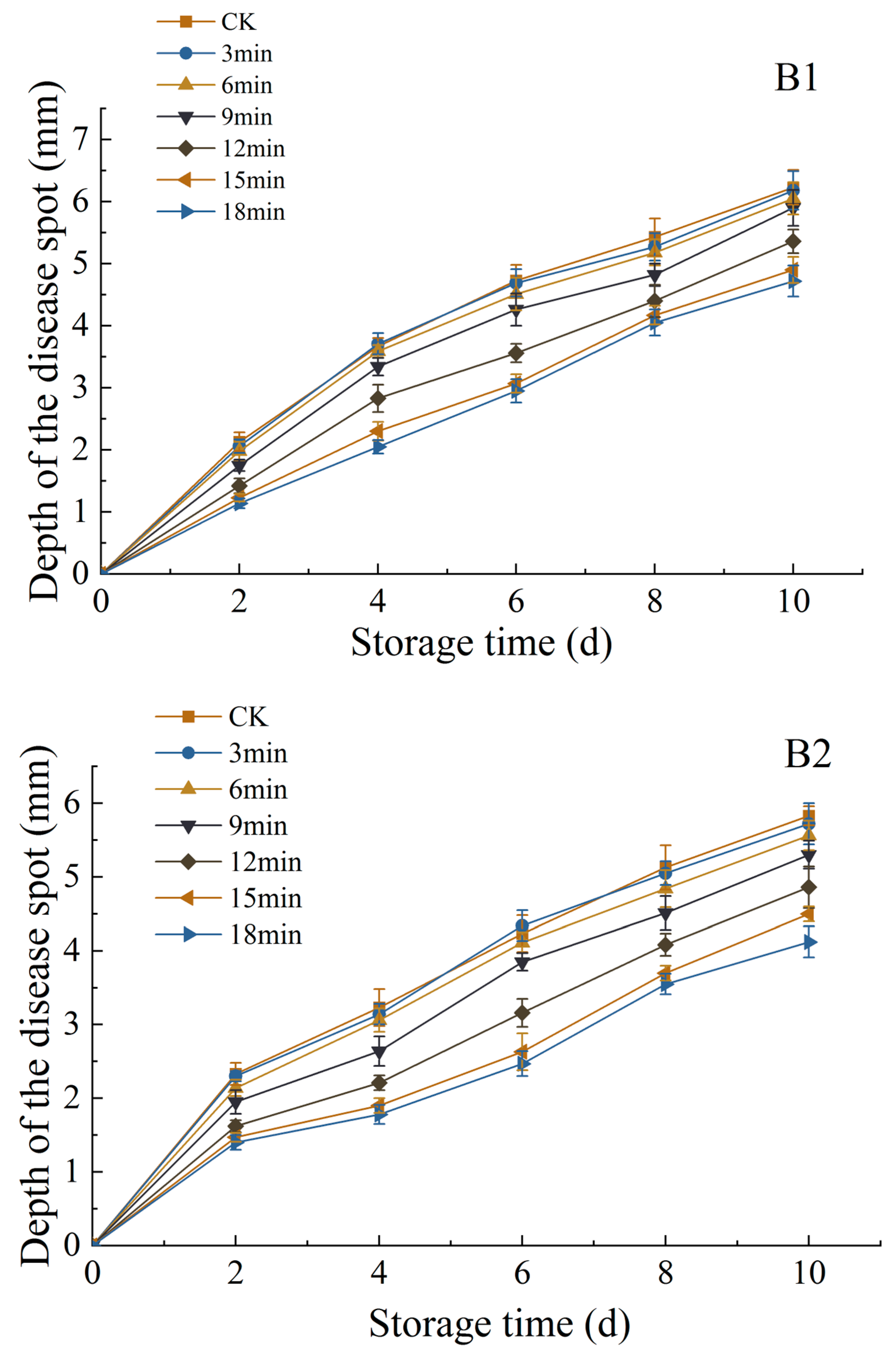
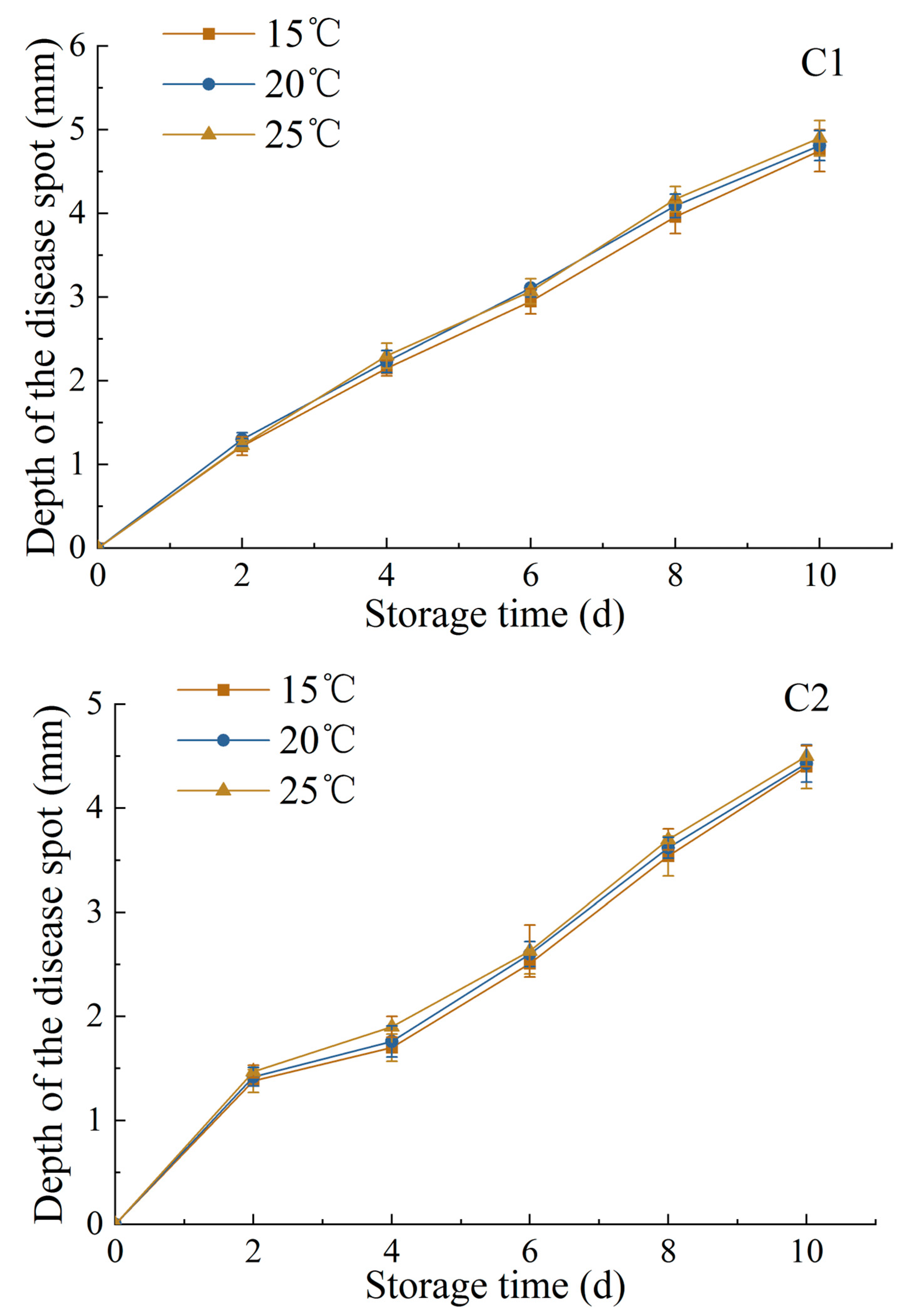
3.1.5. Changes in Diameter and Depth of Disease Spot
3.2. Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Martins, N.; Petropoulos, S.; Ferreira, I.C.F.R. Chemical Composition and Bioactive Compounds of Garlic (Allium Sativum L.) as Affected by Pre- and Post-Harvest Conditions: A Review. Food Chem. 2016, 211, 41–50. [Google Scholar] [CrossRef] [PubMed]
- Beato, V.M.; Orgaz, F.; Mansilla, F.; Montaño, A. Changes in Phenolic Compounds in Garlic (Allium Sativum L.) Owing to the Cultivar and Location of Growth. Plant Foods Hum. Nutr. 2011, 66, 218–223. [Google Scholar] [CrossRef] [PubMed]
- González, R.E.; Soto, V.C.; Sance, M.M.; Camargo, A.B.; Galmarini, C.R. Variability of Solids, Organosulfur Compounds, Pungency and Health-Enhancing Traits in Garlic (Allium Sativum L.) Cultivars Belonging to Different Ecophysiological Groups. J. Agric. Food Chem. 2009, 57, 10282–10288. [Google Scholar] [CrossRef]
- Ichikawa, M.; Mizuno, I.; Yoshida, J.; Ide, N.; Ushijima, M.; Kodera, Y.; Hayama, M.; Ono, K. Pharmacokinetics of Cycloalliin, an Organosulfur Compound Found in Garlic and Onion, in Rats. J. Agric. Food Chem. 2006, 54, 9811–9819. [Google Scholar] [CrossRef] [PubMed]
- Jabbes, N.; Arnault, I.; Auger, J.; Al Mohandes Dridi, B.; Hannachi, C. Agro-Morphological Markers and Organo-Sulphur Compounds to Assess Diversity in Tunisian Garlic Landraces. Sci. Hortic. 2012, 148, 47–54. [Google Scholar] [CrossRef]
- Lee, J.; Harnly, J.M. Free Amino Acid and Cysteine Sulfoxide Composition of 11 Garlic (Allium Sativum L.) Cultivars by Gas Chromatography with Flame Ionization and Mass Selective Detection. J. Agric. Food Chem. 2005, 53, 9100–9104. [Google Scholar] [CrossRef] [PubMed]
- Hirata, S.; Abdelrahman, M.; Yamauchi, N.; Shigyo, M. Diversity Evaluation Based on Morphological, Physiological and Isozyme Variation in Genetic Resources of Garlic (Allium Sativum L.) Collected Worldwide. Genes Genet. Syst. 2016, 91, 161–173. [Google Scholar] [CrossRef]
- Fratianni, F.; Riccardi, R.; Spigno, P.; Ombra, M.N.; Cozzolino, A.; Tremonte, P.; Coppola, R.; Nazzaro, F. Biochemical Characterization and Antimicrobial and Antifungal Activity of Two Endemic Varieties of Garlic (Allium Sativum L.) of the Campania Region, Southern Italy. J. Med. Food 2016, 19, 686–691. [Google Scholar] [CrossRef]
- Li, R.; Wu, W.; Fang, X.; Chen, H.; Han, Y.; Niu, B.; Chen, H.; Gao, H. Antifungal Activity and Mechanism of Perillaldehyde against Penicillium citrinum, a Major Fungal Pathogen of Myrica rubra. Food Sci. 2023, 44, 196–203. [Google Scholar]
- Kong, J.; Zhang, Y.; Ju, J.; Xie, Y.; Guo, Y.; Cheng, Y.; Qian, H.; Quek, S.Y.; Yao, W. Antifungal Effects of Thymol and Salicylic Acid on Cell Membrane and Mitochondria of Rhizopus Stolonifer and Their Application in Postharvest Preservation of Tomatoes. Food Chem. 2019, 285, 380–388. [Google Scholar] [CrossRef]
- Meireles, A.; Giaouris, E.; Simões, M. Alternative Disinfection Methods to Chlorine for Use in the Fresh-Cut Industry. Food Res. Int. 2016, 82, 71–85. [Google Scholar] [CrossRef]
- Rico, D.; Martín-Diana, A.B.; Barat, J.M.; Barry-Ryan, C. Extending and Measuring the Quality of Fresh-Cut Fruit and Vegetables: A Review. Trends Food Sci. Technol. 2007, 18, 373–386. [Google Scholar] [CrossRef]
- Martínez-Vaz, B.M.; Fink, R.C.; Diez-Gonzalez, F.; Sadowsky, M.J. Enteric Pathogen-Plant Interactions: Molecular Connections Leading to Colonization and Growth and Implications for Food Safety. Microb. Environ. 2014, 29, 123–135. [Google Scholar] [CrossRef] [PubMed]
- Yu, H.; Neal, J.A.; Sirsat, S.A. Consumers’ Food Safety Risk Perceptions and Willingness to Pay for Fresh-Cut Produce with Lower Risk of Foodborne Illness. Food Control 2018, 86, 83–89. [Google Scholar] [CrossRef]
- Herman, K.M.; Hall, A.J.; Gould, L.H. Outbreaks Attributed to Fresh Leafy Vegetables, United States, 1973–2012. Epidemiol. Infect. 2015, 143, 3011–3021. [Google Scholar] [CrossRef]
- Sirsat, S.; Neal, J. Microbial Profile of Soil-Free versus In-Soil Grown Lettuce and Intervention Methodologies to Combat Pathogen Surrogates and Spoilage Microorganisms on Lettuce. Foods 2013, 2, 488–498. [Google Scholar] [CrossRef]
- Francis, G.A.; Gallone, A.; Nychas, G.J.; Sofos, J.N.; Colelli, G.; Amodio, M.L.; Spano, G. Factors Affecting Quality and Safety of Fresh-Cut Produce. Crit. Rev. Food Sci. Nutr. 2012, 52, 595–610. [Google Scholar] [CrossRef]
- Bull, R.J.; Crook, J.; Whittaker, M.; Cotruvo, J.A. Therapeutic Dose as the Point of Departure in Assessing Potential Health Hazards from Drugs in Drinking Water and Recycled Municipal Wastewater. Regul. Toxicol. Pharmacol. 2011, 60, 1–19. [Google Scholar] [CrossRef]
- Legay, C.; Rodriguez, M.J.; Sérodes, J.B.; Levallois, P. Estimation of Chlorination By-Products Presence in Drinking Water in Epidemiological Studies on Adverse Reproductive Outcomes: A Review. Sci. Total Environ. 2010, 408, 456–472. [Google Scholar] [CrossRef]
- Kim, J.-G.; Yousef, A.E.; Dave, S. Application of Ozone for Enhancing the Microbiological Safety and Quality of Foods: A Review. J. Food Prot. 1999, 62, 1071–1087. [Google Scholar] [CrossRef]
- Cullen, P.J.; Valdramidis, V.P.; Tiwari, B.K.; Patil, S.; Bourke, P.; O’Donnell, C.P. Ozone Processing for Food Preservation: An Overview on Fruit Juice Treatments. Ozone Sci. Eng. 2010, 32, 166–179. [Google Scholar] [CrossRef]
- Khadre, M.A.; Yousef, A.E. Sporicidal Action of Ozone and Hydrogen Peroxide: A Comparative Study. Int. J. Food Microbiol. 2001, 71, 131–138. [Google Scholar] [CrossRef]
- Zou, H.; Xu, L.; Xu, Z.; Xie, W.; Wang, Y.; Liao, X.; Kong, X. Effects of Ultra-High Temperature Treatment and Packages on Baked Purple Sweet Potato Nectar. LWT 2018, 94, 129–135. [Google Scholar] [CrossRef]
- Gibson, K.E.; Almeida, G.; Jones, S.L.; Wright, K.; Lee, J.A. Inactivation of Bacteria on Fresh Produce by Batch Wash Ozone Sanitation. Food Control 2019, 106, 106747. [Google Scholar] [CrossRef]
- Tzortzakis, N.; Chrysargyris, A. Postharvest Ozone Application for the Preservation of Fruits and Vegetables. Food Rev. Int. 2017, 33, 270–315. [Google Scholar] [CrossRef]
- Miller, F.A.; Silva, C.L.M.; Brandão, T.R.S. A Review on Ozone-Based Treatments for Fruit and Vegetables Preservation. Food Eng. Rev. 2013, 5, 77–106. [Google Scholar] [CrossRef]
- Horvitz, S.; Cantalejo, M.J. Application of Ozone for the Postharvest Treatment of Fruits and Vegetables. Crit. Rev. Food Sci. Nutr. 2014, 54, 312–339. [Google Scholar] [CrossRef]
- Akata, I.; Torlak, E.; Erci, F. Efficacy of Gaseous Ozone for Reducing Microflora and Foodborne Pathogens on Button Mushroom. Postharvest Biol. Technol. 2015, 109, 40–44. [Google Scholar] [CrossRef]
- Öztekin, S.; Zorlugenç, B.; Zorlugenç, F.K. Effects of Ozone Treatment on Microflora of Dried Figs. J. Food Eng. 2006, 75, 396–399. [Google Scholar] [CrossRef]
- Nadas, A.; Olmo, M.; Garcia, J.M. Growth of Botrytis Cinerea and Strawberry Quality in Ozone-Enriched Atmospheres. J Food Sci. 2003, 68, 1798–1802. [Google Scholar] [CrossRef]
- Boonkorn, P.; Gemma, H.; Sugaya, S.; Setha, S.; Uthaibutra, J.; Whangchai, K. Impact of High-Dose, Short Periods of Ozone Exposure on Green Mold and Antioxidant Enzyme Activity of Tangerine Fruit. Postharvest Biol. Technol. 2012, 67, 25–28. [Google Scholar] [CrossRef]
- Sarig, P.; Zahavi, T.; Zutkhi, Y.; Yannai, S.; Lisker, N.; Ben-Arie, R. Ozone for Control of Post-Harvest Decay of Table Grapes Caused ByRhizopus Stolonifer. Physiol. Mol. Plant Pathol. 1996, 48, 403–415. [Google Scholar] [CrossRef]
- Palou, L.; Crisosto, C.H.; Smilanick, J.L.; Adaskaveg, J.E.; Zoffoli, J.P. Effects of Continuous 0.3 Ppm Ozone Exposure on Decay Development and Physiological Responses of Peaches and Table Grapes in Cold Storage. Postharvest Biol. Technol. 2002, 24, 39–48. [Google Scholar] [CrossRef]
- Tzortzakis, N.; Singleton, I.; Barnes, J. Impact of Low-Level Atmospheric Ozone-Enrichment on Black Spot and Anthracnose Rot of Tomato Fruit. Postharvest Biol. Technol. 2008, 47, 1–9. [Google Scholar] [CrossRef]
- Wang, X.; Liu, R.; Yang, Y.; Zhang, M. Isolation, Purification and Identification of Antioxidants in an Aqueous Aged Garlic Extract. Food Chem. 2015, 187, 37–43. [Google Scholar] [CrossRef] [PubMed]
- Minas, I.S.; Karaoglanidis, G.S.; Manganaris, G.A.; Vasilakakis, M. Effect of Ozone Application during Cold Storage of Kiwifruit on the Development of Stem-End Rot Caused by Botrytis Cinerea. Postharvest Biol. Technol. 2010, 58, 203–210. [Google Scholar] [CrossRef]
- Moscoso-Ramírez, P.A.; Palou, L. Effect of Ethylene Degreening on the Development of Postharvest Penicillium Molds and Fruit Quality of Early Season Citrus Fruit. Postharvest Biol. Technol. 2014, 91, 1–8. [Google Scholar] [CrossRef]
- Diao, W.-R.; Zhang, L.-L.; Feng, S.-S.; Xu, J.-G. Chemical Composition, Antibacterial Activity, and Mechanism of Action of the Essential Oil from Amomum Kravanh. J. Food Prot. 2014, 77, 1740–1746. [Google Scholar] [CrossRef]
- Yin, X.; Luo, Y.; Fan, H.; Feng, L.; Shen, H. Effect of Freeze-Chilled Treatment on Flavor of Grass Carp (Ctenopharyngodon Idellus) Fillets and Soups During Short-Term Storage. J. Aquat. Food Prod. Technol. 2016, 25, 777–787. [Google Scholar] [CrossRef]
- Xu, Q.; Xing, Y.; Che, Z.; Guan, T.; Zhang, L.; Bai, Y.; Gong, L. Effect of Chitosan Coating and Oil Fumigation on the Microbiological and Quality Safety of Fresh-Cut Pear: Microbiological Safety of Fresh-Cut Pear. J. Food Saf. 2013, 33, 179–189. [Google Scholar] [CrossRef]
- Xing, Y.; Xu, Q.; Li, X.; Che, Z.; Yun, J. Antifungal Activities of Clove Oil Against Rhizopus Nigricans, Aspergillus Flavus and Penicillium Citrinum in Vitro and in Wounded Fruit Test: Antifungal Activities of Clove Oil. J. Food Saf. 2012, 32, 84–93. [Google Scholar] [CrossRef]
- Xing, Y.; Xu, Q.; Yang, S.; Chen, C.; Tang, Y.; Sun, S.; Zhang, L.; Che, Z.; Li, X. Preservation Mechanism of Chitosan-Based Coating with Cinnamon Oil for Fruits Storage Based on Sensor Data. Sensors 2016, 16, 1111. [Google Scholar] [CrossRef]
- Salve, A.R.; Pegu, K.; Arya, S.S. Comparative Assessment of High-Intensity Ultrasound and Hydrodynamic Cavitation Processing on Physico-Chemical Properties and Microbial Inactivation of Peanut Milk. Ultrason. Sonochem. 2019, 59, 104728. [Google Scholar] [CrossRef] [PubMed]
- Martiñon, M.E.; Moreira, R.G.; Castell-Perez, M.E.; Gomes, C. Development of a Multilayered Antimicrobial Edible Coating for Shelf-Life Extension of Fresh-Cut Cantaloupe (Cucumis Melo L.) Stored at 4 °C. LWT—Food Sci. Technol. 2014, 56, 341–350. [Google Scholar] [CrossRef]
- Ong, M.K.; Ali, A. Antifungal Action of Ozone against Colletotrichum Gloeosporioides and Control of Papaya Anthracnose. Postharvest Biol. Technol. 2015, 100, 113–119. [Google Scholar] [CrossRef]
- Antony-Babu, S.; Singleton, I. Effects of Ozone Exposure on the Xerophilic Fungus, Eurotium Amstelodami IS-SAB-01, Isolated from Naan Bread. Int. J. Food Microbiol. 2011, 144, 331–336. [Google Scholar] [CrossRef]
- Ozkan, R.; Smilanick, J.L.; Karabulut, O.A. Toxicity of Ozone Gas to Conidia of Penicillium Digitatum, Penicillium Italicum, and Botrytis Cinerea and Control of Gray Mold on Table Grapes. Postharvest Biol. Technol. 2011, 60, 47–51. [Google Scholar] [CrossRef]
- Palou, L.; Smilanick, J.L.; Crisosto, C.H.; Mansour, M. Effect of Gaseous Ozone Exposure on the Development of Green and Blue Molds on Cold Stored Citrus Fruit. Plant Dis. 2001, 85, 632–638. [Google Scholar] [CrossRef]
- Tzortzakis, N.; Singleton, I.; Barnes, J. Deployment of Low-Level Ozone-Enrichment for the Preservation of Chilled Fresh Produce. Postharvest Biol. Technol. 2007, 43, 261–270. [Google Scholar] [CrossRef]
- Yeoh, W.K.; Ali, A.; Forney, C.F. Effects of Ozone on Major Antioxidants and Microbial Populations of Fresh-Cut Papaya. Postharvest Biol. Technol. 2014, 89, 56–58. [Google Scholar] [CrossRef]
- Aguayo, E.; Escalona, V.H.; Artés, F. Effect of Cyclic Exposure to Ozone Gas on Physicochemical, Sensorial and Microbial Quality of Whole and Sliced Tomatoes. Postharvest Biol. Technol. 2006, 39, 169–177. [Google Scholar] [CrossRef]
- Zhou, Q. Discussion on the Ozone Disinfection and Sterilization Technology Applied in the Food Industry. China Food Saf. Mag. 2023, 9, 155–157. [Google Scholar] [CrossRef]







Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, H.; Fan, X.; Cao, L.; Wang, X.; Liu, X.; Huang, J.; Xing, Y.; Luo, W. Antifungal Properties of Ozone Treatment against P. citrinum and R. stolonifera in Fresh-Peeled Garlic. Coatings 2023, 13, 1931. https://doi.org/10.3390/coatings13111931
Liu H, Fan X, Cao L, Wang X, Liu X, Huang J, Xing Y, Luo W. Antifungal Properties of Ozone Treatment against P. citrinum and R. stolonifera in Fresh-Peeled Garlic. Coatings. 2023; 13(11):1931. https://doi.org/10.3390/coatings13111931
Chicago/Turabian StyleLiu, Hong, Xiangfeng Fan, Lin Cao, Xiaomin Wang, Xiaocui Liu, Jie Huang, Yage Xing, and Wanmin Luo. 2023. "Antifungal Properties of Ozone Treatment against P. citrinum and R. stolonifera in Fresh-Peeled Garlic" Coatings 13, no. 11: 1931. https://doi.org/10.3390/coatings13111931




