Progress in Fruit and Vegetable Preservation: Plant-Based Nanoemulsion Coatings and Their Evolving Trends
Abstract
:1. Introduction
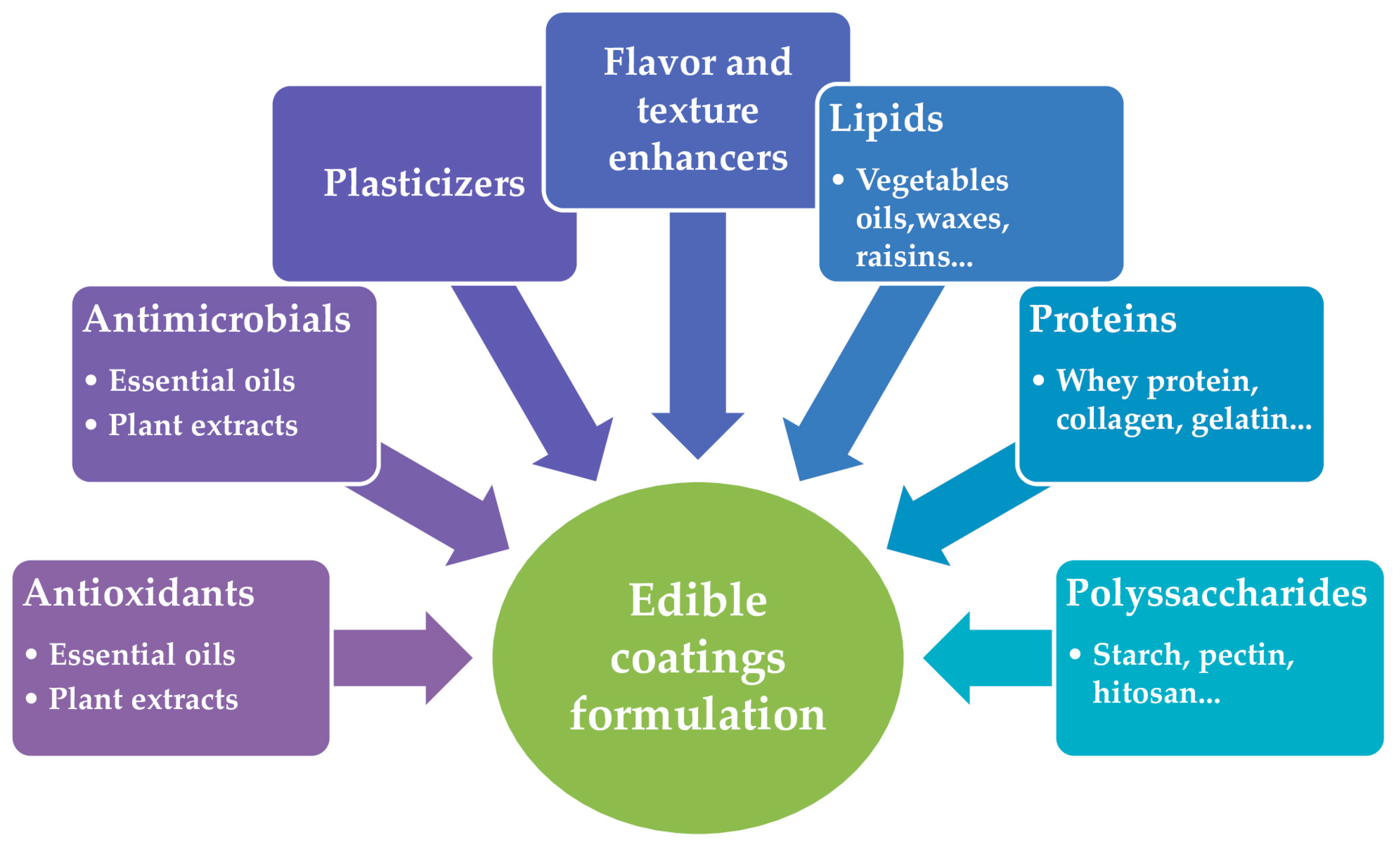
2. Edible Coatings
3. Nanoemulsions
3.1. The Main Features of Nanoemulsion-Based Coatings
3.2. Nanoemulsion-Based Edible Coatings’ Polymers
3.2.1. Coatings Polymers
3.2.2. Polysaccharide-Based Coatings
3.2.3. Protein-Based Coatings
3.2.4. Lipid-Based Coatings
3.2.5. Composite Coatings
3.3. Nanoemulsion-Based Coatings’ Active Compounds
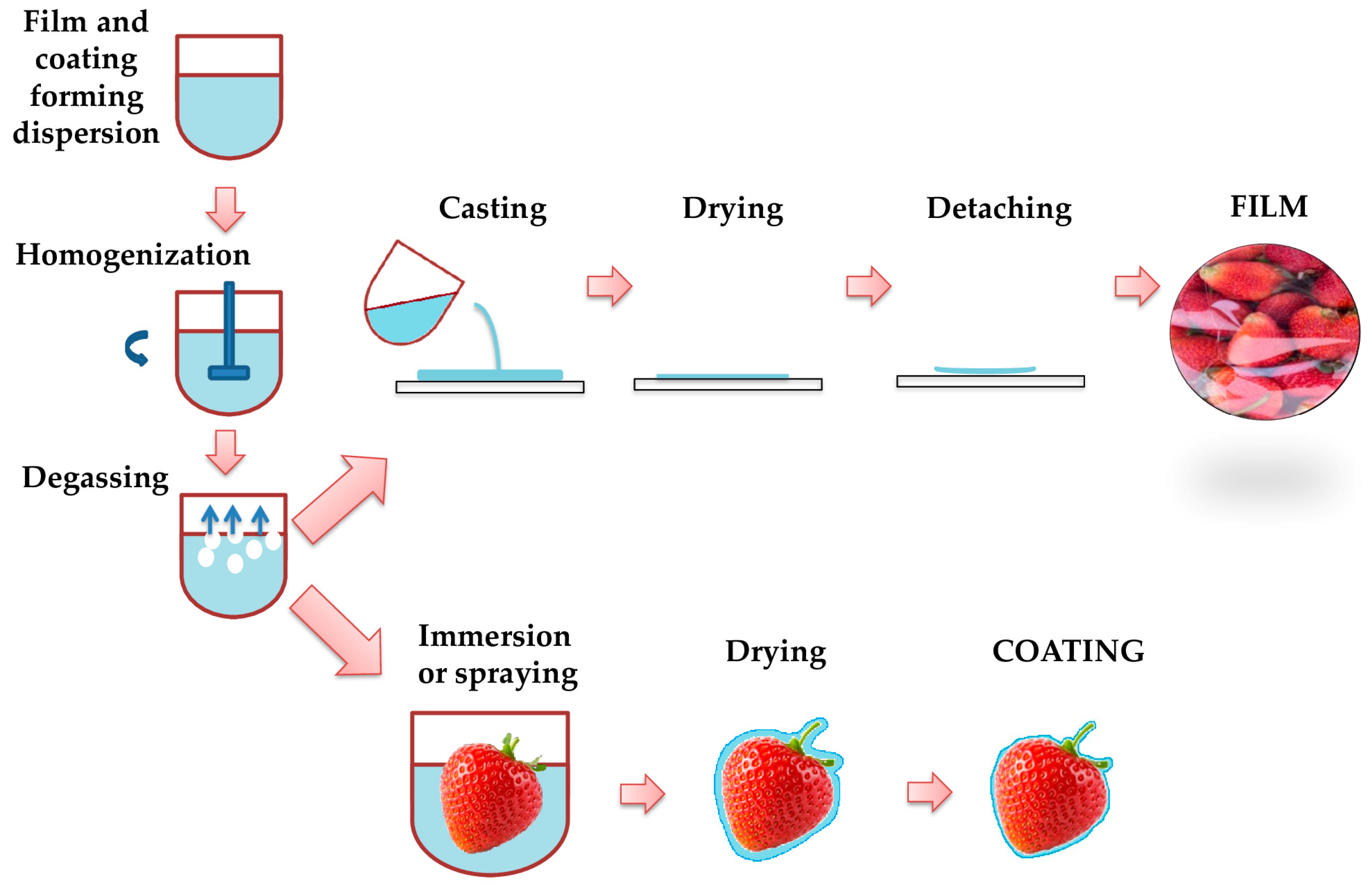
3.4. Nanoemulsion Coating Production Methods
3.5. Nanoemulsion Coating Application Method
| Target Fruits/Vegetable | Nanoemulsion Active Compound | Nanoemulsion Polymer | Producing Technique | Application Method | Ref. |
|---|---|---|---|---|---|
| Papaya | Ginger oil | Carnauba wax and hydroxypropyl methylcellulose | Ultra-Turrax homogenizer | Hand-coating procedure | [77] |
| Oregano EO * | Sodium alginate | Ultrasonication | Dipping | [78] | |
| Eucalyptus staigeriana, Lippia sidoides, and Pimenta pseudocaryophyllus leaf extracts | Carboxymethylcellulose | / | [79] | ||
| Citral oil | / | Phase-inversion method | [80] | ||
| Okra | Basil oil and aqueous extract of Sapindus mukorossi | Alginate | Ultrasonication | Dipping | [81] |
| Apple | Rosemary EO | Water chestnut starch | Ultra-Turrax homogenizer, followed by ultrasonication | Dipping | [82] |
| Lemongrass oil | Carnauba–shellac wax | Ultra-Turrax homogenizer, then high-pressure homogenizer | [83] | ||
| Cinnamon EO * | Pectine from apple pomace | Ultra-Turrax homogenizer | [84] | ||
| Lemon EO | Aloe vera gel and hydroxypropyl methylcellulose | Spraying | [85] | ||
| Apricot fruits | Pomegranate peel extract | Chitosan | Ultrasonication | Dipping | [86] |
| Tomato | Sweet orange EO | Sodium alginate | Ultrasonication | Dipping | [87] |
| Thymol | Quinoa-chitosan | Spontaneous emulsification, ultrasound, and a combination of both methods | [88] | ||
| Cardamom EO | Carboxymethyl cellulose | Ultrasonication | [89] | ||
| Strawberry | Lime, fennel, and lavender | Yam starch | Ultra-Turrax homogenizer | Spraying | [90] |
| Cymbopogon martinii and Mentha spicata EO | Arrowroot starch, cellulose nanocrystals, carnauba wax | Dipping | [91] | ||
| Fresh-cut red bell pepper | Tea tree oil | Chitosan | Magnetic stirrer | Dipping | [92] |
| Cherry | Eryngium campestre L. EO | Chitosan | Sonication with an ultrasonic bath, then Ultra-Turrax homogenizer | Dipping | [93] |
| Pineapple | Eugenol (clove EO) and Aloe vera gel | Chitosan | Spontaneous emulsification and ultrasonication | Spraying | [94] |
| Citral and sesame oil | Sodium alginate | Ultrasonication | Dipping | [95] | |
| Citrus fruits | Eugenol, carvacrol and cinnamaldehyde | Sodium alginate with citric acid, sucrose ester, Vitamin C, and potassium sorbate | Ultra-Turrax homogenizer, then passed through a microfludizer | Applied on the surface of the peel with a soft brush | [96] |
| Salacca fruits | Orange oil | Carnauba wax | Homogenizer | Spraying | [97] |
| Blackberries | Lactic and acetic acid | Chitosan | Ultrasonication | Spraying | [98] |
| Grape berries | Lemongrass oil | Carnauba | Ultra-Turrax homogenizer, then high-pressure homogenizer | Dipping | [7] |
| Garambullo fruits | Tomato oily extract | Gelatin | Ultra-Turrax homogenizer | Dipping | [99] |
| Peach | Aloe vera gel extract | Chitosan | / | Dipping | [100] |
| Fresh-cut kiwi fruits | Opuntia ficus-indica Mucilage | Aloe arborescens gel | Ultra-Turrax homogenizer | Dipping | [101] |
| Ascorbic acid and vanillin | Alginate and carboxymethylcellulose | Ultra-Turrax homogenizer, followed by ultrasonication | [102] | ||
| Pear | Lemon peel EO | Guar gum and chitosan | / | Dipping | [103] |
| Jujube fruits | / | Aloe vera gel, carboxymethyl cellulose, and pectin | / | Dipping | [104] |
| Guava | Cyclea barbata leaves extract | Alginate | / | Dipping | [105] |
| Capsicum fruits | Mulberry leaf extract | Pectin | / | Dipping | [106] |
| Guava fruits | Ginger extract and garlic extract | Gum arabic and aloe vera gel coating | / | Dipping | [13] |
| Melon | Citral oil | / | Phase-inversion method | Dipping | [80] |
| Citral | Chitosan and carboxymethyl cellulose | Ultrasonication | [107] | ||
| Lettuce | Oregano oil | / | Ultrasonication | Dipping | [108] |
4. Studies of Nanoemulsion Coating Applications on Fruit/Vegetable Samples
4.1. pH
4.2. Total Phenolic Content
4.3. Colour
4.4. Firmness
4.5. Weight Loss
4.6. Shelf Life
4.7. Acidity
4.8. Total Soluble Solids
4.9. Vitamin C
4.10. Microbial Analyses
5. Is industrialization and Wider Application of Plant-Based Nanoemulsion Coatings for Fruits and Vegetables Possible?
5.1. Optimization of Nanoemulsion Coating Formulations
5.2. Application of Coatings
5.3. Research Needs
6. Future Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Tripathi, A.D.; Sharma, R.; Agarwal, A.; Haleem, D.R. Nanoemulsions Based Edible Coatings with Potential Food Applications. Int. J. Biobased Plast. 2021, 3, 112–125. [Google Scholar] [CrossRef]
- Sessa, M.; Ferrari, G.; Donsì, F. Novel Edible Coating Containing Essential Oil Nanoemulsions to Prolong the Shelf Life of Vegetable Products. Chem. Eng. Trans. 2015, 43, 55–60. [Google Scholar] [CrossRef]
- Ramos, M.; Mellinas, C.; Solaberrieta, I.; Garrigós, M.C.; Jiménez, A. Emulsions Incorporated in Polysaccharide-Based Active Coatings for Fresh and Minimally Processed Vegetables. Foods 2021, 10, 665. [Google Scholar] [CrossRef] [PubMed]
- Tomić, A.; Šovljanski, O.; Erceg, T. Insight on Incorporation of Essential Oils as Antimicrobial Substances in Biopolymer-Based Active Packaging. Antibiotics 2023, 12, 1473. [Google Scholar] [CrossRef]
- Hassan, B.; Chatha, S.A.S.; Hussain, A.I.; Zia, K.M.; Akhtar, N. Recent Advances on Polysaccharides, Lipids and Protein Based Edible Films and Coatings: A Review. Int. J. Biol. Macromol. 2018, 109, 1095–1107. [Google Scholar] [CrossRef]
- Jose, A.; Pareek, S.; Radhakrishnan, E.K. Advances in Edible Fruit Coating Materials. In Advances in Agri-Food Biotechnology; Sharma, T.R., Deshmukh, R., Sonah, H., Eds.; Springer: Singapore, 2020; pp. 391–408. ISBN 9789811528743. [Google Scholar]
- Kim, I.-H.; Oh, Y.A.; Lee, H.; Song, K.B.; Min, S.C. Grape Berry Coatings of Lemongrass Oil-Incorporating Nanoemulsion. LWT Food Sci. Technol. 2014, 58, 1–10. [Google Scholar] [CrossRef]
- Gupta, C.; Prakash, D. Chapter 10—Safety of Fresh Fruits and Vegetables. In Food Safety and Human Health; Singh, R.L., Mondal, S., Eds.; Academic Press: Cambridge, MA, USA, 2019; pp. 249–283. ISBN 978-0-12-816333-7. [Google Scholar]
- Bajaj, K.; Adhikary, T.; Gill, P.P.S.; Kumar, A. Edible Coatings Enriched with Plant-Based Extracts Preserve Postharvest Quality of Fruits: A Review. Prog. Organ. Coat. 2023, 182, 107669. [Google Scholar] [CrossRef]
- Liyanapathiranage, A.; Dassanayake, R.S.; Gamage, A.; Karri, R.R.; Manamperi, A.; Evon, P.; Jayakodi, Y.; Madhujith, T.; Merah, O. Recent Developments in Edible Films and Coatings for Fruits and Vegetables. Coatings 2023, 13, 1177. [Google Scholar] [CrossRef]
- Shewfelt, R.L.; Prussia, S.E.; Sparks, S.A. Chapter 2—Challenges in Handling Fresh Fruits and Vegetables. In Postharvest Handling, 3rd ed.; Florkowski, W.J., Shewfelt, R.L., Brueckner, B., Prussia, S.E., Eds.; Academic Press: San Diego, CA, USA, 2014; pp. 11–30. ISBN 978-0-12-408137-6. [Google Scholar]
- Travičić, V.; Cvanić, T.; Ćetković, G. Plant-Based Nano-Emulsions as Edible Coatings in the Extension of Fruits and Vegetables Shelf Life: A Patent Review. Foods 2023, 12, 2535. [Google Scholar] [CrossRef]
- Anjum, M.A.; Akram, H.; Zaidi, M.; Ali, S. Effect of Gum Arabic and Aloe Vera Gel Based Edible Coatings in Combination with Plant Extracts on Postharvest Quality and Storability of ‘Gola’ Guava Fruits. Sci. Hortic. 2020, 271, 109506. [Google Scholar] [CrossRef]
- Rios, D.A.d.S.; Nakamoto, M.M.; Braga, A.R.C.; da Silva, E.M.C. Food Coating Using Vegetable Sources: Importance and Industrial Potential, Gaps of Knowledge, Current Application, and Future Trends. Appl. Food Res. 2022, 2, 100073. [Google Scholar] [CrossRef]
- Hasan, S.M.K.; Ferrentino, G.; Scampicchio, M. Nanoemulsion as Advanced Edible Coatings to Preserve the Quality of Fresh-Cut Fruits and Vegetables: A Review. Int. J. Food Sci. Technol. 2020, 55, 1–10. [Google Scholar] [CrossRef]
- Vargas, M.; Pastor, C.; Albors, A.; Chiralt, A.; González-Martínez, C. Development of Edible Coatings for Fresh Fruits and Vegetables: Possibilities and Limitations. Fresh Prod. 2008, 2, 32–40. [Google Scholar]
- Galus, S.; Kadzińska, J. Food Applications of Emulsion-Based Edible Films and Coatings. Trends Food Sci. Technol. 2015, 45, 273–283. [Google Scholar] [CrossRef]
- Silva-Weiss, A.; Ihl, M.; Sobral, P.J.A.; Gómez-Guillén, M.C.; Bifani, V. Natural Additives in Bioactive Edible Films and Coatings: Functionality and Applications in Foods. Food Eng. Rev. 2013, 5, 200–216. [Google Scholar] [CrossRef]
- Mahajan, B.V.C.; Tandon, R.; Kapoor, S.; Sidhu, M.K. Natural Coatings for Shelf-Life Enhancement and Quality Maintenance of Fresh Fruits and Vegetables—A Review. J. Postharvest Technol. 2018, 6, 12–26. [Google Scholar]
- Otoni, C.G.; Avena-Bustillos, R.J.; Azeredo, H.M.C.; Lorevice, M.V.; Moura, M.R.; Mattoso, L.H.C.; McHugh, T.H. Recent Advances on Edible Films Based on Fruits and Vegetables-A Review: Fruit and Vegetable Edible Films. Compr. Rev. Food Sci. Food Saf. 2017, 16, 1151–1169. [Google Scholar] [CrossRef]
- Trajkovska Petkoska, A.; Daniloski, D.; D’Cunha, N.M.; Naumovski, N.; Broach, A.T. Edible Packaging: Sustainable Solutions and Novel Trends in Food Packaging. Food Res. Int. 2021, 140, 109981. [Google Scholar] [CrossRef]
- Paidari, S.; Zamindar, N.; Tahergorabi, R.; Kargar, M.; Ezzati, S.; Shirani, N.; Musavi, S.H. Edible Coating and Films as Promising Packaging: A Mini Review. Food Meas. 2021, 15, 4205–4214. [Google Scholar] [CrossRef]
- Zambrano-Zaragoza, M.L.; González-Reza, R.; Mendoza-Muñoz, N.; Miranda-Linares, V.; Bernal-Couoh, T.F.; Mendoza-Elvira, S.; Quintanar-Guerrero, D. Nanosystems in Edible Coatings: A Novel Strategy for Food Preservation. Int. J. Mol. Sci. 2018, 19, 705. [Google Scholar] [CrossRef]
- Valdés, A.; Ramos, M.; Beltrán, A.; Jiménez, A.; Garrigós, M.C. State of the Art of Antimicrobial Edible Coatings for Food Packaging Applications. Coatings 2017, 7, 56. [Google Scholar] [CrossRef]
- Dhall, R.K. Advances in Edible Coatings for Fresh Fruits and Vegetables: A Review. Crit. Rev. Food Sci. Nutr. 2013, 53, 435–450. [Google Scholar] [CrossRef] [PubMed]
- Han, J.H. Chapter 9—Edible Films and Coatings: A Review. In Innovations in Food Packaging, 2nd ed.; Han, J.H., Ed.; Food Science and Technology; Academic Press: San Diego, CA, USA, 2014; pp. 213–255. ISBN 978-0-12-394601-0. [Google Scholar]
- Ungureanu, C.; Tihan, G.; Zgârian, R.; Pandelea (Voicu), G. Bio-Coatings for Preservation of Fresh Fruits and Vegetables. Coatings 2023, 13, 1420. [Google Scholar] [CrossRef]
- Pham, T.T.; Nguyen, L.L.P.; Dam, M.S.; Baranyai, L. Application of Edible Coating in Extension of Fruit Shelf Life: Review. AgriEngineering 2023, 5, 520–536. [Google Scholar] [CrossRef]
- Matloob, A.; Ayub, H.; Mohsin, M.; Ambreen, S.; Khan, F.A.; Oranab, S.; Rahim, M.A.; Khalid, W.; Nayik, G.A.; Ramniwas, S.; et al. A Review on Edible Coatings and Films: Advances, Composition, Production Methods, and Safety Concerns. ACS Omega 2023, 8, 28932–28944. [Google Scholar] [CrossRef] [PubMed]
- Ezhilarasi, P.N.; Karthik, P.; Chhanwal, N.; Anandharamakrishnan, C. Nanoencapsulation Techniques for Food Bioactive Components: A Review. Food Bioprocess Technol. 2013, 6, 628–647. [Google Scholar] [CrossRef]
- McClements, D.J.; Jafari, S.M. Chapter 1—General Aspects of Nanoemulsions and Their Formulation. In Nanoemulsions; Jafari, S.M., McClements, D.J., Eds.; Academic Press: Cambridge, MA, USA, 2018; pp. 3–20. ISBN 978-0-12-811838-2. [Google Scholar]
- Tan, C.; McClements, D.J. Application of Advanced Emulsion Technology in the Food Industry: A Review and Critical Evaluation. Foods 2021, 10, 812. [Google Scholar] [CrossRef]
- Chellaram, C.; Murugaboopathi, G.; John, A.A.; Sivakumar, R.; Ganesan, S.; Krithika, S.; Priya, G. Significance of Nanotechnology in Food Industry. APCBEE Procedia 2014, 8, 109–113. [Google Scholar] [CrossRef]
- Imam, S.S.; Jahangir, M.A.; Gilani, S.J.; Zafar, A.; Alshehri, S. Nanoemulsions as Delivery Vehicle for Nutraceuticals and Improving Food Nutrition Properties. In Nanoemulsions in Food Technology; CRC Press: Boca Raton, FL, USA, 2021; ISBN 978-1-00-312112-1. [Google Scholar]
- Wang, Q.; Chen, W.; Zhu, W.; McClements, D.J.; Liu, X.; Liu, F. A Review of Multilayer and Composite Films and Coatings for Active Biodegradable Packaging. npj Sci. Food 2022, 6, 18. [Google Scholar] [CrossRef]
- Singh, Y.; Meher, J.G.; Raval, K.; Khan, F.A.; Chaurasia, M.; Jain, N.K.; Chourasia, M.K. Nanoemulsion: Concepts, Development and Applications in Drug Delivery. J. Controll. Release 2017, 252, 28–49. [Google Scholar] [CrossRef]
- Acevedo-Fani, A.; Soliva-Fortuny, R.; Martín-Belloso, O. Nanoemulsions as Edible Coatings. Curr. Opin. Food Sci. 2017, 15, 43–49. [Google Scholar] [CrossRef]
- Rashid, F.; Ahmed, Z.; Ameer, K.; Amir, R.M.; Khattak, M. Optimization of Polysaccharides-Based Nanoemulsion Using Response Surface Methodology and Application to Improve Postharvest Storage of Apple (Malus domestica). Food Meas. 2020, 14, 2676–2688. [Google Scholar] [CrossRef]
- Jeevahan, J.; Chandrasekaran, M. Nanoedible Films for Food Packaging: A Review. J. Mater. Sci. 2019, 54, 12290–12318. [Google Scholar] [CrossRef]
- Hernalsteens, S. Edible Films and Coatings Made up of Fruits and Vegetables. In Biopolymer Membranes and Films; Elsevier: Amsterdam, The Netherlands, 2020; pp. 575–588. ISBN 978-0-12-818134-8. [Google Scholar]
- Zareie, Z.; Tabatabaei Yazdi, F.; Mortazavi, S.A. Development and Characterization of Antioxidant and Antimicrobial Edible Films Based on Chitosan and Gamma-Aminobutyric Acid-Rich Fermented Soy Protein. Carbohydr. Polym. 2020, 244, 116491. [Google Scholar] [CrossRef] [PubMed]
- Xue, R.; Zhao, H.; An, Z.-W.; Wu, W.; Jiang, Y.; Li, P.; Huang, C.-X.; Shi, D.; Li, R.K.Y.; Hu, G.-H.; et al. Self-Healable, Solvent Response Cellulose Nanocrystal/Waterborne Polyurethane Nanocomposites with Encryption Capability. ACS Nano 2023, 17, 5653–5662. [Google Scholar] [CrossRef] [PubMed]
- Guerreiro, A.C.; Gago, C.M.L.; Faleiro, M.L.; Miguel, M.G.C.; Antunes, M.D.C. The Effect of Edible Coatings on the Nutritional Quality of ‘Bravo de Esmolfe’ Fresh-Cut Apple through Shelf-Life. LWT 2017, 75, 210–219. [Google Scholar] [CrossRef]
- Martínez-Hernández, G.B.; Amodio, M.L.; Colelli, G. Carvacrol-Loaded Chitosan Nanoparticles Maintain Quality of Fresh-Cut Carrots. Innov. Food Sci. Emerg. Technol. 2017, 41, 56–63. [Google Scholar] [CrossRef]
- Kumar, R.; Ghoshal, G.; Goyal, M. Effect of Basil Leaves Extract on Modified Moth Bean Starch Active Film for Eggplant Surface Coating. LWT 2021, 145, 111380. [Google Scholar] [CrossRef]
- Gomes, M.d.S.; Cardoso, M.d.G.; Guimarães, A.C.G.; Guerreiro, A.C.; Gago, C.M.L.; Vilas Boas, E.V.d.B.; Dias, C.M.B.; Manhita, A.C.C.; Faleiro, M.L.; Miguel, M.G.C.; et al. Effect of Edible Coatings with Essential Oils on the Quality of Red Raspberries over Shelf-Life. J. Sci. Food Agric. 2017, 97, 929–938. [Google Scholar] [CrossRef]
- Sharma, S.; Ramana Rao, T.V. Xanthan Gum Based Edible Coating Enriched with Cinnamic Acid Prevents Browning and Extends the Shelf-Life of Fresh-Cut Pears. LWT Food Sci. Technol. 2015, 62, 791–800. [Google Scholar] [CrossRef]
- Lima, Á.M.; Cerqueira, M.A.; Souza, B.W.S.; Santos, E.C.M.; Teixeira, J.A.; Moreira, R.A.; Vicente, A.A. New Edible Coatings Composed of Galactomannans and Collagen Blends to Improve the Postharvest Quality of Fruits—Influence on Fruits Gas Transfer Rate. J. Food Eng. 2010, 97, 101–109. [Google Scholar] [CrossRef]
- Feng, Z.; Wu, G.; Liu, C.; Li, D.; Jiang, B.; Zhang, X. Edible Coating Based on Whey Protein Isolate Nanofibrils for Antioxidation and Inhibition of Product Browning. Food Hydrocoll. 2018, 79, 179–188. [Google Scholar] [CrossRef]
- Li, J.; Sun, Q.; Sun, Y.; Chen, B.; Wu, X.; Le, T. Improvement of Banana Postharvest Quality Using a Novel Soybean Protein Isolate/Cinnamaldehyde/Zinc Oxide Bionanocomposite Coating Strategy. Sci. Hortic. 2019, 258, 108786. [Google Scholar] [CrossRef]
- Boyacı, D.; Iorio, G.; Sozbilen, G.S.; Alkan, D.; Trabattoni, S.; Pucillo, F.; Farris, S.; Yemenicioğlu, A. Development of Flexible Antimicrobial Zein Coatings with Essential Oils for the Inhibition of Critical Pathogens on the Surface of Whole Fruits: Test of Coatings on Inoculated Melons. Food Packag. Shelf Life 2019, 20, 100316. [Google Scholar] [CrossRef]
- Baswal, A.K.; Dhaliwal, H.S.; Singh, Z.; Mahajan, B.; Kalia, A.; Gill, K.S. Influence of Carboxy Methylcellulose, Chitosan and Beeswax Coatings on Cold Storage Life and Quality of Kinnow Mandarin Fruit. Sci. Hortic. 2020, 260, 108887. [Google Scholar] [CrossRef]
- Ahmed, Z.F.R.; Palta, J.P. Postharvest Dip Treatment with a Natural Lysophospholipid plus Soy Lecithin Extended the Shelf Life of Banana Fruit. Postharvest Biol. Technol. 2016, 113, 58–65. [Google Scholar] [CrossRef]
- Mellinas, C.; Valdés, A.; Ramos, M.; Burgos, N.; Garrigós, M.d.C.; Jiménez, A. Active Edible Films: Current State and Future Trends. J. Appl. Polym. Sci. 2016, 133, 42631. [Google Scholar] [CrossRef]
- Saberi, B.; Golding, J.B. Postharvest Application of Biopolymer-Based Edible Coatings to Improve the Quality of Fresh Horticultural Produce. In Polymers for Food Applications; Gutiérrez, T.J., Ed.; Springer International Publishing: Cham, Switzerland, 2018; pp. 211–250. ISBN 978-3-319-94625-2. [Google Scholar]
- Moeini, A.; Pedram, P.; Fattahi, E.; Cerruti, P.; Santagata, G. Edible Polymers and Secondary Bioactive Compounds for Food Packaging Applications: Antimicrobial, Mechanical, and Gas Barrier Properties. Polymers 2022, 14, 2395. [Google Scholar] [CrossRef]
- Kouhi, M.; Prabhakaran, M.P.; Ramakrishna, S. Edible Polymers: An Insight into Its Application in Food, Biomedicine and Cosmetics. Trends Food Sci. Technol. 2020, 103, 248–263. [Google Scholar] [CrossRef]
- Zubair, M.; Pradhan, R.A.; Arshad, M.; Ullah, A. Recent Advances in Lipid Derived Bio-Based Materials for Food Packaging Applications. Macromol. Mater. Eng. 2021, 306, 2000799. [Google Scholar] [CrossRef]
- Kumar, L.; Ramakanth, D.; Akhila, K.; Gaikwad, K.K. Edible Films and Coatings for Food Packaging Applications: A Review. Environ. Chem. Lett. 2022, 20, 875–900. [Google Scholar] [CrossRef]
- Kyei, S.K.; Eke, W.I.; Darko, G.; Akaranta, O. Natural Polyhydroxy Resins in Surface Coatings: A Review. J. Coat. Technol. Res. 2022, 19, 775–794. [Google Scholar] [CrossRef]
- Roy, S.; Rhim, J.-W. Fabrication of Pectin/Agar Blended Functional Film: Effect of Reinforcement of Melanin Nanoparticles and Grapefruit Seed Extract. Food Hydrocoll. 2021, 118, 106823. [Google Scholar] [CrossRef]
- Donsì, F. Chapter 11—Applications of Nanoemulsions in Foods. In Nanoemulsions; Jafari, S.M., McClements, D.J., Eds.; Academic Press: Cambridge, MA, USA, 2018; pp. 349–377. ISBN 978-0-12-811838-2. [Google Scholar]
- Yaashikaa, P.R.; Kamalesh, R.; Senthil Kumar, P.; Saravanan, A.; Vijayasri, K.; Rangasamy, G. Recent Advances in Edible Coatings and Their Application in Food Packaging. Food Res. Int. 2023, 173, 113366. [Google Scholar] [CrossRef]
- Kahramanoğlu, İ.; Panfilova, O.; Kesimci, T.G.; Bozhüyük, A.U.; Gürbüz, R.; Alptekin, H. Control of Postharvest Gray Mold at Strawberry Fruits Caused by Botrytis Cinerea and Improving Fruit Storability through Origanum onites L. and Ziziphora clinopodioides L. Volatile Essential Oils. Agronomy 2022, 12, 389. [Google Scholar] [CrossRef]
- Shahbaz, M.U.; Arshad, M.; Mukhtar, K.; Nabi, B.G.; Goksen, G.; Starowicz, M.; Nawaz, A.; Ahmad, I.; Walayat, N.; Manzoor, M.F.; et al. Natural Plant Extracts: An Update about Novel Spraying as an Alternative of Chemical Pesticides to Extend the Postharvest Shelf Life of Fruits and Vegetables. Molecules 2022, 27, 5152. [Google Scholar] [CrossRef]
- Tajkarimi, M.M.; Ibrahim, S.A.; Cliver, D.O. Antimicrobial Herb and Spice Compounds in Food. Food Control 2010, 21, 1199–1218. [Google Scholar] [CrossRef]
- Sánchez-González, L.; Vargas, M.; González-Martínez, C.; Chiralt, A.; Cháfer, M. Use of Essential Oils in Bioactive Edible Coatings: A Review. Food Eng. Rev. 2011, 3, 1–16. [Google Scholar] [CrossRef]
- Pandey, V.K.; Islam, R.U.; Shams, R.; Dar, A.H. A Comprehensive Review on the Application of Essential Oils as Bioactive Compounds in Nano-Emulsion Based Edible Coatings of Fruits and Vegetables. Appl. Food Res. 2022, 2, 100042. [Google Scholar] [CrossRef]
- Mustafa, I.F.; Hussein, M.Z. Synthesis and Technology of Nanoemulsion-Based Pesticide Formulation. Nanomaterials 2020, 10, 1608. [Google Scholar] [CrossRef]
- Espitia, P.J.P.; Fuenmayor, C.A.; Otoni, C.G. Nanoemulsions: Synthesis, Characterization, and Application in Bio-Based Active Food Packaging. Compr. Rev. Food Sci. Food Saf. 2019, 18, 264–285. [Google Scholar] [CrossRef] [PubMed]
- Guerra-Rosas, M.I.; Morales-Castro, J.; Ochoa-Martínez, L.A.; Salvia-Trujillo, L.; Martín-Belloso, O. Long-Term Stability of Food-Grade Nanoemulsions from High Methoxyl Pectin Containing Essential Oils. Food Hydrocoll. 2016, 52, 438–446. [Google Scholar] [CrossRef]
- Rao, J.; McClements, D.J. Food-Grade Microemulsions and Nanoemulsions: Role of Oil Phase Composition on Formation and Stability. Food Hydrocoll. 2012, 29, 326–334. [Google Scholar] [CrossRef]
- Ribeiro, A.M.; Estevinho, B.N.; Rocha, F. Preparation and Incorporation of Functional Ingredients in Edible Films and Coatings. Food Bioprocess Technol. 2021, 14, 209–231. [Google Scholar] [CrossRef]
- Kamal, I. Edible Films and Coatings: Classification, Preparation, Functionality and Applications—A Review. Arch. Organ. Inorg. Chem. Sci. 2020, 4, 501–509. [Google Scholar] [CrossRef]
- Andrade, R.; Skurtys, O.; Osorio, F. Atomizing Spray Systems for Application of Edible Coatings. Compr. Rev. Food Sci. Food Saf. 2012, 11, 323–337. [Google Scholar] [CrossRef]
- Priya, K.; Thirunavookarasu, N.; Chidanand, D.V. Recent Advances in Edible Coating of Food Products and Its Legislations: A Review. J. Agric. Food Res. 2023, 12, 100623. [Google Scholar] [CrossRef]
- Miranda, M.; Sun, X.; Marín, A.; dos Santos, L.C.; Plotto, A.; Bai, J.; Benedito Garrido Assis, O.; David Ferreira, M.; Baldwin, E. Nano- and Micro-Sized Carnauba Wax Emulsions-Based Coatings Incorporated with Ginger Essential Oil and Hydroxypropyl Methylcellulose on Papaya: Preservation of Quality and Delay of Post-Harvest Fruit Decay. Food Chem. X 2022, 13, 100249. [Google Scholar] [CrossRef]
- Tabassum, N.; Aftab, R.A.; Yousuf, O.; Ahmad, S.; Zaidi, S. Application of Nanoemulsion Based Edible Coating on Fresh-Cut Papaya. J. Food Eng. 2023, 355, 111579. [Google Scholar] [CrossRef]
- Zillo, R.R.; da Silva, P.P.M.; de Oliveira, J.; da Glória, E.M.; Spoto, M.H.F. Carboxymethylcellulose Coating Associated with Essential Oil Can Increase Papaya Shelf Life. Sci. Hortic. 2018, 239, 70–77. [Google Scholar] [CrossRef]
- Luciano, W.A.; Pimentel, T.C.; Bezerril, F.F.; Barão, C.E.; Marcolino, V.A.; de Siqueira Ferraz Carvalho, R.; dos Santos Lima, M.; Martín-Belloso, O.; Magnani, M. Effect of Citral Nanoemulsion on the Inactivation of Listeria Monocytogenes and Sensory Properties of Fresh-Cut Melon and Papaya during Storage. Int. J. Food Microbiol. 2023, 384, 109959. [Google Scholar] [CrossRef] [PubMed]
- Gundewadi, G.; Rudra, S.G.; Sarkar, D.J.; Singh, D. Nanoemulsion Based Alginate Organic Coating for Shelf Life Extension of Okra. Food Packag. Shelf Life 2018, 18, 1–12. [Google Scholar] [CrossRef]
- Bashir, O.; Amin, T.; Hussain, S.Z.; Naik, H.R.; Goksen, G.; Wani, A.W.; Manzoor, S.; Malik, A.R.; Wani, F.J.; Proestos, C. Development, Characterization and Use of Rosemary Essential Oil Loaded Water-Chestnut Starch Based Nanoemulsion Coatings for Enhancing Post-Harvest Quality of Apples Var. Golden Delicious. Curr. Res. Food Sci. 2023, 7, 100570. [Google Scholar] [CrossRef] [PubMed]
- Jo, W.-S.; Song, H.-Y.; Song, N.-B.; Lee, J.-H.; Min, S.C.; Song, K.B. Quality and Microbial Safety of ‘Fuji’ Apples Coated with Carnauba-Shellac Wax Containing Lemongrass Oil. LWT Food Sci. Technol. 2014, 55, 490–497. [Google Scholar] [CrossRef]
- Naqash, F.; Masoodi, F.a.; Ayob, O.; Parvez, S. Effect of Active Pectin Edible Coatings on the Safety and Quality of Fresh-Cut Apple. Int. J. Food Sci. Technol. 2022, 57, 57–66. [Google Scholar] [CrossRef]
- Farina, V.; Passafiume, R.; Tinebra, I.; Palazzolo, E.; Sortino, G. Use of Aloe Vera Gel-Based Edible Coating with Natural Anti-Browning and Anti-Oxidant Additives to Improve Post-Harvest Quality of Fresh-Cut ‘Fuji’ Apple. Agronomy 2020, 10, 515. [Google Scholar] [CrossRef]
- Gull, A.; Bhat, N.; Wani, S.M.; Masoodi, F.A.; Amin, T.; Ganai, S.A. Shelf Life Extension of Apricot Fruit by Application of Nanochitosan Emulsion Coatings Containing Pomegranate Peel Extract. Food Chem. 2021, 349, 129149. [Google Scholar] [CrossRef]
- Das, S.; Vishakha, K.; Banerjee, S.; Mondal, S.; Ganguli, A. Sodium Alginate-Based Edible Coating Containing Nanoemulsion of Citrus Sinensis Essential Oil Eradicates Planktonic and Sessile Cells of Food-Borne Pathogens and Increased Quality Attributes of Tomatoes. Int. J. Biol. Macromol. 2020, 162, 1770–1779. [Google Scholar] [CrossRef]
- Robledo, N.; López, L.; Bunger, A.; Tapia, C.; Abugoch, L. Effects of Antimicrobial Edible Coating of Thymol Nanoemulsion/Quinoa Protein/Chitosan on the Safety, Sensorial Properties, and Quality of Refrigerated Strawberries (Fragaria × Ananassa) Under Commercial Storage Environment. Food Bioprocess Technol. 2018, 11, 1566–1574. [Google Scholar] [CrossRef]
- Das, S.K.; Vishakha, K.; Das, S.; Chakraborty, D.; Ganguli, A. Carboxymethyl Cellulose and Cardamom Oil in a Nanoemulsion Edible Coating Inhibit the Growth of Foodborne Pathogens and Extend the Shelf Life of Tomatoes. Biocatal. Agric. Biotechnol. 2022, 42, 102369. [Google Scholar] [CrossRef]
- Gómez-Contreras, P.; Figueroa-Lopez, K.J.; Hernández-Fernández, J.; Cortés Rodríguez, M.; Ortega-Toro, R. Effect of Different Essential Oils on the Properties of Edible Coatings Based on Yam (Dioscorea rotundata L.) Starch and Its Application in Strawberry (Fragaria vesca L.) Preservation. Appl. Sci. 2021, 11, 11057. [Google Scholar] [CrossRef]
- Oliveira Filho, J.G.d.; Albiero, B.R.; Calisto, Í.H.; Bertolo, M.R.V.; Oldoni, F.C.A.; Egea, M.B.; Bogusz Junior, S.; de Azeredo, H.M.C.; Ferreira, M.D. Bio-Nanocomposite Edible Coatings Based on Arrowroot Starch/Cellulose Nanocrystals/Carnauba Wax Nanoemulsion Containing Essential Oils to Preserve Quality and Improve Shelf Life of Strawberry. Int. J. Biol. Macromol. 2022, 219, 812–823. [Google Scholar] [CrossRef]
- Sathiyaseelan, A.; Saravanakumar, K.; Mariadoss, A.V.A.; Ramachandran, C.; Hu, X.; Oh, D.-H.; Wang, M.-H. Chitosan-Tea Tree Oil Nanoemulsion and Calcium Chloride Tailored Edible Coating Increase the Shelf Life of Fresh Cut Red Bell Pepper. Prog. Organ. Coat. 2021, 151, 106010. [Google Scholar] [CrossRef]
- Arabpoor, B.; Yousefi, S.; Weisany, W.; Ghasemlou, M. Multifunctional Coating Composed of Eryngium Campestre L. Essential Oil Encapsulated in Nano-Chitosan to Prolong the Shelf-Life of Fresh Cherry Fruits. Food Hydrocoll. 2021, 111, 106394. [Google Scholar] [CrossRef]
- Basumatary, I.B.; Mukherjee, A.; Katiyar, V.; Dutta, J.; Kumar, S. Chitosan-Based Active Coating for Pineapple Preservation: Evaluation of Antimicrobial Efficacy and Shelf-Life Extension. LWT 2022, 168, 113940. [Google Scholar] [CrossRef]
- Prakash, A.; Baskaran, R.; Vadivel, V. Citral Nanoemulsion Incorporated Edible Coating to Extend the Shelf Life of Fresh Cut Pineapples. LWT 2020, 118, 108851. [Google Scholar] [CrossRef]
- Yang, R.; Miao, J.; Shen, Y.; Cai, N.; Wan, C.; Zou, L.; Chen, C.; Chen, J. Antifungal Effect of Cinnamaldehyde, Eugenol and Carvacrol Nanoemulsion against Penicillium Digitatum and Application in Postharvest Preservation of Citrus Fruit. LWT 2021, 141, 110924. [Google Scholar] [CrossRef]
- Phothisuwan, S.; Koomhin, P.; Matan, N.; Matan, N. Quality Maintenance of Salacca Fruit with a Carnauba Wax Coating Containing Orange Oil and Detection of Sensory Perception Improvement with Electroencephalography to Appraise Brain Responses. LWT 2021, 147, 111628. [Google Scholar] [CrossRef]
- Vilaplana, R.; Guerrero, K.; Guevara, J.; Valencia-Chamorro, S. Chitosan Coatings to Control Soft Mold on Fresh Blackberries (Rubus Glaucus Benth.) during Postharvest Period. Sci. Hortic. 2020, 262, 109049. [Google Scholar] [CrossRef]
- López-Palestina, C.U.; Aguirre-Mancilla, C.L.; Raya-Pérez, J.C.; Ramírez-Pimentel, J.G.; Gutiérrez-Tlahque, J.; Hernández-Fuentes, A.D. The Effect of an Edible Coating with Tomato Oily Extract on the Physicochemical and Antioxidant Properties of Garambullo (Myrtillocactus geometrizans) Fruits. Agronomy 2018, 8, 248. [Google Scholar] [CrossRef]
- Aboryia, M.S.; El-Gioushy, S.F.; Sami, R.; Aljumayi, H.; Alyamani, A.; Almasoudi, A.; Gawish, M.S. Synergistic Effect of Dipping in Aloe Vera Gel and Mixing with Chitosan or Calcium Chloride on the Activities of Antioxidant Enzymes and Cold Storage Potential of Peach (Prunus persica L.) Fruits. Coatings 2022, 12, 498. [Google Scholar] [CrossRef]
- Sortino, G.; Inglese, P.; Farina, V.; Passafiume, R.; Allegra, A. The Use of Opuntia Ficus-Indica Mucilage and Aloe Arborescens as Edible Coatings to Improve the Physical, Chemical, and Microbiological Properties of ‘Hayward’ Kiwifruit Slices. Horticulturae 2022, 8, 219. [Google Scholar] [CrossRef]
- Manzoor, S.; Gull, A.; Wani, S.M.; Ganaie, T.A.; Masoodi, F.A.; Bashir, K.; Malik, A.R.; Dar, B.N. Improving the Shelf Life of Fresh Cut Kiwi Using Nanoemulsion Coatings with Antioxidant and Antimicrobial Agents. Food Biosci. 2021, 41, 101015. [Google Scholar] [CrossRef]
- Iftikhar, A.; Rehman, A.; Usman, M.; Ali, A.; Ahmad, M.M.; Shehzad, Q.; Fatim, H.; Mehmood, A.; Moiz, A.; Shabbir, M.A.; et al. Influence of Guar Gum and Chitosan Enriched with Lemon Peel Essential Oil Coatings on the Quality of Pears. Food Sci. Nutr. 2022, 10, 2443–2454. [Google Scholar] [CrossRef]
- Moradinezhad, F.; Naeimi, A.; Farhangfar, H. Influence of Edible Coatings on Postharvest Quality of Fresh Chinese Jujube Fruits during Refrigerated Storage. J. Hortic. Postharvest Res. 2018, 1, 1–14. [Google Scholar] [CrossRef]
- Utama, N.A.; Pranata, I.A.; Pramesi, P.C. Maintaining Physicochemical and Sensory Properties of Guava Var. Getas Merah Using Alginate and Cyclea Barbata Leaveas Powder as Edible Coating. Adv. Hortic. Sci. 2022, 36, 135–144. [Google Scholar] [CrossRef]
- Shivangi, S.; Dorairaj, D.; Negi, P.S.; Shetty, N.P. Development and Characterisation of a Pectin-Based Edible Film That Contains Mulberry Leaf Extract and Its Bio-Active Components. Food Hydrocoll. 2021, 121, 107046. [Google Scholar] [CrossRef]
- Arnon-Rips, H.; Porat, R.; Poverenov, E. Enhancement of Agricultural Produce Quality and Storability Using Citral-Based Edible Coatings; the Valuable Effect of Nano-Emulsification in a Solid-State Delivery on Fresh-Cut Melons Model. Food Chem. 2019, 277, 205–212. [Google Scholar] [CrossRef]
- Bhargava, K.; Conti, D.S.; da Rocha, S.R.P.; Zhang, Y. Application of an Oregano Oil Nanoemulsion to the Control of Foodborne Bacteria on Fresh Lettuce. Food Microbiol. 2015, 47, 69–73. [Google Scholar] [CrossRef]
- Suhag, R.; Kumar, N.; Petkoska, A.T.; Upadhyay, A. Film Formation and Deposition Methods of Edible Coating on Food Products: A Review. Food Res. Int. 2020, 136, 109582. [Google Scholar] [CrossRef]
- Mahmud, N.; Islam, J.; Tahergorabi, R. Marine Biopolymers: Applications in Food Packaging. Processes 2021, 9, 2245. [Google Scholar] [CrossRef]
- Chettri, S.; Sharma, N.; Mohite, A.M. Edible Coatings and Films for Shelf-Life Extension of Fruit and Vegetables. Biomater. Adv. 2023, 154, 213632. [Google Scholar] [CrossRef] [PubMed]
- Maringgal, B.; Hashim, N.; Mohamed Amin Tawakkal, I.S.; Muda Mohamed, M.T. Recent Advance in Edible Coating and Its Effect on Fresh/Fresh-Cut Fruits Quality. Trends Food Sci. Technol. 2020, 96, 253–267. [Google Scholar] [CrossRef]
- Sousa Gallagher, M.J.; Mahajan, P.V. 22—The Stability and Shelf Life of Fruit and Vegetables. In Food and Beverage Stability and Shelf Life; Kilcast, D., Subramaniam, P., Eds.; Woodhead Publishing Series in Food Science, Technology and Nutrition; Woodhead Publishing: Sawston, UK, 2011; pp. 641–656. ISBN 978-1-84569-701-3. [Google Scholar]
- Abdalla, G.; Mussagy, C.U.; Sant’Ana Pegorin Brasil, G.; Scontri, M.; da Silva Sasaki, J.C.; Su, Y.; Bebber, C.; Rocha, R.R.; de Sousa Abreu, A.P.; Goncalves, R.P.; et al. Eco-Sustainable Coatings Based on Chitosan, Pectin, and Lemon Essential Oil Nanoemulsion and Their Effect on Strawberry Preservation. Int. J. Biol. Macromol. 2023, 249, 126016. [Google Scholar] [CrossRef] [PubMed]
- McClements, D.J.; Rao, J. Food-Grade Nanoemulsions: Formulation, Fabrication, Properties, Performance, Biological Fate, and Potential Toxicity. Crit. Rev. Food Sci. Nutr. 2011, 51, 285–330. [Google Scholar] [CrossRef] [PubMed]





| Nanoemulsion Polymer(s) | Active Compound | Functional Role | Fruits/Vegetable | Ref. |
|---|---|---|---|---|
| Polysaccharide-based | ||||
| Pectin | Ascorbic acid, citric acid, and sodium chlorite | Decreased microbial deterioration; little effect on the nutritional or sensory value. | Apple | [43] |
| Chitosan | Carvacrol | Prevented microbial growth for 13 days at 5 °C. | Cut carrot | [44] |
| Starch | Basil leaf extract | Extended the shelf life of eggplant up to 16 days; maintained firmness; reduced moisture loss; prevented the growth of total soluble solids, and prevented colour changes. | Eggplant | [45] |
| Alginate | Lemon EO * or orange EO | Inhibited bacterial and fungal growth for 15 days of storage. | Red raspberry | [46] |
| Xanthan | Cinnamic acid | Slowed down the process of browning; increased their shelf-life by up to 4 days and 8 days of storage. | Fresh-cut pear | [47] |
| Protein-based | ||||
| Collagen | / | Reduced the rate of gas transport, which made them useful instruments for extending shelf life. | Mangoes and apples | [48] |
| Whey protein | / | Maintained various fruit qualities, such as the total phenolic content, browning, and product weight loss. | Apples | [49] |
| Soy | Cinnamaldehyde | Proved to be an effective antioxidant and moisture barrier. | Banana | [50] |
| Zein | Carvacrol, thymol, and eugenol EO | Reduced growth of some major pathogens on the peel surfaces of melons. | Melon | [51] |
| Lipid-based | ||||
| Carnauba wax | Lemongrass oil | Prevented foodborne pathogens (Salmonella typhimurium and Escherichia coli) and prolonged shelf life. | Grape berries | [7] |
| Beeswax | / | Preserved different fruits’ quality metrics and taste characteristics. | Mandarin | [52] |
| Soy lecithin | Lysophosphatidylethanolamine | Improved the shelf life of banana fruits; provided normal colour development and low ethylene production. | Banana fruits | [53] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cvanić, T.; Šovljanski, O.; Popović, S.; Erceg, T.; Vulić, J.; Čanadanović-Brunet, J.; Ćetković, G.; Travičić, V. Progress in Fruit and Vegetable Preservation: Plant-Based Nanoemulsion Coatings and Their Evolving Trends. Coatings 2023, 13, 1835. https://doi.org/10.3390/coatings13111835
Cvanić T, Šovljanski O, Popović S, Erceg T, Vulić J, Čanadanović-Brunet J, Ćetković G, Travičić V. Progress in Fruit and Vegetable Preservation: Plant-Based Nanoemulsion Coatings and Their Evolving Trends. Coatings. 2023; 13(11):1835. https://doi.org/10.3390/coatings13111835
Chicago/Turabian StyleCvanić, Teodora, Olja Šovljanski, Senka Popović, Tamara Erceg, Jelena Vulić, Jasna Čanadanović-Brunet, Gordana Ćetković, and Vanja Travičić. 2023. "Progress in Fruit and Vegetable Preservation: Plant-Based Nanoemulsion Coatings and Their Evolving Trends" Coatings 13, no. 11: 1835. https://doi.org/10.3390/coatings13111835







