Effect of Bias Voltage on Structure, Mechanical Properties, and High-Temperature Water Vapor Corrosion of AlCrNbSiTi High Entropy Alloy Coatings
Abstract
:1. Introduction
2. Experiments Details
2.1. Coating Preparation
2.2. Coating Characterization
2.2.1. Morphology and Structure
2.2.2. Residual Stress Test
2.2.3. Mechanical Properties
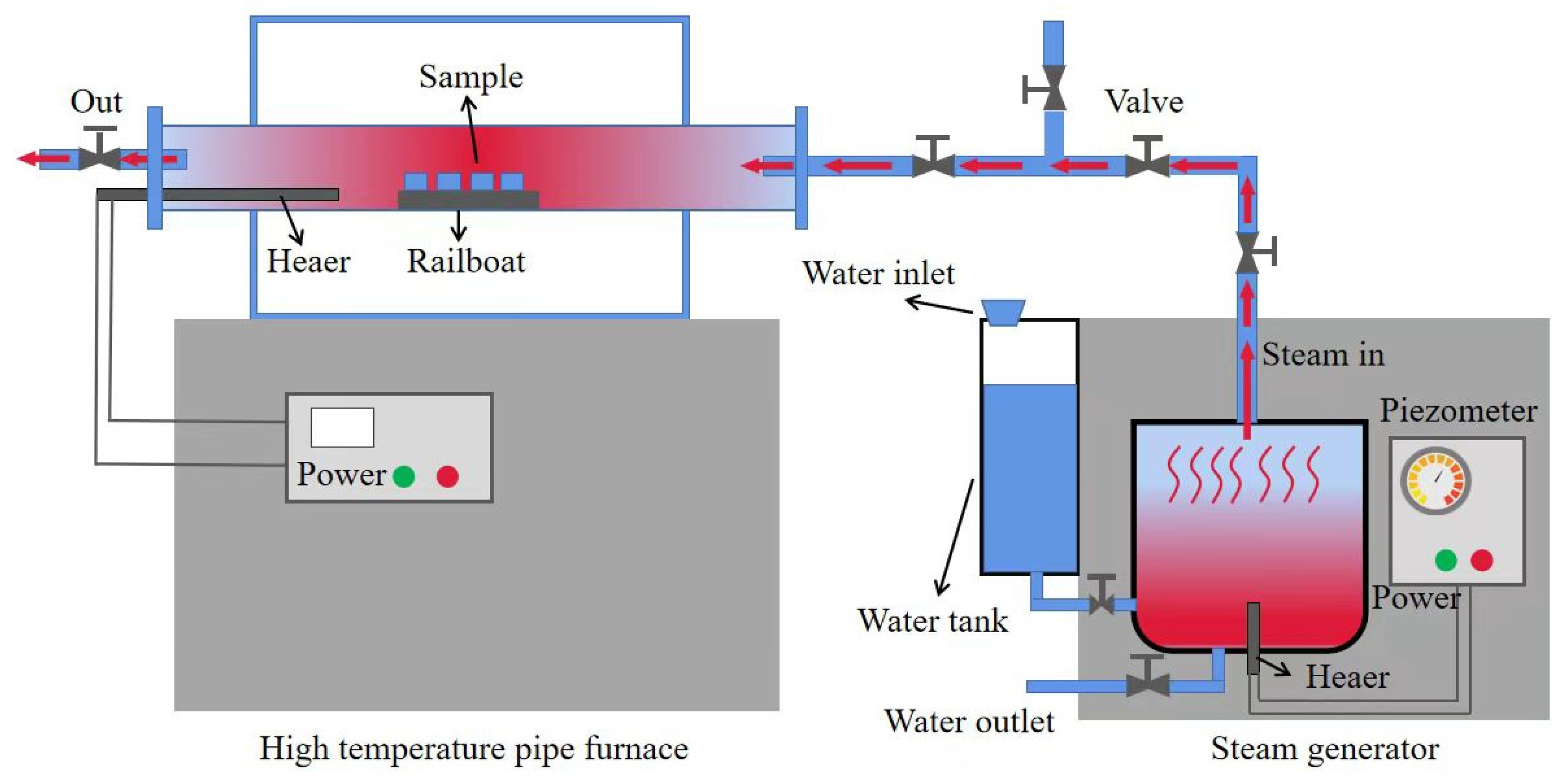
2.2.4. Water Vapor Corrosion Test
3. Results and Discussion
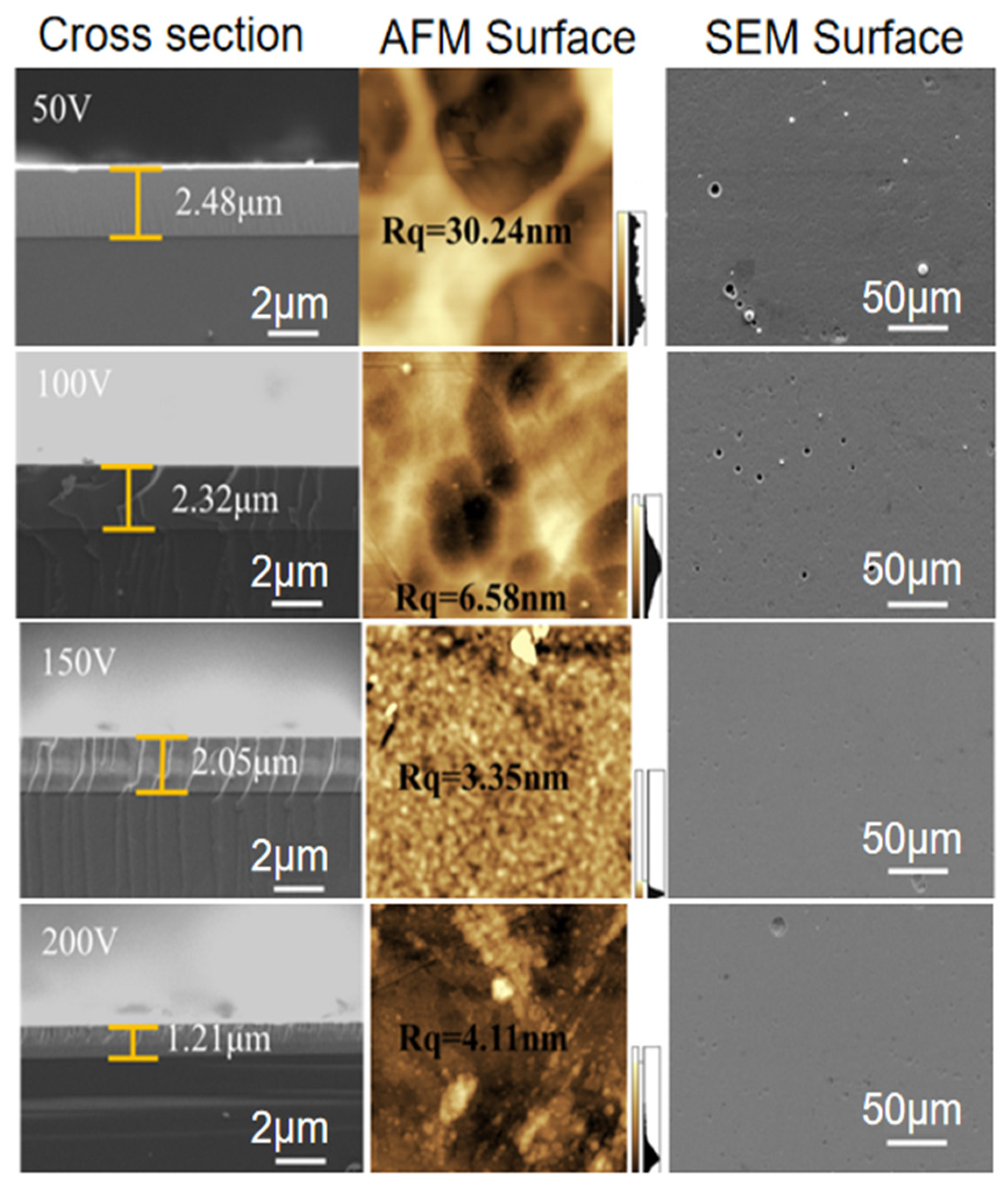
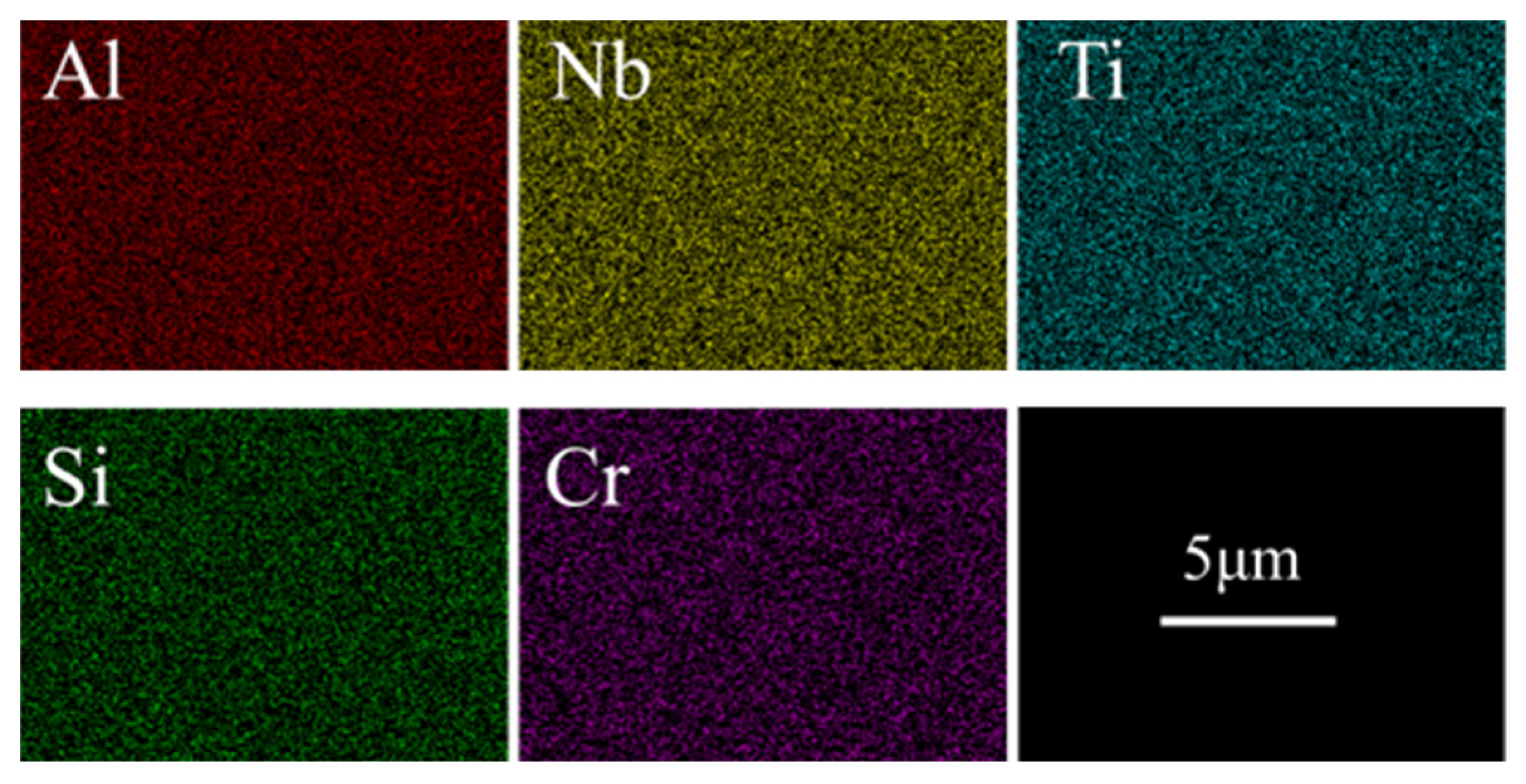
3.1. Coating Morphology
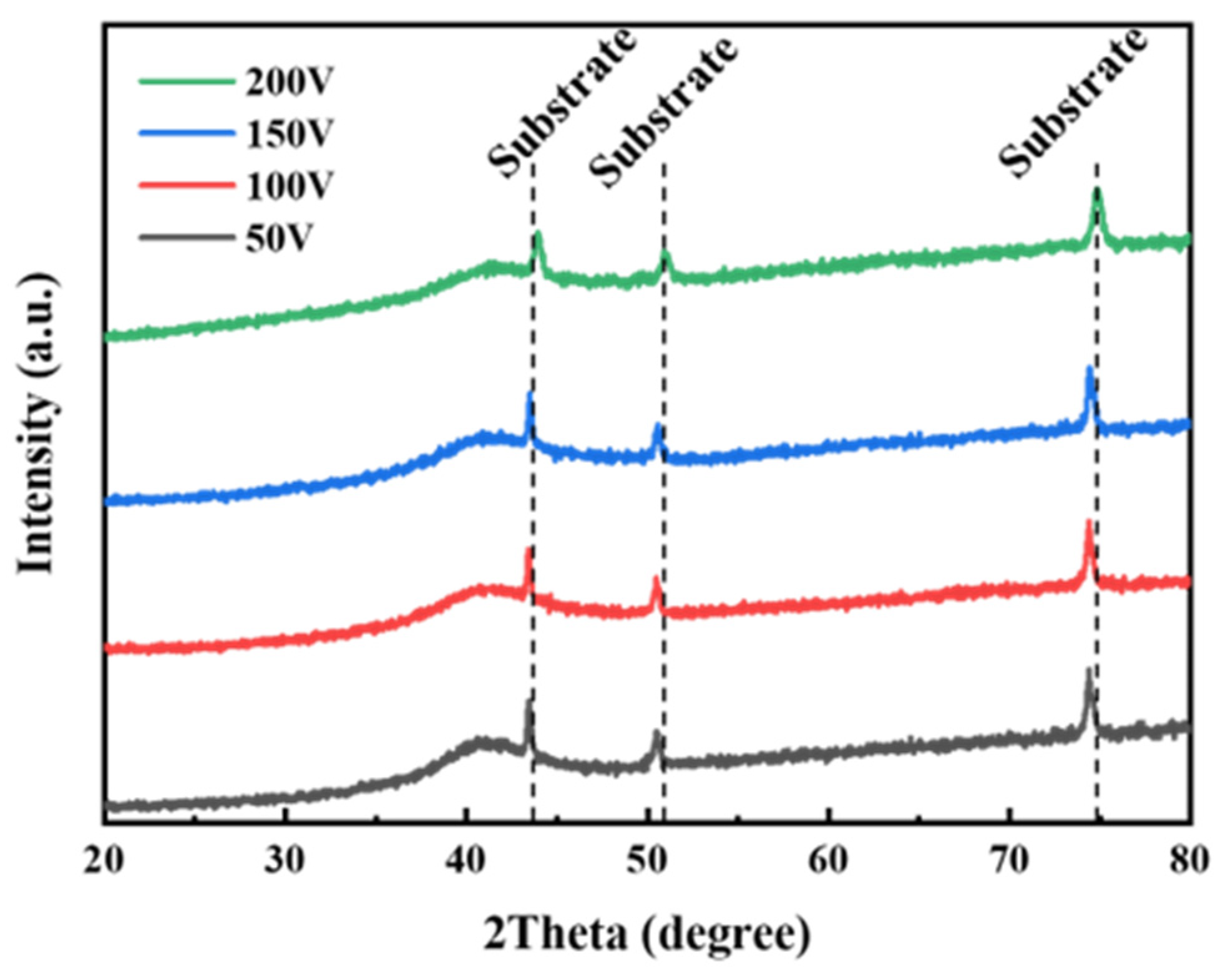
3.2. Crystal Structure of the Coating
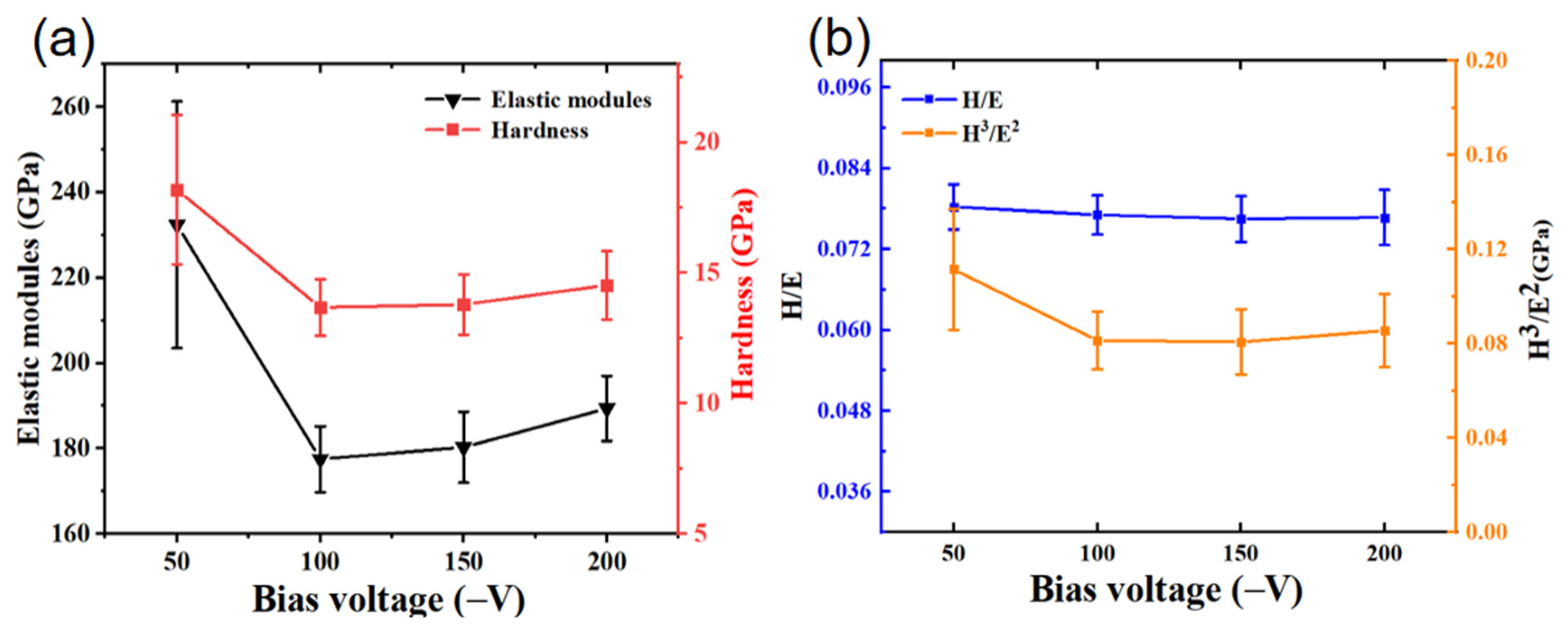
3.3. Mechanical Properties of the Coating
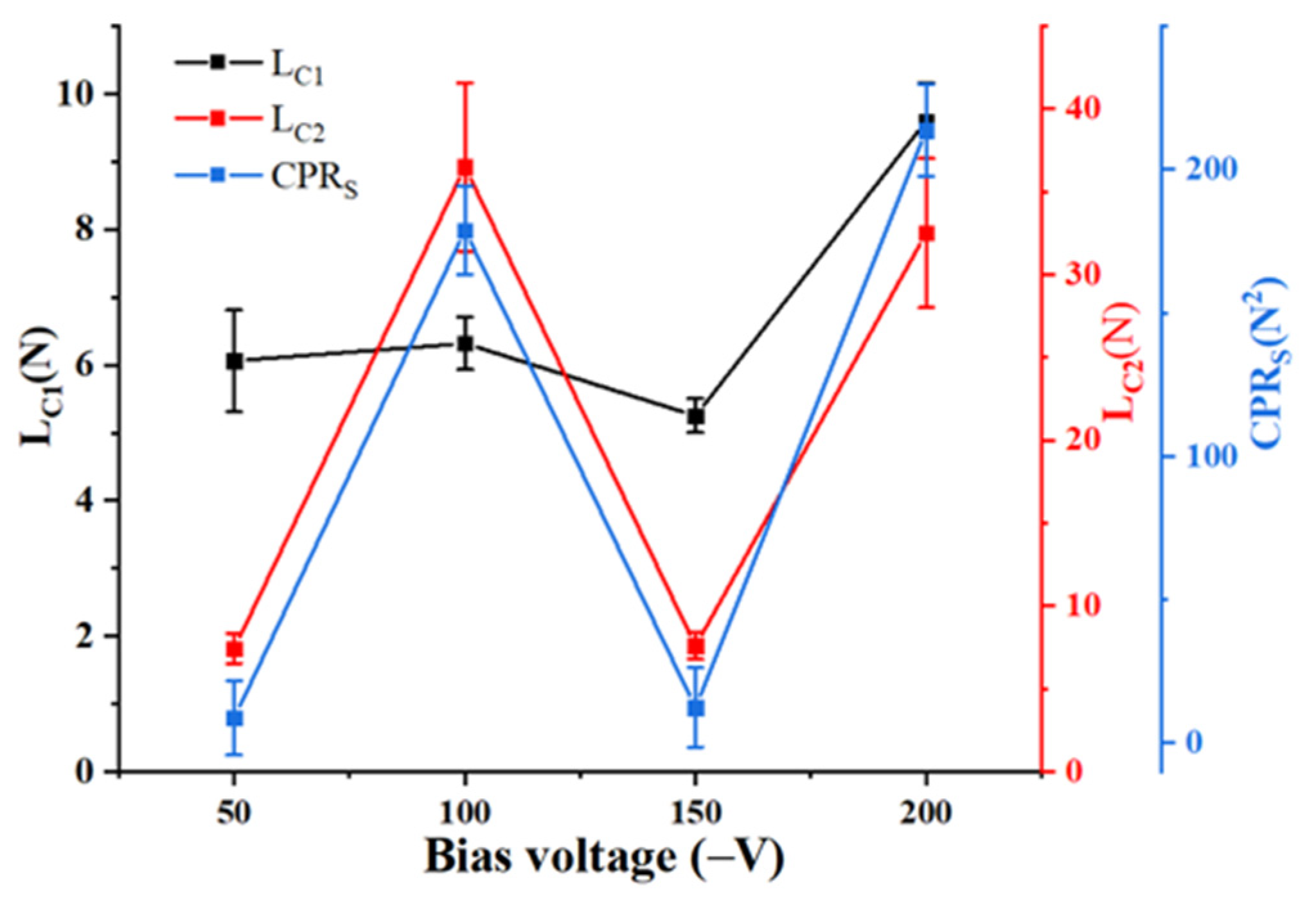
3.4. Coating Scratch Toughness
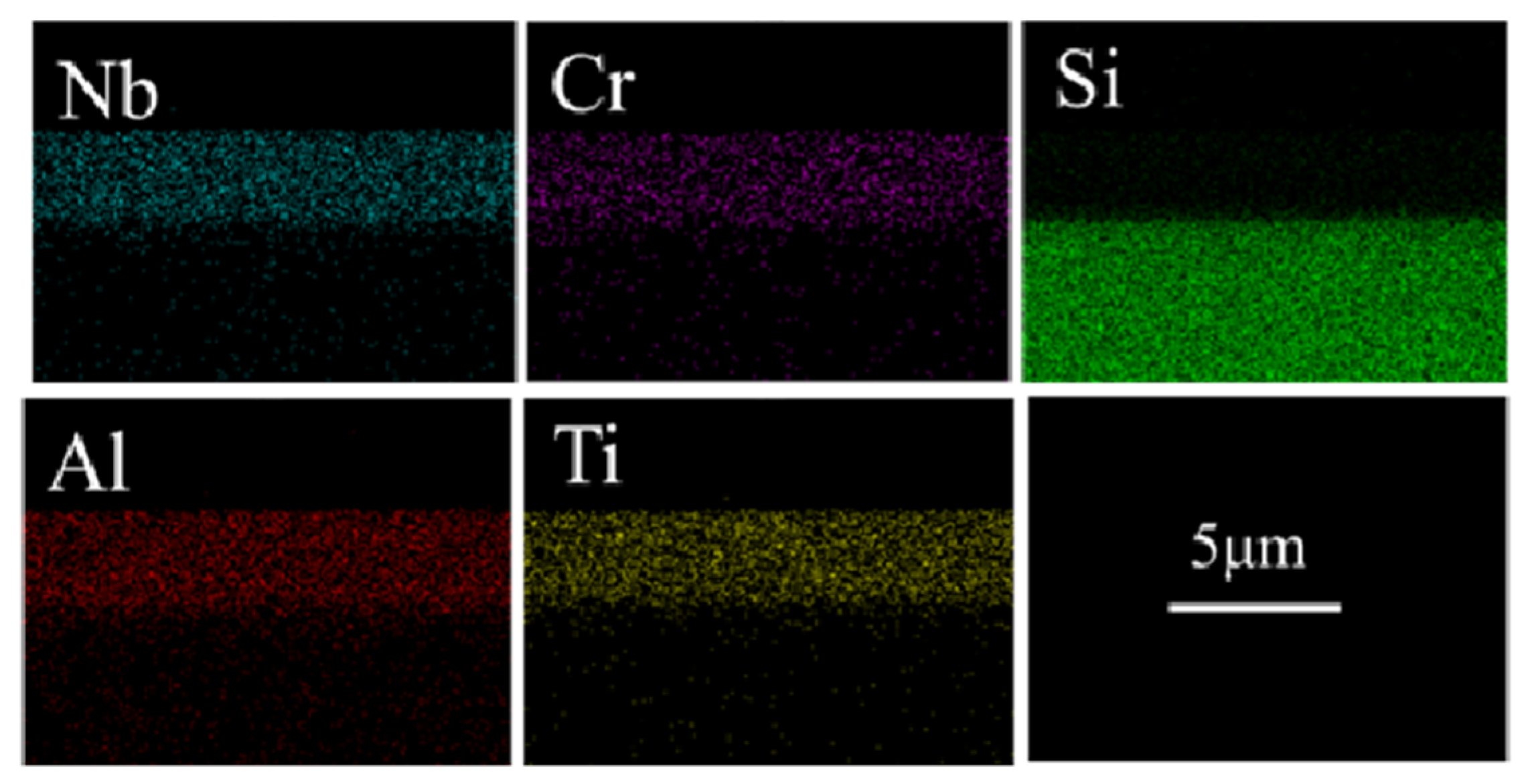
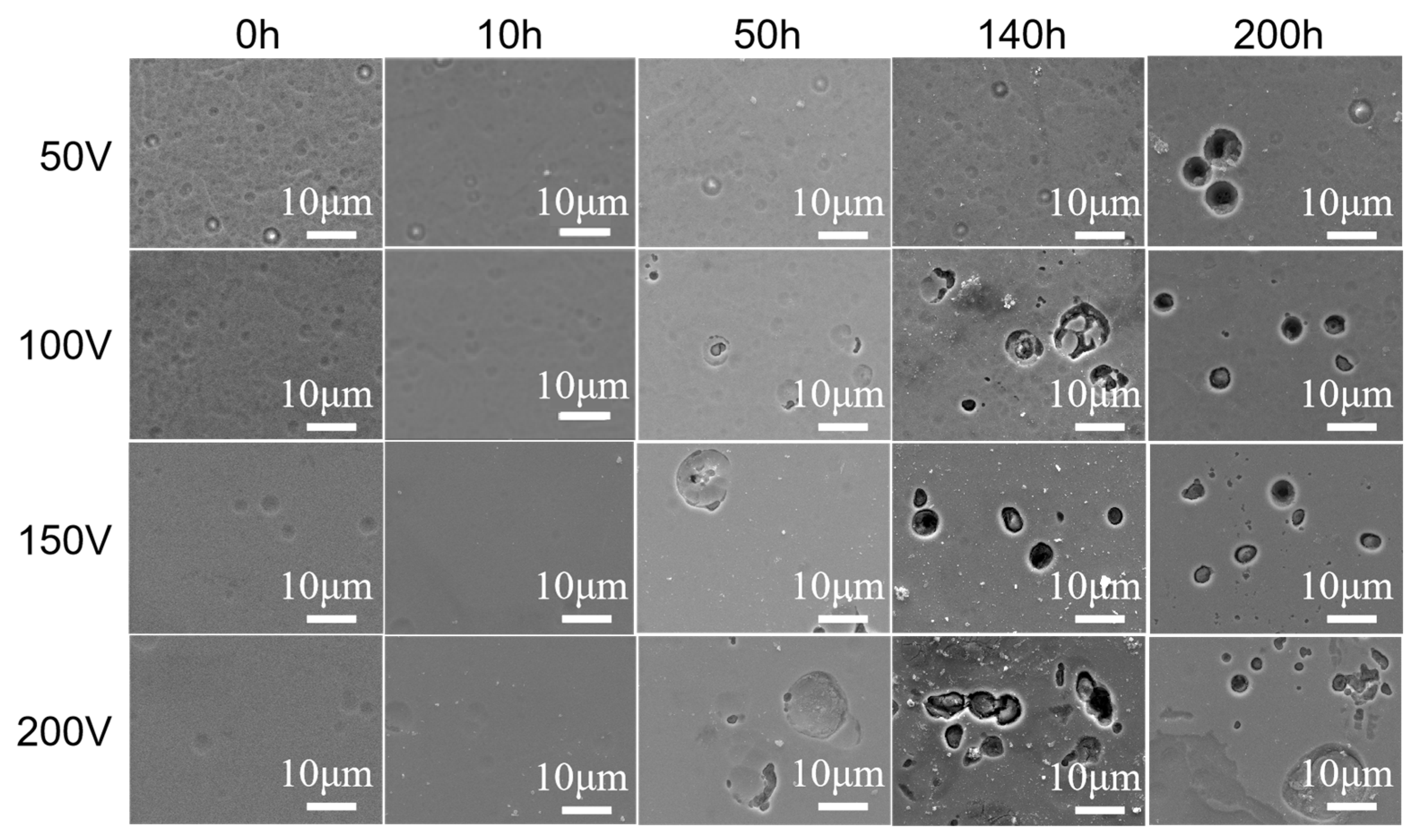
3.5. Corrosion Resistance against Water Vapor
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Hirano, M.; Yonomoto, T.; Ishigaki, M.; Watanabe, N.; Maruyama, Y.; Sibamoto, Y.; Watanabe, T.; Moriyama, K. Insights from review and analysis of the Fukushima Dai-ichi accident. J. Nucl. Sci. Technol. 2012, 49, 1–17. [Google Scholar] [CrossRef]
- Duan, Z.; Yang, H.; Satoh, Y.; Murakami, K.; Kano, S.; Zhao, Z.; Shen, J.; Abe, H. Current status of materials development of nuclear fuel cladding tubes for light water reactors. Nucl. Eng. Des. 2017, 316, 131–150. [Google Scholar] [CrossRef]
- Zinkle, S.; Terrani, K.; Gehin, J.; Ott, L.; Snead, L. Accident tolerant fuels for LWRs: A perspective. J. Nucl. Mater. 2014, 448, 374–379. [Google Scholar] [CrossRef]
- Zinkle, S.J.; Was, G.S. Materials challenges in nuclear energy. Acta Mater. 2013, 61, 735–758. [Google Scholar] [CrossRef]
- Bischoff, J.; Vauglin, C.; Delafoy, C.; Barberis, P.; Vassault, J.P.; Perche, D.; Guerin, B.; Brachet, J.C. Development of Cr-coated zirconium alloy cladding for enhanced accident tolerance. In Proceedings of the TOP FUEL 2016, Boise, ID, USA, 11–15 September 2016. [Google Scholar]
- Schuster, F.; Lomello, F.; Billard, A.; Velisa, G.; Monsifrot, E.; Bischoff, J.; Ambard, A.; Brachet, J.-C.; Lesaux, M.; Leflem, M.; et al. On-going studies at CEA on chromium coated zirconium based nuclear fuel claddings for enhanced accident tolerant LWRS fuel. In Proceedings of the Top FUEL 2015, Zurich, Switzerland, 13–19 September 2015. [Google Scholar]
- Park, J.-H.; Kim, H.-G.; Park, J.-Y.; Jung, Y.-I.; Park, D.-J.; Koo, Y.-H. High temperature steam-oxidation behavior of arc ion plated Cr coatings for accident tolerant fuel claddings. Surf. Coat. Technol. 2015, 280, 256–259. [Google Scholar] [CrossRef]
- Zhong, W.; Mouche, P.A.; Han, X.; Heuser, B.J.; Mandapaka, K.K.; Was, G.S. Performance of iron–chromium–aluminum alloy surface coatings on Zircaloy 2 under high-temperature steam and normal BWR operating conditions. J. Nucl. Mater. 2016, 470, 327–338. [Google Scholar] [CrossRef]
- Brova, M.J.; Alat, E.; Pauley, M.A.; Sherbondy, R.; Motta, A.T.; Wolfe, D.E. Undoped and ytterbium-doped titanium aluminum nitride coatings for improved oxidation behavior of nuclear fuel cladding. Surf. Coat. Technol. 2017, 331, 163–171. [Google Scholar] [CrossRef]
- Meng, C.; Yang, L.; Wu, Y.; Tan, J.; Dang, W.; He, X.; Ma, X. Study of the oxidation behavior of CrN coating on Zr alloy in air. J. Nucl. Mater. 2019, 515, 354–369. [Google Scholar] [CrossRef]
- Koski, K.; Hölsä, J.; Juliet, P. Surface defects and arc generation in reactive magnetron sputtering of aluminium oxide thin films. Surf. Coat. Technol. 1999, 115, 163–171. [Google Scholar] [CrossRef]
- Zhao, P.; Zhu, J.; Yang, K.; Li, M.; Shao, G.; Lu, H.; Ma, Z.; Wang, H.; He, J. Outstanding wear resistance of plasma sprayed high-entropy monoboride composite coating by inducing phase structural cooperative mechanism. Appl. Surf. Sci. 2023, 616, 156516. [Google Scholar] [CrossRef]
- Guo, Q.; Hou, H.; Pan, Y.; Pei, X.; Song, Z.; Liaw, P.K.; Zhao, Y. Hardening-softening of Al0.3CoCrFeNi high-entropy alloy under nanoindentation. Mater. Des. 2023, 231, 112050. [Google Scholar] [CrossRef]
- Wang, K.; Zhu, J.; Wang, H.; Yang, K.; Zhu, Y.; Qing, Y.; Ma, Z.; Gao, L.; Liu, Y.; Wei, S.; et al. Air plasma-sprayed high-entropy (Y0.2Yb0.2Lu0.2Eu0.2Er0.2)3Al5O12 coating with high thermal protection performance. J. Adv. Ceram. 2022, 11, 1571–1582. [Google Scholar] [CrossRef]
- Čekada, M.; Panjan, P.; Kek-Merl, D.; Panjan, M.; Kapun, G. SEM study of defects in PVD hard coatings. Vacuum 2007, 82, 252–256. [Google Scholar] [CrossRef]
- Mitterer, C.; Heuzè, O.; Derflinger, V.H. Substrate and coating damage by arcing during sputtering. Surf. Coat. Technol. 1997, 89, 233–238. [Google Scholar] [CrossRef]
- Zhang, S.; Sun, D.; Fu, Y.; Du, H.; Zhang, Q. Effect of sputtering target power density on topography and residual stress during growth of nanocomposite nc-TiN/a-SiNx thin films. Diam. Relat. Mater. 2004, 13, 1777–1784. [Google Scholar] [CrossRef]
- Lai, C.-H.; Lin, S.-J.; Yeh, J.-W.; Davison, A. Effect of substrate bias on the structure and properties of multi-element (AlCrTaTiZr)N coatings. J. Phys. D Appl. Phys. 2006, 39, 4628. [Google Scholar] [CrossRef]
- Ren, B.; Liu, Z.; Shi, L.; Cai, B.; Wang, M. Structure and properties of (AlCrMnMoNiZrB0.1)Nx coatings prepared by reactive DC sputtering. Appl. Surf. Sci. 2011, 257, 7172–7178. [Google Scholar] [CrossRef]
- Zhao, Y.; Chen, S.; Chen, Y.; Wu, S.; Xie, W.; Yan, W.; Wang, S.; Liao, B.; Zhang, S. Super-hard and anti-corrosion (AlCrMoSiTi)Nx high entropy nitride coatings by multi-arc cathodic vacuum magnetic filtration deposition. Vacuum 2022, 195, 110685. [Google Scholar] [CrossRef]
- Lackner, J.M.; Major, L.; Kot, M. Microscale interpretation of tribological phenomena in Ti/TiN soft-hard multilayer coatings on soft austenite steel substrates. Bull. Pol. Acad. Sci. Tech. Sci. 2011, 59, 343–355. [Google Scholar] [CrossRef]
- Sharifahmadian, O.; Mahboubi, F. A comparative study of microstructural and tribological properties of N-DLC/DLC double layer and single layer coatings deposited by DC-pulsed PACVD process. Ceram. Int. 2019, 45, 7736–7742. [Google Scholar] [CrossRef]
- Baer, D.R.; Thevuthasan, S. Chapter 16—Characterization of Thin Films and Coatings. In Handbook of Deposition Technologies for Films and Coatings, 3rd ed.; Martin, P.M., Ed.; William Andrew Publishing: Boston, MA, USA, 2010; pp. 749–864. [Google Scholar]




| Elements | Zone A (at.%) | Zone B (at.%) | Zone C (at.%) |
|---|---|---|---|
| Al | 9.7 | 7.3 | 0 |
| Si | 14.5 | 6.4 | 0 |
| Ti | 21.0 | 14.5 | 1.6 |
| Cr | 41.3 | 27.1 | 1.5 |
| Nb | 12.9 | 10.4 | 0 |
| O | 0 | 0 | 8.4 |
| W | 0.6 | 23.7 | 54.9 |
| Co | 0 | 10.5 | 33.5 |
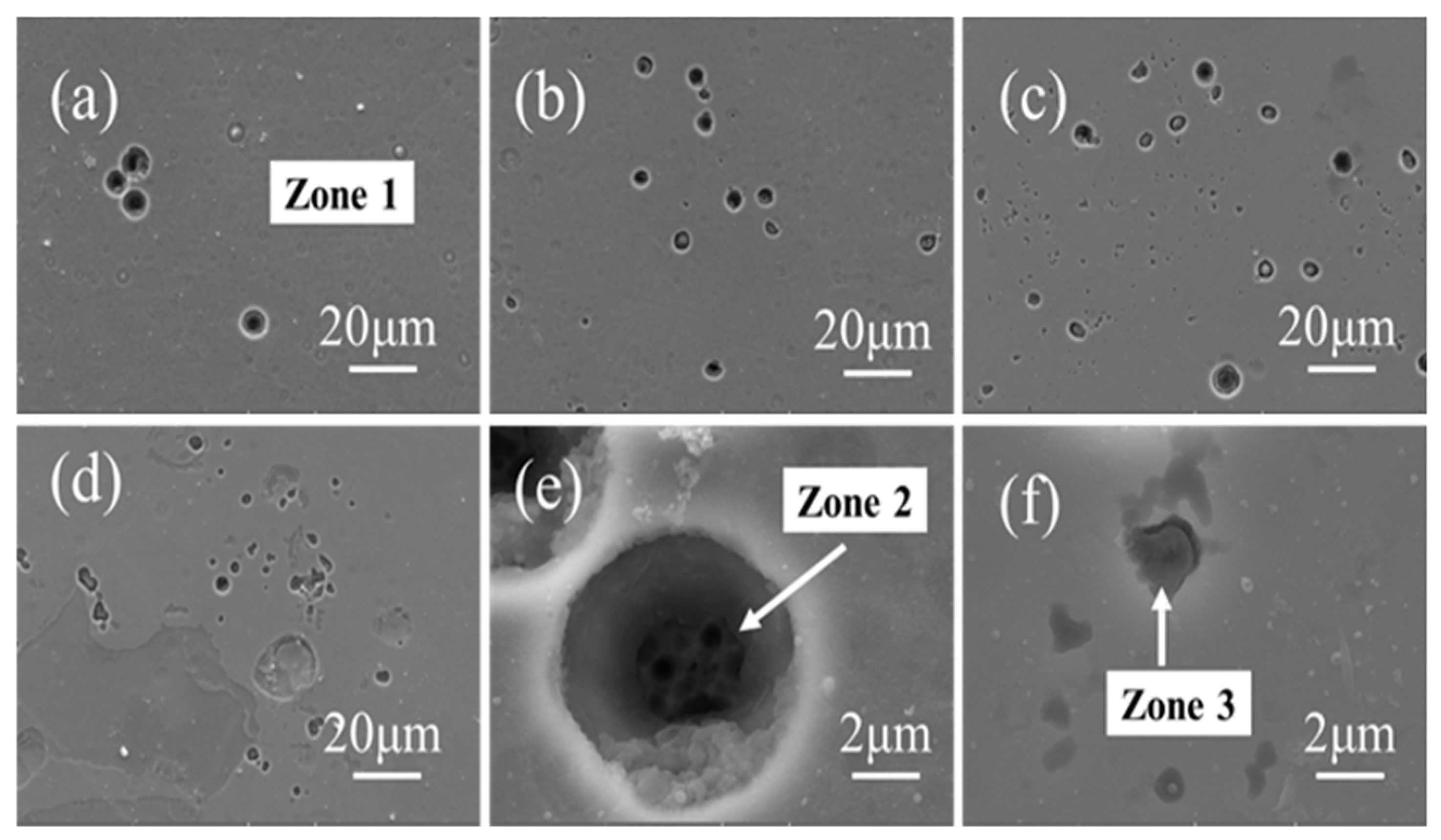
| Elements | Zone 1 (at.%) | Zone 2 (at.%) | Zone 3 (at.%) |
|---|---|---|---|
| Al | 20.1 | 2.6 | 5.7 |
| Si | 11.5 | 2.3 | 11.4 |
| Ti | 18.2 | 2.2 | 22.3 |
| Cr | 18.5 | 16.0 | 25.6 |
| Nb | 9.8 | 0.9 | 13.4 |
| O | 21.9 | 0 | 21.6 |
| Mn | 0 | 11.0 | 0 |
| Fe | 0 | 65.0 | 0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, X.; Zeng, Z.; Wang, H.; Bai, H.; Li, W.; Li, Y.; Wang, Z.; Chen, Y.; Yang, B. Effect of Bias Voltage on Structure, Mechanical Properties, and High-Temperature Water Vapor Corrosion of AlCrNbSiTi High Entropy Alloy Coatings. Coatings 2023, 13, 1948. https://doi.org/10.3390/coatings13111948
Wang X, Zeng Z, Wang H, Bai H, Li W, Li Y, Wang Z, Chen Y, Yang B. Effect of Bias Voltage on Structure, Mechanical Properties, and High-Temperature Water Vapor Corrosion of AlCrNbSiTi High Entropy Alloy Coatings. Coatings. 2023; 13(11):1948. https://doi.org/10.3390/coatings13111948
Chicago/Turabian StyleWang, Xuanzheng, Zhong Zeng, Haobin Wang, Haiping Bai, Wentao Li, Yonghao Li, Ziwei Wang, Yanming Chen, and Bing Yang. 2023. "Effect of Bias Voltage on Structure, Mechanical Properties, and High-Temperature Water Vapor Corrosion of AlCrNbSiTi High Entropy Alloy Coatings" Coatings 13, no. 11: 1948. https://doi.org/10.3390/coatings13111948





