Effects and Consequences of an Alkali-Induced Cathodic Environment on Coating Aging
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.1.1. Free Membranes
2.1.2. Coated Steel
2.2. Exposure Conditions
2.2.1. Free Membranes
2.2.2. Coated Steel
2.3. Gravimetric Measurements
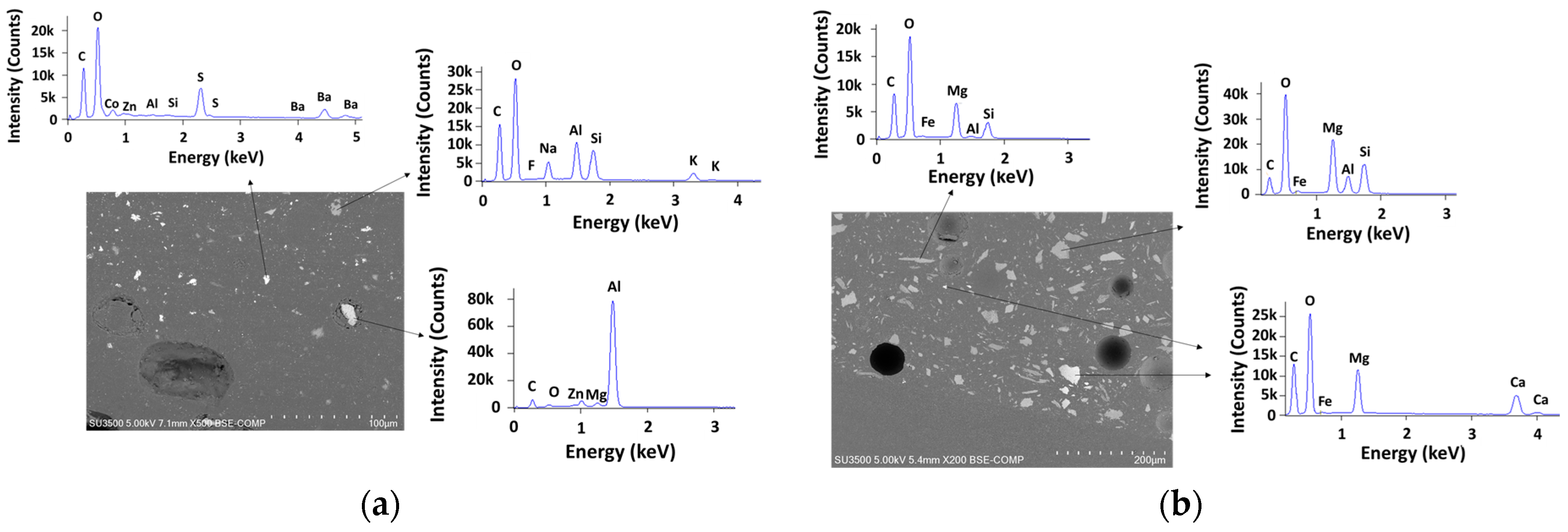
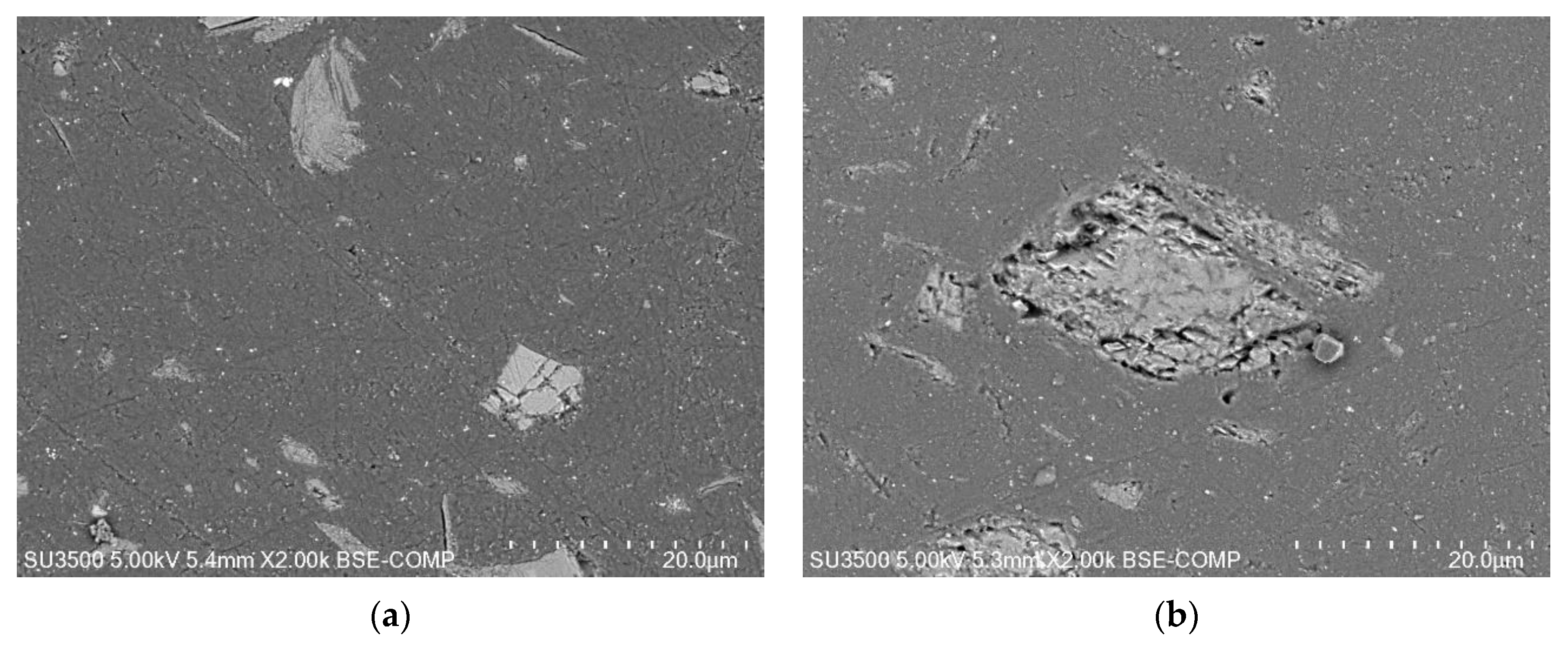
2.4. Characterization
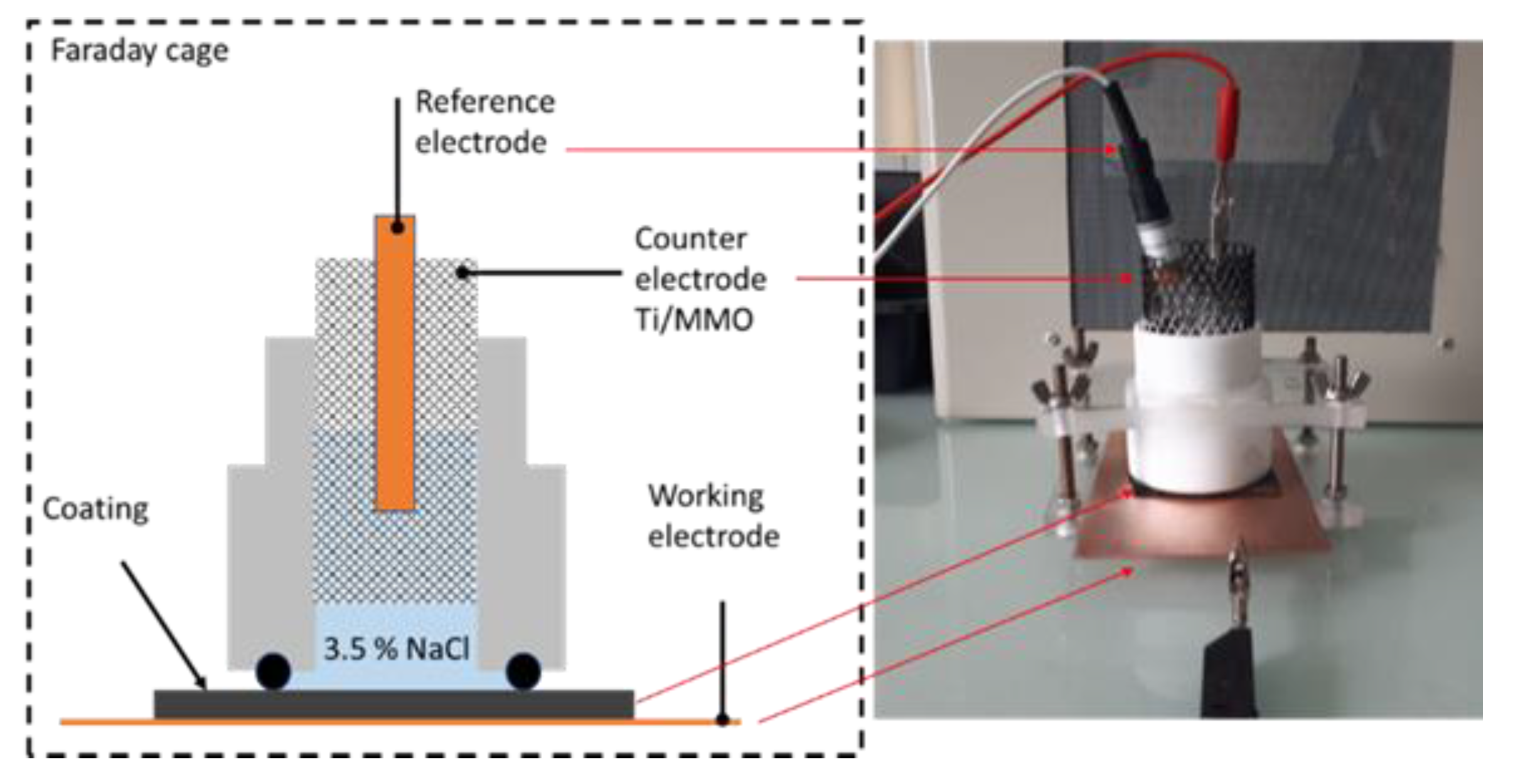
2.4.1. Electrochemical Impedance Spectroscopy (EIS)
2.4.2. Adhesion Test on Coated Samples
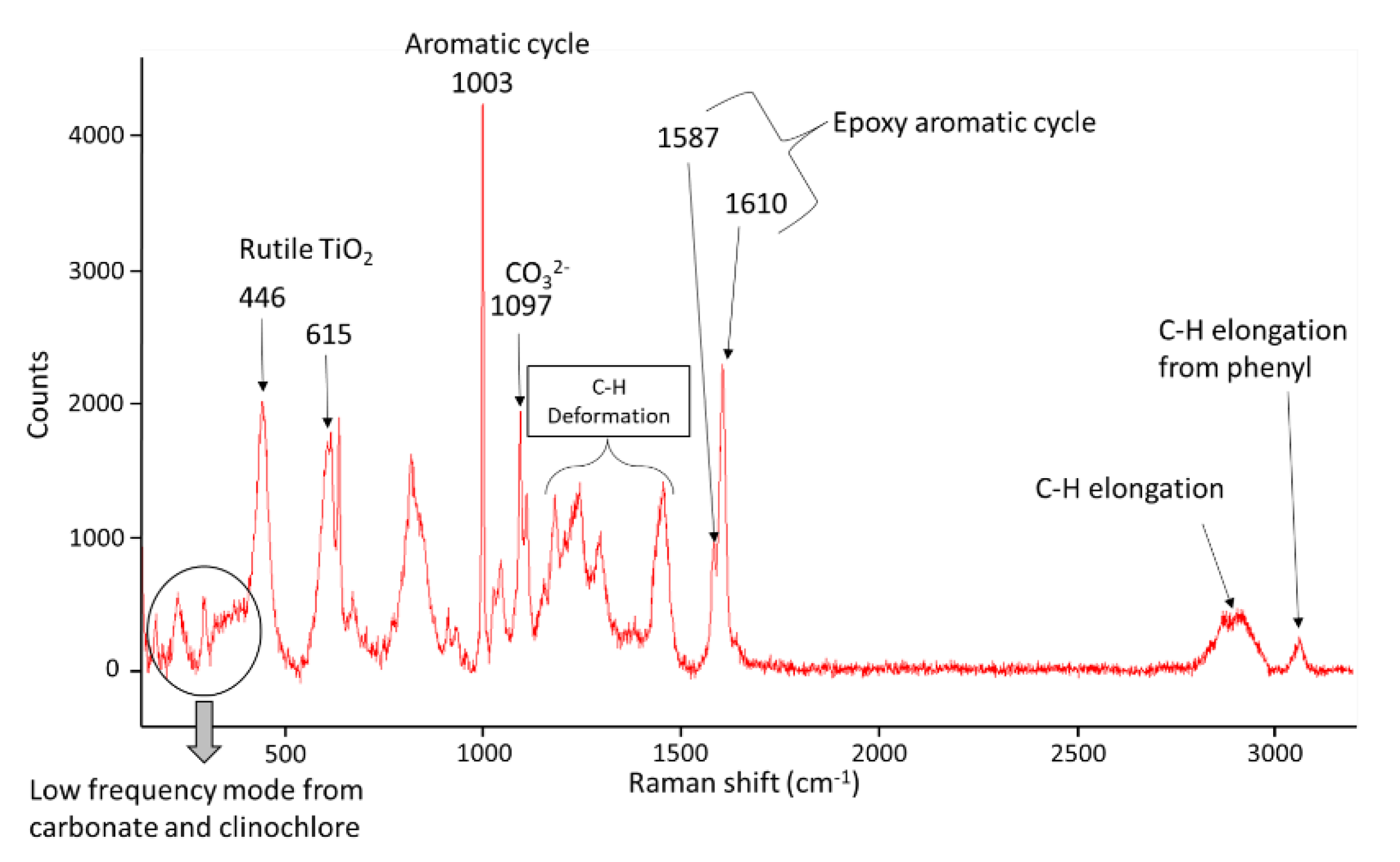
2.4.3. Characterization of the Aging of the Free Membranes
3. Results
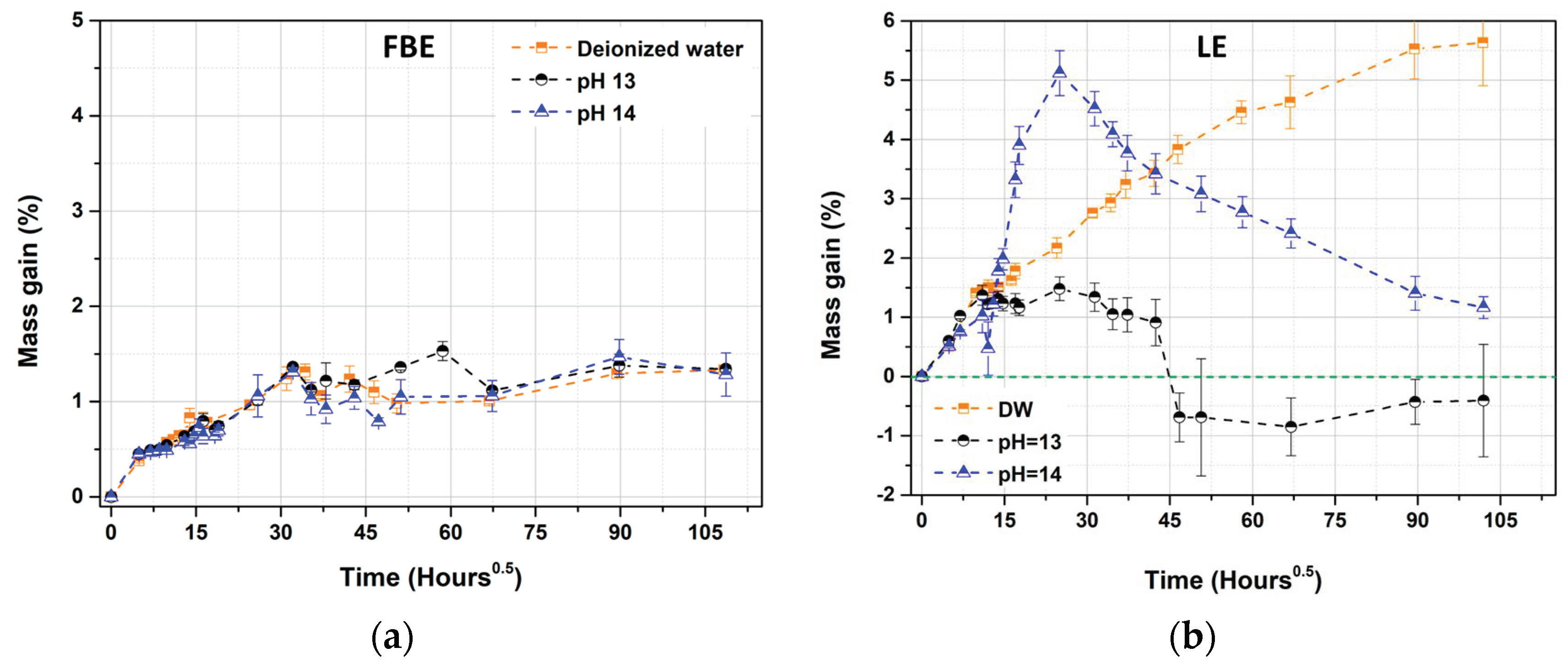
3.1. Aging Effect in Deionized Water
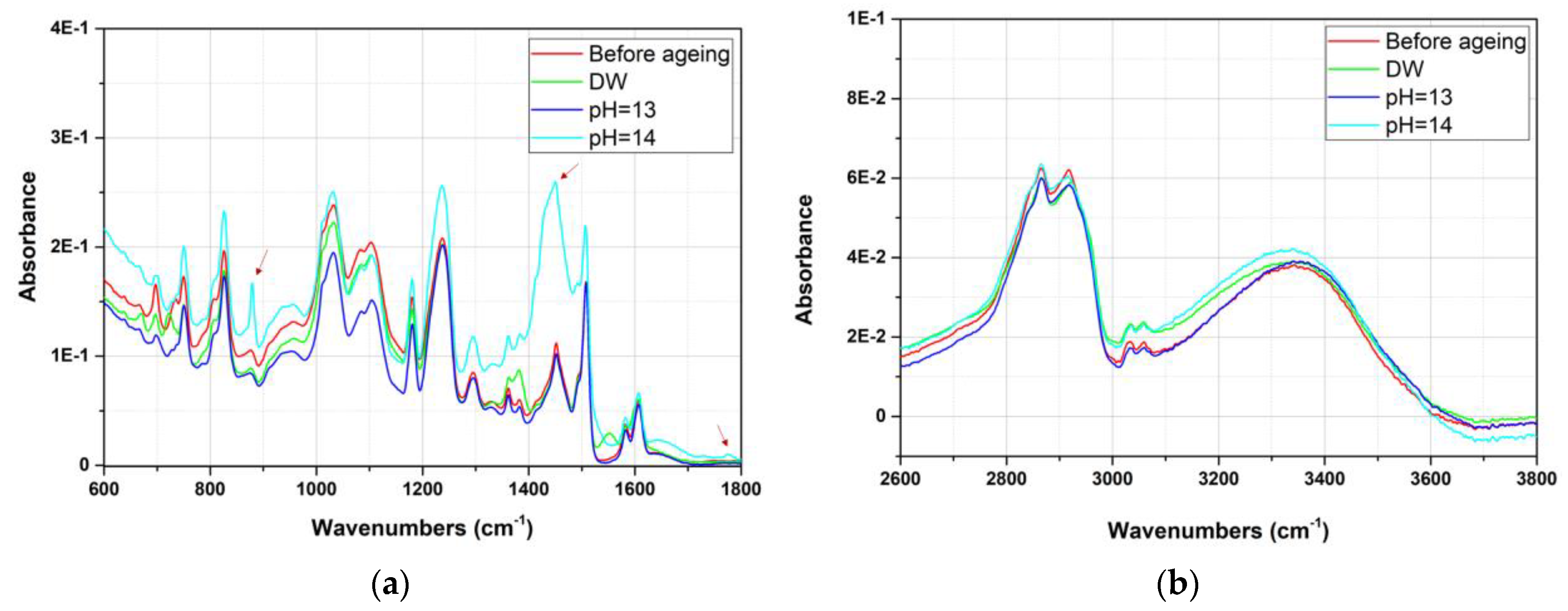
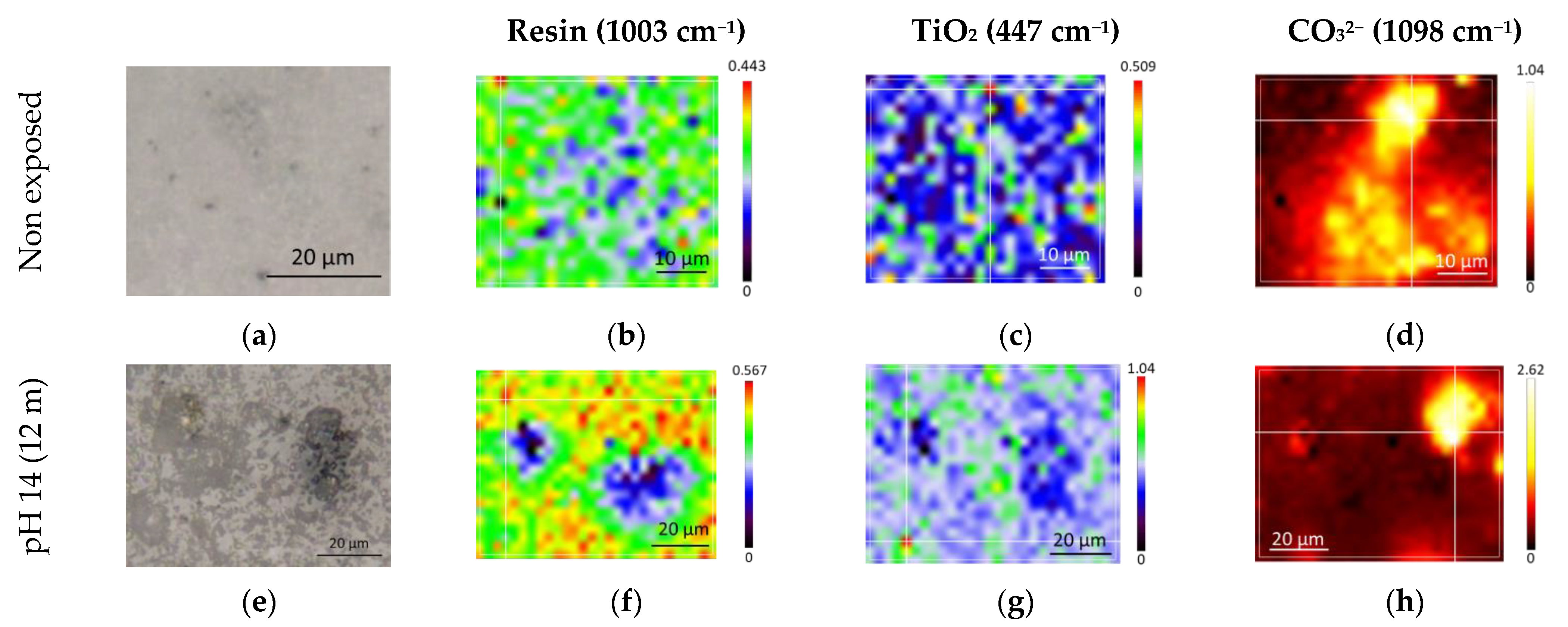
3.2. Aging Effect of Alkaline Solution at Different pH
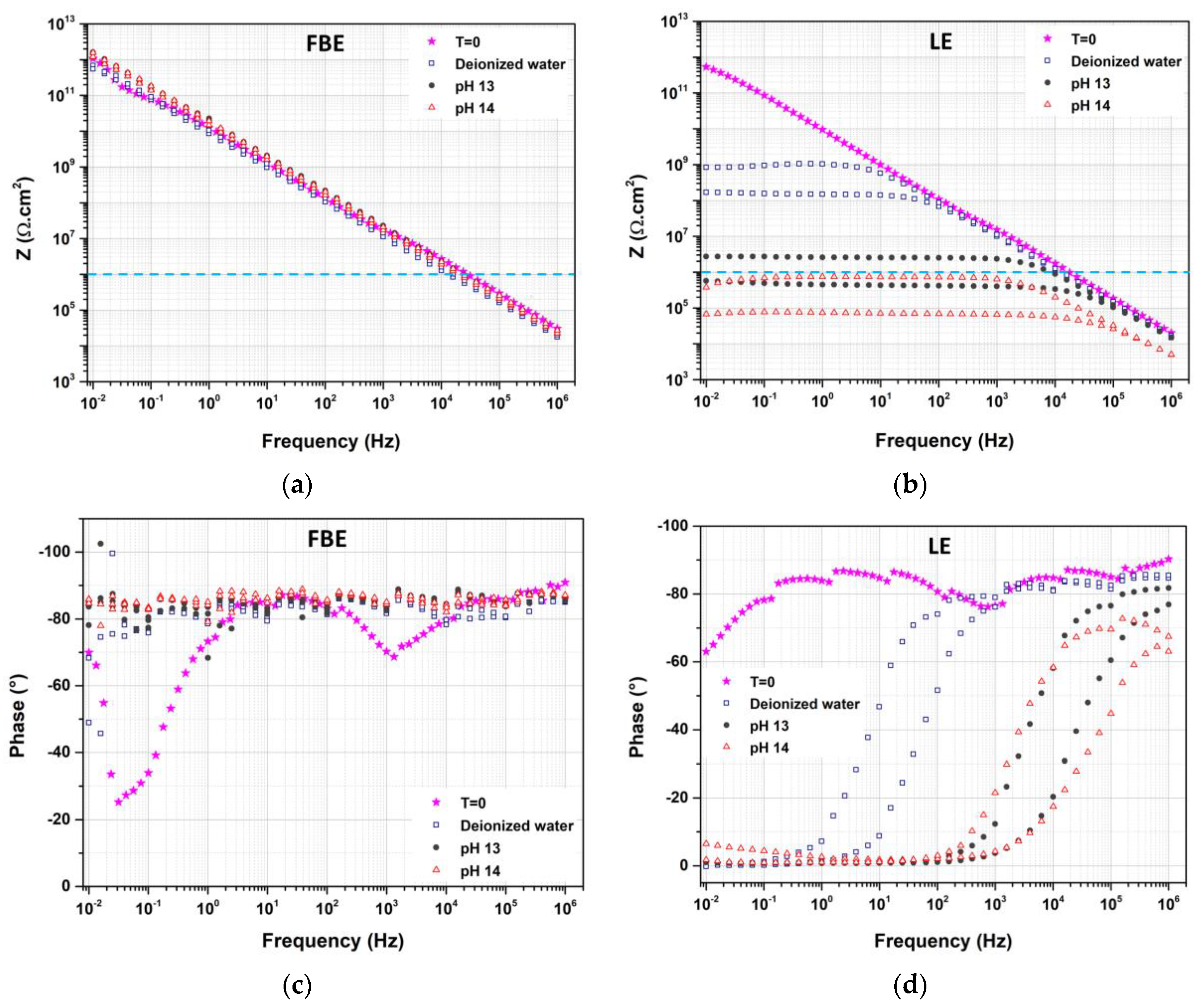
3.3. Impact of Aging in Alkaline Media on Coating Performance
3.3.1. Free Membrane Configuration
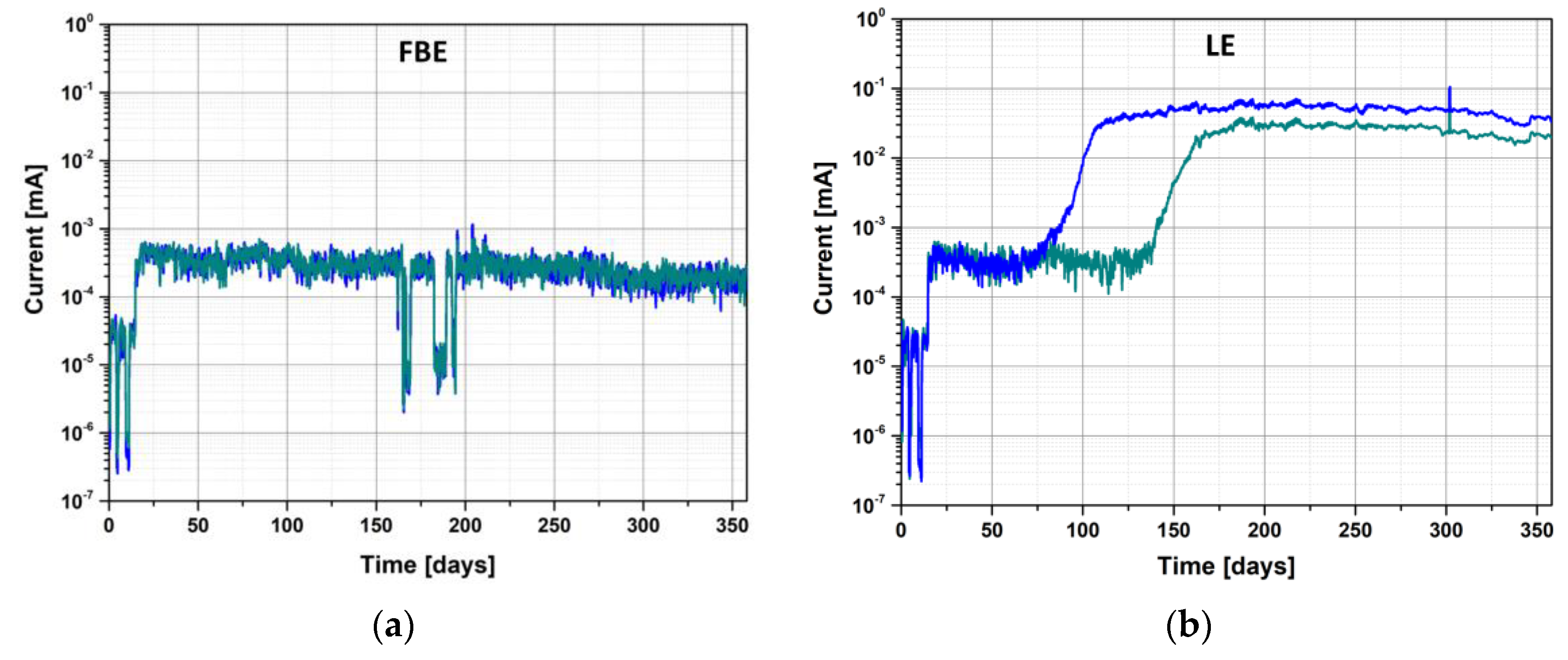
3.3.2. Coated Steel Configuration
4. Discussion
5. Conclusions
- The free membrane approach and methodology have been proven to an interesting way to show degradation under immersion and have a correlation with coated steel behavior.
- For this FBE, no firm influence of the pH on polymer degradation was observed (for either a free membrane and coated steel).
- This LE was sensitive to aging, even in deionized water, in which the leaching of mineral fillers was highlighted. The degradation of the LE was accelerated in the alkaline solution. The leaching of mineral fillers led to a loss of barrier properties, ultimately resulting in the weakening of the polymer–steel interface, as illustrated by interfacial failure. These conclusions show the importance of considering coating aging resulting from alkalinity induced by CP.
- The decrease in coating resistance is expected to further accelerate aging through an increase CP current demand and related local alkalinity. It should be noted that the results obtained and presented applied only to precise formulations of LE and FBE; however, the proposed chemical method appears relevant and shows the necessity of considering such specific aging in regards to coating specifications and improvements.
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Koch, G.; Varney, J.; Thompson, N.; Moghissi, O.; Gould, M.; Payer, J. International Measures of Prevention, Application, and Economics of Corrosion Technologies Study. NACE Int. 2016, 216, 2–3. [Google Scholar]
- Thomas, N.L. The Barrier Properties of Paint Coatings. Prog. Org. Coat. 1991, 19, 101–121. [Google Scholar] [CrossRef]
- Pélissier, K.; Thierry, D. Powder and High-Solid Coatings as Anticorrosive Solutions for Marine and Offshore Applications? A Review. Coatings 2020, 10, 916. [Google Scholar] [CrossRef]
- Bierwagen, G.P. Reflections on Corrosion Control by Organic Coatings. Prog. Org. Coat. 1996, 28, 43–48. [Google Scholar] [CrossRef]
- Legghe, E.; Aragon, E.; Bélec, L.; Margaillan, A.; Melot, D. Correlation between Water Diffusion and Adhesion Loss: Study of an Epoxy Primer on Steel. Prog. Org. Coat. 2009, 66, 276–280. [Google Scholar] [CrossRef]
- Negele, O.; Funke, W. Internal Stress and Wet Adhesion of Organic Coatings. Prog. Org. Coat. 1996, 28, 285–289. [Google Scholar] [CrossRef]
- Perera, D.Y. On Adhesion and Stress in Organic Coatings. Prog. Org. Coat. 1996, 28, 21–23. [Google Scholar] [CrossRef]
- Kehr, A.; Dabiri, M.; Hislop, R.; Devadass, M. Dual-Layer Fusion Bonded Epoxy (FBE) Coatings Protect Pipelines. In Proceedings of the Indopipe Conference, Jakarta, Indonesia, 30 May–1 June 2006; pp. 3–5. [Google Scholar]
- Sauvant-Moynot, V.; Kittel, J.; Melot, D.; Roche, M. Three Layer Polyolefin Coatings: How the FBE Primer Properties Govern the Long Term Adhesion. In Proceedings of the 17th International Conference on Pipeline Protection, Edinburgh, UK, 17 October 2007. [Google Scholar]
- Sørensen, P.A.; Kiil, S.; Dam-Johansen, K.; Weinell, C.E. Influence of Substrate Topography on Cathodic Delamination of Anticorrosive Coatings. Prog. Org. Coat. 2009, 64, 142–149. [Google Scholar] [CrossRef]
- Knudsen, O.Ø.; Forsgren, A. Corrosion Control through Organic Coatings, 2nd ed.; Philip, A., Schweitzer, P.E., Eds.; CRC Press: Boca Raton, FL, USA, 2017; ISBN 9781498760737. [Google Scholar]
- Nazarov, A.; Thierry, D. Application of Scanning Kelvin Probe in the Study of Protective Paints. Front. Mater. 2019, 6, 192. [Google Scholar] [CrossRef]
- Diler, E.; Roustan, M. Corrosion Rate of Buried Steel after Cathodic Protection Interruption. In Proceedings of the 18th Pipeline Technology Conference, Berlin, Germany, 11 May 2023. [Google Scholar]
- Diler, E.; Larché, N.; Leballeur, C.; Thierry, D.; Vittonato, J.; Agel, E.; Castillon, F.; Fontaine, S.; Campaignolle, X. Influence of the Coupon Surface and Geometry on the Cathodic Protection Current Density Demand in Soils. In Proceedings of the 7th Days Cathodic Protection & Associated Coatings, Juan Les Pins, France, 7–10 June 2021. [Google Scholar]
- Angst, U.M. A Critical Review of the Science and Engineering of Cathodic Protection of Steel in Soil and Concrete. Corrosion 2019, 75, 1420–1433. [Google Scholar] [CrossRef]
- Hammond, J.S.; Holubka, J.W.; deVries, J.E.; Dickie, R.A. The Application of X-Ray Photo-Electron Spectroscopy to a Study of Interfacial Composition in Corrosion-Induced Paint de-Adhesion. Corros. Sci. 1981, 21, 239–253. [Google Scholar] [CrossRef]
- Löffler, T. Alkali Resistance of Coatings—Will Tests of Cathodic Disbondment Provide Reliable Statements to This Question? In Proceedings of the 17th Pipeline Technology Conference, Berlin, Germany, 7–10 March 2022. [Google Scholar]
- Xu, M.; Lam, C.N.C.; Wong, D.; Asselin, E. Evaluation of the Cathodic Disbondment Resistance of Pipeline Coatings—A Review. Prog. Org. Coat. 2020, 146, 105728. [Google Scholar] [CrossRef]
- Holub, J.; Wong, D.T.; Tan, M. Analysis of CDT Methods And Factors Affecting Cathodic Disbondment. In Proceedings of the CORROSION 2007, Nashville, TN, USA, 11–15 March 2007. [Google Scholar]
- Thompson, I.; Saithala, J.R. Review of Pipeline Coating Systems from an Operator’s Perspective. Corros. Eng. Sci. Technol. 2016, 51, 118–135. [Google Scholar] [CrossRef]
- Diler, E.; Larche, N.; Vittonato, J.; Castillon, F.; Fontaine, S.; Kerzerho, T.; Lucas, P.; Agel, E. Cathodic Protection Shielding and Prevention of the Corrosion under Disbonded Coating in Soils. In Proceedings of the AMPP 2023, Denver, CO, USA, 23 March 2023; p. 1948. [Google Scholar]
- ISO 4624:2016; Paints and Varnishes—Pull-off Test for Adhesion. ISO Standard: Brussels, Belgium, 2016.
- Davies, P.; Sohier, L.; Cognard, J.Y.; Bourmaud, A.; Choqueuse, D.; Rinnert, E.; Créac’hcadec, R. Influence of Adhesive Bond Line Thickness on Joint Strength. Int. J. Adhes. Adhes. 2009, 29, 724–736. [Google Scholar] [CrossRef]
- Vašková, H.; Křesálek, V. Quasi Realtime Monitoring of Epoxy Resin Crosslinking via Raman Microscopy. Int. J. Math. Models Methods Appl. Sci. 2011, 5, 1197–1204. [Google Scholar]
- Diniz, F.B.; De Andrade, G.F.; Martins, C.R.; De Azevedo, W.M. A Comparative Study of Epoxy and Polyurethane Based Coatings Containing Polyaniline-DBSA Pigments for Corrosion Protection on Mild Steel. Prog. Org. Coat. 2013, 76, 912–916. [Google Scholar] [CrossRef]
- Wilmshurst, J.K.; Bernstein, H.J. The Infrared and Raman Spectra of Toluene, Toluene-α-D3, m-Xylene, and m-Xylene-Aα′-D6. Can. J. Chem. 1957, 35, 911–925. [Google Scholar] [CrossRef]
- Vedad, J.; Reilly, L.; Desamero, R.Z.B.; Mojica, E.R.E. Quantitative Analysis of Xylene Mixtures Using a Handheld Raman Spectrometer. In Raman Spectroscopy in the Undergraduate Curriculum; ACS Symposium Series; ACS Publications: Washington, DC, USA, 2018; Volume 1305, pp. 129–152. [Google Scholar] [CrossRef]
- Strelchuk, V.V.; Budzulyak, S.I.; Budzulyak, I.M.; Ilnytsyy, R.V.; Kotsyubynskyy, V.O.; Segin, M.Y.; Yablon, L.S. Raman Spectroscopy of the Laser Irradiated Titanium Dioxide. Semicond. Phys. Quantum Electron. Optoelectron. 2010, 13, 309–313. [Google Scholar] [CrossRef]
- Gautam, S.K.; Singh, F.; Sulania, I.; Singh, R.G.; Kulriya, P.K.; Pippel, E. Micro-Raman Study on the Softening and Stiffening of Phonons in Rutile Titanium Dioxide Film: Competing Effects of Structural Defects, Crystallite Size, and Lattice Strain. J. Appl. Phys. 2014, 115, 143504. [Google Scholar] [CrossRef]
- Hong, L.V.; Le, N.T.H.; Thuan, N.C.; Thanh, N.D.; Nghia, N.X.; Phuc, N.X. Observation of the Phase Formation in TiO2 Nano Thin Film by Raman Scattering. J. Raman Spectrosc. 2005, 36, 946–949. [Google Scholar] [CrossRef]
- Dufresne, W.J.B.; Rufledt, C.J.; Marshall, C.P. Raman Spectroscopy of the Eight Natural Carbonate Minerals of Calcite Structure. J. Raman Spectrosc. 2018, 49, 1999–2007. [Google Scholar] [CrossRef]
- Kleppe, A.K.; Jephcoat, A.P.; Welch, M.D. The Effect of Pressure upon Hydrogen Bonding in Chlorite: A Raman Spectroscopic Study of Clinochlore to 26.5 GPa. Am. Mineral. 2003, 88, 567–573. [Google Scholar] [CrossRef]
- Ulian, G.; Moro, D.; Valdrè, G. Infrared and Raman spectroscopic features of Clinochlore Mg6Si4O10(OH)8: A density functional theory contribution. Appl. Clay Sci. 2020, 197, 105779. [Google Scholar] [CrossRef]
- NF EN 1484; Water Analysis Guidelines for the Determination of Total Organic Carbon (TOC) and Dissolved Organic Carbon (DOC). AFNOR: Paris, France, 1997.
- NF ISO 15923-1; Water Quality—Determination of Selected Parameters by Discrete Analysis Systems—Part 1: Amonium, Nitrate, Nitrite, Chloride, Orthophosphate, Sulfate and Silicate with Photometric Detection. AFNOR: Paris, France, 2014.
- NF EN ISO 15587-1; Water Quality—Digestion for the Determination of Selected Elements in Water—Part 1: Aqua Regia Digestion. AFNOR: Paris, France, 2002.
- NF EN ISO 11885; Water Quality—Determination of Selected Elements by Inductively Coupled Plasma Optical Emission Spectrometry (ICP-OES). AFNOR: Paris, France, 2009.
- Capiel, G.; Uicich, J.; Fasce, D.; Montemartini, P.E. Diffusion and Hydrolysis Effects during Water Aging on an Epoxy-Anhydride System. Polym. Degrad. Stab. 2018, 153, 165–171. [Google Scholar] [CrossRef]
- Fredj, N.; Cohendoz, S.; Mallarino, S.; Feaugas, X.; Touzain, S. Evidencing Antagonist Effects of Water Uptake and Leaching Processes in Marine Organic Coatings by Gravimetry and EIS. Prog. Org. Coat. 2010, 67, 287–295. [Google Scholar] [CrossRef]
- Xiao, G.Z.; Shanahan, M.E.R. Irreversible Effects of Hygrothermal Aging on DGEBA/DDA Epoxy Resin. J. Appl. Polym. Sci. 1998, 69, 363–369. [Google Scholar] [CrossRef]
- Colin, X.; Verdu, J. Humid Ageing of Organic Matrix Composites. In Solid Mechanics and Its Applications; Springer: Dordrecht, The Netherlands, 2014; Volume 208, pp. 47–114. [Google Scholar] [CrossRef]
- Antoon, M.K.; Koenig, J.L. Irreversible Effects of Moisture on the Epoxy Matrix in Glass-Reinforced Composites. J. Polym. Sci. Polym. Phys. 1981, 19, 197–212. [Google Scholar] [CrossRef]
- Linde, E.; Giron, N.H.; Celina, M.C. Water Diffusion with Temperature Enabling Predictions for Sorption and Transport Behavior in Thermoset Materials. Polymer 2018, 153, 653–667. [Google Scholar] [CrossRef]
- Del Grosso Destreri, M.; Vogelsang, J.; Fedrizzi, L.; Deflorian, F. Water Up-Take Evaluation of New Waterborne and High Solid Epoxy Coatings. Part II: Electrochemical Impedance Spectroscopy. Prog. Org. Coat. 1999, 37, 69–81. [Google Scholar] [CrossRef]
- Scott, P.; Lees, J.M. Water, Salt Water, and Alkaline Solution Uptake in Epoxy Thin Films. J. Appl. Polym. Sci. 2013, 130, 1898–1908. [Google Scholar] [CrossRef]
- Vosgien Lacombre, C.; Bouvet, G.; Trinh, D.; Mallarino, S.; Touzain, S. Water Uptake in Free Films and Coatings Using the Brasher and Kingsbury Equation: A Possible Explanation of the Different Values Obtained by Electrochemical Impedance Spectroscopy and Gravimetry. Electrochim. Acta 2017, 231, 162–170. [Google Scholar] [CrossRef]
- Nguyen, V.N.; Perrin, F.X.; Vernet, J.L. Water Permeability of Organic/Inorganic Hybrid Coatings Prepared by Sol–Gel Method: A Comparison between Gravimetric and Capacitance Measurements and Evaluation of Non-Fickian Sorption Models. Corros. Sci. 2005, 47, 397–412. [Google Scholar] [CrossRef]
- Mittermayr, F.; Klammer, D.; Köhler, S.; Dietzel, M. Dolomite Dissolution in Alkaline Cementious Media. In Proceedings of the EGU General Assembly, Vienna, Austria, 2–7 May 2010. [Google Scholar]
- Yin, X.; Liu, Y.; Miao, Y.; Xian, G. Water Absorption, Hydrothermal Expansion, and Thermomechanical Properties of a Vinylester Resin for Fiber-Reinforced Polymer Composites Subjected to Water or Alkaline Solution Immersion. Polymers 2019, 11, 505. [Google Scholar] [CrossRef] [PubMed]
- Roggero, A.; Villareal, L.; Caussé, N.; Santos, A.; Pébère, N. Correlation between the Physical Structure of a Commercially Formulated Epoxy Paint and Its Electrochemical Impedance Response. Prog. Org. Coat. 2020, 146, 105729. [Google Scholar] [CrossRef]
- Wang, B.; Li, D.; Xian, G.; Li, C. Effect of Immersion in Water or Alkali Solution on the Structures and Properties of Epoxy Resin. Polymers 2021, 13, 1902. [Google Scholar] [CrossRef] [PubMed]
- Bouvet, G.; Dang, N.; Cohendoz, S.; Feaugas, X.; Mallarino, S.; Touzain, S. Impact of Polar Groups Concentration and Free Volume on Water Sorption in Model Epoxy Free Films and Coatings. Prog. Org. Coat. 2016, 96, 32–41. [Google Scholar] [CrossRef]
- Morsch, S.; Lyon, S.; Greensmith, P.; Smith, S.D.; Gibbon, S.R. Water Transport in an Epoxy–Phenolic Coating. Prog. Org. Coat. 2015, 78, 293–299. [Google Scholar] [CrossRef]
- Grzechnik, A.; Simon, P.; Gillet, P.; McMillan, P. An Infrared Study of MgCO3 at High Pressure. Phys. B Condens. Matter 1999, 262, 67–73. [Google Scholar] [CrossRef]
- Tian, L.; Tahmasebi, A.; Yu, J. An Experimental Study on Thermal Decomposition Behavior of Magnesite. J. Therm. Anal. Calorim. 2014, 118, 1577–1584. [Google Scholar] [CrossRef]
- NF EN ISO 21809-3; Industries Du Pétrole et Du Gaz Naturel—Revêtements Externes Des Conduites Enterrées Ou Immergées Utilisées Dans Les Systèmes de Transport Par Conduites—Partie 3: Revêtements Des Joints Soudés Sur Site—Industries Du Pétrole. ISO: Geneva, Switzerland, 2016.
- AFNOR NF EN 10290; Tubes et Raccords En Acier Pour Canalisations Enterrées et Immergées—Revêtements Externes En Polyuréthane Ou Polyuréthane Modifié Liquides. AFNOR: Paris, France, 2002.
- NF EN 12068:1999; Protection Cathodique—Revêtements Organiques Extérieurs Pour La Protection Contre La Corrosion de Tubes En Acier Enterrés Ou Immergés En Conjonction Avec La Protection Cathodique—Bandes et Matériaux Rétractables. European Committee for Standardization: Brussels, Belgium, 1999.
- Akbarinezhad, E.; Bahremandi, M.; Faridi, H.R.; Rezaei, F. Another Approach for Ranking and Evaluating Organic Paint Coatings via Electrochemical Impedance Spectroscopy. Corros. Sci. 2009, 51, 356–363. [Google Scholar] [CrossRef]
- Castela, A.S.L.; Simões, A.M.; Ferreira, M.G.S. E.I.S. Evaluation of Attached and Free Polymer Films. Prog. Org. Coat. 2000, 38, 1–7. [Google Scholar] [CrossRef]
- Leidheiser, H. Electrical and Electrochemical Measurements as Predictors of Corrosion at the Metal–Organic Coating Interface. Prog. Org. Coat. 1979, 7, 79–104. [Google Scholar] [CrossRef]
- Kendig, M.; Scully, J. Basic Aspects of Electrochemical Impedance Application for the Life Prediction of Organic Coatings on Metals. Corrosion 1990, 46, 22–29. [Google Scholar] [CrossRef]
- Croll, S.G. Electrolyte Transport in Polymer Barrier Coatings: Perspectives from Other Disciplines. Prog. Org. Coat. 2018, 124, 41–48. [Google Scholar] [CrossRef]
- Shreepathi, S.; Naik, S.M.; Vattipalli, M.R. Water Transportation through Organic Coatings: Correlation between Electrochemical Impedance Measurements, Gravimetry, and Water Vapor Permeability. J. Coat. Technol. Res. 2012, 9, 411–422. [Google Scholar] [CrossRef]
- Vosgien Lacombre, C.; Bouvet, G.; Trinh, D.; Mallarino, S.; Touzain, S. Effect of Pigment and Temperature onto Swelling and Water Uptake during Organic Coating Ageing. Prog. Org. Coat. 2018, 124, 249–255. [Google Scholar] [CrossRef]
- Kittel, J.; Celati, N.; Keddam, M.; Takenouti, H. Influence of the Coating–Substrate Interactions on the Corrosion Protection: Characterisation by Impedance Spectroscopy of the Inner and Outer Parts of a Coating. Prog. Org. Coat. 2003, 46, 135–147. [Google Scholar] [CrossRef]
- Kittel, J.; Celati, N.; Keddam, M.; Takenouti, H. New Methods for the Study of Organic Coatings by EIS: New Insights into Attached and Free Films. Prog. Org. Coat. 2001, 41, 93–98. [Google Scholar] [CrossRef]
- Chen, M.; Yu, Q.S.; Reddy, C.M.; Yasuda, H.K. Model Study Investigating the Role of Interfacial Factors in Electrochemical Impedance Spectroscopy Measurements. Corrosion 2000, 56, 709–721. [Google Scholar] [CrossRef]
- Dolatzadeh, F.; Jalili, M.M.; Moradian, S. Influence of Various Loadings of Hydrophilic or Hydrophobic Silica Nanoparticles on Water Uptake and Porosity of a Polyurethane Coating. Mater. Corros. 2013, 64, 609–618. [Google Scholar] [CrossRef]






| Carbon | Manganese | Phosphorous | Sulphur | Silicon |
|---|---|---|---|---|
| 0.22% max | 1.60% max | 0.05% max | 0.05% max | 0.05% max |
| Coating | Solution | Temperature | Replicate | Duration | Comments |
|---|---|---|---|---|---|
| FBE | Deionized water (DW) DW + NaOH at pH 13 (4 g·L−1) DW + NaOH at pH 14 (50 g·L−1) | RT | 3 | 490 days | Two sides exposed |
| LE |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pélissier, K.; Diler, E.; Dossot, M.; Carteret, C.; Vittonato, J.; Castillon, F.; Fontaine, S.; Kerzerho, T.; Larché, N.; Lucas, P. Effects and Consequences of an Alkali-Induced Cathodic Environment on Coating Aging. Coatings 2023, 13, 1949. https://doi.org/10.3390/coatings13111949
Pélissier K, Diler E, Dossot M, Carteret C, Vittonato J, Castillon F, Fontaine S, Kerzerho T, Larché N, Lucas P. Effects and Consequences of an Alkali-Induced Cathodic Environment on Coating Aging. Coatings. 2023; 13(11):1949. https://doi.org/10.3390/coatings13111949
Chicago/Turabian StylePélissier, Krystel, Erwan Diler, Manuel Dossot, Cédric Carteret, Jean Vittonato, François Castillon, Sylvain Fontaine, Thierry Kerzerho, Nicolas Larché, and Patrice Lucas. 2023. "Effects and Consequences of an Alkali-Induced Cathodic Environment on Coating Aging" Coatings 13, no. 11: 1949. https://doi.org/10.3390/coatings13111949
APA StylePélissier, K., Diler, E., Dossot, M., Carteret, C., Vittonato, J., Castillon, F., Fontaine, S., Kerzerho, T., Larché, N., & Lucas, P. (2023). Effects and Consequences of an Alkali-Induced Cathodic Environment on Coating Aging. Coatings, 13(11), 1949. https://doi.org/10.3390/coatings13111949







