Effect of Process Pressure on Carbon-Based Thin Films Deposited by Cathodic Arc on Stainless Steel for Bipolar Plates
Abstract
:1. Introduction
2. Materials and Methods
3. Results
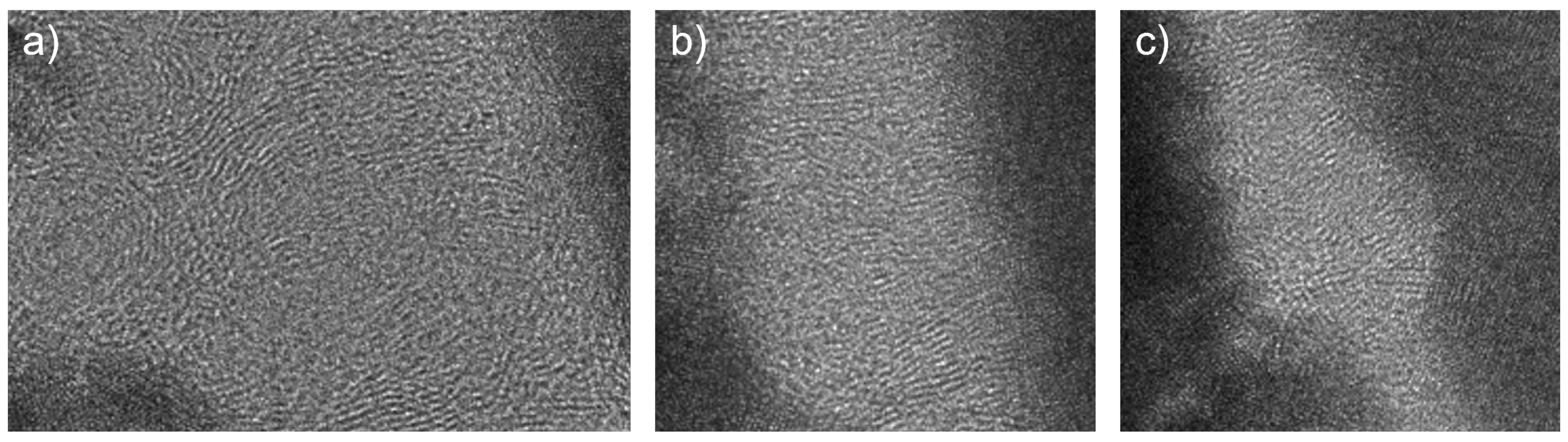
3.1. Structural Characterization
3.2. Interfacial Contact Resistance
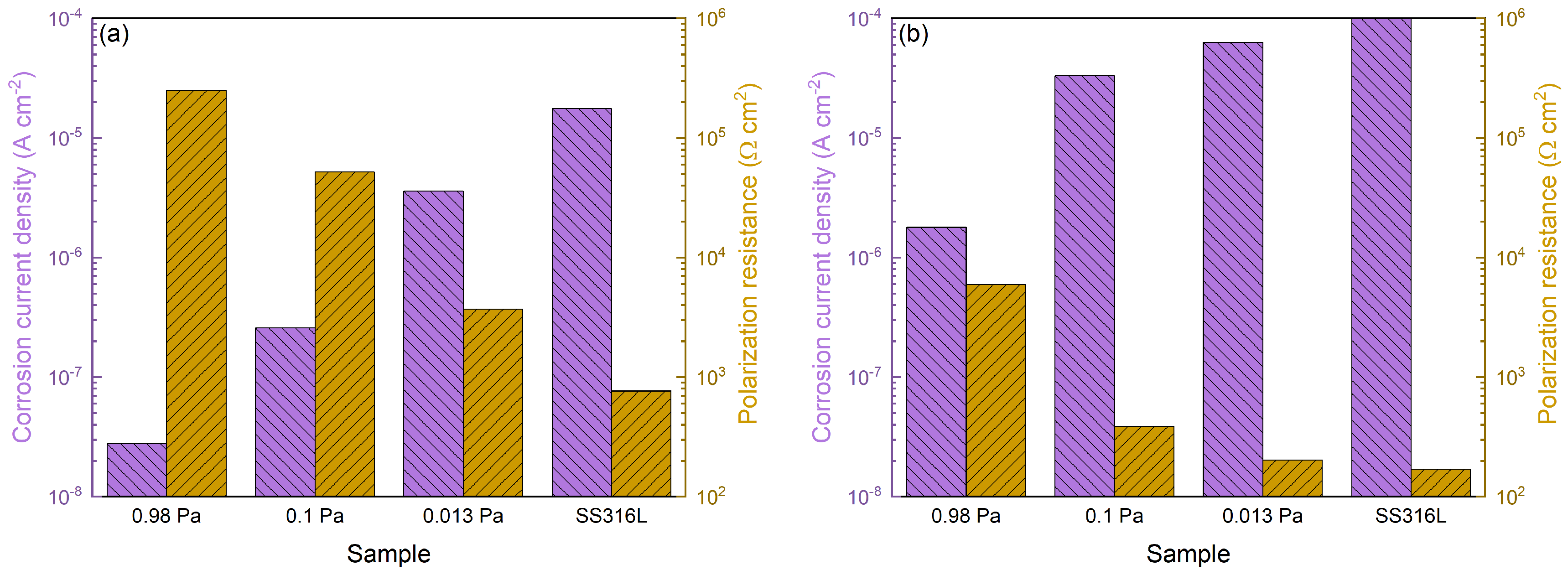
3.3. Electrochemical Characterization
4. Conclusions
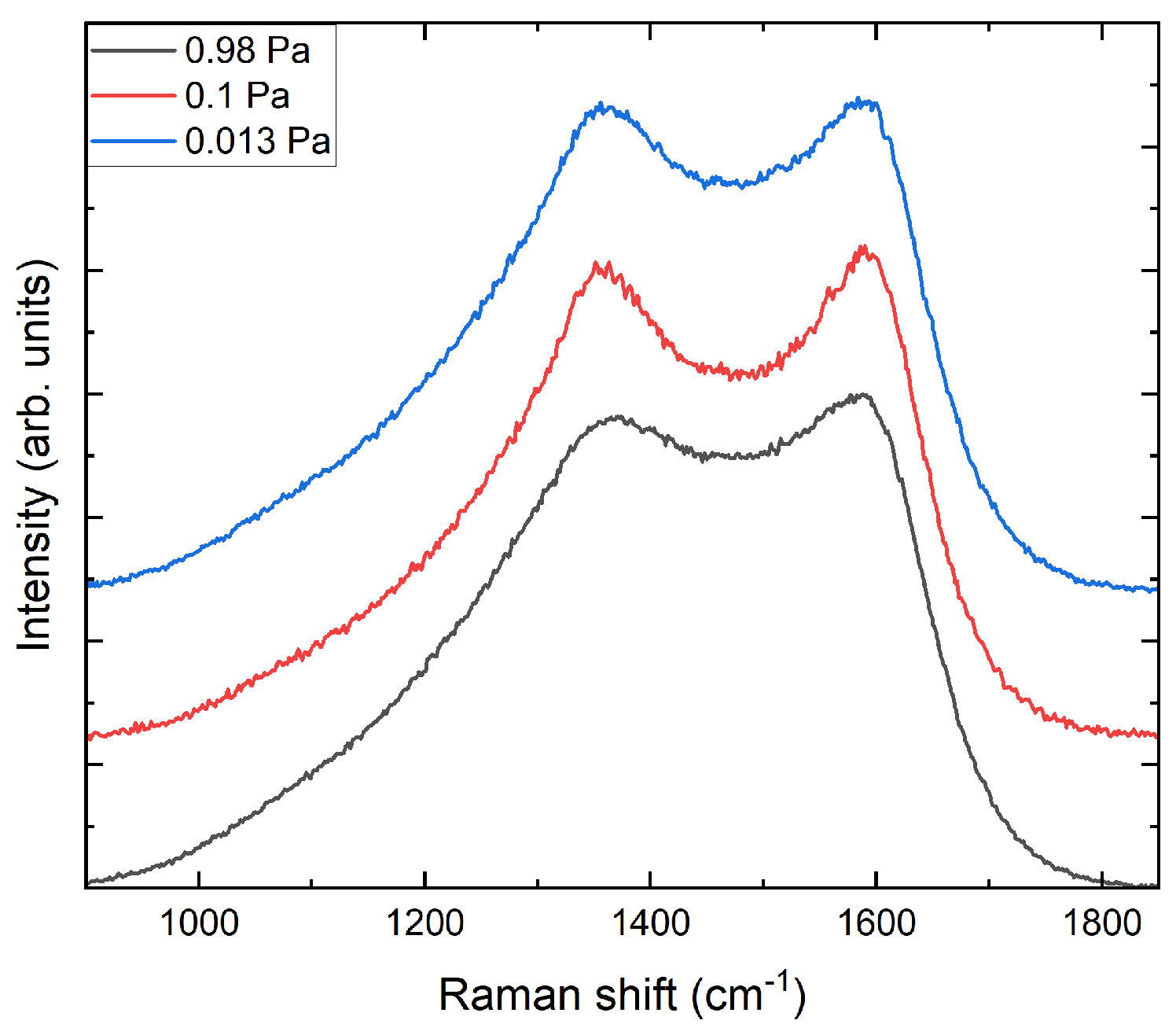
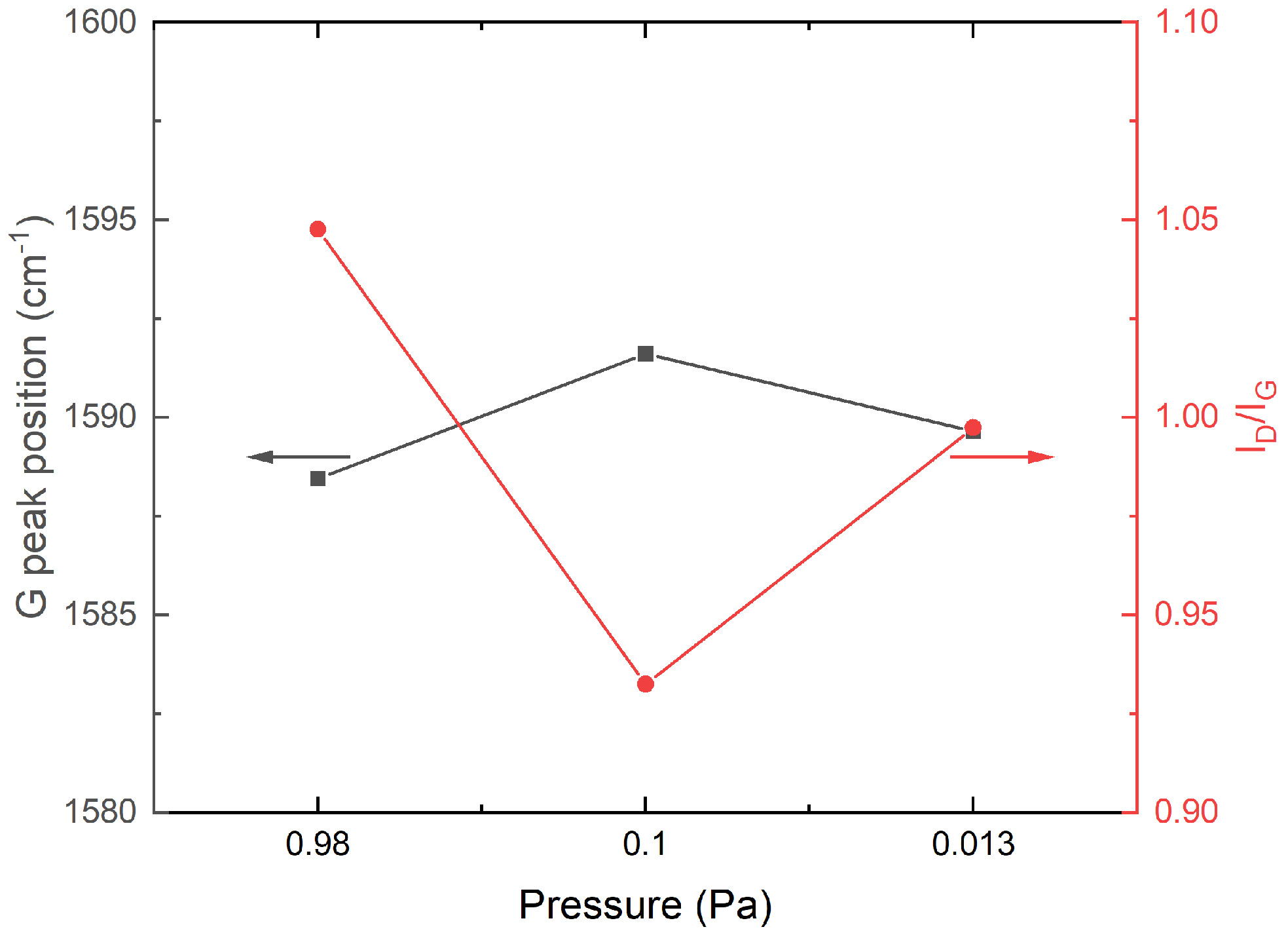
- The evaluated Raman spectra indicate the structural differences between the carbon layer variants. Based on the characteristics and G peak position, the carbon layer variants can be classified as highly sp2-bonded carbon.
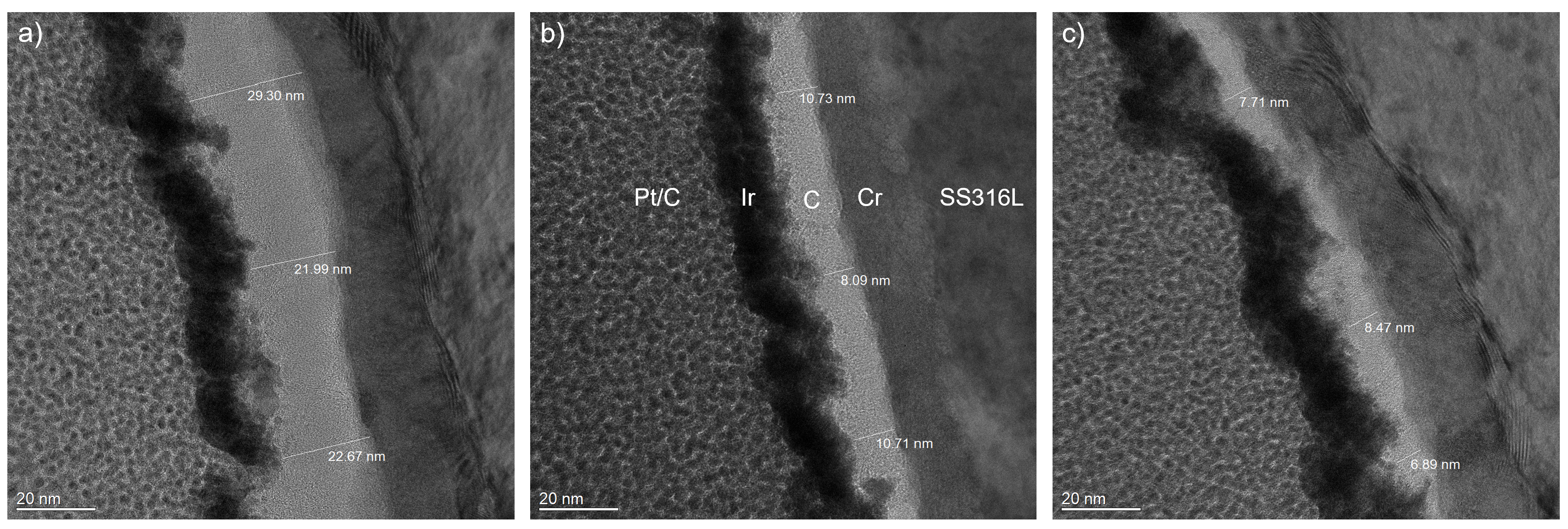
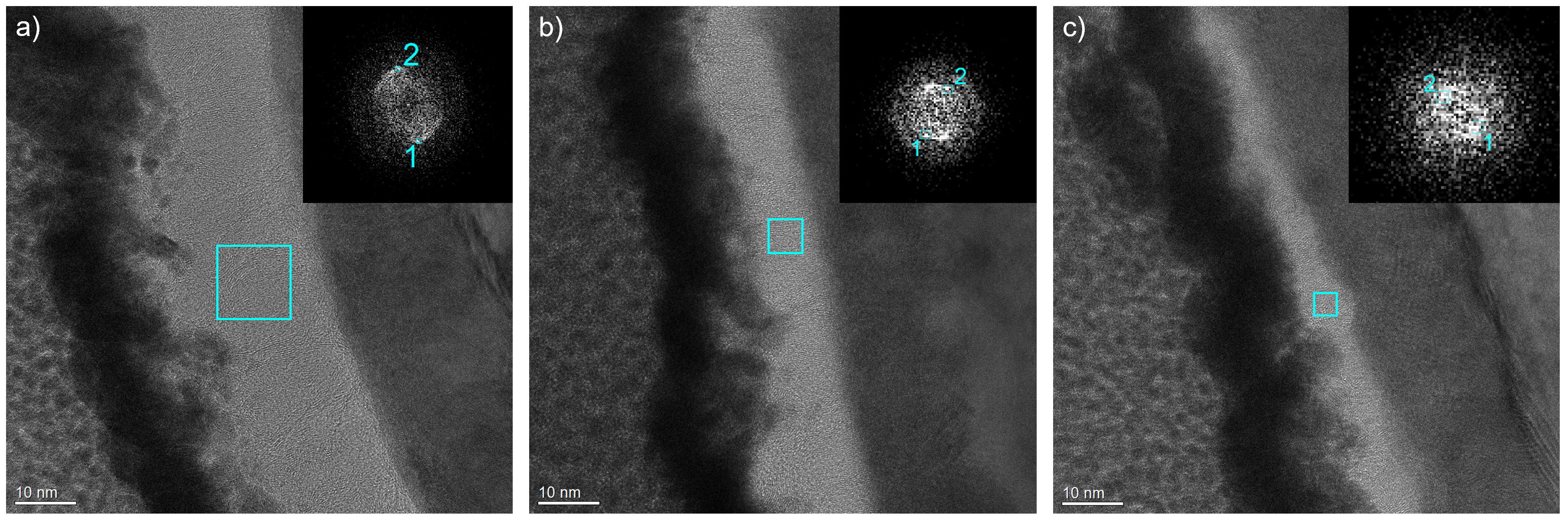
- The investigated cross-sectional microstructures show mainly perpendicular graphitic layers, and the thickness of the carbon top layer increases with higher process pressure. For the carbon top layer deposited at 0.98 Pa, structural distortions, such as round-shaped features, can be observed, which agrees with the Raman results.
- Both the contact resistance and electrochemical properties of the SS316L substrate are greatly improved.
- The process pressure has a minor effect on the interfacial contact resistance, and there is also no apparent influence due to the thickness differences between the coating variants.
- The corrosion resistance is considerably affected by the process pressure, and the 0.98 Pa sample exhibits the best corrosion resistance among the specimens, which is likely attributed to the different microstructure.
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gröger, O.; Gasteiger, H.; Suchsland, J. Review-Electromobility: Batteries or Fuel Cells? J. Electrochem. Soc. 2015, 162, 2605–2622. [Google Scholar] [CrossRef]
- Alaswad, A.; Omran, A.; Sodre, J.R.; Wilberforce, T.; Pignatelli, G.; Dassisti, M.; Baroutaji, A.; Olabi, A.G. Technical and Commercial Challenges of Proton-Exchange Membrane (PEM) Fuel Cells. Energies 2021, 14, 012108. [Google Scholar] [CrossRef]
- Tawfik, H.; Hung, Y.; Mahajan, D. Metal bipolar plates for PEM fuel cell—A review. J. Power Sources 2007, 163, 755–767. [Google Scholar] [CrossRef]
- Mohr, P. Optimierung von Brennstoffzellen-Bipolarplatten für die Automobile Anwendung. Ph.D. Thesis, University Duisburg-Essen, Duisburg, Germany, 2018. [Google Scholar]
- United States Department of Energy. Hydrogen and Fuel Cell Technologies Office Multi-Year Research, Development, and Demonstration Plan; Technical Report; U.S. Department of Energy: Washington, DC, USA, 2017. [Google Scholar]
- Davies, D.; Adcock, P.; Turpin, M.; Rowen, S. Stainless steel as a bipolar plate material for solid polymer fuel cells. J. Power Sources 2000, 86, 237–242. [Google Scholar] [CrossRef]
- Song, Y.; Zhang, C.; Ling, C.; Han, M.; Yong, R.; Sun, D.; Chen, J. Review on current research of materials, fabrication and application for bipolar plate in proton exchange membrane fuel cell. Int. J. Hydrogen Energy 2020, 45, 29832–29847. [Google Scholar] [CrossRef]
- Yang, Y.; Guo, L.J.; Liu, H. Corrosion Characteristics of SS316l as Bipolar Plate Material in PEMFC Cathode Environments with Different Acidities. Int. J. Hydrogen Energy 2011, 36, 1654–1663. [Google Scholar] [CrossRef]
- Papadias, D.; Ahluwalia, R.; Thomson, J.; Meyer, H.; Brady, M.; Wang, H.; Turner, J.; Mukundan, R.; Borup, R. Degradation of SS316L bipolar plates in simulated fuel cell environment: Corrosion rate, barrier film formation kinetics and contact resistance. J. Power Sources 2015, 273, 1237–1249. [Google Scholar] [CrossRef]
- Mittal, V.O.; Kunz, H.R.; Fenton, J.M. Membrane Degradation Mechanisms in PEMFCs. J. Electrochem. Soc. 2007, 154, B652. [Google Scholar] [CrossRef]
- Liang, P.; Qiu, D.; Peng, L.; Yi, P.; Lai, X.; Ni, J. Contact resistance prediction of proton exchange membrane fuel cell considering fabrication characteristics of metallic bipolar plates. Energy Convers. Manag. 2018, 169, 334–344. [Google Scholar] [CrossRef]
- Liu, R.; Jia, Q.; Zhang, B.; Lai, Z.; Chen, L. Protective coatings for metal bipolar plates of fuel cells: A review. Int. J. Hydrogen Energy 2022, 47, 22915–22937. [Google Scholar] [CrossRef]
- Schultrich, B. Tetrahedrally Bonded Amorphous Carbon Films I, 1st ed.; Springer: Berlin/Heidelberg, Germany, 2018. [Google Scholar] [CrossRef]
- Yi, P.; Zhang, D.; Qiu, D.; Peng, L.; Lai, X. Carbon-based coatings for metallic bipolar plates used in proton exchange membrane fuel cells. Int. J. Hydrogen Energy 2019, 44, 6813–6843. [Google Scholar] [CrossRef]
- Yi, P.; Peng, L.; Feng, L.; Gan, P.; Lai, X. Performance of a proton exchange membrane fuel cell stack using conductive amorphous carbon-coated 304 stainless steel bipolar plates. J. Power Sources 2010, 195, 7061–7066. [Google Scholar] [CrossRef]
- Larijani, M.; Yari, M.; Afshar, A.; Jafarian, M.; Eshghabadi, M. A comparison of carbon coated and uncoated 316L stainless steel for using as bipolar plates in PEMFCs. J. Alloys Compd. 2011, 509, 7400–7404. [Google Scholar] [CrossRef]
- Husby, H.; Kongstein, O.; Oedegaard, A.; Seland, F. Carbon-polymer composite coatings for PEM fuel cell bipolar plates. Int. J. Hydrogen Energy 2014, 39, 951–957. [Google Scholar] [CrossRef]
- Bi, F.; Li, X.; Yi, P.; Hou, K.; Peng, L.; Lai, X. Characteristics of Amorphous Carbon Films to Resist High Potential Impact in PEMFCs Bipolar Plates for Automotive Application. Int. J. Hydrogen Energy 2017, 42, 14279–14289. [Google Scholar] [CrossRef]
- Lu, C.; Shi, F.; Jin, J.; Peng, X. Study on the Properties of Vertical Carbon Nanotube Films Grown on Stainless Steel Bipolar Plates. Materials 2019, 12, 899. [Google Scholar] [CrossRef]
- Park, J.H.; Wada, T.; Naruse, Y.; Hagio, T.; Kamimoto, Y.; Ryoichi, I. Electrodeposition of Ni-W/CNT Composite Plating and Its Potential as Coating for PEMFC Bipolar Plate. Coatings 2020, 10, 1095. [Google Scholar] [CrossRef]
- Asri, N.; Husaini, T.; Sulong, A.; Majlan, E.; Daud, W. Coating of stainless steel and titanium bipolar plates for anticorrosion in PEMFC: A review. Int. J. Hydrogen Energy 2019, 42, 9135–9148. [Google Scholar] [CrossRef]
- Xu, Z.; Qiu, D.; Yi, P.; Peng, L.; Lai, X. Towards mass applications: A review on the challenges and developments in metallic bipolar plates for PEMFC. Prog. Nat. Sci. Mater. Int. 2020, 30, 815–824. [Google Scholar] [CrossRef]
- Chauhan, S.; Ponkshe, S. A Review of Coated Metallic Bipolar Plates for Proton Exchange Membrane Fuel Cell PEMFC. In Proceedings of the WCX SAE World Congress Experience, Detroit, MI, USA, 5–7 April 2022. [Google Scholar] [CrossRef]
- Huang, Y. Corrosion Resistance, Interfacial Contact Resistance, and Hydrophobicity of 316L Stainless Steel Bipolar Plates Coated with TiN/Amorphous Carbon Double Layer under Different Carbon Target Currents. Coatings 2023, 13, 1494. [Google Scholar] [CrossRef]
- Vetter, J.; Mueller, J.; Erkens, G. Domino Platform: PVD Coaters for Arc Evaporation and High Current Pulsed Magnetron Sputtering. IOP Conf. Ser. Mater. Sci. Eng. 2012, 39, 012004. [Google Scholar] [CrossRef]
- Gudmundsson, J.T.; Anders, A.; von Keudell, A. Foundations of physical vapor deposition with plasma assistance. Plasma Sources Sci. Technol. 2022, 31, 083001. [Google Scholar] [CrossRef]
- Anders, A. A review comparing cathodic arcs and high power impulse magnetron sputtering (HiPIMS). Surf. Coatings Technol. 2014, 257, 308–325. [Google Scholar] [CrossRef]
- Ohring, M. Materials Science of Thin Films: Depositon and Structure, 2nd ed.; Elsevier: Amsterdam, The Netherlands, 2002. [Google Scholar] [CrossRef]
- Anders, A. A structure zone diagram including plasma-based deposition and ion etching. Thin Solid Film. 2010, 518, 4087–4090. [Google Scholar] [CrossRef]
- Steinhorst, M.; Giorgio, M.; Roch, T.; Leyens, C. Bias Voltage Dependency of Structural and Bipolar Plate-Related Properties of Cathodic Arc-Deposited Carbon-Based Coatings. Acs Appl. Mater. Interfaces 2023, 15, 51704–51712. [Google Scholar] [CrossRef] [PubMed]
- Yi, P.; Zhang, W.; Bi, F.; Peng, L.; Lai, X. Microstructure and Properties of a-C Films Deposited Under Different Argon Flow Rate on Stainless Steel Bipolar Plates for Proton Exchange Membrane Fuel Cells. J. Power Sources 2019, 410–411, 188–195. [Google Scholar] [CrossRef]
- Vetter, J. 60 years of DLC coatings: Historical highlights and technical review of cathodic arc processes to synthesize various DLC types, and their evolution for industrial applications. Surf. Coat. Technol. 2014, 257, 213–240. [Google Scholar] [CrossRef]
- Robertson, J. Diamond-like amorphous carbon. Mater. Sci. Eng. R Rep. 2002, 37, 129–281. [Google Scholar] [CrossRef]
- Ferrari, A.; Robertson, J. Raman spectroscopy of amorphous, nanostructured, diamond-like carbon, and nanodiamond. Philos. Trans. R. Soc. London Ser. A Math. Phys. Eng. Sci. 2004, 362, 2477–2512. [Google Scholar] [CrossRef]
- Ferrari, A.; Robertson, J. Interpretation of Raman spectra of disordered and amorphous carbon. Phys. Rev. B 2000, 61, 14095–14107. [Google Scholar] [CrossRef]
- Gregoire, J.M.; Lobovsky, M.B.; Heinz, M.F.; DiSalvo, F.J.; van Dover, R.B. Resputtering Phenomena and Determination of Composition in Codeposited Films. Phys. Rev. B 2007, 76, 195437. [Google Scholar] [CrossRef]
- Tritremmel, C. Comparison of Magnetron Sputtering and Arc Evaporation by Al-Cr-N Hard Coatings. Ph.D. Thesis, University of Leoben, Leoben, Austria, 2007. [Google Scholar]
- Su, Y.L.; Kao, W.H.; Chang, G.Y. Tribological, Anti-corrosion, and Electrical Conductivity Properties of CrCx Coatings Deposited on Stainless Steel 316L and Used as Metal Bipolar Plates for Fuel Cells. J. Mater. Eng. Perform. 2023, 32, 3739–3754. [Google Scholar] [CrossRef]
- Hamann, C.; Hamnett, A.; Vielstich, W. Electrochemistry, 2nd ed.; Wiley-VCH: Weinheim, Germany, 2007. [Google Scholar]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Steinhorst, M.; Giorgio, M.; Roch, T.; Leyens, C. Effect of Process Pressure on Carbon-Based Thin Films Deposited by Cathodic Arc on Stainless Steel for Bipolar Plates. Coatings 2023, 13, 1962. https://doi.org/10.3390/coatings13111962
Steinhorst M, Giorgio M, Roch T, Leyens C. Effect of Process Pressure on Carbon-Based Thin Films Deposited by Cathodic Arc on Stainless Steel for Bipolar Plates. Coatings. 2023; 13(11):1962. https://doi.org/10.3390/coatings13111962
Chicago/Turabian StyleSteinhorst, Maximilian, Maurizio Giorgio, Teja Roch, and Christoph Leyens. 2023. "Effect of Process Pressure on Carbon-Based Thin Films Deposited by Cathodic Arc on Stainless Steel for Bipolar Plates" Coatings 13, no. 11: 1962. https://doi.org/10.3390/coatings13111962
APA StyleSteinhorst, M., Giorgio, M., Roch, T., & Leyens, C. (2023). Effect of Process Pressure on Carbon-Based Thin Films Deposited by Cathodic Arc on Stainless Steel for Bipolar Plates. Coatings, 13(11), 1962. https://doi.org/10.3390/coatings13111962





