The Influence of Lyophobicity and Lyophilicity of Film-Forming Systems on the Properties of Tin Oxide Films
Abstract
:1. Introduction
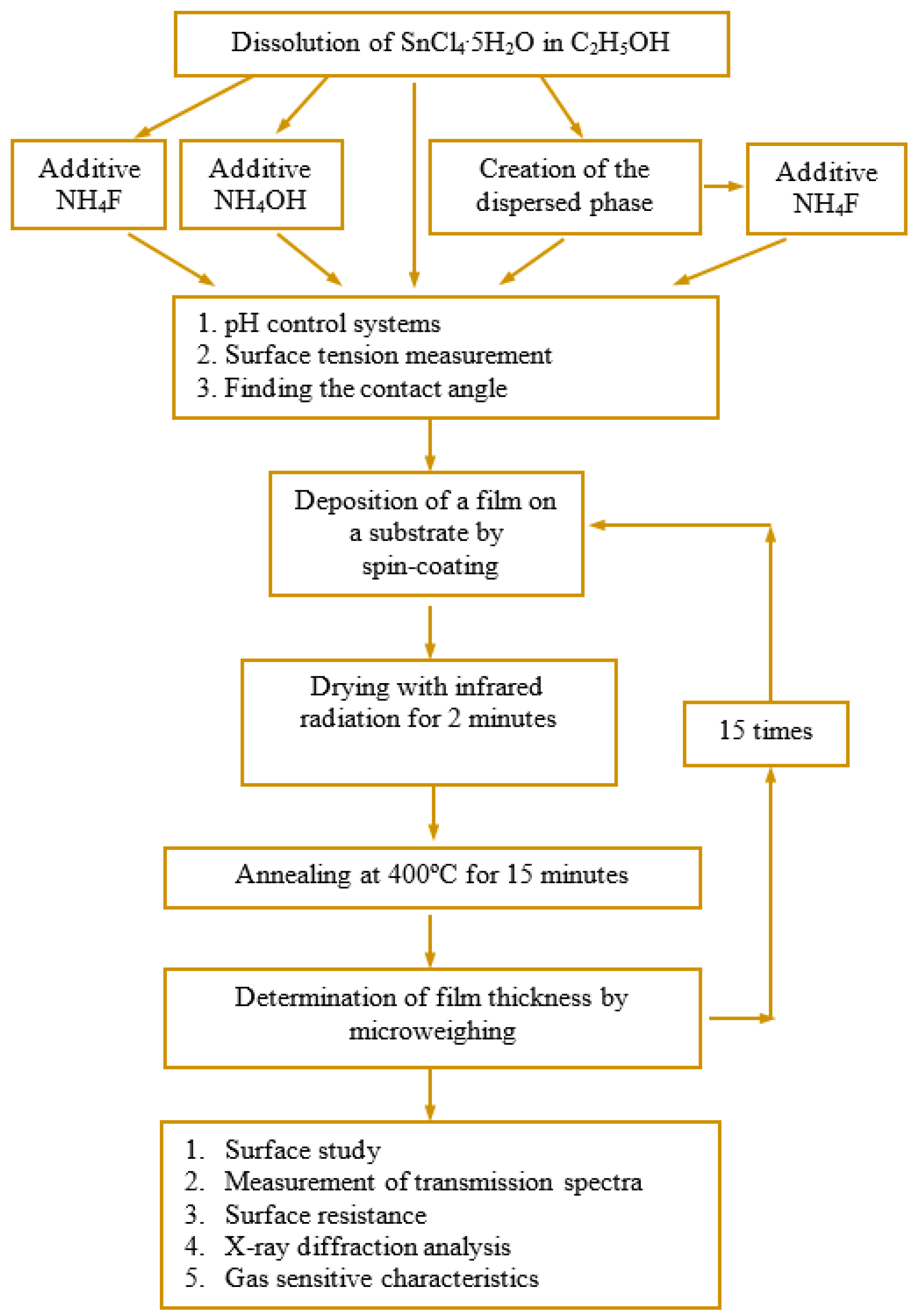
2. Materials and Methods
- SnCl4/EtOH: SnCl4·5H2O was dissolved in ethanol. The concentration of tin ions in the system was 0.13 mol/L.
- SnCl4/EtOH/NH4F: The fluorinating agent, NH4F, was added to the SnCl4/EtOH system. The ratio of tin ions to fluorine ions was 10/4. The NH4F crystals were dissolved after being stirred for 2 h at 140 rpm and concurrently heated to 35 °C.
- SnCl4/EtOH/NH4OH: 20% alcohol solution of ammonia was added to the SnCl4/EtOH system until the pH level reached the same as that of the SnCl4/EtOH/NH4F system (pH = 1.80).
- SnO2/EtOH: A concentrated aqueous ammonia solution was added to the SnCl4/EtOH system until the complete precipitation of tin hydroxide. The solvent and water associated with tin oxide was removed by heating to 100 °C and stirring at a speed of 160 rpm. After drying, a white powder was obtained. Ethanol was used to fill the tin oxide powder to obtain a solution with a tin oxide concentration of 0.13 mol/L. The vessel’s contents were stirred at a speed of 100 rpm without any heating until the precipitate transitioned into solution. The procedure lasted for 4 h.
- Film-forming system containing SnO2 in the form of a dispersed phase with the addition of NH4F (SnO2/EtOH/NH4F): crystals of ammonium fluoride were added to the system containing SnO2 in the form of a dispersed phase. The ratio of tin ions to fluorine ions was 10/4.
3. Results and Discussion
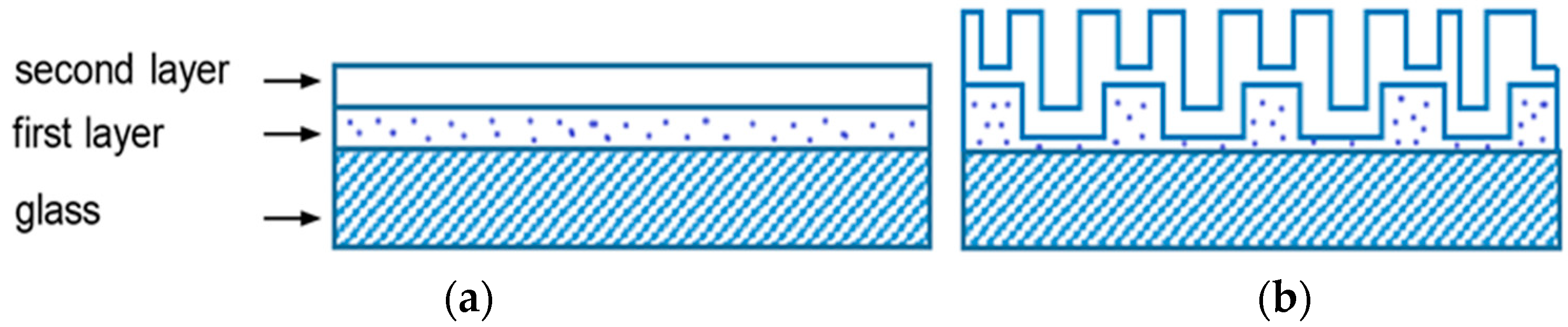
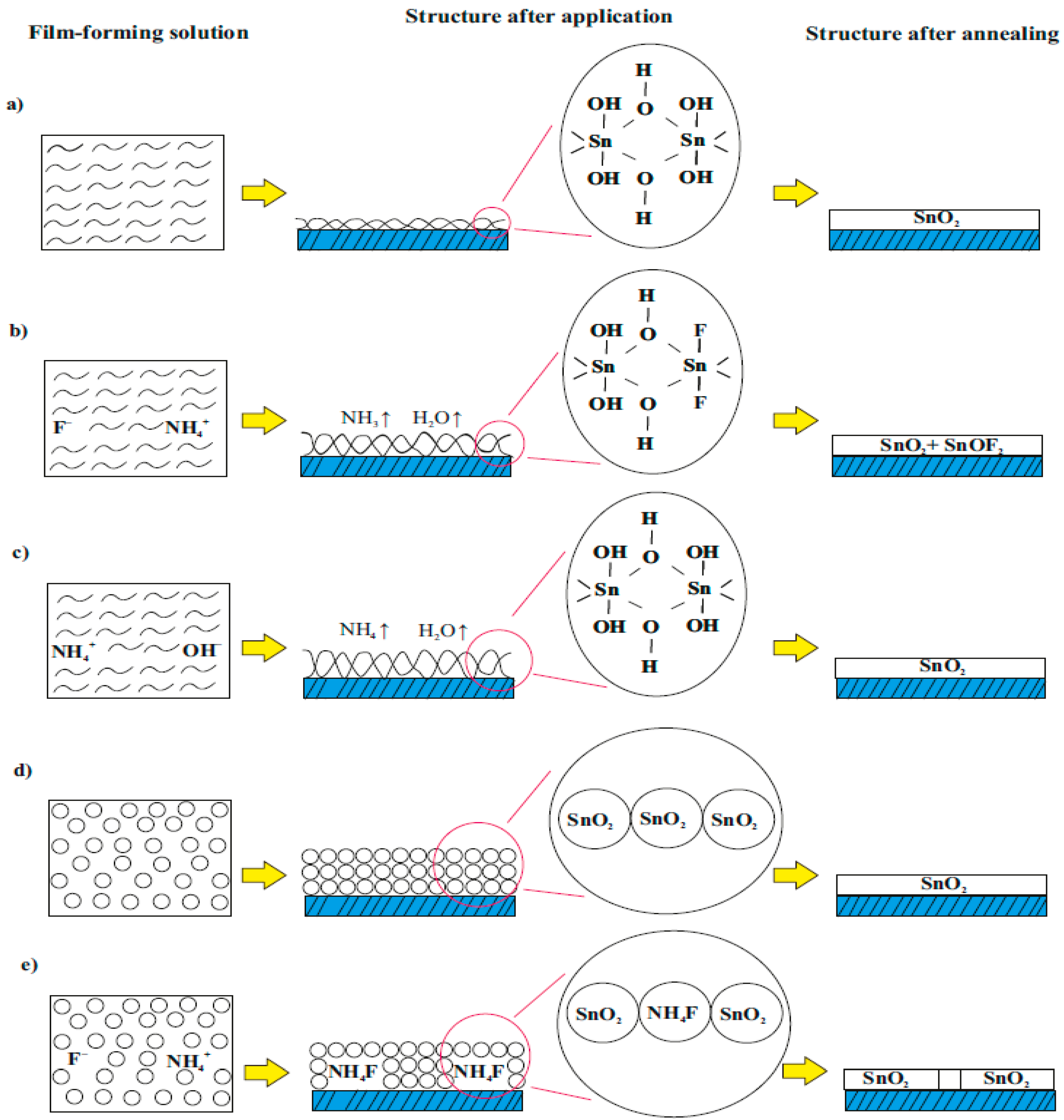
3.1. Formation of Films
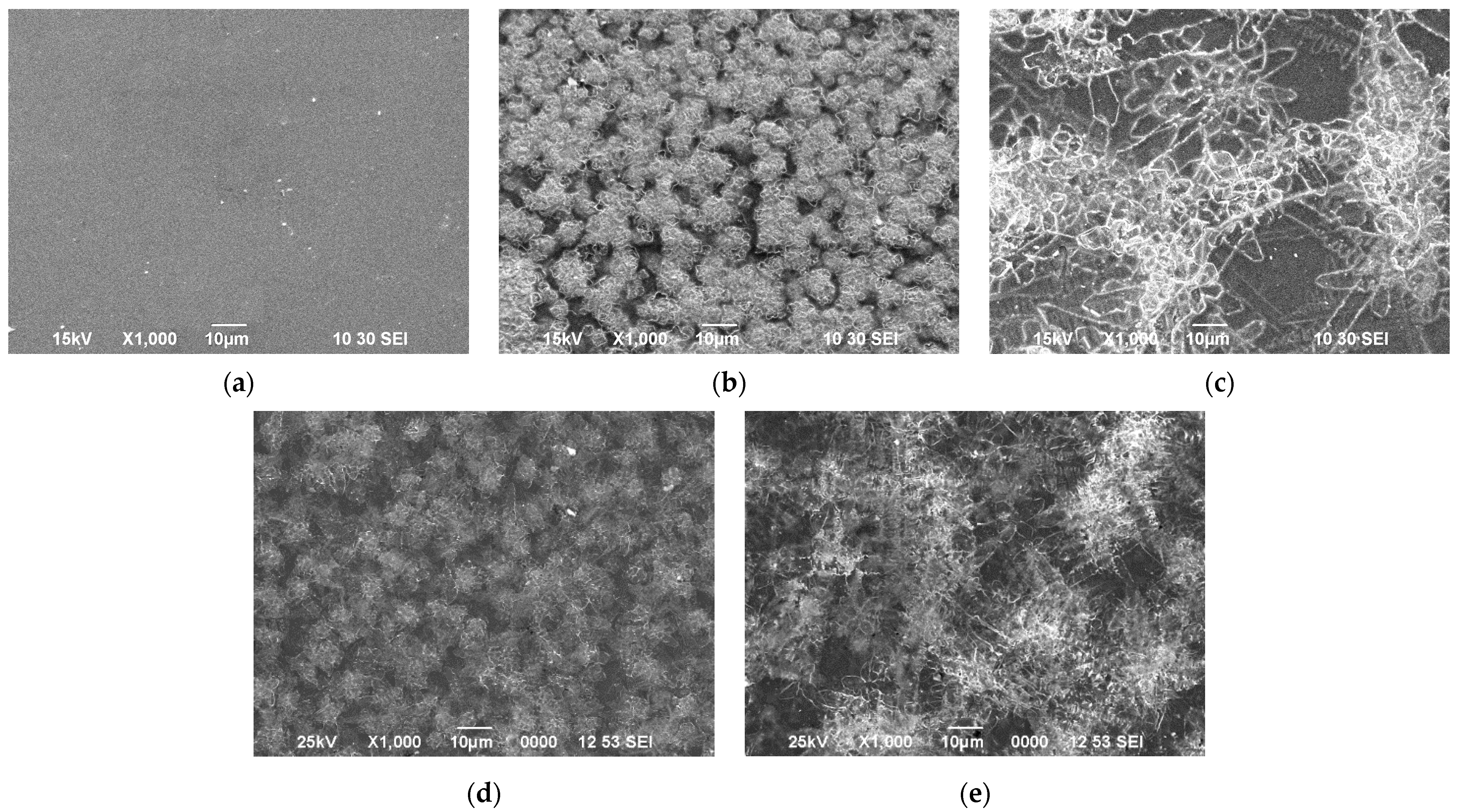
3.2. Film Structure
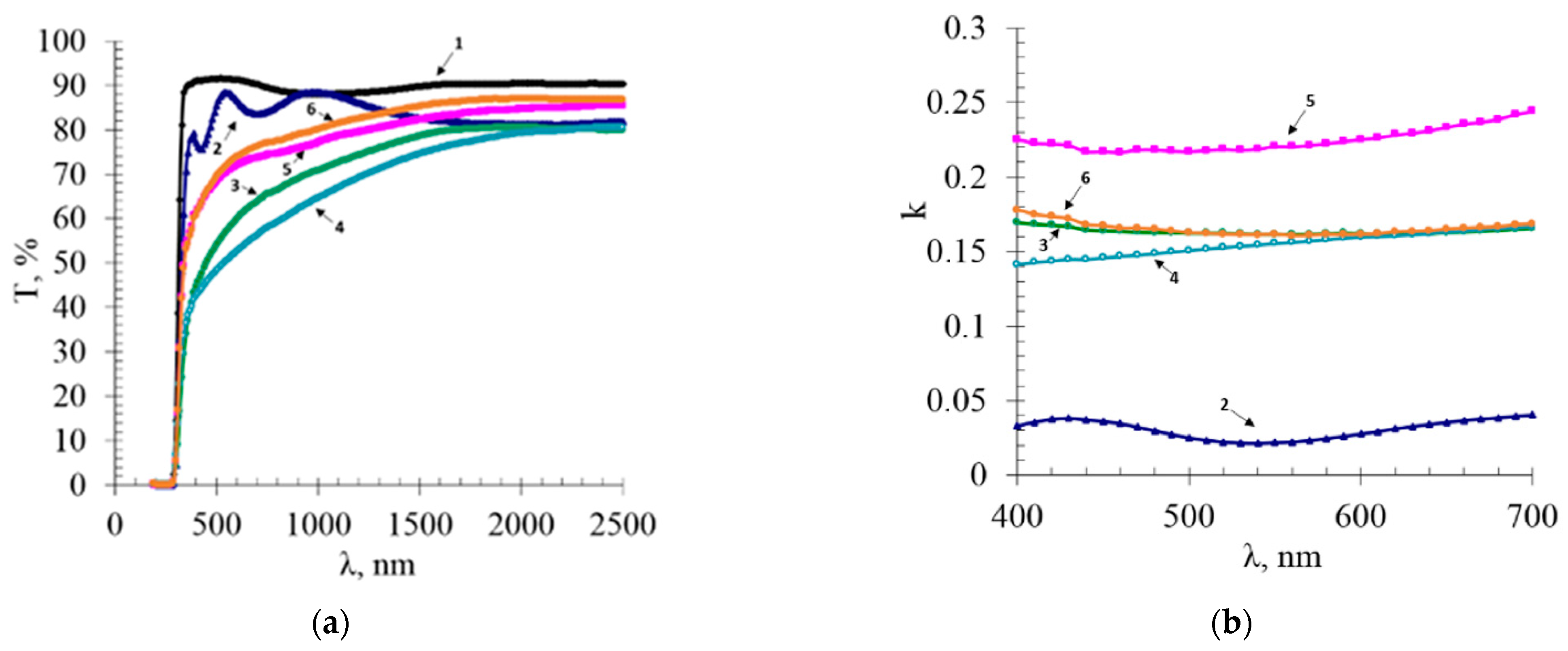
3.3. Optical Spectra and Surface Resistance
- for minimum:
- for maximum:
2NH4F → NH4(HF2) + NH3↑.
NH4(HF2) → NH3↑ + 2HF↑.
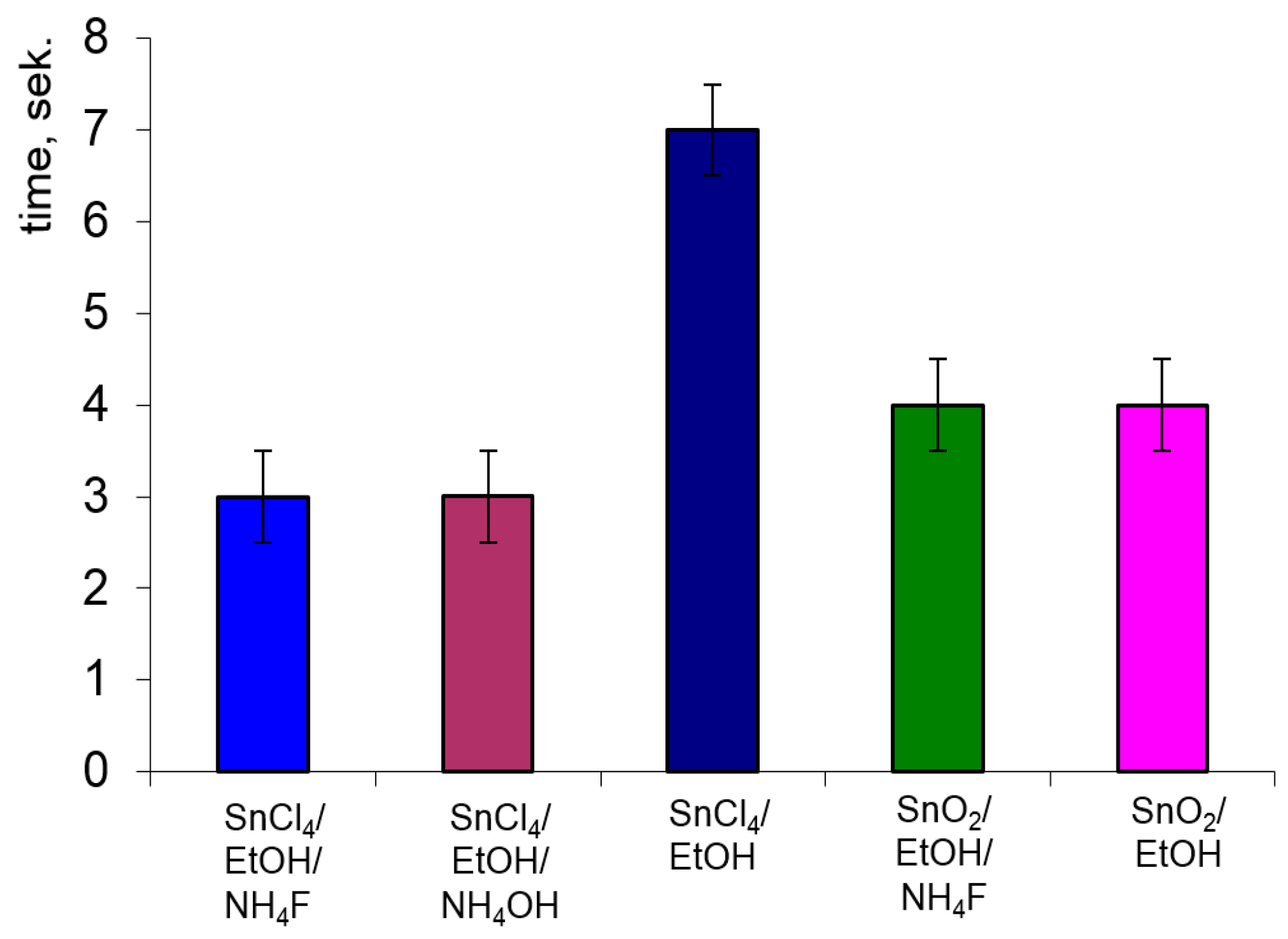
3.4. Sensitivity to Ethanol
(T < 300 °C).
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ramanauskas, R.; Iljinas, A.; Marcinauskas, L.; Milieska, M.; Kavaliauskas, Z.; Gecevicius, G.; Capas, V. Deposition and application of Indium-Tin-Oxide films for defrosting windscreens. Coatings 2022, 12, 670. [Google Scholar] [CrossRef]
- Chen, Z.N.; Chen, S.M. Efficient and stable quantum-dot light-emitting diodes enabled by Tin Oxide multifunctional electron transport layer. Adv. Opt. Mater. 2022, 10, 2102404. [Google Scholar] [CrossRef]
- Donercark, E.; Guler, S.; Ciftpinar, E.H.; Kabacelik, I.; Koc, M.; Ercelebi, A.C.; Turan, R. Impact of oxygen partial pressure during Indium Tin Oxide sputtering on the performance of silicon heterojunction solar cells. Mater. Sci. Eng. B Adv. Funct. Solid State Mater. 2022, 281, 115750. [Google Scholar] [CrossRef]
- Kuznetsova, S.; Knalipova, O.; Chen, Y.W.; Kozik, V. The joint effect of doping with tin(IV) and heat treatment on the transparency and conductivity of films based on titanium dioxide as photoelectrodes of sensitized solar cells. Nanosyst. Phys. Chem. Math. 2022, 13, 193–205. [Google Scholar] [CrossRef]
- Villarreal, C.C.; Sandoval, J.I.; Ramnani, P.; Terse-Thakoor, T.; Vi, D.; Mulchandani, A. Graphene compared to fluorine-doped tin oxide as transparent conductor in ZnO dye-sensitized solar cells. J. Environ. Chem. Eng. 2022, 10, 107551. [Google Scholar] [CrossRef]
- Pozov, S.M.; Andritsos, K.; Theodorakos, I.; Georgiou, E.; Ioakeimidis, A.; Kabla, A.; Melamed, S.; de la Vega, F.; Zergioti, I.; Choulis, S.A. Indium Tin Oxide-Free inverted organic photo-voltaics using laser- induced forward transfer silver nanoparticle embedded metal grids. ACS Appl. Electron. Mater. 2022, 4, 2689–2698. [Google Scholar] [CrossRef]
- Ambardekar, V.; Bhowmick, T.; Bandyopadhyay, P.P. Understanding on the hydrogen detection of plasma sprayed tin oxide/tungsten oxide (SnO2/WO3) sensor. Int. J. Hydrogen Energy 2022, 47, 15120–15131. [Google Scholar] [CrossRef]
- Grushevskaya, E.A.; Ibraimova, S.A.; Dmitriyeva, E.A.; Lebedev, I.A.; Mukhamedshina, D.M.; Fedosimova, A.I.; Serikkanov, A.S.; Temiraliev, A.T. Sensitivity to ethanol vapour of thin films SnO2 doped with fluorine. Eurasian Chem. Technol. J. 2019, 21, 13–17. [Google Scholar] [CrossRef]
- Mukhamedshina, D.M.; Fedosimova, A.I.; Dmitriyeva, E.A.; Lebedev, I.A.; Grushevskaya, E.A.; Ibraimova, S.A.; Serikkanov, A.S. Influence of plasma treatment on physical properties of thin SnO2 films obtained from SnCl4 solutions with additions of NH4F and NH4OH. Eurasian Chem. Technol. J. 2019, 21, 57–61. [Google Scholar] [CrossRef]
- Lee, Y.G.; Choi, W.S. Effect of PGMEA addition on Zinc-Tin-Oxide thin-film transistor fabricated by inkjet-printing process. Adv. Eng. Mater. 2022, 24, 2200128. [Google Scholar] [CrossRef]
- Jin, R.S.; Shi, K.L.; Qiu, B.B.; Huang, S.H. Photoinduced-reset and multilevel storage transistor memories based on antimony-doped tin oxide nanoparticles floating gate. Nanotechnology 2022, 33, 025201. [Google Scholar] [CrossRef] [PubMed]
- Tang, Y.L.; Huang, C.H.; Nomura, K. Vacuum-free liquid-metal-printed 2D Indium-Tin Oxide thin-film transistor for oxide inverters. ACS Nano 2022, 16, 3280–3289. [Google Scholar] [CrossRef]
- Huang, S.Y.; Liu, Y.N.; Yang, F.; Wang, Y.; Yu, T.; Ma, D.L. Metal nanowires for transparent conductive electrodes in flexible chromatic devices: A review. Environ. Chem. Lett. 2022, 20, 3005–3037. [Google Scholar] [CrossRef]
- Gilshtein, E.; Bolat, S.; Sevilla, G.T.; Cabas-Vidani, A.; Clemens, F.; Graule, T.; Tiwari, A.N.; Romanyuk, Y.E. Inkjet-printed conductive ITO patterns for transparent security systems. Adv. Mater. Technol. 2020, 5, 2000369. [Google Scholar] [CrossRef]
- Kim, J.; Murdoch, B.J.; Partridge, J.G.; Xing, K.; Qi, D.C.; Lipton-Duffin, J.; McConville, C.F.; van Embden, J.; Gaspera, E.D. Ultrasonic spray pyrolysis of antimony-doped tin oxide transparent conductive coatings. Adv. Mater. Interfaces 2020, 7, 2000655. [Google Scholar] [CrossRef]
- Shilova, O.A.; Gubanova, N.N.; Matveev, V.A.; Ivanova, A.G.; Arsentiev, M.Y.; Pugachev, K.E.; Ivankova, E.M.; Kruchinina, I.Y. Processes of film-formation and crystallization in catalytically active ‘spin-on glass’ silica films containing Pt and Pd nanoparticles. J. Mol. Liq. 2019, 288, 110996. [Google Scholar] [CrossRef]
- Gubanova, N.N.; Matveev, V.A.; Shilova, O.A. Bimetallic Pt/Pd nanoparticles in sol–gel-derived silica films and xerogels. J. Sol Gel Sci. Technol. 2019, 92, 367–375. [Google Scholar] [CrossRef]
- Muhammad, A.; Hassan, Z.; Mohammad, S.M.; Rajamanickam, S.; Abed, S.M.; Ashiq, M.G.B. Realization of UV-C absorption in ZnO nanostructures using fluorine and silver co-doping. Colloid Interface Sci. Commun. 2022, 47, 100588. [Google Scholar] [CrossRef]
- Wu, K.D.; Feng, Y.F.; Xie, Y.D.; Zhang, J.M.; Xiong, D.P.; Chen, L.; Feng, Z.Y.; Wen, K.H.; He, M. Ti3C2/fluorine-doped carbon as anode material for high performance potassium-ion batteries. J. Alloys Compd. 2023, 938, 168430. [Google Scholar] [CrossRef]
- Guo, Y.; Tang, Z.H.; Liu, Y.; Lu, Y.; Wu, W.W.; Yin, Z.D.; Wu, X.H. Boosting the multi-electron reaction capability of Na4MnCr(PO4)3 by a fluorine doping strategy for sodium-ion batteries. J. Alloys Compd. 2023, 937, 168429. [Google Scholar] [CrossRef]
- Chen, Y.L.; Huang, Y.P.; Tian, H.L.; Ye, L.Q.; Li, R.P.; Chen, C.C.; Dai, Z.X.; Huang, D. Fluorine-doped BiVO4 photocatalyst: Preferential cleavage of C−N bond for green degradation of glyphosate. J. Environ. Sci. 2023, 127, 60–68. [Google Scholar] [CrossRef]
- Nusupov, K.K.; Beisenkhanov, N.B.; Valitova, I.V.; Dmitrieva, E.A.; Zhumagaliuly, D.; Shilenko, E.A. Structural studies of thin silicon layers repeatedly implanted by carbon ions. Phys. Solid State 2006, 48, 1255–1267. [Google Scholar] [CrossRef]
- Lu, K.; Wang, Z.Y.; Wu, Y.X.; Zhai, X.W.; Wang, C.X.; Li, J.; Wang, Z.M.; Li, X.Y.; He, Y.X.; An, T. Synergistic effect of F doping and WO3 loading on electrocatalytic oxygen evolution. Chem. Eng. J. 2023, 451, 138590. [Google Scholar] [CrossRef]
- Ye, F.; Zeng, J.J.; Cai, X.M.; Su, X.Q.; Wang, B.; Wang, H.; Roy, V.A.L.; Tian, X.Q.; Li, J.W.; Zhang, D.P. Doping cuprous oxide with fluorine and its band gap narrowing. J. Alloys Compd. 2017, 721, 64–69. [Google Scholar] [CrossRef]
- Ding, Y.F.; Yin, S.F.; Cai, M.Q. Enhanced photocatalytic toluene oxidation performance induced by two types of cooperative fluorine doping in polymeric carbon nitride with the first-principles calculations. J. Colloid Interface Sci. 2023, 630, 452–459. [Google Scholar] [CrossRef] [PubMed]
- Almaev, A.V.; Kopyev, V.V.; Novikov, V.A.; Chikiryaka, A.V.; Yakovlev, N.N.; Usseinov, A.B.; Karipbayev, Z.T.; Akilbekov, A.T.; Koishybayeva, Z.K.; Popov, A.I. ITO Thin Films for Low-Resistance Gas Sensors. Materials 2023, 16, 342. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.S.; Wang, G.; Zhi, H.; Dong, J.; Hao, J.; Zhang, X.; Wang, J.; Li, D.T.; Liu, B.S. Synthesis of the Porous ZnO Nanosheets and TiO2/ZnO/FTO Composite films by a low-temperature hydrothermal method and their applications in photocatalysis and electrochromism. Coatings 2022, 12, 695. [Google Scholar] [CrossRef]
- Niu, F.J.; Wang, D.G.; Williams, L.J.; Nayak, A.; Li, F.; Chen, X.Y.; Troian-Gautier, L.; Huang, Q.; Liu, Y.M.; Brennaman, M.K.; et al. A Semiconductor-mediator-catalyst artificial photosynthetic system for photoelectrochemical water oxidation. Chem. A Eur. J. 2022, 28, e202102630. [Google Scholar] [CrossRef]
- Yang, S.H.; Ke, X.; Zhang, M.L.; Luo, D.X. Decoration of PdAg dual-metallic alloy nanoparticles on Z-Scheme α-Fe2O3/CdS for manipulable products via photocatalytic reduction of carbon dioxide. Front. Chem. 2022, 10, 937543. [Google Scholar] [CrossRef]
- Han, D.; Wu, C.; Zhao, Y.; Xiao, L.; Zhao, Z. Ion implantation-modified fluorine-doped tin oxide by zirconium with continuously tunable work function and its application in perovskite solar cells. ACS Appl. Mater. Interfaces 2017, 9, 42029–42034. [Google Scholar] [CrossRef]
- Latif, H.; Liu, J.; Mo, D.; Wang, R.; Zeng, J.; Zhai, P.F.; Sattar, A. Effect of target morphology on morphological, optical and electrical properties of FTO thin film deposited by pulsed laser deposition for mapbbr3 perovskite solar cell. Surf. Interfaces 2021, 24, 101117. [Google Scholar] [CrossRef]
- Afzaal, M.; Yates, H.M.; Walter, A.; Nicolay, S. Improved FTO/NiOx interfaces for inverted planar triple-cation perovskite solar cells. IEEE J. Photovolt. 2019, 9, 1302–1308. [Google Scholar] [CrossRef]
- Yang, X.; Liu, R.; Lei, Y.; Li, P.; Wang, K.; Zheng, Z.; Wang, D. Dual Influence of reduction annealing on diffused Hematite/FTO junction for enhanced photoelectrochemical water oxidation. ACS Appl. Mater. Interfaces 2016, 8, 16476–16485. [Google Scholar] [CrossRef]
- Mu, S.; Shi, Q. Photoelectrochemical properties of bare fluorine doped tin oxide and its electrocatalysis and photoelectrocatalysis toward cysteine oxidation. Electrochim. Acta 2016, 195, 59–67. [Google Scholar] [CrossRef]
- Cirocka, A.; Zarzeczańska, D.; Wcisło, A. Good choice of electrode material as the key to creating electrochemical sensors—Characteristics of carbon materials and transparent conductive oxides (TCO). Materials 2021, 14, 4743. [Google Scholar] [CrossRef]
- Burnat, D.; Sezemsky, P.; Lechowicz, K.; Koba, M.; Janczuk-Richter, M.; Janik, M.; Stranak, V.; Niedziółka-Jönsson, J.; Bogdanowicz, R.; Śmietana, M. Functional fluorine-doped tin oxide coating for opto-electrochemical label-free biosensors. Sens. Actuators B Chem. 2022, 367, 132145. [Google Scholar] [CrossRef]
- Nascimento, R.A.S.; Mulato, M. Mechanisms of ion detection for fet-sensors using FTO: Role of cleaning process, ph sequence and electrical resistivity. Mater. Res. 2017, 20, 1369–1379. [Google Scholar] [CrossRef]
- Huang, G.-K.; Gupta, S.; Lee, C.-Y.; Tai, N.-H. Acid-treated carbon nanotubes/polypyrrole/fluorine-doped tin oxide electrodes with high sensitivity for saliva glucose sensing. Diam. Relat. Mater. 2022, 129, 109385. [Google Scholar] [CrossRef]
- Liu, J.W.; Minh, D.Q.; Amberg, G. Thermohydrodynamics of boiling in binary compressible fluids. Phys. Rev. E 2015, 92, 043017. [Google Scholar] [CrossRef]
- Shchekin, A.K.; Gosteva, L.A.; Lebedeva, T.S. Thermodynamic properties of stable and unstable vapor shells around lyophobic nanoparticles. Phys. A Stat. Mech. Its Appl. 2020, 560, 125105. [Google Scholar] [CrossRef]
- Liang, Y.E.; Weng, Y.H.; Hsieh, I.F.; Tsao, H.K.; Sheng, Y.J. Attractive encounter of a nanodrop toward a nanoprotrusion. J. Phys. Chem. C 2017, 121, 7923–7930. [Google Scholar] [CrossRef]
- Klym, H.; Karbovnyk, I.; Piskunov, S.; Popov, A.I. Positron Annihilation Lifetime Spectroscopy Insight on Free Volume Conversion of Nanostructured MgAl2O4 Ceramics. Nanomaterials 2021, 11, 3373. [Google Scholar] [CrossRef]
- GOST 5962-13; Rectified Ethyl Alcohol from Food Raw Materials. Russian GOST: Moscow, Russia, 2014.
- Sui, R.; Charpentier, P.A.; Marriott, R.A. Metal oxide-related dendritic structures: Self-assembly and applications for sensor, catalysis, energy conversion and beyond. Nanomaterials 2021, 11, 1686. [Google Scholar] [CrossRef] [PubMed]
- Małgorzata, Z.; Tadeusz, K.; Beata, S.; Paweł, S. Investigation of the dendritic structure influence on the electrical and mechanical properties diversification of the continuously casted copper strand. Materials 2020, 13, 5513. [Google Scholar] [CrossRef]
- Murzalinov, D.; Dmitriyeva, E.; Lebedev, I.; Bondar, E.A.; Fedosimova, A.I.; Kemelbekova, A. The effect of pH solution in the sol–gel process on the structure and properties of thin SnO2 films. Processes 2022, 10, 1116. [Google Scholar] [CrossRef]
- Temiraliev, A.; Tompakova, N.; Fedosimova, A.; Dmitriyeva, E.; Lebedev, I.; Grushevskaya, E.; Mukashev, B.; Serikkanov, A. Birth and fusion in a sol-gel process with low diffusion. Eurasian Phys. Tech. J. 2020, 17, 132–137. [Google Scholar] [CrossRef]
- Shilova, O.A. Fractals, morphogenesis and triply periodic minimal surfaces in sol–gel-derived thin films. J. Sol. Gel. Sci. Technol. 2020, 95, 599–608. [Google Scholar] [CrossRef]
- Chen, X.; Bai, R.; Huang, M. Optical properties of amorphous Ta2O5 thin films deposited by RF magnetron sputtering. Opt. Mater. 2019, 97, 109404. [Google Scholar] [CrossRef]
- Zuo, Y.; Guo, L.; Liu, W.; Ding, J. Measurement of the scattering matrix and extinction coefficient of the chaff corridor. IEEE Access 2020, 8, 206755–206769. [Google Scholar] [CrossRef]
- Dmitrieva, E.A.; Mukhamedshina, D.M.; Beisenkhanov, N.B.; Mit’, K.A. The effect of NH4F and NH4OH on the structure and physical properties of thin SnO2 films synthesized by the sol-gel method. Glass Phys. Chem. 2014, 40, 31–36. [Google Scholar] [CrossRef]
- Mukhamedshina, D.M.; Mit’, K.A.; Beisenkhanov, N.B.; Dmitriyeva, E.A.; Valitova, I.V. Influence of plasma treatments on the microstructure and electrophysical properties of SnOx thin films synthesized by magnetron sputtering and sol–gel technique. J. Mater. Sci. Mater. Electron. 2008, 19, 382–387. [Google Scholar] [CrossRef]
- Tarighi, A.; Mashreghi, A. Dependence of photovoltaic properties of spray-pyrolyzed F-doped SnO2 thin film on spray solution preparation method. J. Electron. Mater. 2019, 48, 7827–7835. [Google Scholar] [CrossRef]
- Miranda, H.; Velumani, S.; Samudio Pérez, C.A.; Krause, J.C.; D’Souza, F.; De Obaldía, E.; Ching-Prado, E. Effects of changes on temperature and fluorine concentration in the structural, optical and electrical properties of SnO2:F thin films. J. Mater. Sci. Mater. Electron. 2019, 30, 15563–15581. [Google Scholar] [CrossRef]
- Dmitriyeva, E.A.; Mukhamedshina, D.M.; Mit, K.A.; Lebedev, I.A.; Girina, I.I.; Fedosimova, A.I.; Grushevskya, E.A. Doping of fluorine of tin dioxide films synthesized by sol-gel method. News Natl. Acad. Sci. Repub. Kazakhstan 2019, 433, 73–79. [Google Scholar] [CrossRef]
- Smorokov, A.A.; Kantaev, A.S.; Borisov, V.A. Research of titanomagnetite concentrate decomposition by means of ammonium fluoride and ammonium hydrogen fluoride. AIP Conf. Proc. 2019, 2143, 020022. [Google Scholar] [CrossRef]
- Konan, F.K.; Hartiti, B.; Batan, A.; Aka, B. X-ray diffraction, XPS, and Raman spectroscopy of coated ZnO:Al (1—7 at%) nanoparticles. E J. Surf. Sci. Nanotechnol. 2019, 17, 163–168. [Google Scholar] [CrossRef]
- Konan, F.K.; Ncho, J.S.; Nkuissi, H.J.T.; Hartiti, B.; Boko, A. Influence of the precursor concentration on the morphological and structural properties of zinc oxide (ZnO). Mater. Chem. Phys. 2019, 229, 330–333. [Google Scholar] [CrossRef]
- Kim, K.W.; Cho, P.S.; Kim, S.J.; Lee, J.H.; Kang, C.Y.; Kim, J.S.; Yoon, S.J. The selective detection of C2H5OH using SnO2–ZnO thin film gas sensors prepared by combinatorial solution deposition. Sens. Actuators B 2007, 123, 318–324. [Google Scholar] [CrossRef]
- Gong, H.; Wang, Y.J.; Teo, S.C.; Huang, L. Interaction between thin-film tin oxide gas sensor and five organic vapors. Sens. Actuators B 1999, 54, 232–235. [Google Scholar] [CrossRef]
- Li, X.; Gua, Z.; Cho, J.; Sun, H.; Kurup, P. Tin–copper mixed metal oxide nanowires: Synthesis and sensor response to chemical vapors. Sens. Actuators B 2011, 158, 199–207. [Google Scholar] [CrossRef]
- Motsoeneng, R.G.; Kortidis, I.; Ray, S.S.; Motaung, D.E. Designing SnO2 nanostructure-based sensors with tailored selectivity toward propanol and ethanol vapors. ACS Omega 2019, 4, 13696–13709. [Google Scholar] [CrossRef] [PubMed]



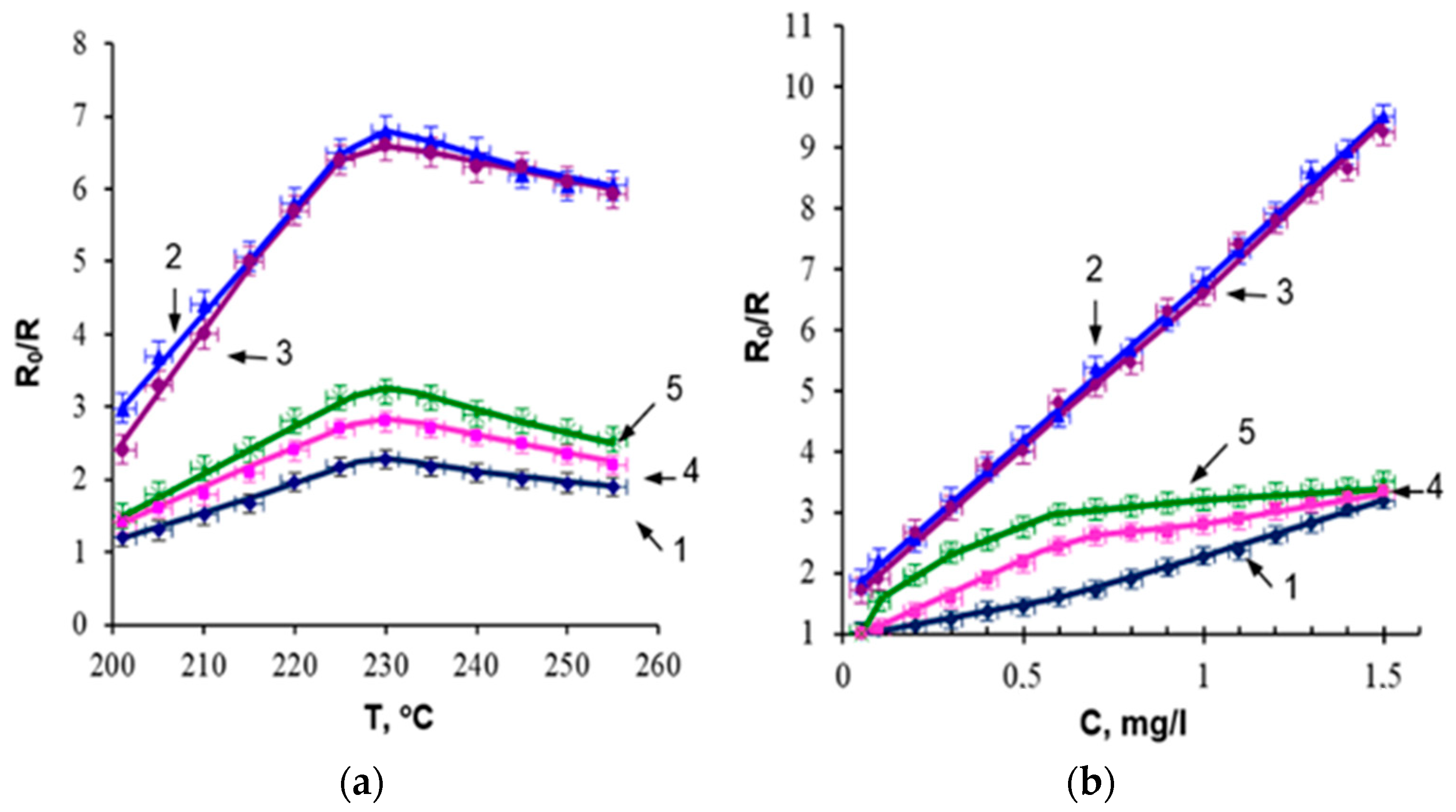
| The Composition of the Film-Forming System | Approximation Function | Approximation Confidence Level (R2) |
|---|---|---|
| SnCl4/EtOH | y = 16.755x − 4.2498 | 0.9994 |
| SnCl4/EtOH/NH4F | y = 0.7716x2 + 1.0978x + 0.7338 | 0.9989 |
| SnCl4/EtOH/NH4OH | y = 0.476x2 + 2.4008x − 1.1223 | 0.9978 |
| SnO2/EtOH | y = 0.6135x2 − 3.3234x + 2.3959 | 0.9958 |
| SnO2/EtOH/NH4F | y = 0.3788x2 − 1.8943x + 1.3543 | 0.9926 |
| The Composition of the Film-Forming System | Rsh, kOm/sq. | ρ, Om · cm | 1/ρ, Om−1 · cm−1 | Φ, 10−7 Om−1 |
|---|---|---|---|---|
| SnCl4/EtOH | 15.6 ± 1.4 | 0.390 ± 0.035 | 2.6 ± 0.2 | 146.8 ± 3.4 |
| SnCl4/EtOH/NH4F | 6.7 ± 0.6 | 0.097 ± 0.008 | 10.3 ± 0.8 | 6.3 ± 0.6 |
| SnCl4/EtOH/NH4OH | 15.4 ± 1.6 | 0.255 ± 0.026 | 3.9 ± 0.4 | 0.9 ± 0.1 |
| SnO2/EtOH | 78.9 ± 6.9 | 0.512 ± 0.044 | 1.9 ± 0.2 | 4.5 ± 0.4 |
| SnO2/EtOH/NH4F | 69.4 ± 8.3 | 0.590 ± 0.070 | 1.7 ± 0.2 | 6.2 ± 0.5 |
| The Composition of the Film-Forming System | SnO2 Crystallitis Sizes on Planes | ||
|---|---|---|---|
| (110) | (101) | (211) | |
| SnCl4/EtOH | 5.1 ± 0.3 nm | 6.3 ± 0.3 nm | 5.1 ± 0.3 nm |
| SnCl4/EtOH/NH4F | 4.0 ± 0.2 nm | 6.1 ± 0.3 nm | 5.2 ± 0.3 nm |
| SnCl4/EtOH/NH4OH | 3.6 ± 0.2 nm | 3.7 ± 0.2 nm | 3.5 ± 0.2 nm |
| SnO2/EtOH | 4.0 ± 0.2 nm | 4.6 ± 0.2 nm | 3.8 ± 0.2 nm |
| SnO2/EtOH/NH4F | 4.1± 0.2 nm | 4.5 ± 0.2 nm | 3.8 ± 0.2 nm |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dmitriyeva, E.; Lebedev, I.; Bondar, E.; Fedosimova, A.; Temiraliev, A.; Murzalinov, D.; Ibraimova, S.; Nurbaev, B.; Elemesov, K.; Baitimbetova, B. The Influence of Lyophobicity and Lyophilicity of Film-Forming Systems on the Properties of Tin Oxide Films. Coatings 2023, 13, 1990. https://doi.org/10.3390/coatings13121990
Dmitriyeva E, Lebedev I, Bondar E, Fedosimova A, Temiraliev A, Murzalinov D, Ibraimova S, Nurbaev B, Elemesov K, Baitimbetova B. The Influence of Lyophobicity and Lyophilicity of Film-Forming Systems on the Properties of Tin Oxide Films. Coatings. 2023; 13(12):1990. https://doi.org/10.3390/coatings13121990
Chicago/Turabian StyleDmitriyeva, Elena, Igor Lebedev, Ekaterina Bondar, Anastasia Fedosimova, Abzal Temiraliev, Danatbek Murzalinov, Sayora Ibraimova, Bedebek Nurbaev, Kasym Elemesov, and Bagila Baitimbetova. 2023. "The Influence of Lyophobicity and Lyophilicity of Film-Forming Systems on the Properties of Tin Oxide Films" Coatings 13, no. 12: 1990. https://doi.org/10.3390/coatings13121990







