Effect of Ultrasonic Cleaning after Laser Texturizing of Surface of AISI 316L Steel on the Degree of Wetting and Corrosion Resistance
Abstract
:1. Introduction
2. Effect of Laser Texturing on Corrosion Resistance
- -
- a negatively charged region is formed at the boundary between the aqueous environment and the hydrophobic coating, which leads to a decrease in the content of corrosive anions;
- -
- the hydrophobic coating serves as a corrosion inhibitor, preventing the adsorption of aggressive ions that initiate corrosion processes on the metal surface;
- -
- textured multimodal roughness, like the hydrophobic coating, acts as a barrier to electron transfer between the metal surface and the electrolyte solution.
- -
- it is a direct measurement method, as it is based on measuring the mass loss of the material;
- -
- it allows us to obtain fairly accurate results, since this method involves weighing the objects of study before and after corrosion tests. Despite the long testing time, this method is used as a verification method in arbitration analyses.
3. Materials and Methods
- -
- samples with the original surface—3 pieces;
- -
- samples on the surface of which molecular layers of surfactants were formed—3 pieces;
- -
- samples modified using laser equipment and subsequently processed in a surfactant emulsion—15 pieces;
- -
- samples subjected to ultrasonic cleaning after laser surface modification and subsequent treatment in a surfactant emulsion—15 pieces.
3.1. Manufacturing of Specimens and Surface Preparation
3.2. Selection of a Model Environment for Corrosion Research
- -
- acceleration of the corrosion process should not be caused by a change in its mechanism;
- -
- to most effectively accelerate the corrosion process, it is necessary to identify the main controlling factor and act on it. For example, if the corrosive activity of one of the components of the medium clearly prevails, in model environments, it is advisable to increase its concentration, while controlling the preservation of the unchanged mechanism of the corrosion process.
- -
- model of river water containing 30 mg/dm3 NaCl and 70 mg/dm3 Na2SO4 (chloride content—18.2 mg/dm3, sulfates—47.32 mg/dm3);
- -
- model of demineralized water with a pH equal to 9.8–9.9—for heaters of heating supply systems operating on softened or demineralized water;
- -
- model of district water used in heating systems contaminated with chlorides and sulfates, containing 14.8 mg/dm3 NaCl and 56.2 mg/dm3 Na2SO4 (chloride content—9 mg/dm3, sulfates—38 mg/dm3), with a solution pH of 9.1 ± 0.1 when testing low-alloy and low-carbon steels.
3.3. Corrosion Studies
4. Results
5. Conclusions
- Modification of the steel surface via the laser texturizing of its relief and, with subsequent treatment, with surfactants promotes the achievement of a hydrophobic state. In this case, an increase in the value of the water contact angle occurs with increasing laser fluence. Within the range of laser fluence from 50 to 300 J/cm2, the value of the water contact angle changes from 67 to 152°, respectively.
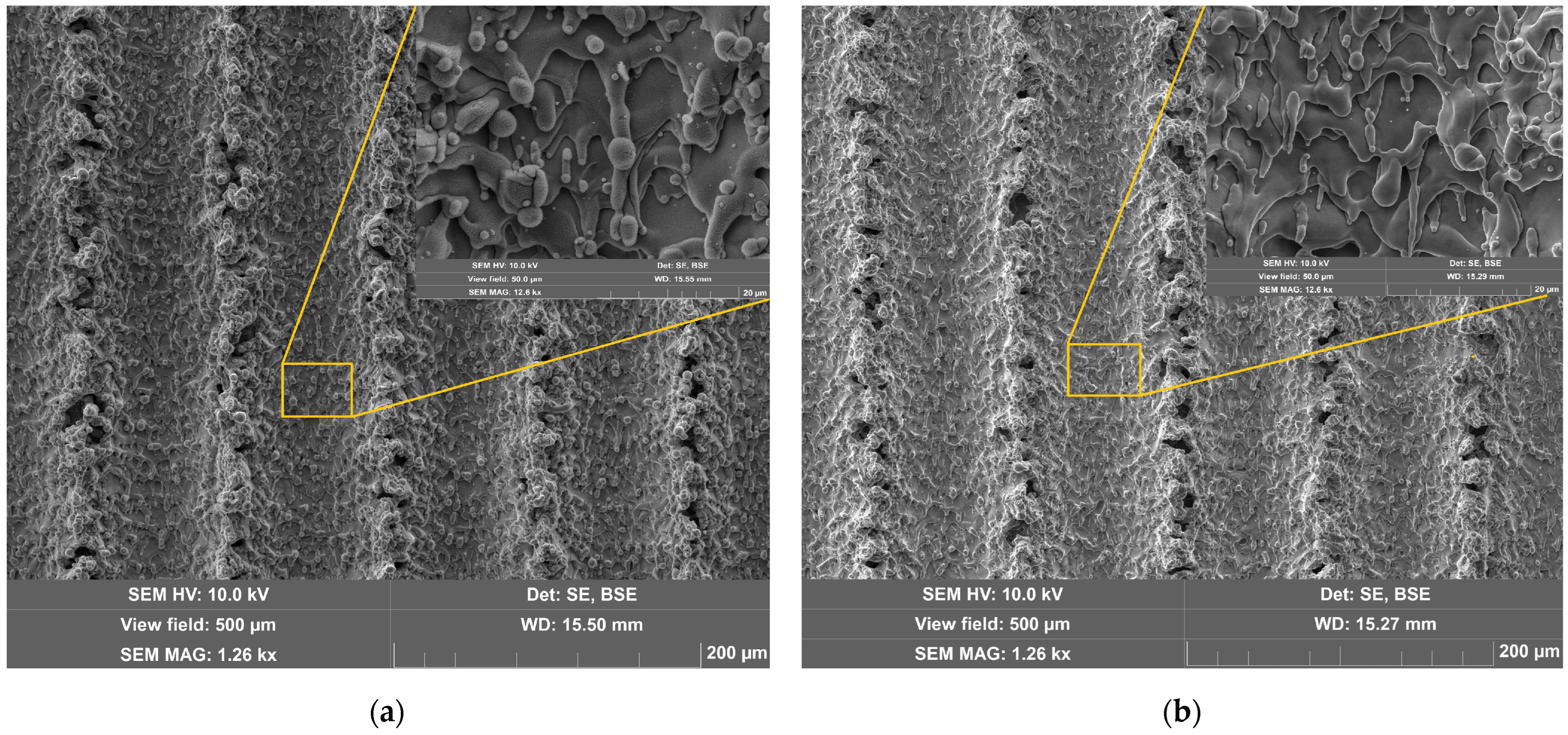
- Ultrasonic cleaning of steel surfaces leads to the removal of nano-/micro-sized particles formed during laser texturizing and forming multimodal roughness. It is noted that the mass fraction of these particles increases with increasing laser fluence during laser surface treatment. Thus, at laser fluences between 50 and 300 J/cm2, the mass removal ranges from 17 to 109 ppm. It is suggested that this is caused by the increasing number of returning particles ablated during laser texturizing, which have a low adhesion to the surface.
- Ultrasonic cleaning after the laser texturizing of steel surfaces contributes to achieving a higher degree of hydrophobicity. The increase in the water contact angle ranges from 6 to 81% in the studied range of laser fluence. The maximum value of the water contact angle was 161° at a laser fluence of 300 J/cm2. This effect can be explained by the fact that, as a result of ultrasonic cleaning, the textured relief of the steel surface becomes more structured, resulting in a decrease in the proportion of the contact area of the steel surface with the liquid. This thesis is consistent, among other things, with the Cassie–Baxter model of surface wettability.
- Additional ultrasonic cleaning of steel surfaces modified by laser texturizing leads to a decrease in the rate of processes of up to six times. It is suggested that this may be due to the removal, via ultrasonic cleaning, of particles formed by the return of some of the ablated material with a poor adhesion to the surface. This, in turn, leads to a decrease in the surface contact area with the aggressive medium and, as a consequence, to a decrease in the corrosion rate.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Rodič, P.; Kapun, B.; Milošev, I. Superhydrophobic Aluminium Surface to Enhance Corrosion Resistance and Obtain Self-Cleaning and Anti-Icing Ability. Molecules 2022, 27, 1099. [Google Scholar] [CrossRef] [PubMed]
- Mohamed, A.M.; Abdullah, A.M.; Younan, N.A. Corrosion behavior of superhydrophobic surfaces: A review. Arab. J. Chem. 2015, 8, 749–765. [Google Scholar] [CrossRef]
- Zhong, Z.; Ma, W.; Yao, S.; Niu, J.; Xu, X. Condensation heat transfer between a vertical superhydrophobic aluminum surface and moist air under natural convection. Appl. Therm. Eng. 2023, 229, 120591. [Google Scholar] [CrossRef]
- Betancur, L.; Mangini, D.; Mantelli, M.; Marengo, M. Experimental Study of Thermal Performance in a Closed Loop Pulsating Heat Pipe with Alternating Superhydrophobic Channels. Therm. Sci. Eng. Prog. 2019, 17, 10036. [Google Scholar] [CrossRef]
- Du, Q.; Zhou, P.; Pan, Y.; Qu, X.; Liu, L.; Yu, H.; Hou, J. Influence of hydrophobicity and roughness on the wetting and flow resistance of water droplets on solid surface: A many-body dissipative particle dynamics study. Chem. Eng. Sci. 2022, 249, 117327. [Google Scholar] [CrossRef]
- Li, B.; Bai, J.; He, J.; Ding, C.; Dai, X.; Ci, W.; Zhu, T.; Liao, R.; Yuan, Y. A Review on Superhydrophobic Surface with Anti-Icing Properties in Overhead Transmission Lines. Coatings 2023, 13, 301. [Google Scholar] [CrossRef]
- He, H.; Guo, Z. Superhydrophobic materials used for anti-icing Theory, application, and development. iScience 2021, 24, 103357. [Google Scholar] [CrossRef] [PubMed]
- Wei, Z.; Zhang, F.; Dai, F.; He, Q. Preparation of a Superhydrophobic Surface by a One-Step Powder Pressing Method with Liquid Silicone Rubber as the Carrier. ACS Omega 2023, 8, 8548–8556. [Google Scholar] [CrossRef]
- Fuchs-Godec, R. A Synergistic Effect between Stearic Acid and (+)-α-Tocopherol as a Green Inhibitor on Ferritic Stainless Steel Corrosion Inhibition in 3.0% NaClSolution. Coatings 2021, 11, 971. [Google Scholar] [CrossRef]
- Ali, H.M.; Qasim, M.A.; Malik, S.; Murtaza, G. Techniques for the Fabrication of Super-Hydrophobic Surfaces and Their Heat Transfer Applications: 14. In Heat Transfer; Volkov, K., Ed.; IntechOpen: Rijeka, Croatian, 2018. [Google Scholar]
- Singh, N.S.; Zhang, J.; Stafford, J.; Anthony, C.; Gao, N. Implementing Superhydrophobic Surfaces within Various Condensation Environments: A Review. Adv. Mater. Interfaces 2021, 8, 2001442. [Google Scholar] [CrossRef]
- Xiao, Z.; Xu, D.; Zhang, W.; Yu, X.; Zhang, Y. Dip-coating of Superhydrophobic Surface on Irregular Substrates for Dropwise Condensation. J. Bionic Eng. 2021, 18, 387–397. [Google Scholar] [CrossRef]
- Subramaniam, A.; Shanthi, J. Spin-coated polymer composite hydrophobic surfaces with self-cleaning performance. Mater. Res. Express 2019, 6, 076412. [Google Scholar]
- Parvate, S.; Dixit, P.; Chattopadhyay, S. Superhydrophobic Surfaces: Insights from Theory and Experiment. J. Phys. Chem. B 2020, 24, 1323–1360. [Google Scholar] [CrossRef] [PubMed]
- Le Brouster, R.; Giboz, J.; Nourdine, A.; Tenchine, L.; Charvin, N.; Mele, P. Origins of the Gain in Hydrophobicity of Polystyrene Linked to the Addition of Tailored Fluorinated Oligo-Polystyrene Additives. ACS Appl. Polym. Mater. 2023, 5, 6966–6975. [Google Scholar] [CrossRef]
- Nishino, T.; Meguro, M.; Nakamae, K.; Matsushita, M.; Ueda, Y. The lowest surface free energy based on –CF3 alignment. Langmuir 1999, 15, 4321–4323. [Google Scholar] [CrossRef]
- Ahmad, Z.; Khan, A.U.; Farooq, R.; Mastoi, N.R.; Saif, T. Hydrophobicity A Green Technique for Enhancing Corrosion Resistance of Alloys. In New Trends in Alloy Development, Characterization and Application; Zaki, A., Ed.; IntechOpen: London, UK, 2015. [Google Scholar]
- Ragutkin, A.V.; Dasaev, M.R.; Kalakutskaya, O.V.; Zilova, O.S.; Trushin, E.S. Creation of Hydrophobic Functional Surfaces of Structural Materials on the Basis of Laser Ablation (Overview). Therm. Eng. 2022, 69, 429–449. [Google Scholar] [CrossRef]
- Min, T. Design and Fabrication of Super-Hydrophobic Surfaces by Laser Micro/Nano-Processing. Ph.D. Thesis, National University of Singapore, Singapore, 2012. [Google Scholar]
- Mahmoudi, B.; Torkamany, M.J.; Aghdam, A.R.S.R.; Sabbaghzade, J. Laser surface hardening of AISI 420 stainless steel treated by pulsed Nd:YAG laser. Mater. Des. 2010, 31, 2553–2560. [Google Scholar] [CrossRef]
- Pantelis, D.I.; Bouyiouri, E.; Kouloumbi, N.; Vassiliou, P.; Koutsomichalis, A. Wear and corrosion resistance of laser surface hardened structural steel. Surf. Coat. Technol. 2002, 161, 125–134. [Google Scholar] [CrossRef]
- Sundqvist, J.; Manninen, T.; Heikkinen, H.-P.; Anttila, S.; Kaplan, A.F.H. Laser surface hardening of 11% Cr ferritic stainless steel and its sensitisation behaviour. Surf. Coat. Technol. 2018, 344, 673–679. [Google Scholar] [CrossRef]
- Telasang, G.; Majumdar, J.D.; Padmanabham, G.; Manna, I. Wear and corrosion behavior of laser surface engineered AISI H13 hot working tool steel. Surf. Coat. Technol. 2015, 261, 69–78. [Google Scholar] [CrossRef]
- de Lara, L.R.; Jagdheesh, R.; Ocaña, J.L. Corrosion resistance of laser patterned ultrahydrophobic aluminium surface. Mater. Lett. 2016, 184, 100–103. [Google Scholar] [CrossRef]
- Trdan, U.; Hocevar, M.; Gregorcic, P. Transition from superhydrophilic to superhydrophobic state of laser textured stainless steel surface and its effect on corrosion resistance. Corros. Sci. 2017, 123, 21–26. [Google Scholar] [CrossRef]
- Emelyanenko, K.A.; Emelyanenko, A.M.; Boinovich, L.B. Laser Obtained Superhydrophobic State for Stainless Steel CorrosionProtection, a Review. Coatings 2023, 13, 194. [Google Scholar] [CrossRef]
- Maharjan, N.; Murugan, V.K.; Zhou, W.; Seita, M. Corrosion behavior of laser hardened 50CrMo4 (AISI 4150) steel: A depth-wise analysis. Appl. Surf. Sci. 2019, 494, 941–951. [Google Scholar] [CrossRef]
- Yang, Z.; Liu, X.; Tian, Y. A Contrastive Investigation on Anticorrosive Performance of Laser-Induced Super-Hydrophobic and Oil-Infused Slippery Coatings. Prog. Org. Coat. 2020, 138, 105313. [Google Scholar] [CrossRef]
- Tang, Y.; Cai, Y.; Wang, L.; Luo, X.; Wang, B.; Song, Q.; Liu, Z. Formation Mechanism of Superhydrophobicity of Stainless Steel by Laser-Assisted Decomposition of Stearic Acid and Its Corrosion Resistance. Opt. Laser Technol. 2022, 153, 108190. [Google Scholar] [CrossRef]
- Grigoriev, S.; Trushin, E.; Likhaeva, A.; Volkov, A.; Dasaev, M. Imparting hydrophobic functionalities to copper surface using laser ablation and water emulsion of surfactants. In Proceedings of the E3S Web of Conferences, Marseille, France, 27 September 2021; Volume 289. [Google Scholar]
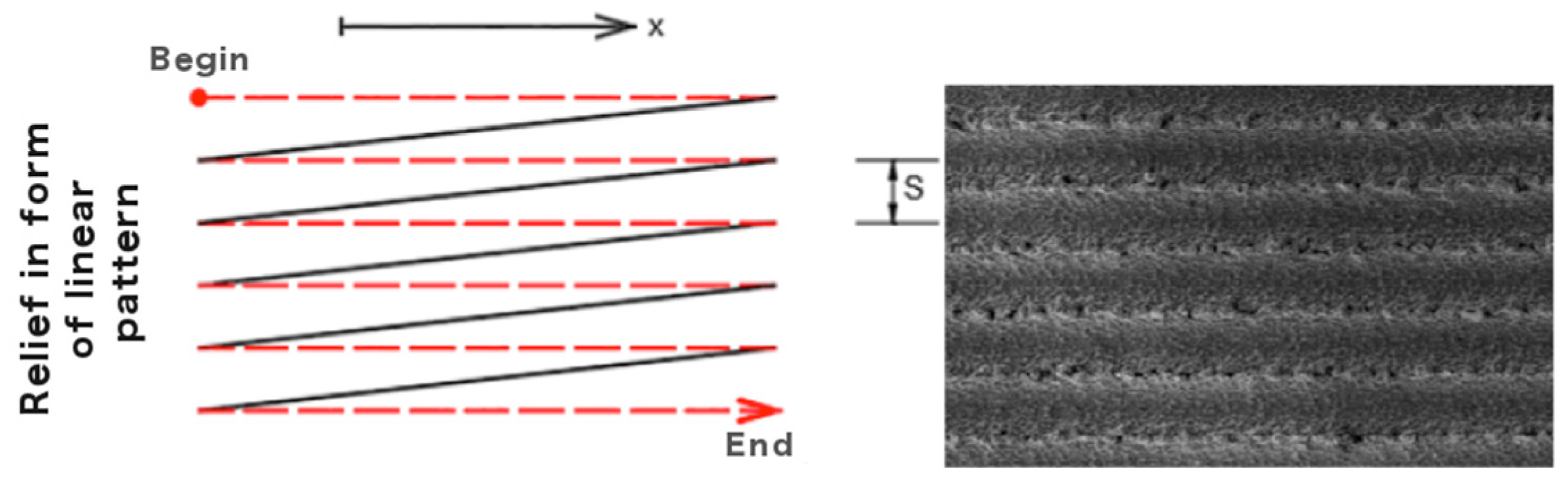



| Laser Output Power, W | Modulation Rate, kHz | Scan Speed, mm/s | Laser Fluence, J/cm2 |
|---|---|---|---|
| 22.4 | 20 | 836 | 50 |
| 387 | 100 | ||
| 255 | 150 | ||
| 191 | 200 | ||
| 127 | 300 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dasaev, M.; Kalakutskaya, O.; Zilova, O.; Mednikov, A. Effect of Ultrasonic Cleaning after Laser Texturizing of Surface of AISI 316L Steel on the Degree of Wetting and Corrosion Resistance. Coatings 2023, 13, 2058. https://doi.org/10.3390/coatings13122058
Dasaev M, Kalakutskaya O, Zilova O, Mednikov A. Effect of Ultrasonic Cleaning after Laser Texturizing of Surface of AISI 316L Steel on the Degree of Wetting and Corrosion Resistance. Coatings. 2023; 13(12):2058. https://doi.org/10.3390/coatings13122058
Chicago/Turabian StyleDasaev, Marat, Olga Kalakutskaya, Olga Zilova, and Alexey Mednikov. 2023. "Effect of Ultrasonic Cleaning after Laser Texturizing of Surface of AISI 316L Steel on the Degree of Wetting and Corrosion Resistance" Coatings 13, no. 12: 2058. https://doi.org/10.3390/coatings13122058




