Physical and Chemical Properties of High-Temperature Silicone-Based Polymer Coatings Applied on Different Surface Roughnesses
Abstract
:1. Introduction
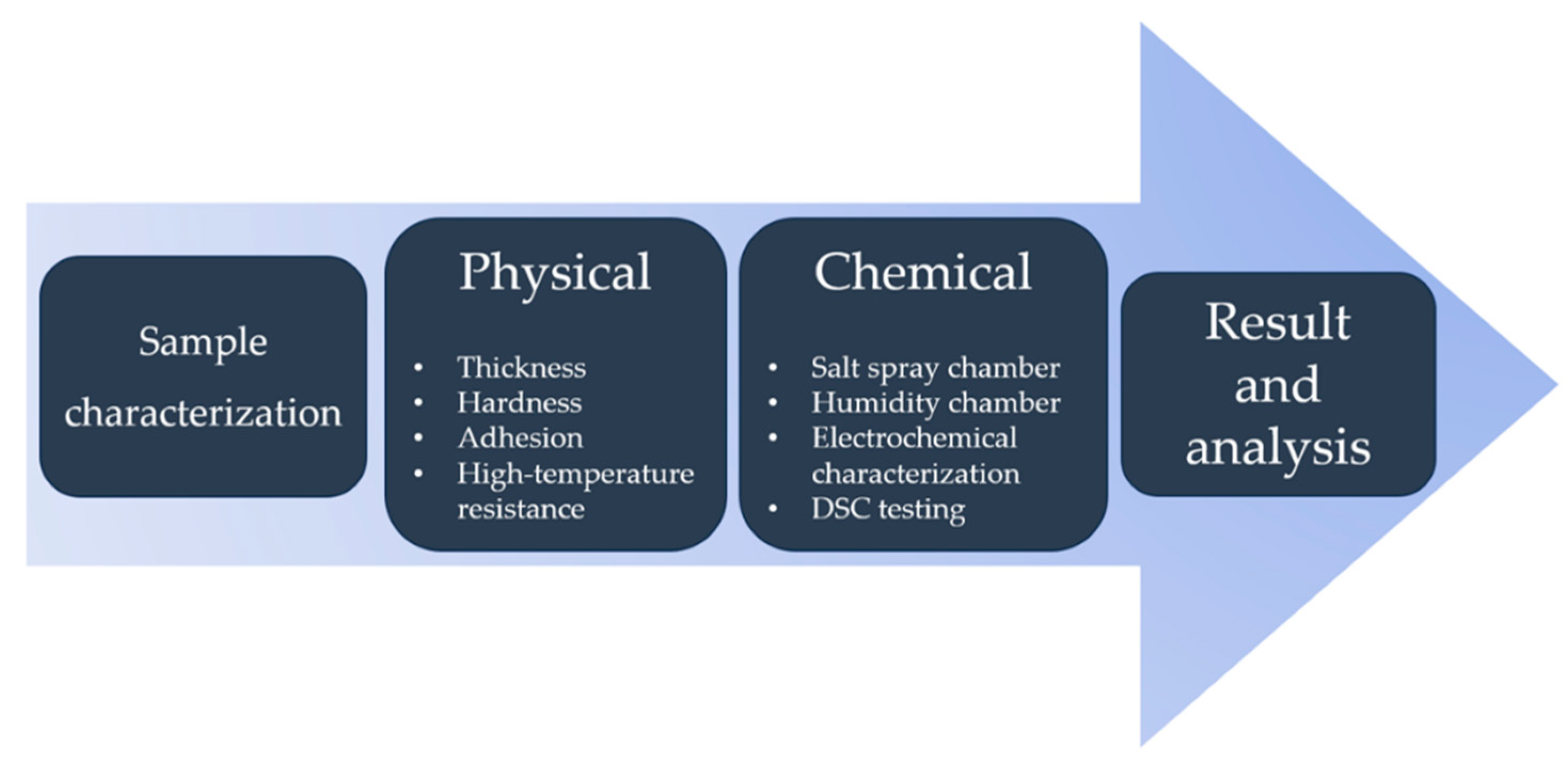
2. Materials and Methods
2.1. Materials
2.2. Preparation of the Coatings
2.3. Dry Film Thickness, Adhesion, and Gloss
2.4. Accelerated Testing in Chambers
2.5. Electrochemical Characterization
2.6. Differential Scanning Calorimetry (DSC)
3. Results and Discussion
3.1. Surface Roughness, Coating Thickness, and Hardness Testing
3.2. Coating Adhesion Testing
3.3. Assessment after Accelerated Testing in Salt Spray Chamber and Humidity Chamber
3.4. High-Temperature Testing
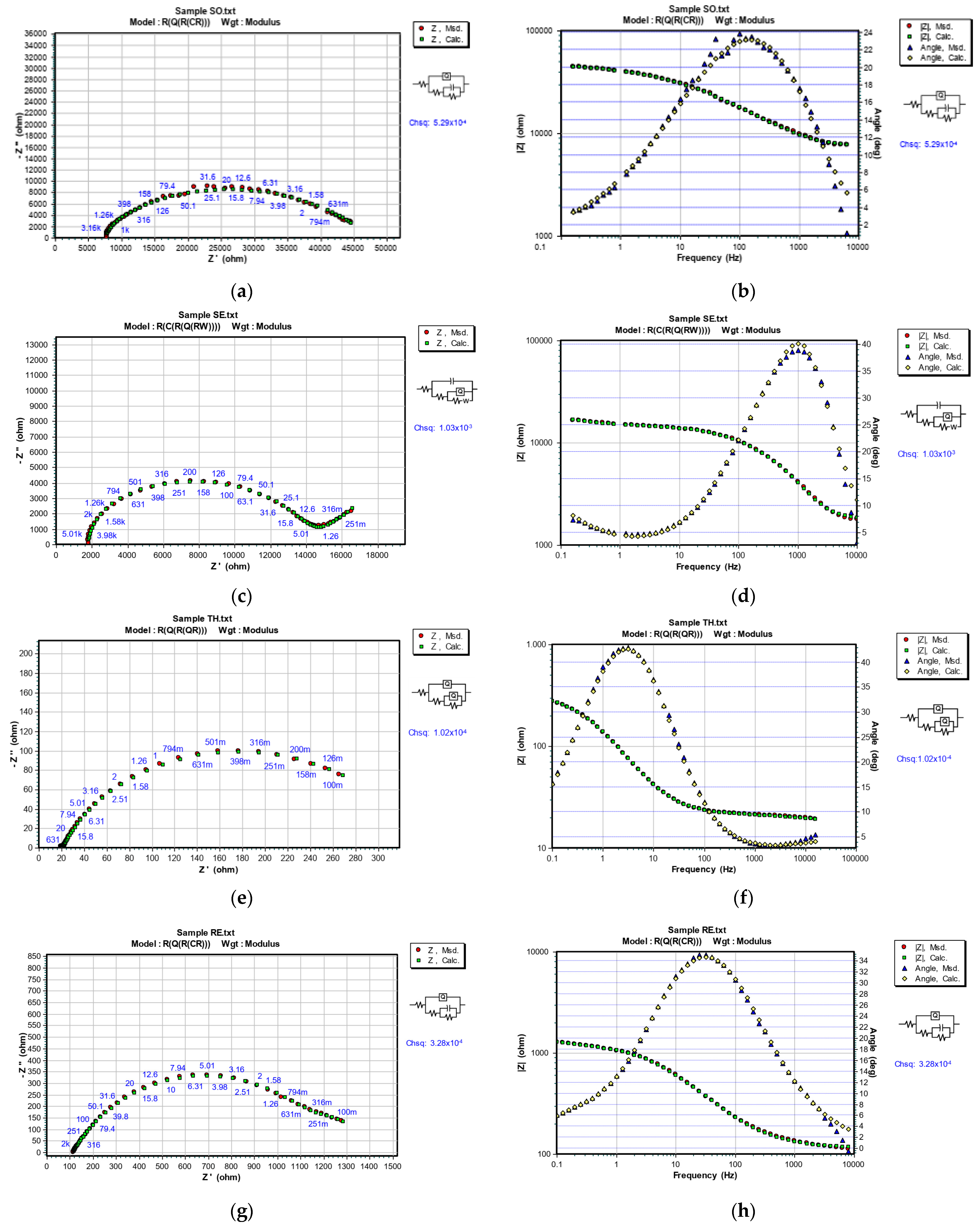
3.5. Electrochemical Characterization
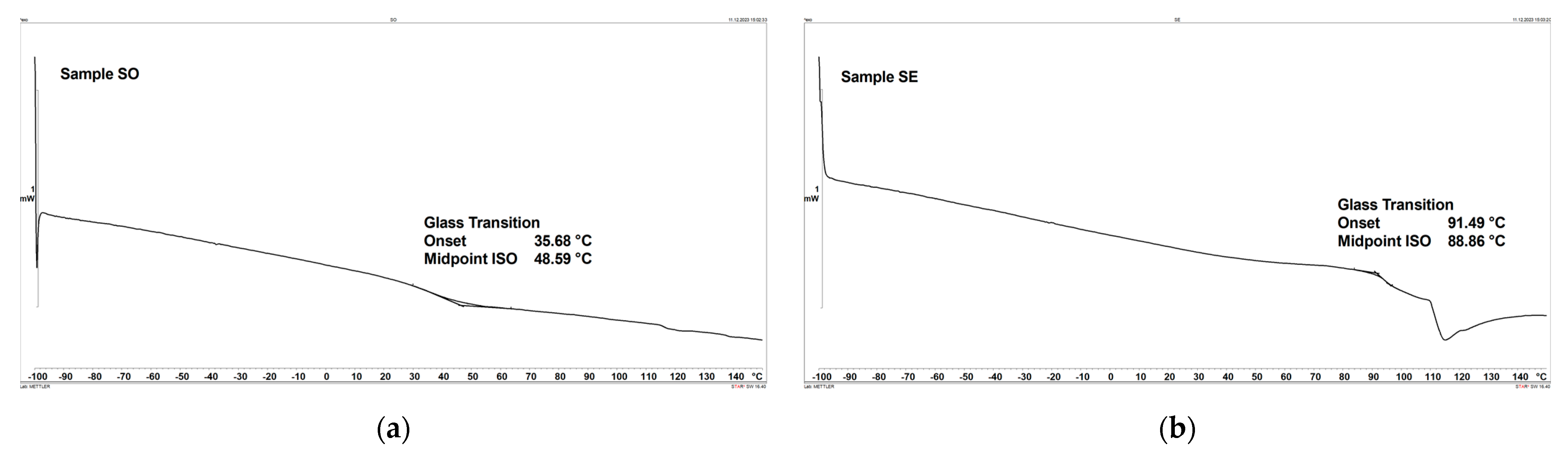
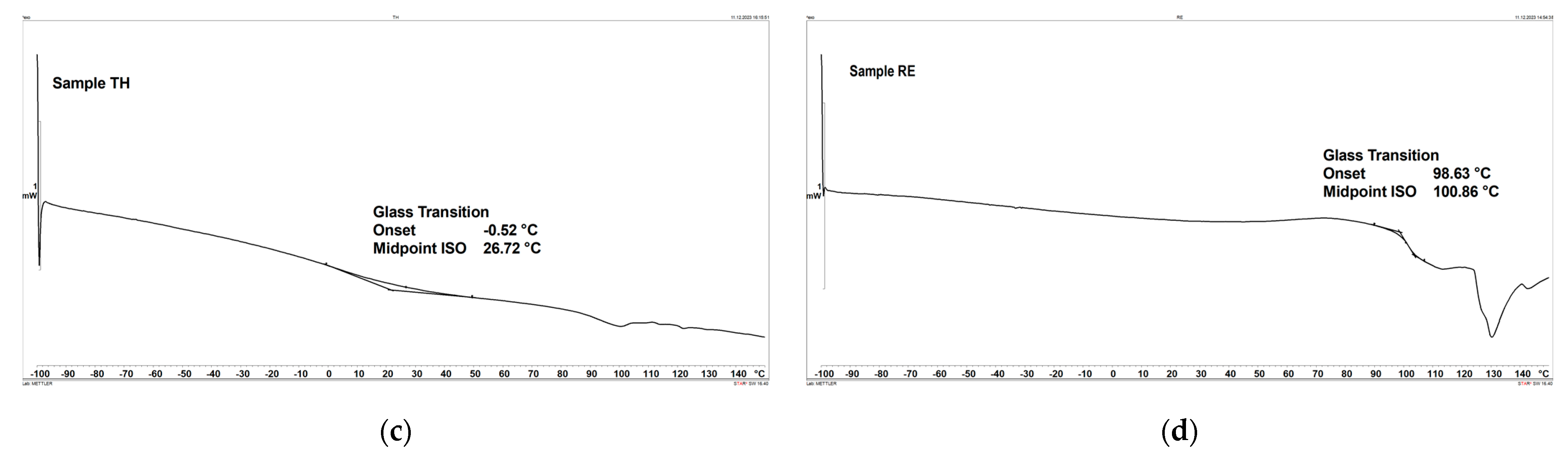
3.6. Differential Scanning Calorimetry (DSC)
4. Conclusions
- The sandblasted samples exhibited significantly better coating adhesion after testing in the salt spray and humidity chambers compared to the shot-blasted samples. After the salt spray chamber test, with the SO and SE samples, there was a complete detachment (classification 5 according to ISO 2409) of the coatings applied on the shot-blasted samples.
- The best-performing coating in the accelerated chamber testing was inorganic zinc ethyl silicate, which not only exhibited excellent properties but also maintained a decent external appearance from an esthetic perspective.
- Coating hardness was equal for all tested coatings, probably due to the great influence of the steel substrate.
- The EIS results show that solvent-based coatings have better barrier properties than water-based coatings. The solvent-based coating SO exhibited the highest resistance, which is 101 Ωcm2 orders higher than water-based coating SE and 102 Ωcm2 orders higher than water-based coating TH.
- The DSC results show that sample RE achieves the highest glass transition temperature, measuring 100.86 °C, while sample TH exhibits the lowest temperature, measuring 26.72 °C.
- The properties and appearances of the coatings are more favorable in humid conditions and high-temperature environments, which align with the real-world application conditions for coating protection of wood-burning stoves.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bose, S. High Temperature Coatings; Butterworth-Heinemann: Oxford, UK, 2017. [Google Scholar]
- Lai, G.Y. High-Temperature Corrosion and Materials Applications. In High-Temperature Corrosion and Materials Applications; ASM International: Detroit, MI, USA, 2007; Volume 2. [Google Scholar]
- Zielecka, M.; Rabajczyk, A.; Cygańczuk, K.; Pastuszka, Ł.; Jurecki, L. Silicone Resin-Based Intumescent Paints. Materials 2020, 13, 4785. [Google Scholar] [CrossRef] [PubMed]
- Mévrel, R. State of the art on high-temperature corrosion-resistant coatings. Mater. Sci. Eng. A 1989, 120, 13–24. [Google Scholar] [CrossRef]
- Bellamy, G.T.; DiDomizio, M.J.; Patel, M.K.; McKinnon, M.B. Characterization of high-temperature paints for infrared thermography in fire research. Fire Saf. J. 2023, 137, 103775. [Google Scholar] [CrossRef]
- Chew, K.W.; Yahaya, A.H.; Arof, A.K. Studies on the properties of silicone resin films on mild steel. Pigment. Resin Technol. 2000, 29, 364–368. [Google Scholar] [CrossRef]
- Mathivanan, L.; Selvaraj, M.; Azim, S.S.; Balakrishnan, K. Evaluation of heat resistant properties of silicone-based coatings by SEM and a.c. Impedance techniques. Prog. Org. Coat. 1996, 28, 113–116. [Google Scholar] [CrossRef]
- Ramakrishnan, M. Epoxy High Temperature Coatings and Considerations. In Proceedings of the Corrosion 2017, New Orleans, LO, USA, 26–30 March 2017; NACE International: Houston, TX, USA, 2017; p. 9430. [Google Scholar]
- Mathivanan, L.; Arof, A.K. The degradation of silicone coatings in high-temperature atmospheres. Anti-Corros. Methods Mater. 1998, 45, 403–412. [Google Scholar] [CrossRef]
- Xu, J.; Long, J.; Zhang, R.; Du, J.; Guan, S.; Wang, Y.; Huang, L.; Wei, H.; Liu, L.; Huang, Y. Greatly improving thermal stability of silicone resins by modification with POSS. Polym. Degrad. Stab. 2020, 174, 109082. [Google Scholar] [CrossRef]
- Gao, H.; Xiong, Y.; Zhang, K.; Cao, S.; Hu, M.; Li, Y.; Zhang, P.; Liu, X. Molecular Dynamics Simulation of Interface Properties between Water-Based Inorganic Zinc Silicate Coating Modified by Organosilicone and Iron Substrate. J. Renew. Mater. 2023, 11, 1715–1729. [Google Scholar] [CrossRef]
- Alsamuraee, A.M.A.; Jaafer, I.H.; Ameen, A.H.; Abdullah, Q.A. Electrochemical impedance spectroscopic evaluation of cor-rosion protection properties of polyurethane/polyvinyl chloride blend coating on steel. Am. J. Sci. Res. 2011, 2, 761–768. [Google Scholar] [CrossRef]
- Stojanović, I.; Logar, M.; Fatović, I.; Alar, V.; Rakela-Ristevski, D. Experimental Study of Atmospherically and Infrared-Dried Industrial Topcoats. Coatings 2023, 13, 1343. [Google Scholar] [CrossRef]
- Chen, J.; Liu, J.; Wang, H.; Li, B.; Hu, Q.; Shao, T.; Yang, R.; Wang, B.; Wan, Q.; Li, Z.; et al. Experimental Study on Neutral Salt Spray Accelerated Corrosion of Metal Protective Coatings for Power-Transmission and Transformation Equipment. Coatings 2023, 13, 480. [Google Scholar] [CrossRef]
- Tsai, P.-Y.; Chen, T.-E.; Lee, Y.-L. Development and Characterization of Anticorrosion and Antifriction Properties for High Performance Polyurethane/Graphene Composite Coatings. Coatings 2018, 8, 250. [Google Scholar] [CrossRef]
- ISO 2409; Paints and Varnishes—Cross-Cut Test. International Organization for Standardization: Geneva, Switzerland, 2020.
- Aračić, S.; Krumes, S.; Matičević, T. Research into the possibility of using the new generation paint systems for the anticorrosion protection of steel structures. Tech. Gaz. 2009, 16, 75–79. [Google Scholar]
- ISO 6270; Paints and Varnishes—Determination of Resistance to Humidity—Part 1: Condensation (Single-Sided Exposure). International Organization for Standardization: Geneva, Switzerland, 2017.
- ISO 4628; Paints and Varnishes—Evaluation of Degradation of Coatings—Designation of Quantity and Size of Defects, and of Intensity of Uniform Changes in Appearance—Part 1: General Introduction and Designation System. International Organization for Standardization: Geneva, Switzerland, 2016.
- Barroso, G.; Li, Q.; Bordia, R.K.; Motz, G. Polymeric and ceramic silicon-based coatings—A review. J. Mater. Chem. A 2019, 7, 1936–1963. [Google Scholar] [CrossRef]
- Indumathy, B.; Sathiyanathan, P.; Prasad, G.; Reza, M.S.; Prabu, A.A.; Kim, H. A Comprehensive Review on Processing, Development and Applications of Organofunctional Silanes and Silane-Based Hyperbranched Polymers. Polymers 2023, 15, 2517. [Google Scholar] [CrossRef]
- Hoang, N.; Khoa, T.A.; Nhung, L.T.; Phuong, P.M.; Binh, T.D.; Hang, T.T.X.; Chi, N.V.; Nguyen, T.-D. Flake ZnAl Alloy as an Effective Pigment in Silicate Coatings for the Corrosion Protection of Steel. Coatings 2022, 12, 1046. [Google Scholar] [CrossRef]
- ISO 2808; Paints and Varnishes—Determination of Film Thickness. International Organization for Standardization: Geneva, Switzerland, 2019.
- ISO 9227; Corrosion Tests in Artificial Atmospheres—Salt Spray Tests. International Organization for Standardization: Geneva, Switzerland, 2022.
- ISO 12944-6; Paints and Varnishes—Corrosion Protection of Steel Structures by Protective Coating Systems—Part 6: Laboratory Performance Test Methods. International Organization for Standardization: Geneva, Switzerland, 2018.
- ISO 8501-1; Preparation of Steel Substrates before Application of Paints and Related Products—Visual Assessment of Surface Cleanliness—Part 1: Rust Grades and Preparation Grades of Uncoated Steel Substrates and of Steel Substrates after Overall Removal of Previous Coatings. International Organization for Standardization: Geneva, Switzerland, 2007.
- Kralj, M.; Pavković, K.; Stojanović, I.; Anđal, J. Adhesion and anticorrosive properties of DTM coating as related to primer coating. Građevinar 2018, 71, 401–408. [Google Scholar] [CrossRef]
- Draganovská, D.; Brezinová, J.; Guzanová, A. Surface Characterization after Blasting. In Tribology of Machine Elements; Pintaude, G., Cousseau, T., Rudawska, A., Eds.; IntechOpen: London, UK, 2022. [Google Scholar] [CrossRef]
- Lyon, S.B.; Bingham, R.; Mills, D.J. Advances in corrosion protection by organic coatings: What we know and what we would like to know. Prog. Org. Coat. 2017, 102, 2–7. [Google Scholar] [CrossRef]
- Ambat, R.; Piotrowska, K. Preventive measures for corrosion in electronics: Intrinsic and extrinsic strategies. In Humidity and Electronics; Ambat, R., Piotrowska, K., Eds.; Woodhead Publishing: Sawston, UK, 2022; pp. 285–337. [Google Scholar] [CrossRef]
- Stojanović, I.; Cindrić, I.; Turkalj, L.; Kurtela, M.; Rakela-Ristevski, D. Durability and Corrosion Properties of Waterborne Coating Systems on Mild Steel Dried under Atmospheric Conditions and by Infrared Radiation. Materials 2022, 15, 8001. [Google Scholar] [CrossRef]
- Šoić, I.; Martinez, S.; Dubravić, M. Gel-Electrolyte EIS setup used for probing of IR Dried/Cured industrial coatings. Prog. Org. Coat. 2019, 137, 105331. [Google Scholar] [CrossRef]
- Park, H.S.; Kim, S.R.; Park, H.J.; Kwak, C.; Hahm, H.S.; Kim, S.K. Preparation and Characterization Of Weather Resistant Silicone/Acrylic Resin Coatings. J. Coat. Technol. 2003, 75, 55–64. [Google Scholar] [CrossRef]




| Coating | Label | Base | Temperature Resistance, °C | Density kg/L | Wet Film Thickness, μm | Dry Film Thickness, μm | Volume Sold, % | Theoretical Spreading Rate |
|---|---|---|---|---|---|---|---|---|
| Solvalitt Black | SO | Silicone acrylic coating | 600 | 1.30 | 50–70 | 20–30 | 43 ± 2 | 21–14 m2/L |
| Thermodur 600 Aqua | TH | Water-based modified silicone resin | 600 | 1.29 | 53–107 | 15–30 | 28 | 11 m2/kg |
| Senotherm UHT 2K-Hydro 3590 | SE | Water-based | 600 | - | 47 | 25 | 53 | 9 m2/kg |
| Resist 78 | RE | Inorganic zinc ethyl silicate | 540 | 2.50 | 70–125 | 50–90 | 72 ± 2 | 14.4–8 m2/L |
| Testing Parameters | Standard | Testing Conditions |
|---|---|---|
| Testing Duration, hours | As agreed | 96 |
| Temperature, °C | 35 ± 2 | 35 ± 0.1 |
| Testing Chamber Volume, liters | min 400 | 450 |
| Air Humidifier Temperature, °C | 45–50 | 47 |
| Compressed Air Pressure, bar | 0.7–1.4 | 0.98 |
| Used Solution | NaCl | NaCl |
| Solution Concentration, % | 5 | 5 |
| Collected Condensate Amount, mL/80 cm2/h | 1.5 ± 0.5 | 2.0 |
| Condensate pH Value at 25 ± 2 °C | 6.5–7.2 | 7.1 |
| Conductivity of Distilled Water, µS/cm at 25 ± 2 °C | max 20 | <10 |
| Sandblasted Samples | ||||
|---|---|---|---|---|
| Sample | (µm) | Shore D Hardness | Cross-Cut | Test Type |
| SO-P1 | 21.2 | 93.6 | Salt chamber | |
| SO-P2 | 22.0 | 92.6 | Humidity chamber | |
| SO-P3 | 15.9 | 93.2 | 0 | Cyclic temperature |
| SE-P1 | 14.4 | 93.8 | Salt chamber | |
| SE-P2 | 15.3 | 94.6 | Humidity chamber | |
| SE-P3 | 17.1 | 94.2 | 0 | Cyclic temperature |
| TH-P1 | 10.9 | 91.6 | Salt chamber | |
| TH-P2 | 11.1 | 93.6 | Humidity chamber | |
| TH-P3 | 11.3 | 92.4 | 0 | Cyclic temperature |
| RE-P1 | 33.9 | 93.8 | Salt chamber | |
| RE-P2 | 31.4 | 93.6 | Humidity chamber | |
| RE-P3 | 31.2 | 93.1 | 0 | Cyclic temperature |
| Shot-Blasted Samples | ||||
|---|---|---|---|---|
| Sample | (µm) | Shore D Hardness | Cross-Cut | Test Type |
| SO-S1 | 26.7 | 93.6 | Salt chamber | |
| SO-S2 | 24.0 | 92.4 | Humidity chamber | |
| SO-S3 | 20.3 | 93.3 | 1 | Cyclic temperature |
| SE-S1 | 17.1 | 93.6 | Salt chamber | |
| SE-S2 | 12.0 | 94.6 | Humidity chamber | |
| SE-S3 | 13.3 | 93.2 | 1 | Cyclic temperature |
| TH-S1 | 10.3 | 92.0 | Salt chamber | |
| TH-S2 | 11.2 | 93.8 | Humidity chamber | |
| TH-S3 | 10.3 | 92.1 | 0 | Cyclic temperature |
| RE-S1 | 27.6 | 93.8 | Salt chamber | |
| RE-S2 | 33.8 | 93.8 | Humidity chamber | |
| RE-S3 | 27.8 | 93.6 | 1 | Cyclic temperature |
| Surface Preparation | Shot-Blasted (S) | Sandblasted (P) | ||
|---|---|---|---|---|
| Coating | Sample Appearance | Grade | Sample Appearance | Grade |
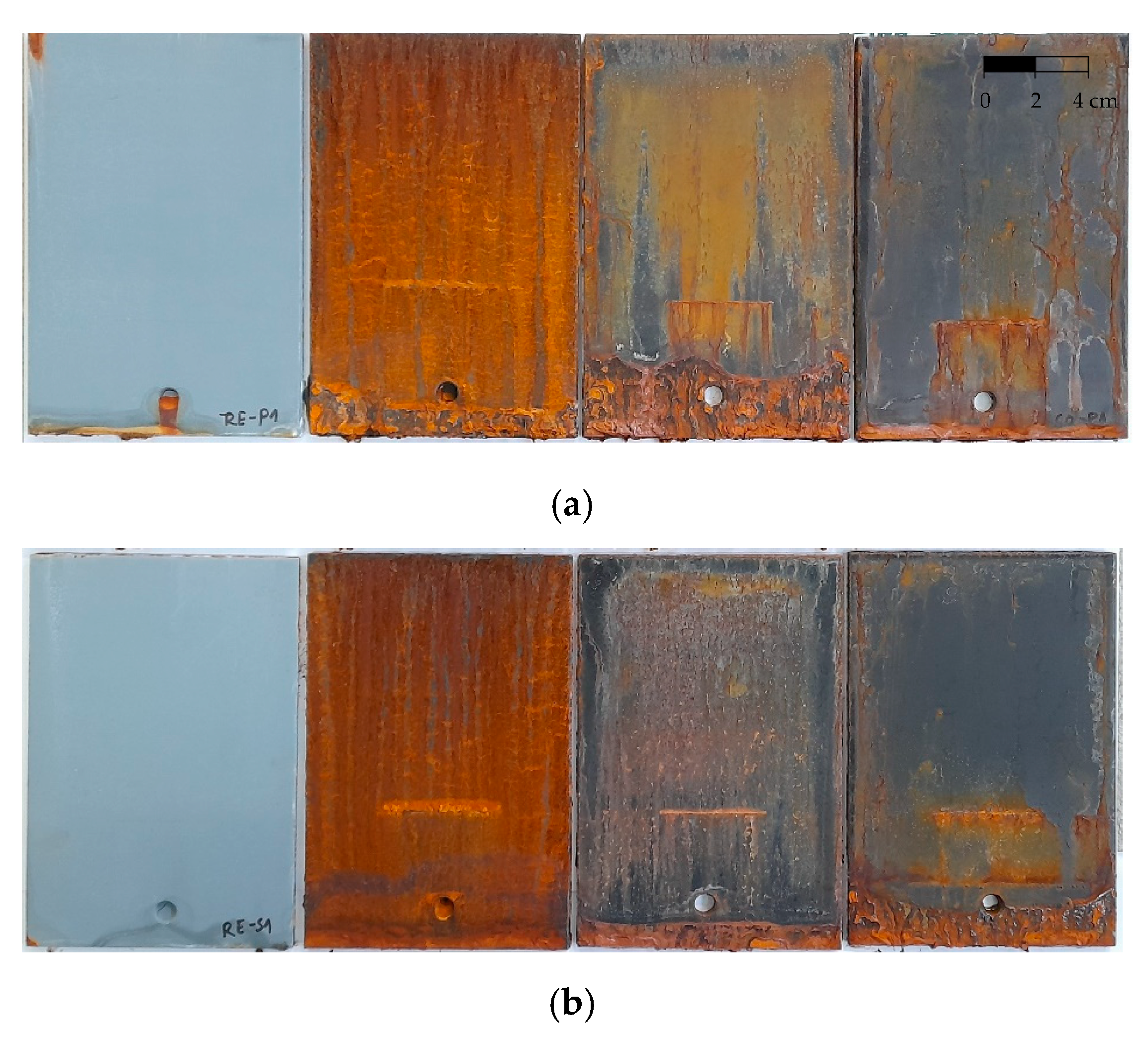
| SO |  | 5 |  | 3 |
| SE |  | 5 |  | 1 |
| TH |  | 3 |  | 1 |
| RE |  | 2 |  | 0 |
| Surface Preparation | Shot-Blasted (S) | Sandblasted (P) | ||
|---|---|---|---|---|
| Coating | Sample Appearance | Grade | Sample Appearance | Grade |
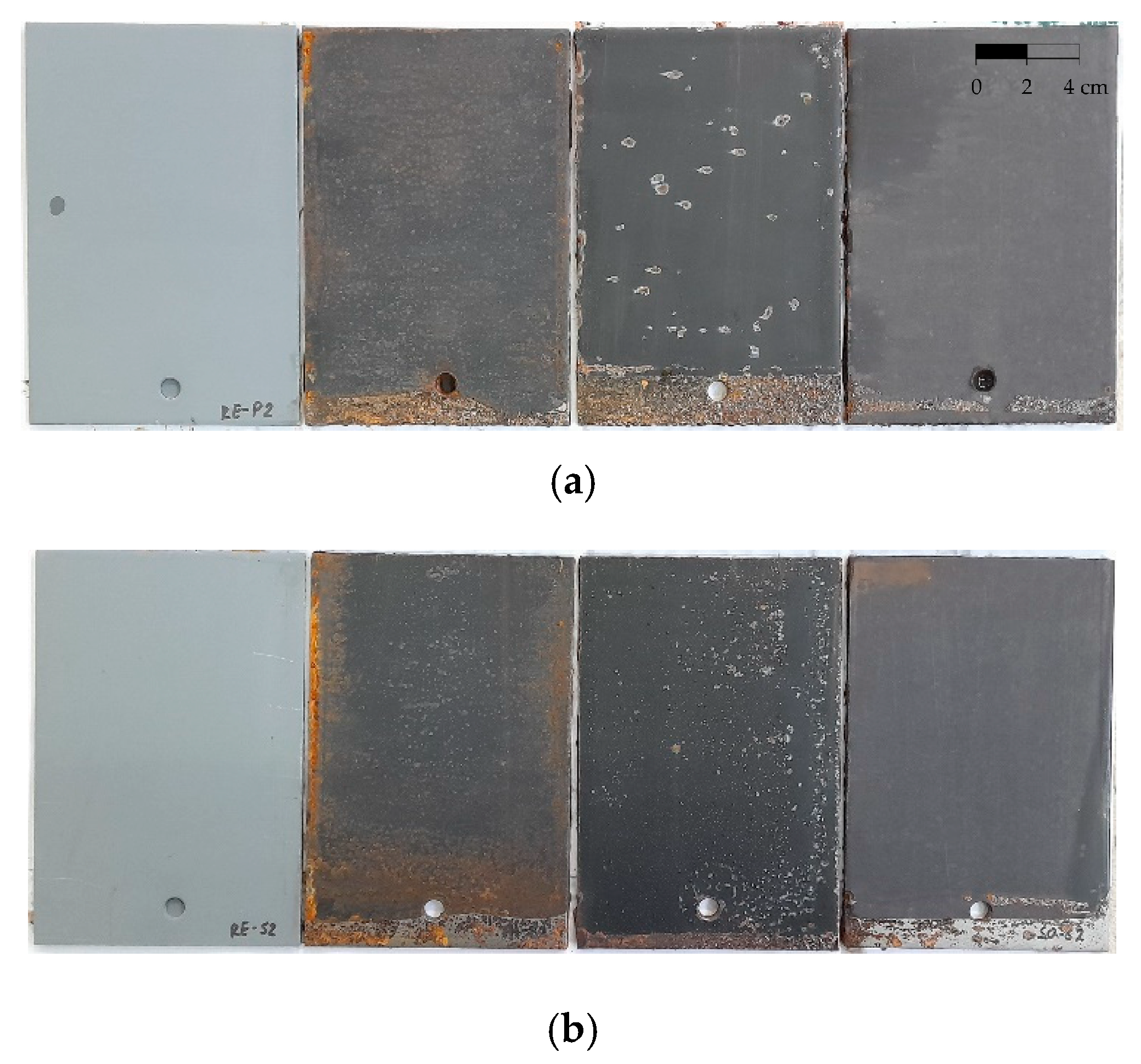
| SO |  | 1 |  | 1 |
| SE |  | 5 |  | 0 |
| TH |  | 3 |  | 0 |
| RE |  | 1 |  | 1 |
| Sample | ISO 4628-2 | ISO 4628-3 | ISO 4628-4 | ISO 4628-8 |
|---|---|---|---|---|
| Blistering | Rusting | Cracking | Corrosion around the Scribe, mm | |
| SO-P1 | 0(S0) | Ri 2 | 0(S0) | 0.134 |
| SO-S1 | 2(S3) | Ri 1 | 0(S0) | 0.100 |
| SE-P1 | 3(S2) | Ri 2 | 0(S0) | 0.192 |
| SE-S1 | 3(S3) | Ri 4 | 0(S0) | 0.165 |
| TH-P1 | 3(S2) | Ri 5 | 0(S0) | 0.150 |
| TH-S1 | 3(S2) | Ri 5 | 0(S0) | 0.175 |
| RE-P1 | 0(S0) | Ri 0 | 0(S0) | 0 |
| RE-S1 | 0(S0) | Ri 0 | 0(S0) | 0 |
| Uzorak | ISO 4628-2 | ISO 4628-3 | ISO 4628-4 |
|---|---|---|---|
| Blistering | Rusting | Cracking | |
| SO-P2 | 0(S0) | Ri 0 | 0(S0) |
| SO-S2 | 0(S0) | Ri 0 | 0(S0) |
| SE-P2 | 0(S0) | Ri 1 | 0(S0) |
| SE-S2 | 3(S2) | Ri 1 | 0(S0) |
| TH-P2 | 4(S2) | Ri 1 | 0(S0) |
| TH-S2 | 3(S2) | Ri 1 | 0(S0) |
| RE-P2 | 0(S0) | Ri 0 | 0(S0) |
| RE-S2 | 0(S0) | Ri 0 | 0(S0) |
| Surface Preparation | Shot-Blasted | Sandblasted | ||
|---|---|---|---|---|
| Coating | Sample Appearance | Grade | Sample Appearance | Grade |
| Solvalit ATM (SO-S3, SO-P3) |  | 0 |  | 0 |
| Senoterm UHT 2K Hydro ATM (SE-S3, SE-P3) |  | 2 |  | 0 |
| Thermodur 600 Aqua ATM (TH-S3, TH-P3) |  | 0 |  | 0 |
| Resist 78 (RE-S3, RE-P3) |  | 1 |  | 0 |
| Sample | RS, (102 Ωcm2) | nc | CPEC, (10−6 Fcm2) | RC, (102 Ωcm2) | ndl | Cdl/CPEdl, (10−7 Fcm2) | Rct, (102 Ωcm2) | W (10−4 Ωcm2s1/2) | DFT (µm) |
|---|---|---|---|---|---|---|---|---|---|
| SO | 71.49 | 0.478 | 2.763 | 9.380 | - | 0.169 | 398.0 | - | 28.0 |
| SE | 18.07 | - | 0.044 | 56.65 | 0.70 | 151.5 | 69.36 | 3.098 | 29.8 |
| TH | 0.136 | 0.409 | 0.059 | 0.872 | 0.78 | 1239 | 3.328 | - | 30.2 |
| RE | 1.133 | 0.800 | 84.03 | 11.39 | - | 8419 | 13.43 | - | 39.1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Stojanović, I.; Škrlec, B.; Kurtela, M.; Alar, V.; Odeljan, M. Physical and Chemical Properties of High-Temperature Silicone-Based Polymer Coatings Applied on Different Surface Roughnesses. Coatings 2023, 13, 2100. https://doi.org/10.3390/coatings13122100
Stojanović I, Škrlec B, Kurtela M, Alar V, Odeljan M. Physical and Chemical Properties of High-Temperature Silicone-Based Polymer Coatings Applied on Different Surface Roughnesses. Coatings. 2023; 13(12):2100. https://doi.org/10.3390/coatings13122100
Chicago/Turabian StyleStojanović, Ivan, Borna Škrlec, Marin Kurtela, Vesna Alar, and Martina Odeljan. 2023. "Physical and Chemical Properties of High-Temperature Silicone-Based Polymer Coatings Applied on Different Surface Roughnesses" Coatings 13, no. 12: 2100. https://doi.org/10.3390/coatings13122100
APA StyleStojanović, I., Škrlec, B., Kurtela, M., Alar, V., & Odeljan, M. (2023). Physical and Chemical Properties of High-Temperature Silicone-Based Polymer Coatings Applied on Different Surface Roughnesses. Coatings, 13(12), 2100. https://doi.org/10.3390/coatings13122100








