A Review of Corrosion-Resistant PEO Coating on Mg Alloy
Abstract
:1. Introduction
2. Growth and Corrosion Process of PEO Coating on Mg Alloy
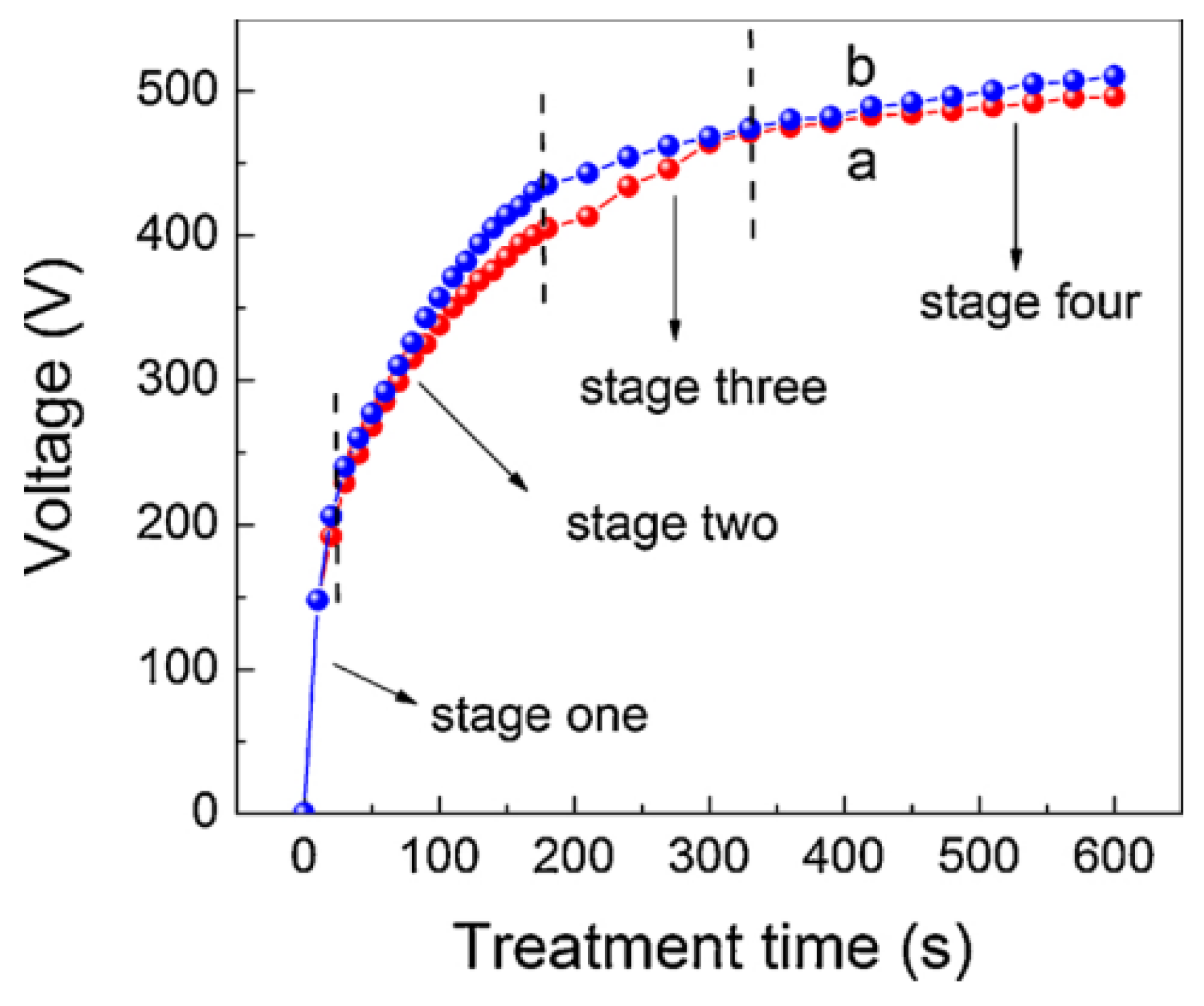
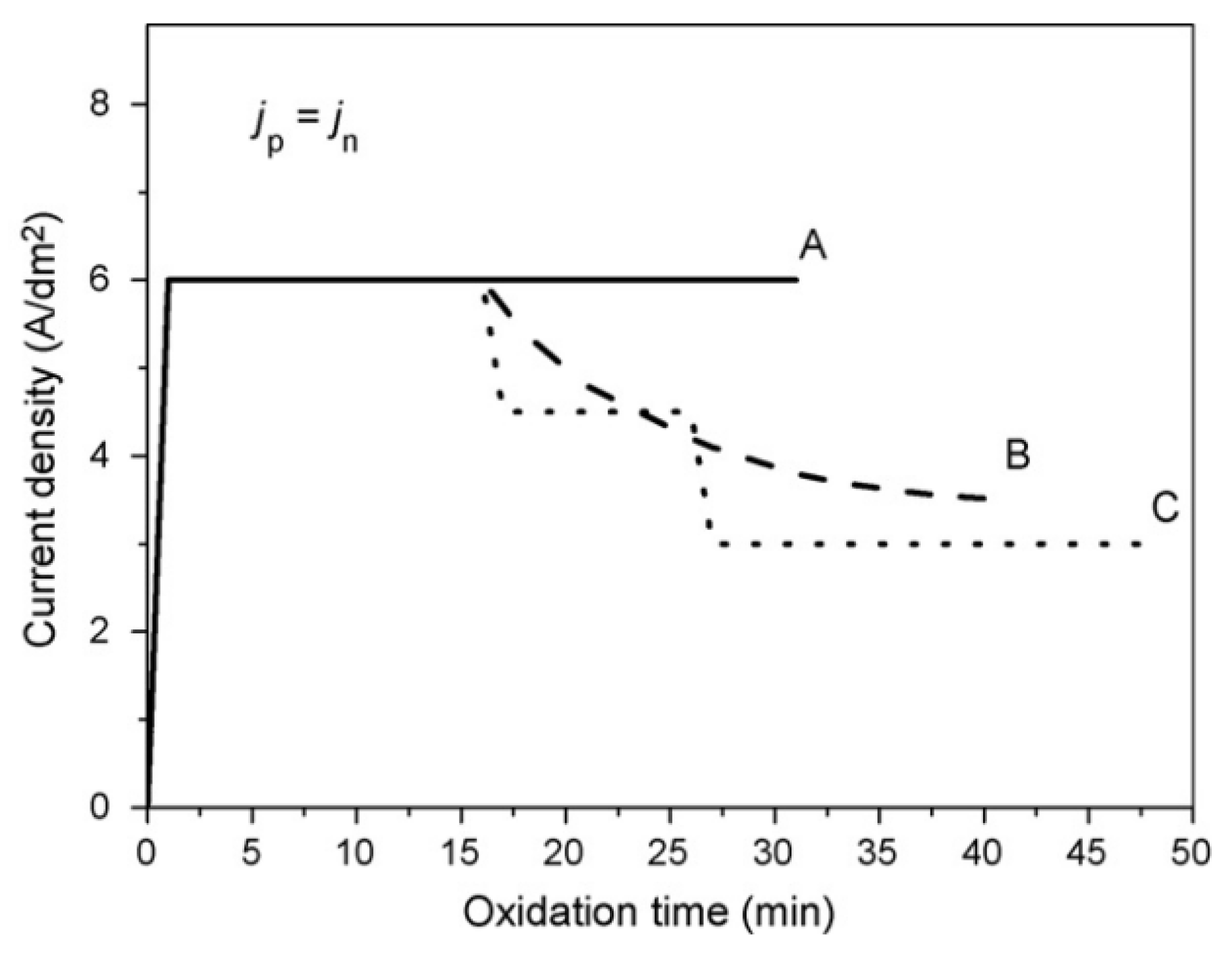
2.1. Growth of PEO Coating
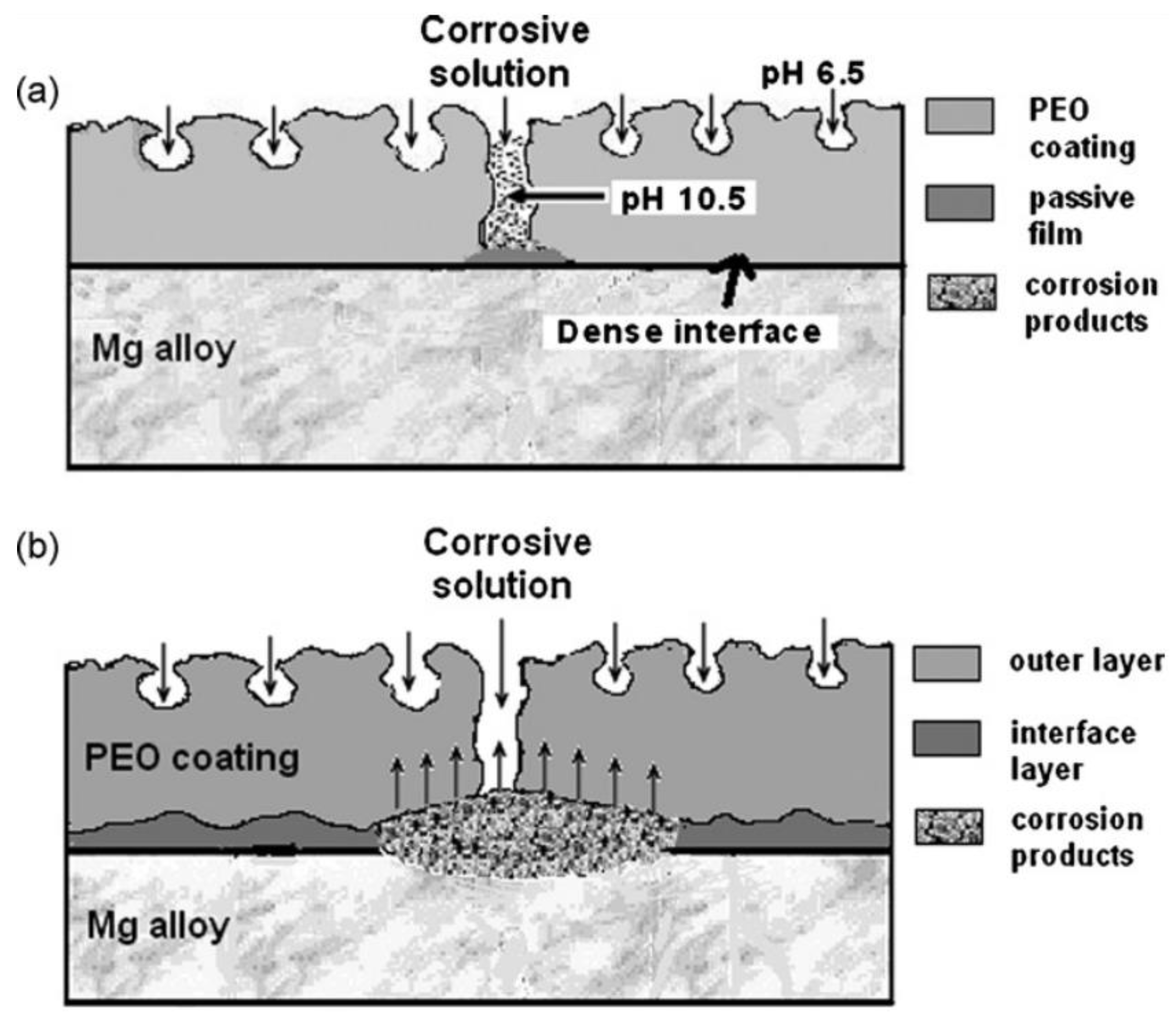
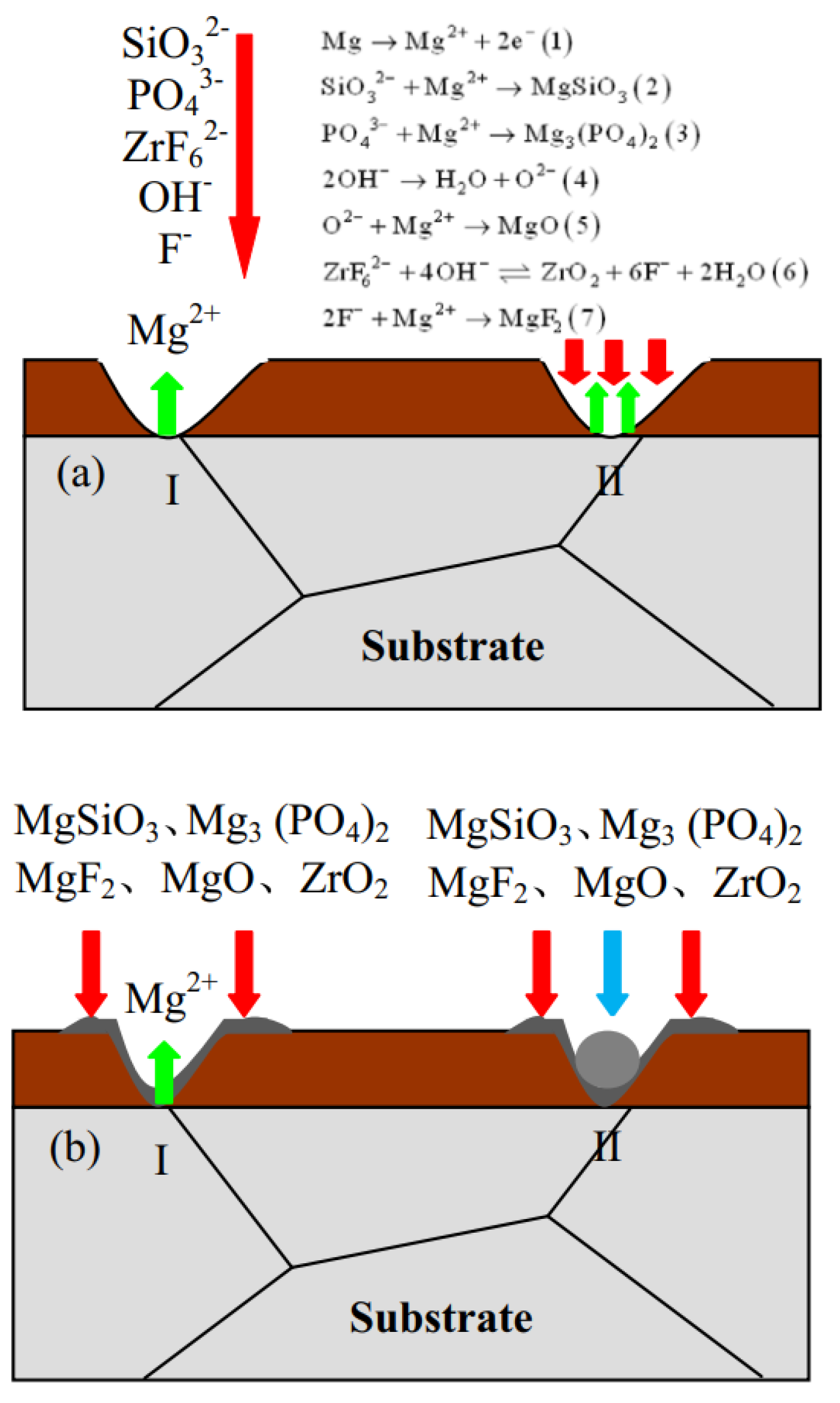
2.2. Corrosion of PEO Coating
3. Improvement of the Coating Structure
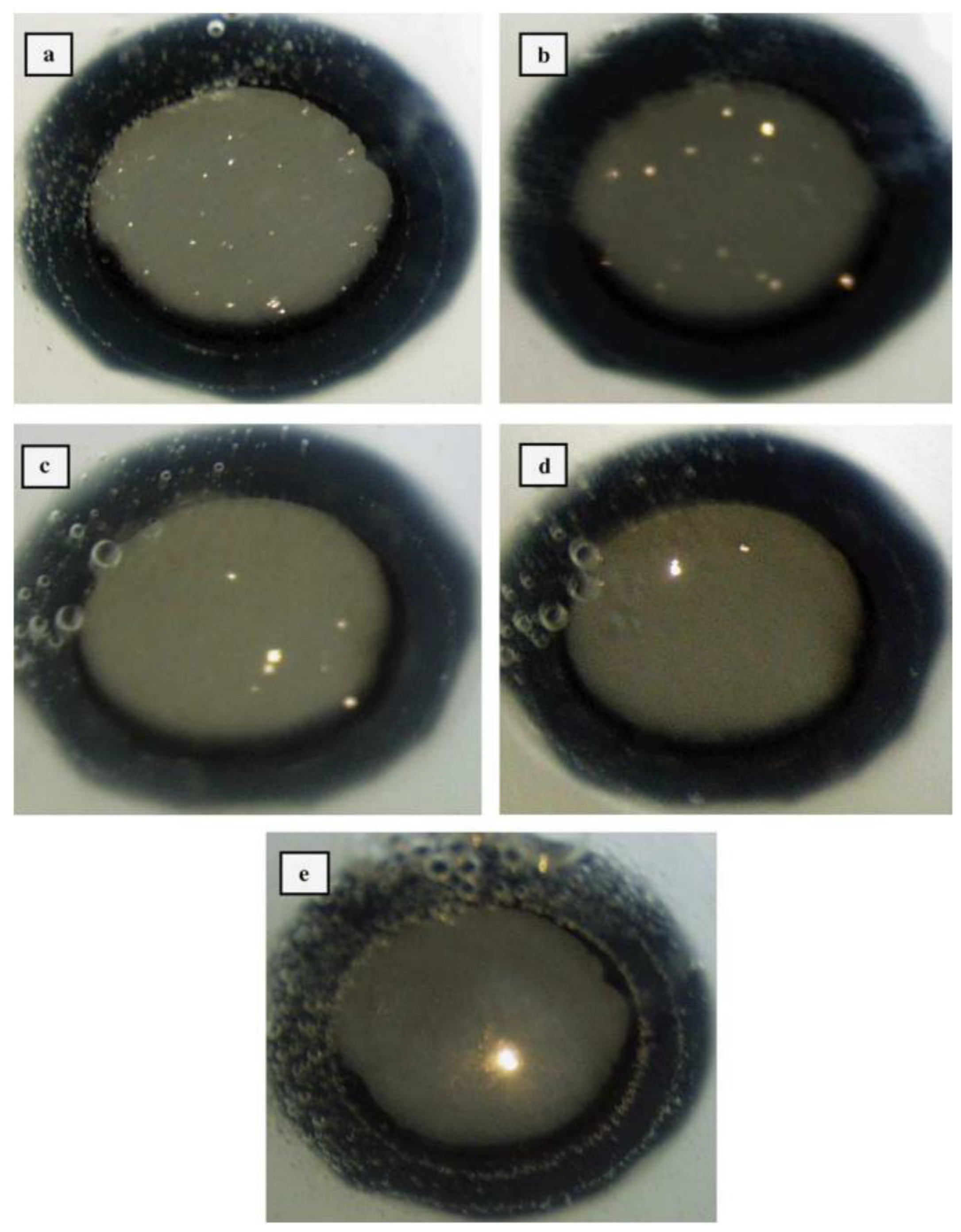
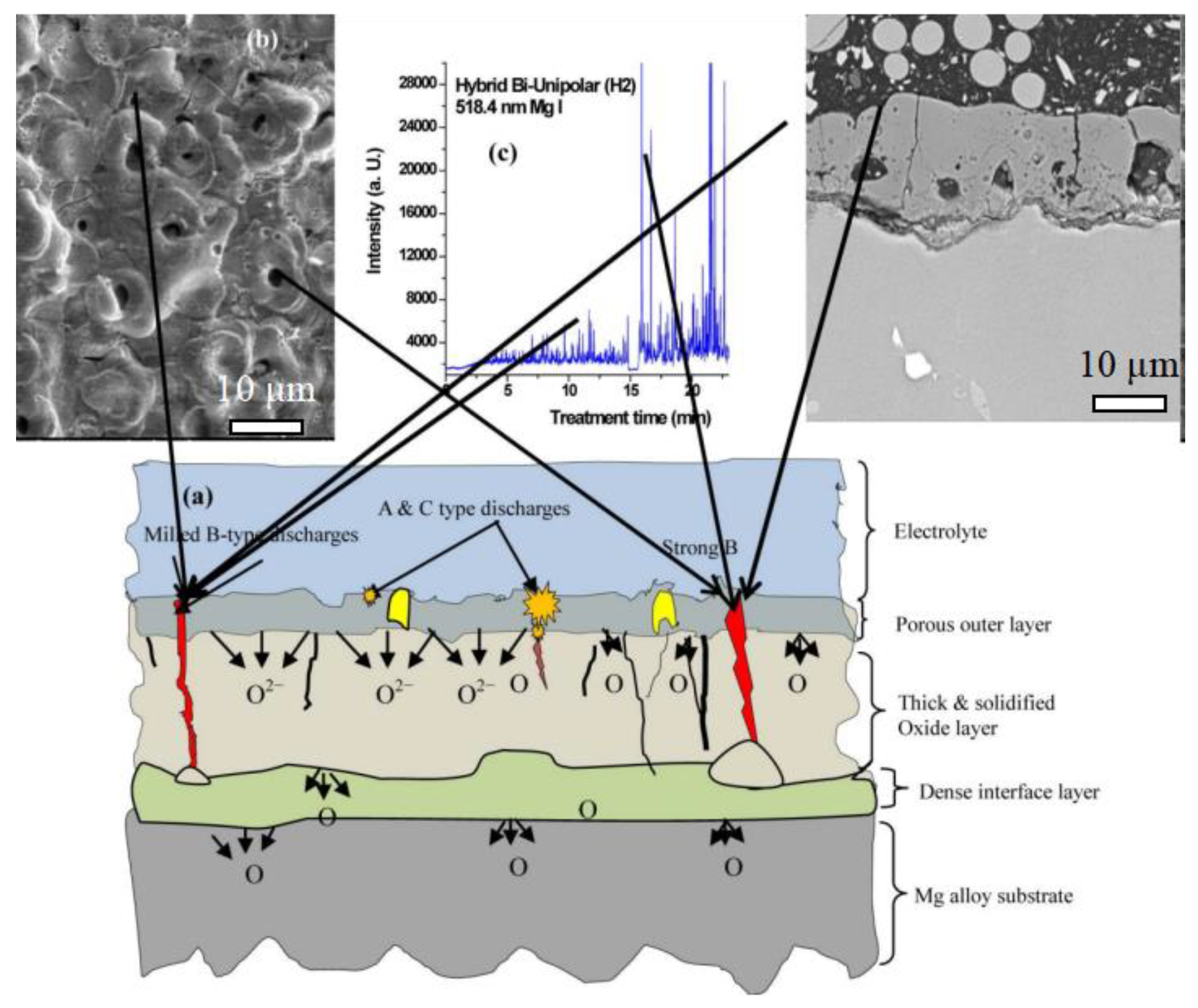
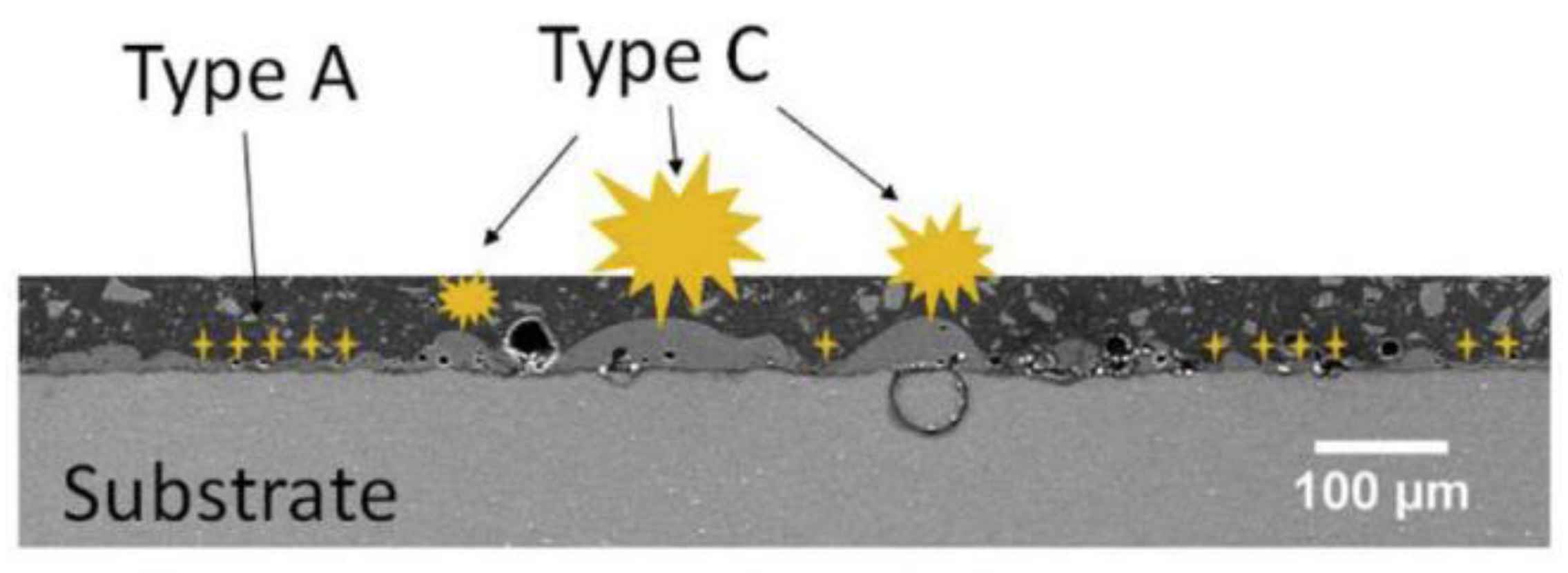
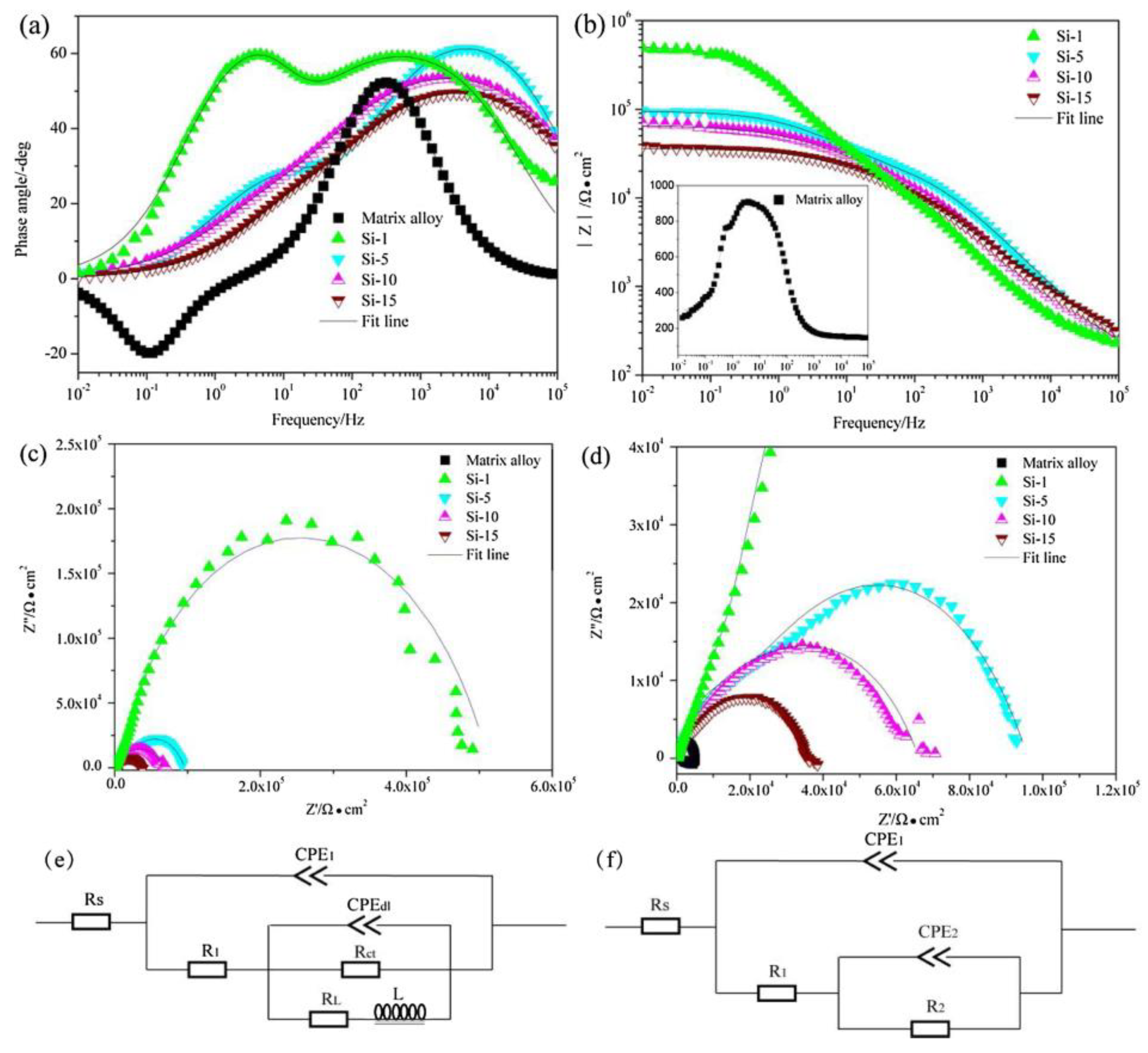
3.1. The Influence of Discharge Behavior on the Coating Structure
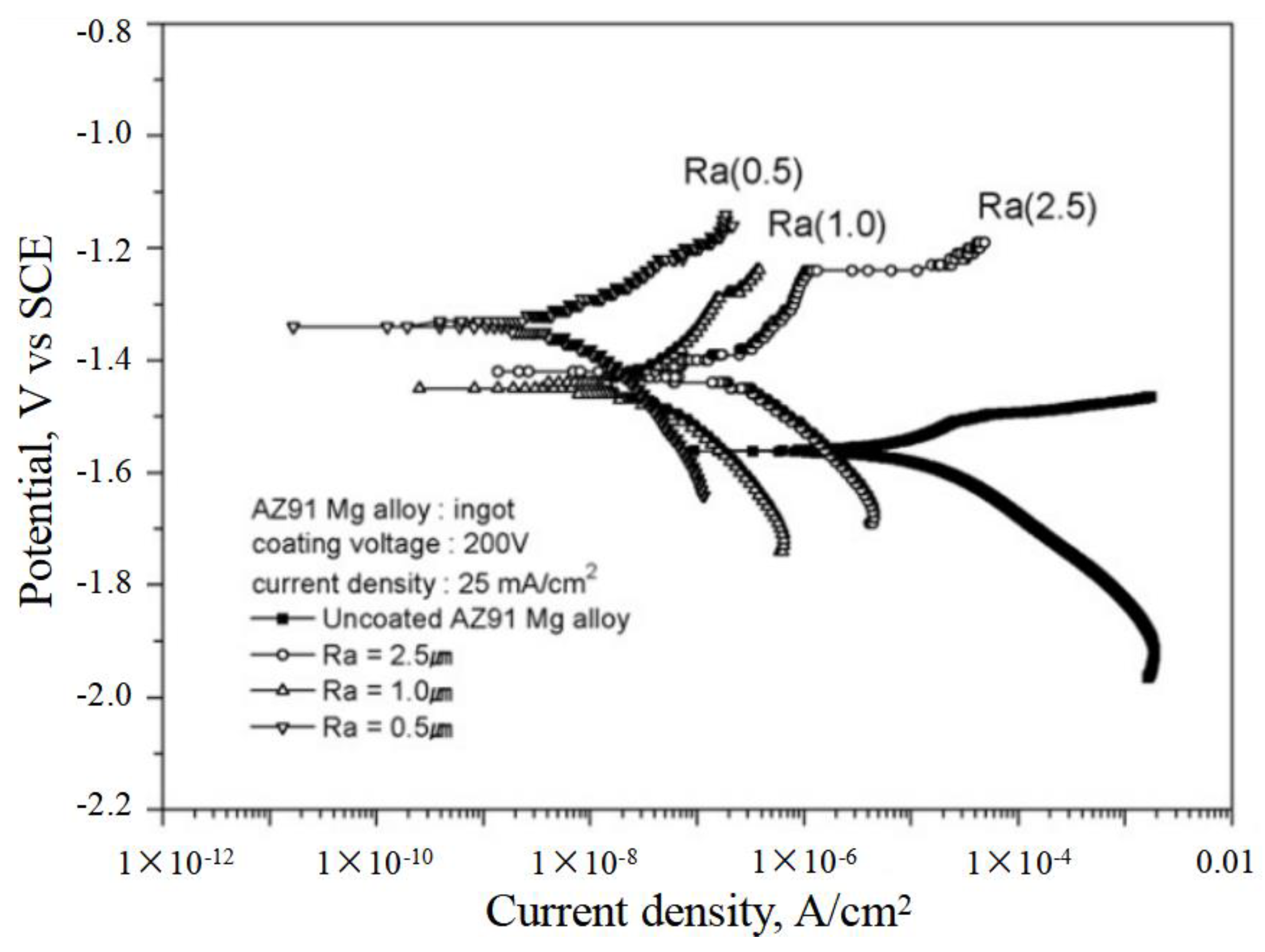
3.2. The Influence of Coating Roughness on the Corrosion
3.3. The Influence of Coating Thickness on Corrosion
3.4. The Influence of Coating Density on Corrosion
3.5. The Influence of Coating Micropores and Defects on Corrosion
4. Improvement of the Coating Phase Composition
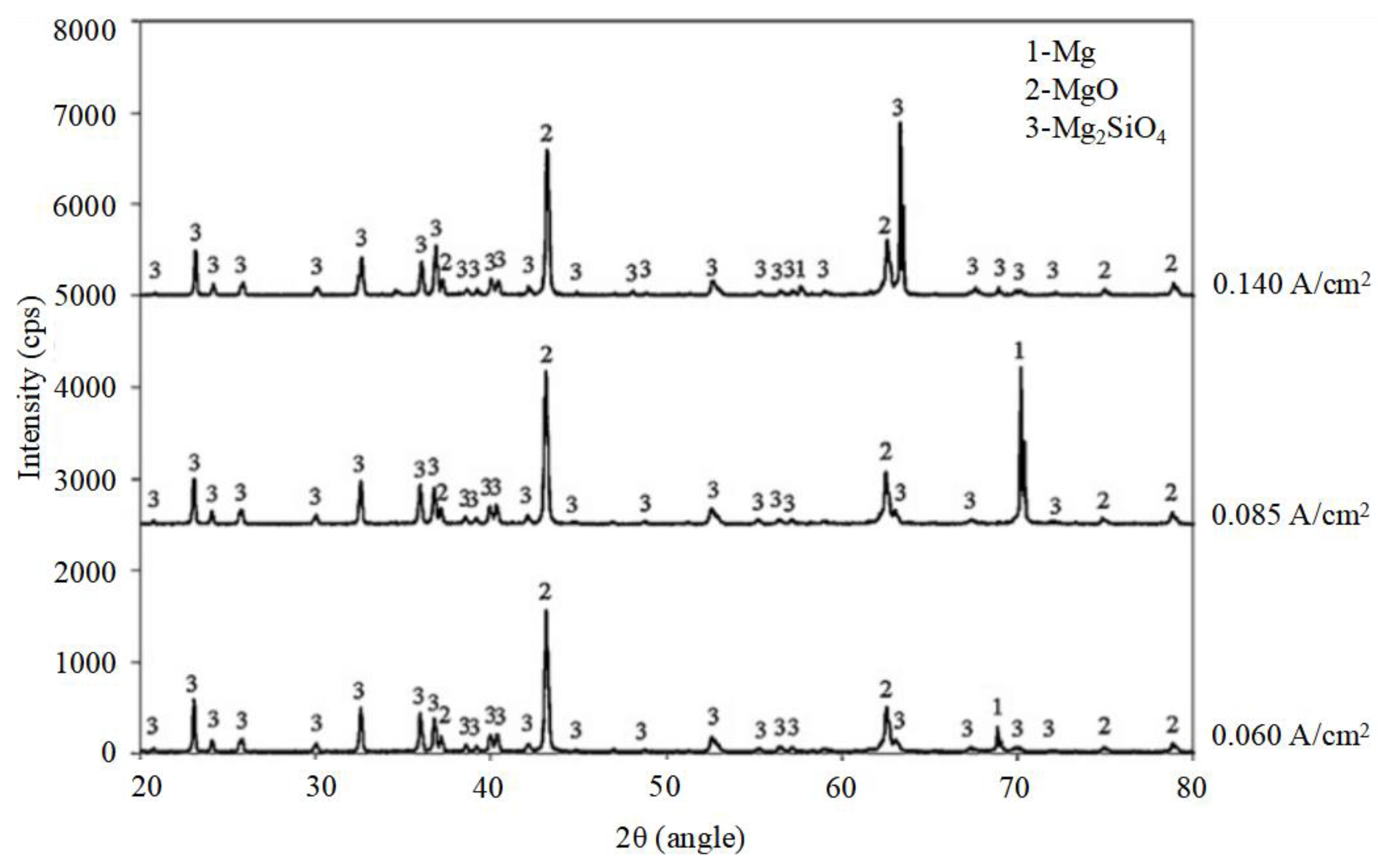
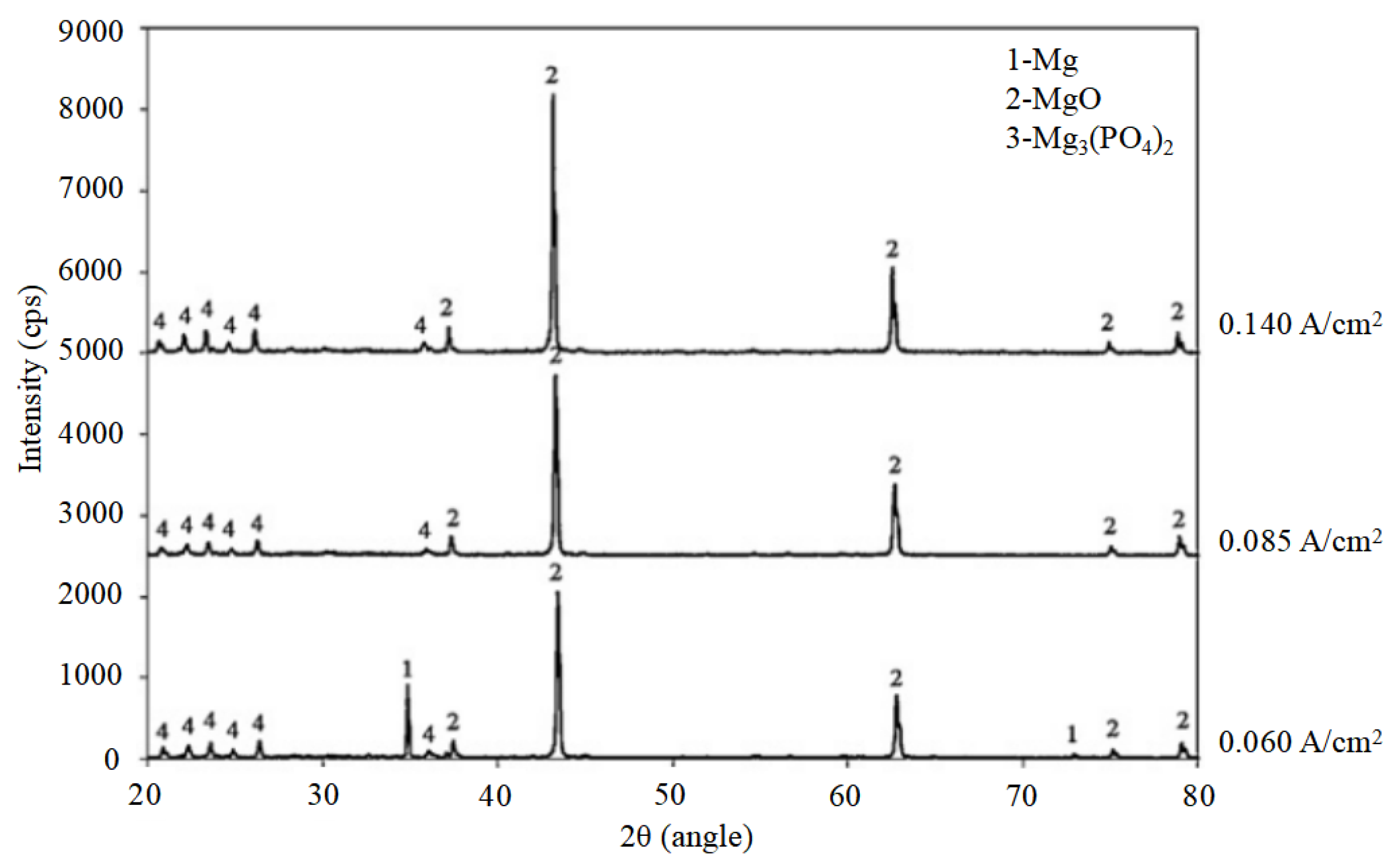
4.1. The Influence of PEO Electrolyte on Corrosion
4.2. The Influence of Added Compounds on Corrosion
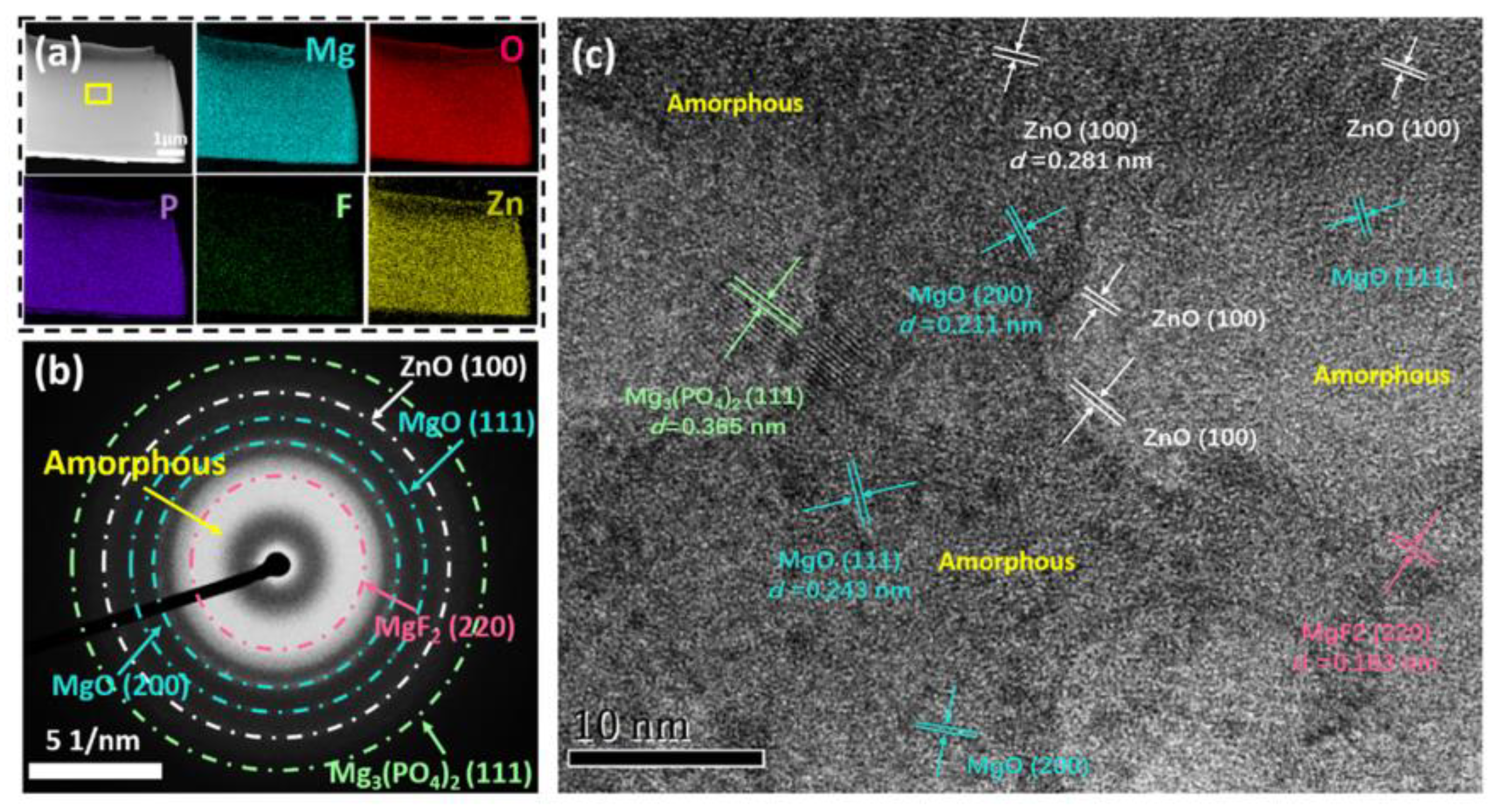
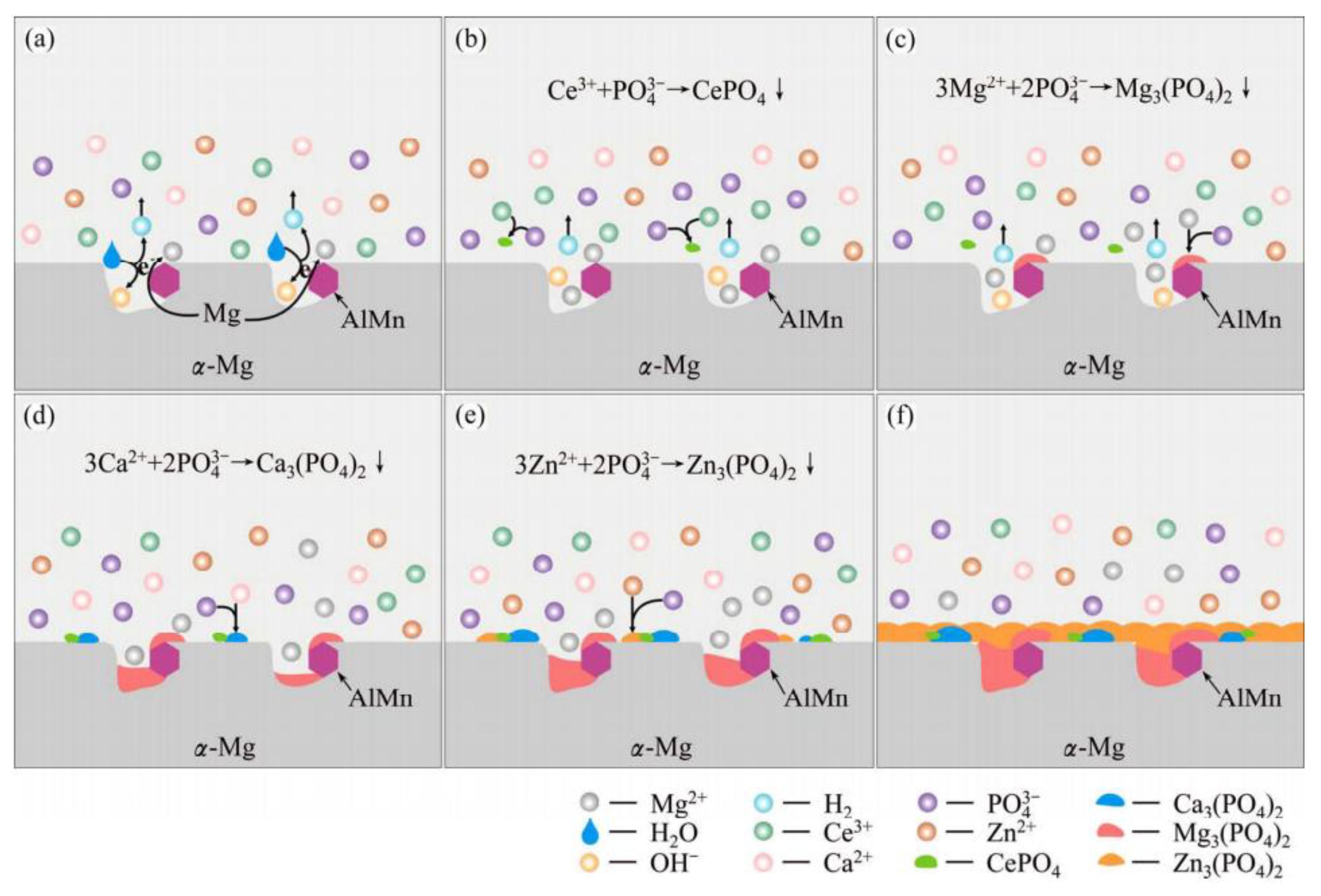
4.3. The Influence of Self-Growing Insoluble Phases on the Corrosion
5. Prospect
- (1)
- PEO mechanism. PEO technology is a complex and comprehensive technology that involves various disciplines such as chemistry, electrochemistry, plasma chemistry, and thermochemistry. While researchers have proposed some discharge processes and film-forming mechanisms of PEO technology, there are limitations, and the specific discharge and film-forming processes of PEO still cannot be clearly explained. Therefore, it is necessary to analyze and improve the theoretical model of PEO from an energy perspective, combining thermodynamic and kinetic theories, as well as advanced spectroscopic methods, in order to better design corrosion-resistant coatings.
- (2)
- Electrolyte and electrical parameters. The selection of electrolyte directly affects the coating growth process on the Mg alloy. For example, phosphate system coatings grow inward, while silicate coatings maintain outward growth. Additives also affect the discharge process, resulting in coatings with different characteristics. The choice of electrical parameters affects the strength of discharge and the speed of film formation. Therefore, it is necessary to establish a large database of PEO coatings on Mg alloy and continuously share research theories and results of existing electrolytes and electrical parameters to improve the reference value of relevant data and refine more effective electrolyte systems for preparing corrosion-resistant coatings.
- (3)
- Power supply and energy consumption. Coatings prepared using bipolar pulse power sources are believed to be uniform and dense, resulting in optimal coating performance. Additionally, they can reduce the energy consumption of PEO processes. However, achieving uniform preparation of large samples is still a challenge, with the current maximum treatment area being only 4 m2. To meet the requirements of preparing uniform corrosion-resistant coatings on a large scale, it is necessary to design and develop an efficient, energy-saving, high-power power supply system suitable for large-area processing.
- (4)
- Strengthening of corrosion-resistant coatings. Various enhancement methods, including improving the crystallinity, density, thickness, roughness, micropores, and cracks of coatings, as well as the study of corrosion-resistant phases, are widely researched. There is relatively little research on the preparation of insoluble metal salts on Mg alloys, such as zinc phosphate and calcium phosphate, which have extremely low solubility product constants. It is necessary to uniformly mix these insoluble metal salts in PEO coatings on Mg alloys to significantly improve the corrosion resistance.
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Parande, G.; Manakari, V.; Sharma Kopparthy, S.D.; Gupta, M. A study on the effect of low-cost eggshell reinforcement on the immersion, damping and mechanical properties of Mg–zinc alloy. Compos. Part B Eng. 2020, 182, 107650. [Google Scholar] [CrossRef]
- Srikanth, N.; Zhong, X.L.; Gupta, M. Enhancing damping of pure Mg using nano-size alumina particulates Materials. Letters 2005, 59, 3851–3855. [Google Scholar]
- Hamid, Z.A.; El-khair, M.T.A.; Hassan, H.B. Synthesis and protection of AM50 Mg alloy and its composites using environmentally pretreatment electrolyte. Surf. Coat. Technol. 2011, 206, 1041–1050. [Google Scholar] [CrossRef]
- Shao, Z.; Cai, Z.; Hu, R.; Wei, S. The study of electroless nickel plating directly on Mg alloy. Surf. Coat. Technol. 2014, 249, 42–47. [Google Scholar] [CrossRef]
- Ji, R.; Peng, G.; Zhang, S.; Li, Z.; Li, J.; Fang, T.; Zhang, Z.; Wang, Y.; He, Y.; Wu, J. The fabrication of a CeO2 coating via cathode plasma electrolytic deposition for the corrosion resistance of AZ31 Mg alloy. Ceram. Int. 2018, 44, 19885–19891. [Google Scholar] [CrossRef]
- Ono, S.; Moronuki, S.; Mori, Y.; Koshi, A.; Liao, J.; Asoh, H. Effect of Electrolyte Concentration on the Structure and Corrosion Resistance of Anodic Films Formed on Mg through Plasma Electrolytic Oxidation. Electrochim. Acta 2017, 240, 415–423. [Google Scholar] [CrossRef]
- Kuo, Y.-L.; Chang, K.-H. Atmospheric pressure plasma enhanced chemical vapor deposition of SiOx films for improved corrosion resistant properties of AZ31 Mg alloys. Surf. Coat. Technol. 2015, 283, 194–200. [Google Scholar] [CrossRef]
- Saei, E.; Ramezanzadeh, B.; Amini, R.; Kalajahi, M.S. Effects of combined organic and inorganic corrosion inhibitors on the nanostructure cerium based conversion coating performance on AZ31 Mg alloy: Morphological and corrosion studies. Corros. Sci. 2017, 127, 186–200. [Google Scholar] [CrossRef]
- Huang, Q.; Liu, L.; Wu, Z.; Ji, S.; Chu, P.K. Corrosion-resistant plasma electrolytic oxidation coating modified by Zinc phosphate and self-healing mechanism in the salt-spray environment. Surf. Coat. Technol. 2020, 384, 125321. [Google Scholar] [CrossRef]
- Luo, D.; Liu, Y.; Yin, X.; Wang, H.; Han, Z.; Ren, L. Corrosion inhibition of hydrophobic coatings fabricated by micro-arc oxidation on an extruded Mge5Sne1Zn alloy substrate. J. Alloys Compd. 2018, 731, 731–738. [Google Scholar] [CrossRef]
- Li, Z.; Ren, Q.; Wang, X.; Kuang, Q.; Ji, D.; Yuan, R.; Jing, X. Effect of phosphate additive on the morphology and anti-corrosion performance of plasma electrolytic oxidation coatings on Mg-lithium alloy. Corros. Sci. 2019, 157, 295–304. [Google Scholar] [CrossRef]
- Verdier, S.; Boinet, M.; Maximovitch, S.; Dalard, F. Formation, structure and composition of anodic films on AM60 Mg alloy obtained by DC plasma anodizing. Corros. Sci. 2005, 47, 1429–1444. [Google Scholar] [CrossRef]
- Durdu, S.; Aytaç, A.; Usta, M. Characterization and corrosion behavior of ceramic coating on Mg by micro-arc oxidation. J. Alloys Compd. 2011, 509, 8601–8606. [Google Scholar] [CrossRef]
- Liu, X.Y.; Luan, B.L. Corrosion and wear properties of PEO coatings formed on AM60B alloy in NaAlO2 electrolytes. Appl. Surf. Sci. 2011, 257, 9135–9141. [Google Scholar] [CrossRef]
- Duan, H.; Yan, C.; Wang, F. Effect of electrolyte additives on performance of plasma electrolytic oxidation films formed on Mg alloy AZ91D. Electrochim. Acta 2007, 52, 3785–3793. [Google Scholar] [CrossRef]
- Gu, Y.; Bandopadhyay, S.; Chen, C.-F.; Guo, Y.; Ning, C. Effect of oxidation time on the corrosion behavior of micro-arc oxidation produced AZ31 Mg alloys in simulated body fluid. J. Alloys Compd. 2012, 543, 109–117. [Google Scholar] [CrossRef]
- Zhang, X.; Zhang, Y.; Lv, Y.; Dong, Z.; Hashimoto, T.; Zhou, X. Enhanced corrosion resistance of AZ31 Mg alloy by one-step formation of PEO/Mg-Al LDH composite coating. Corros. Commun. 2022, 6, 67–83. [Google Scholar] [CrossRef]
- Guo, H.X.; Ying, M.A.; Wang, J.S.; Wang, Y.S.; Hao, Y. Corrosion behavior of micro-arc oxidation coating on AZ91D Mg alloy in NaCl solutions with different concentrations. Trans. Nonferrous Met. Soc. China 2012, 22, 1786–1793. [Google Scholar] [CrossRef]
- Liang, J.; Srinivasan, P.B.; Blawert, C.; Störmer, M.; Dietzel, W. Electrochemical corrosion behaviour of plasma electrolytic oxidation coatings on AM50 Mg alloy formed in silicate and phosphate based electrolytes. Electrochim. Acta 2009, 54, 3842–3850. [Google Scholar] [CrossRef]
- Hussein, R.; Northwood, D.; Nie, X. Processing-Microstructure Relationships in the Plasma Electrolytic Oxidation (PEO) Coating of a Magnesium Alloy. Mater. Sci. Appl. 2014, 5, 124–139. [Google Scholar] [CrossRef]
- Dehnavi, V.; Binns, W.J.; Noël, J.J.; Shoesmith, D.W.; Luan, B.L. Growth behaviour of low-energy plasma electrolytic oxidation coatings on a Magnesium alloy. J. Magnes. Alloys 2018, 6, 229–237. [Google Scholar] [CrossRef]
- Hussein, R.O.; Nie, X.; Northwood, D.O. A Yerokhin and A Matthews, Spectroscopic study of electrolytic plasma and discharging behaviour during the plasma electrolytic oxidation (PEO) process. J. Phys. D Appl. Phys. 2010, 43, 105203. [Google Scholar] [CrossRef]
- Krishna, L.R.; Poshal, G.; Jyothirmayi, A.; Sundararajan, G. Relative hardness and corrosion behavior of micro arc oxidation coatings deposited on binary and ternary Mg alloys. Mater. Des. 2015, 77, 6–14. [Google Scholar] [CrossRef]
- Rapheal, G.; Kumar, S.; Scharnagl, N.; Blawert, C. Effect of current density on the microstructure and corrosion properties of plasma electrolytic oxidation (PEO) coatings on AM50 Mg alloy produced in an electrolyte containing clay additives. Surf. Coat. Technol. 2016, 289, 150–164. [Google Scholar] [CrossRef]
- Wu, D.; Liu, X.; Lu, K.; Zhang, Y.; Wang, H. Influence of C3H8O3 in the electrolyte on characteristics and corrosion resistance of the microarc oxidation coatings formed on AZ91D Mg alloy surface. Appl. Surf. Sci. 2009, 255, 7115–7120. [Google Scholar] [CrossRef]
- Kazanski, B.; Kossenko, A.; Zinigrad, M.; Lugovskoy, A. Fluoride ions as modifiers of the oxide layer produced by plasma electrolytic oxidation on AZ91D Mg alloy. Appl. Surf. Sci. 2013, 287, 461–466. [Google Scholar] [CrossRef]
- Liu, F.; Shan, D.; Song, Y.; Han, E.-H. Effect of additives on the properties of plasma electrolytic oxidation coatings formed on AM50 Mg alloy in electrolytes containing K2ZrF6. Surf. Coat. Technol. 2011, 206, 455–463. [Google Scholar] [CrossRef]
- Yoo, B.; Shin, K.R.; Hwang, D.Y.; Dong, H.L.; Dong, H.S. Effect of surface roughness on leakage current and corrosion resistance of oxide layer on AZ91 Mg alloy prepared by plasma electrolytic oxidation. Appl. Surf. Sci. 2010, 256, 6667–6672. [Google Scholar] [CrossRef]
- Yang, C.; Sheng, L.Y.; Zhao, C.C.; Wu, D.; Zheng, Y.F. Regulating the ablation of nanoparticle-doped MAO coating on Mg alloy by MgF2 passivation layer construction. Mater. Lett. 2024, 355, 135559. [Google Scholar] [CrossRef]
- Chen, D.; Wang, R.; Huang, Z.; Wu, Y.; Zhang, Y.; Wu, G.; Li, D.; Guo, C.; Jiang, G.; Yu, S.; et al. Evolution processes of the corrosion behavior and structural characteristics of plasma electrolytic oxidation coatings on AZ31 Mg alloy. Appl. Surf. Sci. 2018, 434, 326–335. [Google Scholar] [CrossRef]
- Lee, S.-J.; Do, L.H.T. Effects of copper additive on micro-arc oxidation coating of LZ91 Mg-lithium alloy. Surf. Coat. Technol. 2016, 307, 781–789. [Google Scholar] [CrossRef]
- Han, H.; Wang, R.; Wu, Y.; Zhang, X.; Wang, D.; Yang, Z.; Su, Y.; Shen, D.; Nash, P. An investigation of plasma electrolytic oxidation coatings on crevice surface of AZ31 Mg alloy. J. Alloys Compd. 2019, 811, 152010. [Google Scholar] [CrossRef]
- Zhao, J.; Xie, X.; Zhang, C. Effect of the Graphene Oxide Additive on the Corrosion Resistance of the Plasma Electrolytic Oxidation Coating of the AZ31 Mg Alloy. Corros. Sci. 2016, 114, 146–155. [Google Scholar] [CrossRef]
- Tran, Q.-P.; Chin, T.-S.; Kuo, Y.-C.; Jin, C.-X.; Trung, T.; Tuan, C.V.; Dang, D.Q. Diamond powder incorporated oxide layers formed on 6061 Al alloy by plasma electrolytic oxidation. J. Alloys Compd. 2018, 751, 289–298. [Google Scholar] [CrossRef]
- Liu, X.H.; Liu, L.; Dong, S.; Chen, X.B.; Dong, J.B. Towards dense corrosion-resistant plasma electrolytic oxidation coating on Mg-Gd-Y-Zr alloy by using ultra-high frequency pulse current. Surf. Coat. Technol. 2022, 447, 128881. [Google Scholar] [CrossRef]
- Chen, W.-W.; Wang, Z.-X.; Sun, L.; Lu, S. Research of growth mechanism of ceramic coatings fabricated by micro-arc oxidation on Mg alloys at high current mode. J. Magnes. Alloys 2015, 3, 253–257. [Google Scholar] [CrossRef]
- Dou, J.; Wang, C.; Gu, G.; Chen, C. Formation of silicon-calcium-phosphate-containing coating on Mg-Zn-Ca alloy by a two-step micro-arc oxidation technique. Mater. Lett. 2018, 212, 37–40. [Google Scholar] [CrossRef]
- Cui, X.-J.; Liu, C.-H.; Yang, R.-S.; Li, M.-T.; Lin, X.-Z. Self-sealing micro-arc oxidation coating on AZ91D Mg alloy and its formation mechanism. Surf. Coat. Technol. 2015, 269, 228–237. [Google Scholar] [CrossRef]
- Yang, W.; Wang, J.; Xu, D.; Li, J.; Chen, T. Characterization and formation mechanism of grey micro-arc oxidation coatings on Mg alloy. Surf. Coat. Technol. 2015, 283, 281–285. [Google Scholar] [CrossRef]
- Liang, J.; Hu, L.; Hao, J. Improvement of corrosion properties of microarc oxidation coating on Mg alloy by optimizing current density parameters. Appl. Surf. Ence 2007, 253, 6939–6945. [Google Scholar] [CrossRef]
- Zhang, Y.; Wu, Y.; Chen, D.; Wang, R.; Li, D.; Guo, C.; Jiang, G.; Shen, D.; Yu, S.; Nash, P. Micro-structures and growth mechanisms of plasma electrolytic oxidation coatings on aluminium at different current densities. Surf. Coat. Technol. 2017, 321, 236–246. [Google Scholar] [CrossRef]
- Pezzato, L.; Rigon, M.; Martucci, A.; Brunelli, K.; Dabalà, M. Plasma Electrolytic Oxidation (PEO) as pre-treatment for sol-gel coating on aluminum and Mg alloys. Surf. Coat. Technol. 2019, 366, 114–123. [Google Scholar] [CrossRef]
- Srinivasan, P.B.; Blawert, C.; Dietzel, W. Effect of plasma electrolytic oxidation treatment on the corrosion and stress corrosion cracking behaviour of AM50 Mg alloy. Mater. Sci. Eng. A 2008, 494, 401–406. [Google Scholar] [CrossRef]
- Luo, H.; Cai, Q.; Wei, B.; Yu, B.; Li, D.; He, J.; Liu, Z. Effect of (NaPO3)6 concentrations on corrosion resistance of plasma electrolytic oxidation coatings formed on AZ91D Mg alloy. J. Alloys Compd. 2008, 464, 537–543. [Google Scholar] [CrossRef]
- Li, S.; Wu, Y.; Yamamura, K.; Nomura, M.; Fujii, T. Improving the grindability of titanium alloy Ti–6Al–4V with the assistance of ultrasonic vibration and plasma electrolytic oxidation. CIRP Ann.—Manuf. Technol. 2017, 66, 345–348. [Google Scholar] [CrossRef]
- An, L.; Ma, Y.; Liu, Y.; Sun, L.; Wang, S.; Wang, Z. Effects of additives, voltage and their interactions on PEO coatings formed on Mg alloys. Surf. Coat. Technol. 2018, 354, 226–235. [Google Scholar] [CrossRef]
- Liang, J.; Hu, L.; Hao, J. Characterization of microarc oxidation coatings formed on AM60B Mg alloy in silicate and phosphate electrolytes. Appl. Surf. Sci. 2007, 253, 4490–4496. [Google Scholar] [CrossRef]
- Zeng, R.-C.; Hu, Y.; Zhang, F.; Huang, Y.-D.; Wang, Z.-L.; Li, S.-Q.; Han, E.-H. Corrosion resistance of cerium-doped zinc calcium phosphate chemical conversion coatings on AZ31 Mg alloy. Trans. Nonferrous Met. Soc. China 2016, 26, 472–483. [Google Scholar] [CrossRef]
- Li, H.; Lu, S.; Qin, W.; Wu, X. In-situ grown MgO-ZnO ceramic coating with high thermal emittance on Mg alloy by plasma electrolytic oxidation. Acta Astronaut. 2017, 136, 230–235. [Google Scholar] [CrossRef]
- Qin, W.; Li, H.; Lu, S.; Wu, X. Influence of Zr4+ ions on solar absorbance and emissivity of coatings formed on AZ31 Mg alloy by plasma electrolytic oxidation. Surf. Coat. Technol. 2015, 269, 220–227. [Google Scholar]
- Tu, W.; Cheng, Y.; Wang, X.; Zhan, T.; Han, J.; Cheng, Y. Plasma electrolytic oxidation of AZ31 Mg alloy in aluminate-tungstate electrolytes and the coating formation mechanism. J. Alloys Compd. 2017, 725, 199–216. [Google Scholar] [CrossRef]
- Lu, X.; Blawert, C.; Mohedano, M.; Scharnagl, N.; Zheludkevich, M.L.; Kainer, K.U. Influence of electrical parameters on particle uptake during plasma electrolytic oxidation processing of AM50 Mg alloy. Surf. Coat. Technol. 2016, 289, 179–185. [Google Scholar] [CrossRef]
- Yang, C.; Huang, J.; Cui, S.; Fu, R.; Sheng, L.; Xu, D.; Tian, X.; Zheng, Y.; Chu, P.K.; Wu, Z.Z. NaF assisted preparation and the improved corrosion resistance of high content ZnO doped plasma electrolytic oxidation coating on AZ31B alloy. J. Magnes. Alloys, 2023; in press. [Google Scholar] [CrossRef]
- Yang, C.; Cai, H.; Cui, S.H.; Huang, J.; Zhu, J.Y.; Wu, Z.C.; Ma, Z.Y.; Fu, R.K.Y.; Sheng, L.Y.; Tian, X.B.; et al. A zinc-doped coating prepared on the Mg alloy by plasma electrolytic oxidation for corrosion protection. Surf. Coat. Technol. 2022, 433, 128148. [Google Scholar] [CrossRef]
- Liang, J.; Guo, B.; Tian, J.; Liu, H.; Zhou, J.; Liu, W.; Xu, T. Effects of NaAlO2 on structure and corrosion resistance of microarc oxidation coatings formed on AM60B Mg alloy in phosphate–KOH electrolyte. Surf. Coat. Technol. 2005, 199, 121–126. [Google Scholar] [CrossRef]







Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, C.; Chen, P.; Wu, W.; Sheng, L.; Zheng, Y.; Chu, P.K. A Review of Corrosion-Resistant PEO Coating on Mg Alloy. Coatings 2024, 14, 451. https://doi.org/10.3390/coatings14040451
Yang C, Chen P, Wu W, Sheng L, Zheng Y, Chu PK. A Review of Corrosion-Resistant PEO Coating on Mg Alloy. Coatings. 2024; 14(4):451. https://doi.org/10.3390/coatings14040451
Chicago/Turabian StyleYang, Chao, Pinghu Chen, Wenxing Wu, Liyuan Sheng, Yufeng Zheng, and Paul K. Chu. 2024. "A Review of Corrosion-Resistant PEO Coating on Mg Alloy" Coatings 14, no. 4: 451. https://doi.org/10.3390/coatings14040451






