Assessment of AF4 Parylene Cohesion/Adhesion on Si and SiO2 Substrates by Means of Pull-Off Energy
Abstract
:1. Introduction
2. Materials and Methods
2.1. Samples and Pull-Off Tests
2.2. Calculation of Work (Pull-Off Energy) from F/s Curves
2.3. Nanoindentation Measurements
2.4. Raman Spectroscopy Measurements
2.5. XRD Measurements
2.6. AFM Topography Measurements
3. Results and Discussion
3.1. Bond Strength and Pull-Off Energy
3.2. Nanoindentation Measurements
3.3. X-ray Diffractometry and Raman Spectroscopy Measurements
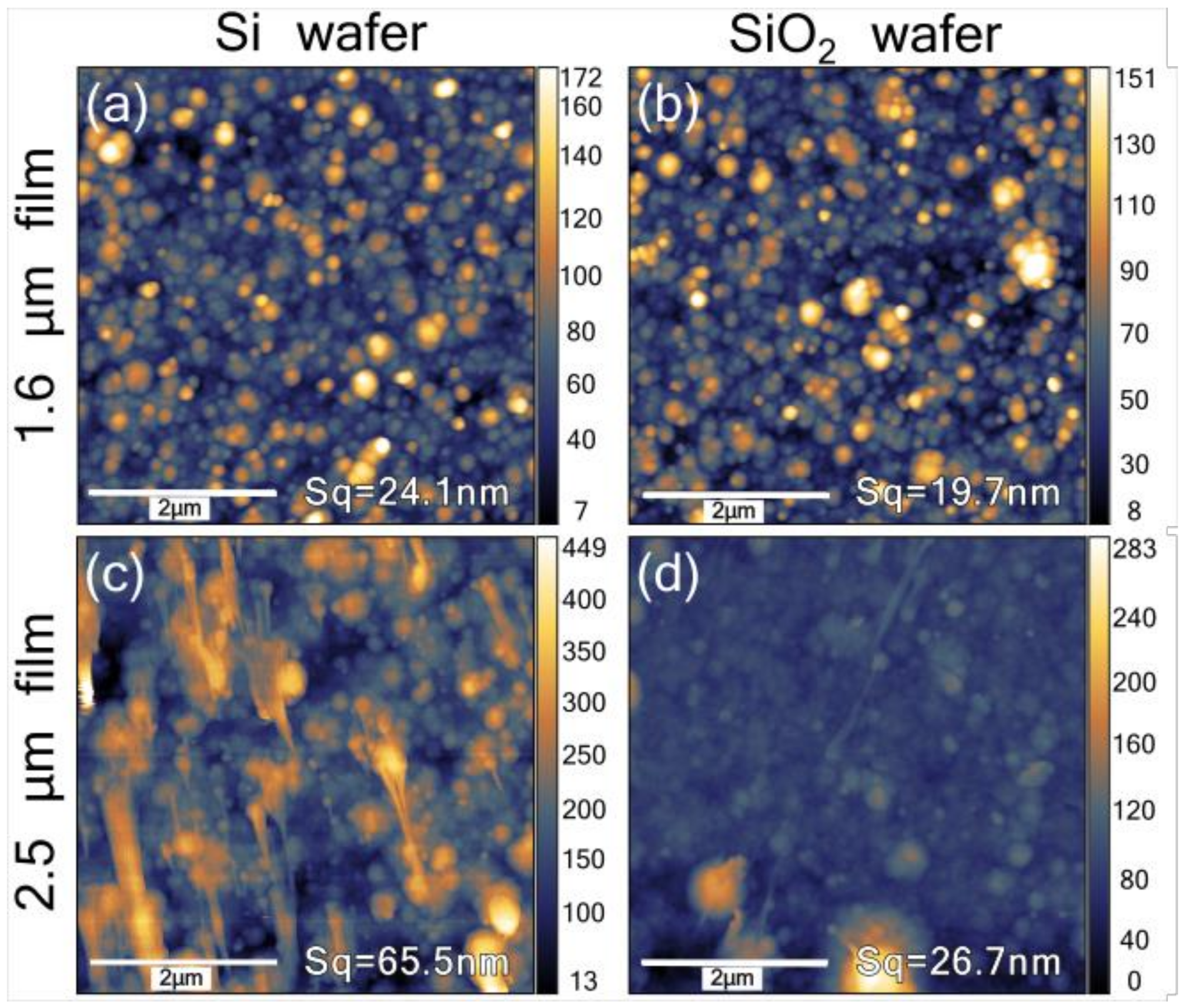
3.4. AFM Topography Measurements
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kim, B.J.; Meng, E. Micromachining of parylene C for bioMEMS. Polym. Adv. Technol. 2016, 27, 564–576. [Google Scholar] [CrossRef]
- Tian, L.; Yin, Y.; Zhao, J.; Jin, H.; Shang, Y.; Yan, S.; Dong, S. Parylene F coatings for combating marine biofouling. Mater. Lett. 2021, 285, 129141. [Google Scholar] [CrossRef]
- Hassler, C.; von Metzen, R.P.; Ruther, P.; Stieglitz, T. Characterization of parylene C as an encapsulation material for implanted neural prostheses. J. Biomed. Mater. B Appl. Biomater. 2010, 93, 267–274. [Google Scholar] [CrossRef]
- Mariello, M.; Kim, K.; Wu, K.; Lacour, S.P.; Leterrier, Y. Recent advances in encapsulation of flexible bioelectronic implants: Materials, Technologies, and Characterization Methods. Adv. Mater. 2022, 34, 2201129. [Google Scholar] [CrossRef] [PubMed]
- Golda-Cepa, M.; Engvall, K.; Hakkarainen, M.; Kotarba, A. Recent progress on parylene C polymer for biomedical applications: A review. Prog. Organ. Coat. 2020, 140, 105493. [Google Scholar] [CrossRef]
- Zhu, J.; Liu, X.; Shi, Q.; He, T.; Sun, Z.; Guo, X.; Lee, C. Development trends and perspectives of future sensors and MEMS/NEMS. Micromachines 2020, 11, 7. [Google Scholar] [CrossRef] [Green Version]
- Kim, B.J.; Meng, E. Review of polymer MEMS micromachining. J. Micromech. Microeng. 2016, 26, 013001. [Google Scholar] [CrossRef]
- Ortigoza-Diaz, J.; Scholten, K.; Larson, C.; Cobo, A.; Hudson, T.; Yoo, J.; Meng, E. Techniques and considerations in the microfabrication of parylene c microelectromechanical systems. Micromachines 2018, 9, 422. [Google Scholar] [CrossRef] [Green Version]
- Selbmann, F.; Baum, M.; Meinecke, C.; Wiemer, M.; Kuhn, H.; Joseph, Y. Low-temperature parylene-based adhesive bonding technology for 150 and 200 mm wafers for fully biocompatible and highly reliable microsystems. ECS J. Solid State Sci. Technol. 2021, 10, 074010. [Google Scholar] [CrossRef]
- Xu, H.; Yang, Z.; Guo, Y.; Xu, Q.; Dou, S.; Jin, Y.; Wang, W. Copolymerization of Parylene C and Parylene F to Enhance Adhesion and Thermal Stability without Coating Performance Degradation; SSRN: Rochester, NY, USA, 2022; pp. 1–14. Available online: https://ssrn.com/abstract=4259971 (accessed on 15 January 2023).
- Seymour, J.P.; Elkasabi, Y.M.; Chen, H.Y.; Lahann, J.; Kipke, D.R. The insulation performance of reactive parylene films in implantable electronic devices. Biomaterials 2009, 30, 6158–6167. [Google Scholar] [CrossRef]
- Hartmann, F.; Jakešová, M.; Mao, G.; Nikić, M.; Kaltenbrunner, M.; Đerek, V.; Głowacki, E.D. Scalable microfabrication of folded parylene-based conductors for stretchable electronics. Adv. Electron. Mater. 2021, 7, 2001236. [Google Scholar] [CrossRef]
- Xie, X.; Rieth, L.; Merugu, S.; Tathireddy, P.; Solzbacher, F. Plasma-assisted atomic layer deposition of Al2O3 and parylene C bi-layer encapsulation for chronic implantable electronics. Appl. Phys. Lett. 2012, 101, 093702. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Marszalek, T.; Gazicki-Lipman, M.; Ulanski, J. Parylene C as a versatile dielectric material for organic field-effect transistors. Beilstein J. Nanotechnol. 2017, 8, 1532–1545. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Trantidou, T.; Tariq, M.; Terracciano, C.M.; Toumazou, C.; Prodromakis, T. Parylene C-based flexible electronics for ph monitoring applications. Sensors 2014, 14, 11629–11639. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Qiu, Y.; Jung, Y.H.; Lee, S.; Shih, T.Y.; Lee, J.; Xu, Y.H.; Ma, Z. Compact parylene-c-coated flexible antenna for WLAN and upper-band UWB applications. Electron. Lett. 2014, 50, 1782–1784. [Google Scholar] [CrossRef]
- Buchwalder, S.; Borzì, A.; Diaz Leon, J.J.; Bourgeois, F.; Nicolier, C.; Nicolay, S.; Burger, J. Thermal analysis of parylene thin films for barrier layer applications. Polymers 2022, 14, 3677. [Google Scholar] [CrossRef]
- Kahouli, A.; Sylvestre, A.; Jomni, F.; André, E.; Garden, J.L.; Yangui, B.; Legrand, J. Dielectric properties of parylene AF4 as low-k material for microelectronic applications. Thin Solid Films 2012, 520, 2493–2497. [Google Scholar] [CrossRef]
- Dolbier, W.R., Jr.; Beach, W.F. Parylene-AF4: A polymer with exceptional dielectric and thermal properties. J. Fluorine Chem. 2003, 122, 97–104. [Google Scholar] [CrossRef]
- Hicks, C.; Duffy, B.; Hargaden, G.C. Synthesis and modification of octafluoro [2.2] paracyclophane (parylene AF4). Org. Chem. Front. 2014, 1, 716–725. [Google Scholar] [CrossRef] [Green Version]
- Senkovich, J.J. Parylene AF-4 via the trapping of a phenoxy leaving group. Chem. Vapor. Depos. 2013, 19, 327–331. [Google Scholar] [CrossRef]
- Lagomarsini, C.; Jean-Mistral, C.; Kachroudi, A.; Monfray, S.; Sylvestre, A. Outstanding performance of parylene polymers as electrets for energy harvesting and high-temperature applications. J. Appl. Polym. Sci. 2019, 137, 48790. [Google Scholar] [CrossRef]
- Karakurt, I.; Zhong, J.; Lin, L. 3D printed flexible triboelectric energy harvesters via conformal coating of parylene AF4. In Proceedings of the 32nd international conference on micro electro mechanical systems (MEMS), Seoul, Republic of Korea, 27–31 January 2019. [Google Scholar]
- Watson, A.M.; Dease, K.; Terrab, S.; Roath, C.; Gopinath, J.T.; Bright, V.M. Focus-tunable low-power electrowetting lenses with thin parylene films. Appl. Opt. 2015, 54, 6224–6229. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sinani, T.; Miskovic, G. Adhesion evaluation of parylene AF4 to silicon and glass substrate. In Proceedings of the 45th international spring seminar on electronics technology (ISSE), Vienna, Austria, 11–15 May 2022; pp. 1–6. [Google Scholar]
- Vencl, A.; Arostegui, S.; Favaro, G.; Zivic, F.; Mrdak, M.; Mitrović, S.; Popovic, V. Evaluation of adhesion/cohesion bond strength of the thick plasma spray coatings by scratch testing on coatings cross-sections. Tribol. Int. 2011, 44, 1281–1288. [Google Scholar] [CrossRef]
- Sickfeld, J. Pull-off test, an internationally standardized method for adhesion testing-assessment of the relevance of test results. In Adhesion Aspects of Polymeric Coatings; Springer: Boston, MA, USA, 1983; pp. 543–567. [Google Scholar]
- Awaja, F.; Gilbert, M.; Kelly, G.; Fox, B.; Pigram, P.J. Adhesion of polymers. Prog. Polymer Sci. 2009, 34, 948–968. [Google Scholar] [CrossRef]
- Harder, T.A.; Yao, T.J.; He, Q.; Shih, C.Y.; Tai, Y.C. Residual stress in thin-film parylene-C. In Proceedings of the MEMS IEEE international conference, Las Vegas, NV, USA, 24 January 2002. [Google Scholar]
- Ramos, N.M.; Simões, M.L.; Delgado, J.M.; Freitas, V.P.D. Applicability of the pull-off test: Teachings from a large sample of in situ tests. In Hygrothermal Behavior, Building Pathology and Durability; Springer: Berlin/Heidelberg, Germany, 2013; pp. 165–181. [Google Scholar]
- Goodno, B.J.; Gere, J.M. Mechanics of Materials; Cengage Learning: Boston, MA, USA, 2020. [Google Scholar]
- Hsu, J.M.; Rieth, L.; Kammer, S.; Orthner, M.; Solzbacher, F. Effect of thermal and deposition processes on surface morphology, crystallinity, and adhesion of Parylene-C. Sens. Mater. 2008, 20, 87–102. [Google Scholar]
- Song, Z.; Park, J.H.; Kim, H.R.; Lee, G.Y.; Kang, M.J.; Kim, M.H.; Pyun, J.C. Carbon electrode obtained via pyrolysis of plasma-deposited parylene-c for electrochemical immunoassays. Analyst 2022, 147, 3783–3794. [Google Scholar] [CrossRef]
- Ramaswamy, S.; Gleason, K.K. Overview of strategies for the cvd of organic films and functional polymer layers. Chem. Vapor Depos. 2009, 15, 77–90. [Google Scholar]
- Mihály, J.; Sterkel, S.; Ortner, H.M.; Kocsis, L.; Hajba, L.; Furdyga, É.; Mink, J. FTIR and FT-Raman spectroscopic study on polymer based high pressure digestion vessels. Croat. Chem. Acta 2006, 79, 5. [Google Scholar]
- Solonenko, D.; Gasiorowski, J.; Apaydin, D.; Oppelt, K.; Nuss, M.; Keawsongsaeng, W.; Thamyongkit, P. Doping-induced polaron formation and solid-state polymerization in benzoporphyrin-oligothiophene conjugated systems. J. Phys. Chem. C 2017, 121, 24397–24407. [Google Scholar] [CrossRef]






| Sample Type | Bond Strength [kPa] | Pull-Off Energy [mJ] |
|---|---|---|
| Si substrate-1.6 μm AF4 | 220 ± 88 kPa | 1.4 ± 0.5 mJ |
| Si substrate-2.5 μm AF4 | 234 ± 85 kPa | 1.7 ± 0.8 mJ |
| SiO2 substrate-1.6 μm AF4 | 303 ± 82 kPa | 3.0 ± 1.0 mJ |
| SiO2 substrate-2.5 μm AF4 | 240 ± 55 kPa | 6.3 ± 1.8 mJ |
| Sample Type | Elastic Modulus [GPa] |
|---|---|
| Si substrate-1.6 μm AF4 | 3.2 ± 0.4 GPa |
| Si substrate-2.5 μm AF4 | 3.8 ± 0.4 GPa |
| SiO2 substrate-1.6 μm AF4 | 5.8 ± 1.0 GPa |
| SiO2 substrate-2.5 μm AF4 | 7.7 ± 2.0 GPa |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sinani, T.; Solonenko, D.; Miskovic, G. Assessment of AF4 Parylene Cohesion/Adhesion on Si and SiO2 Substrates by Means of Pull-Off Energy. Coatings 2023, 13, 237. https://doi.org/10.3390/coatings13020237
Sinani T, Solonenko D, Miskovic G. Assessment of AF4 Parylene Cohesion/Adhesion on Si and SiO2 Substrates by Means of Pull-Off Energy. Coatings. 2023; 13(2):237. https://doi.org/10.3390/coatings13020237
Chicago/Turabian StyleSinani, Taulant, Dmytro Solonenko, and Goran Miskovic. 2023. "Assessment of AF4 Parylene Cohesion/Adhesion on Si and SiO2 Substrates by Means of Pull-Off Energy" Coatings 13, no. 2: 237. https://doi.org/10.3390/coatings13020237





