Activity of Colloidal Silver Solution against Microorganisms Implicated in Ocular Infections
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Microorganisms and Inoculum Preparation
2.3. Killing Activity
2.4. Effectiveness on Biofilm-Embedded Cells
2.5. Light Microscopy
2.6. Statistical Analysis
3. Results
3.1. Killing Activity
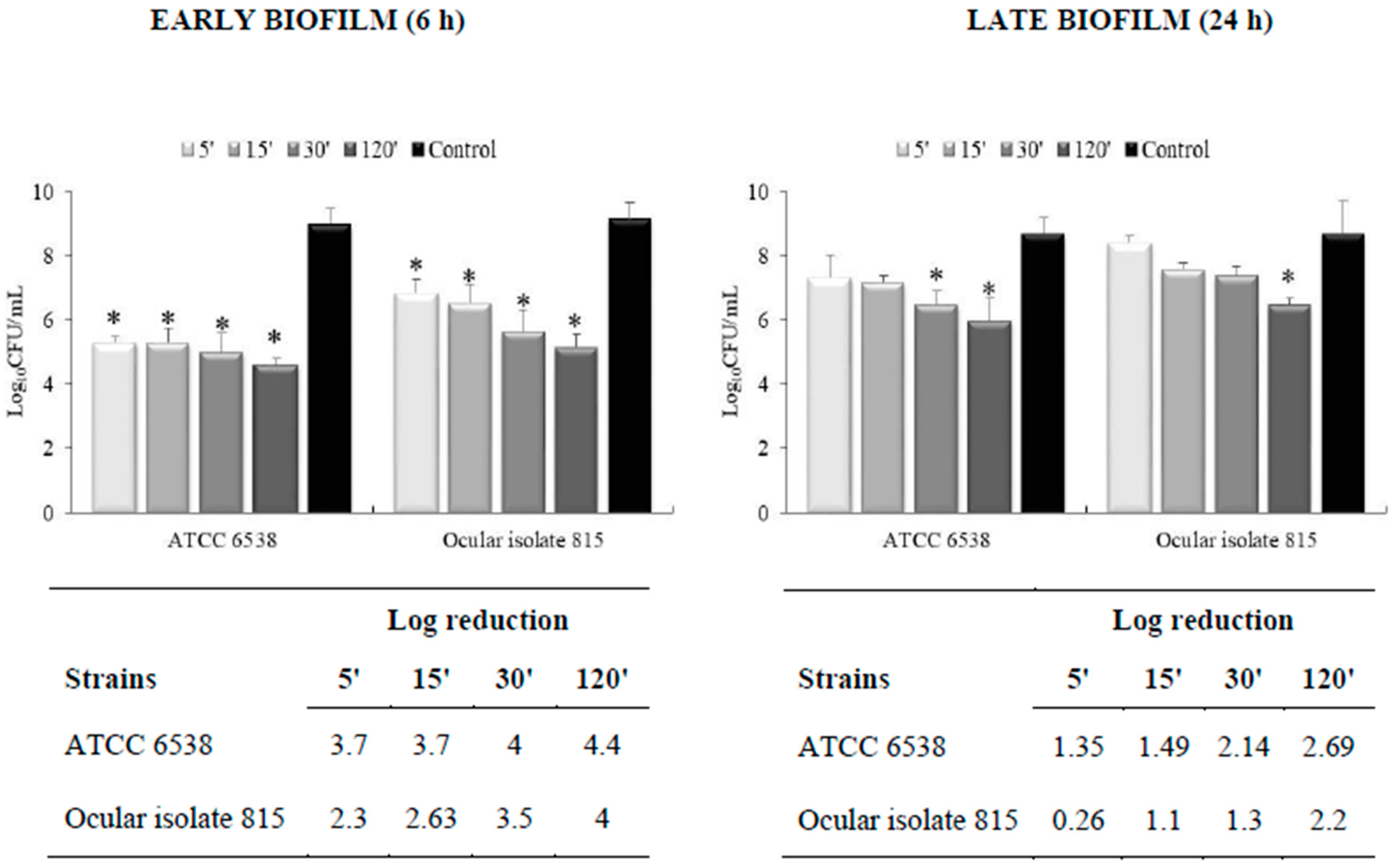
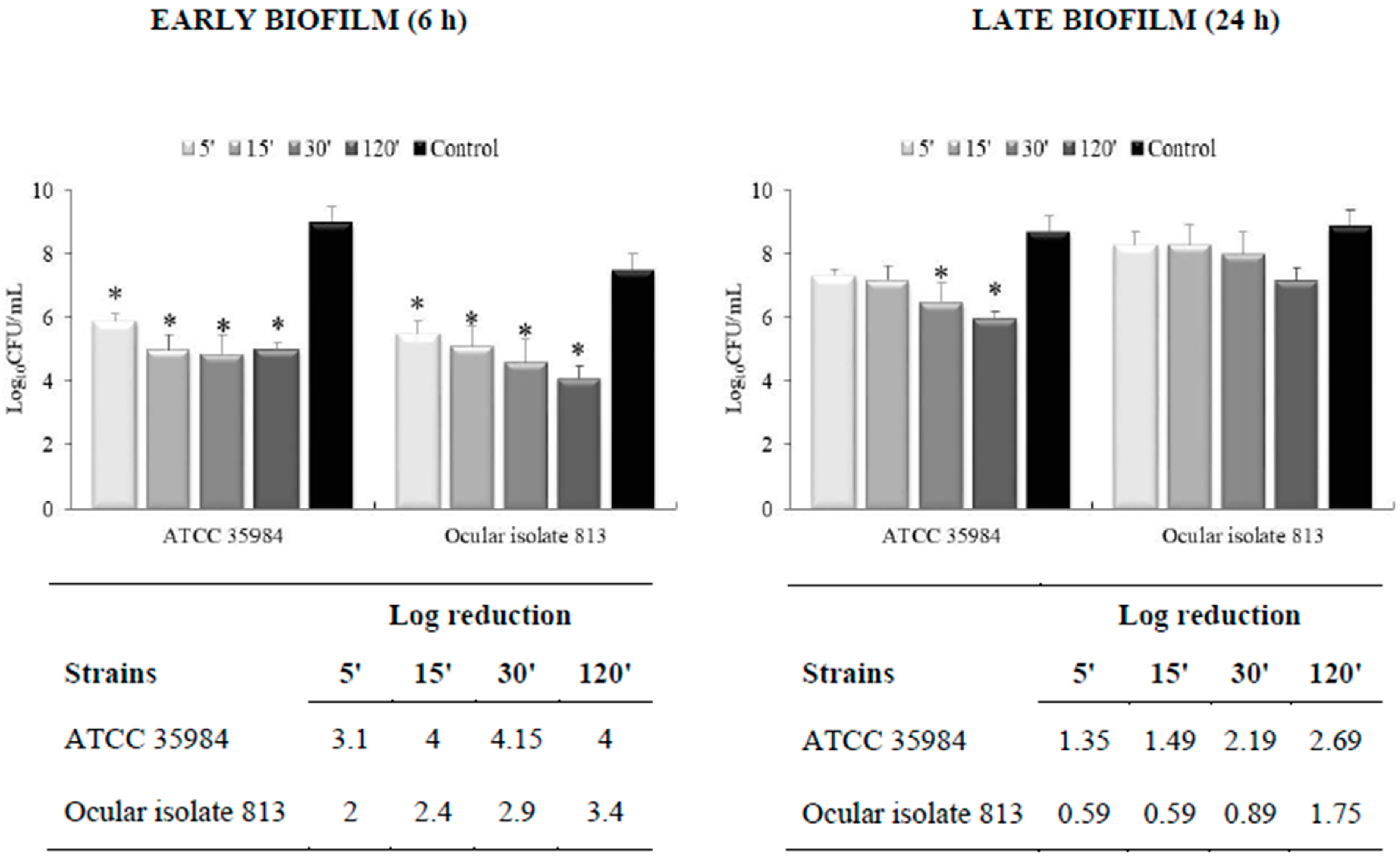
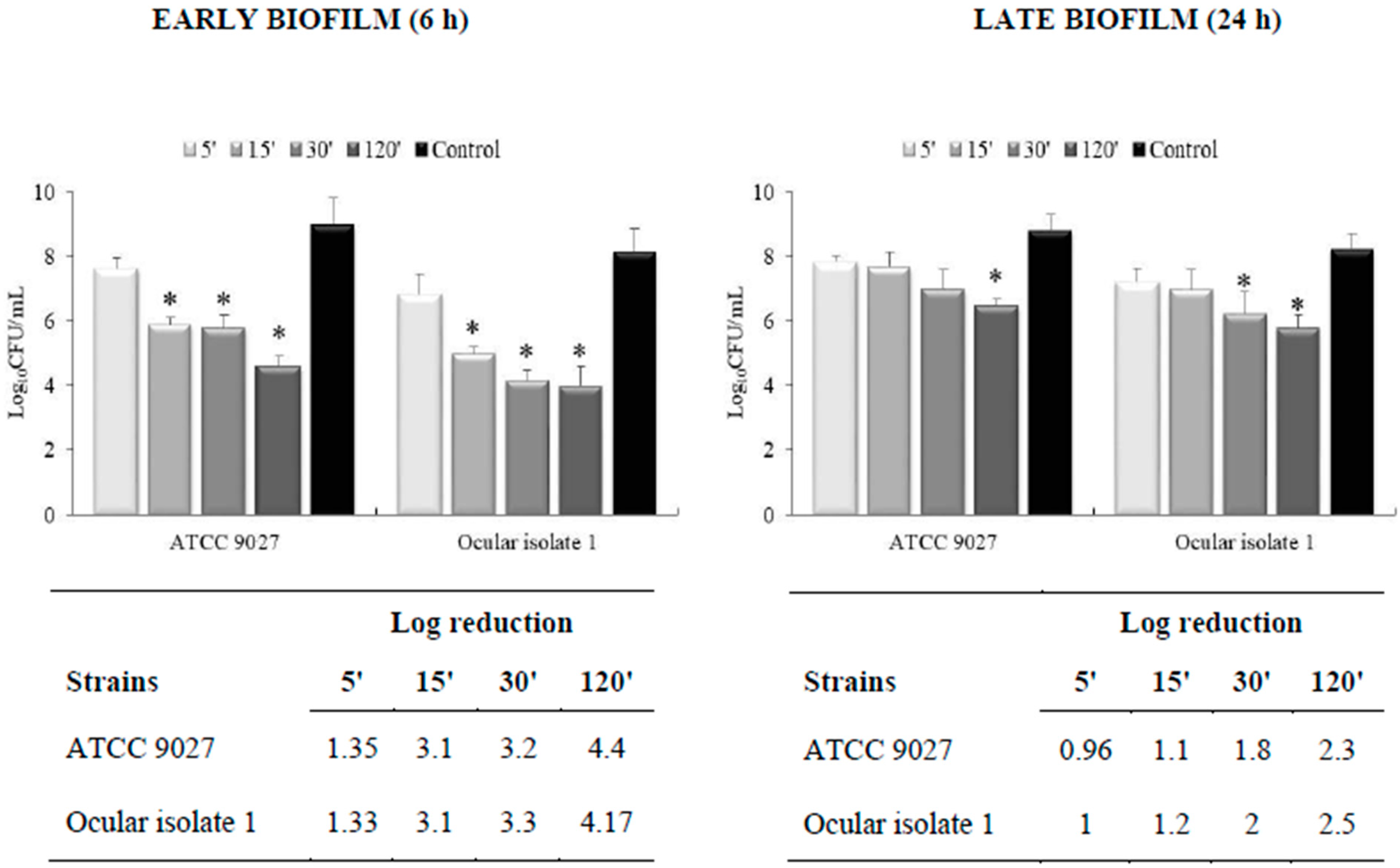
3.2. Effectiveness on Preformed Biofilm
3.3. Light Microscopy
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Durand, M.L. Bacterial and fungal endophthalmitis. Clin. Microbiol. Rev. 2017, 30, 597–613. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Grzybowski, A.; Brona, P.; Kim, S.J. Microbial flora and resistance in ophthalmology: A review. Graefes Arch. Clin. Exp. Ophthalmol. 2017, 255, 851–862. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rynerson, J.M.; Perry, H.D. DEBS—A unification theory for dry eye and blepharitis. Clin. Ophthalmol. 2016, 10, 2455–2467. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Olsen, I. Biofilm-specific antibiotic tolerance and resistance. Eur. J. Clin. Microbiol. Infect. Dis. 2015, 34, 77–86. [Google Scholar] [CrossRef] [PubMed]
- Jung, W.K.; Koo, H.C.; Kim, K.W.; Shin, S.; Kim, S.H.; Park, Y.H. Antibacterial activity and mechanism of action of the silver ion in Staphylococcus aureus and Escherichia coli. Appl. Environ. Microbiol. 2008, 74, 2171–2178. [Google Scholar] [CrossRef] [Green Version]
- Sondi, I.; Salopek-Sondi, B. Silver nanoparticles as antimicrobial agent: A case study on E. coli as a model for Gram-negative bacteria. J. Colloid Interface Sci. 2004, 275, 177–182. [Google Scholar] [CrossRef]
- Gajbhiye, M.; Kesharwani, J.; Ingle, A.; Gade, A.; Rai, M. Fungus-mediated synthesis of silver nanoparticles and their activity against pathogenic fungi in combination with fluconazole. Nanomedicine 2009, 5, 382–386. [Google Scholar] [CrossRef]
- Hendiger, E.B.; Padzik, M.; Sifaoui, I.; Reyes-Batlle, M.; López-Arencibia, A.; Rizo-Liendo, A.; Bethencourt-Estrella, C.J.; Nicolás-Hernández, D.S.; Chiboub, O.; Rodríguez-Expósito, R.L.; et al. Silver nanoparticles as a novel potential preventive agent against Acanthamoeba keratitis. Pathogens 2020, 9, 350. [Google Scholar] [CrossRef]
- Gaikwad, S.; Ingle, A.; Gade, A.; Rai, M.; Falanga, A.; Incoronato, N.; Russo, L.; Galdiero, S.; Galdiero, M. Antiviral activity of mycosynthesized silver nano-particles against herpes simplex virus and human parainfluenza virus type 3. Int. J. Nanomed. 2013, 8, 4303–4314. [Google Scholar]
- Maillard, J.Y.; Denyer, S.P. Focus on silver. In European Wound Management Association (EWMA) Position Document: The Role of Topical Antimicrobials in Managing Wound Infection; MEP Ltd.: London, UK, 2006. [Google Scholar]
- Russell, A.D.; Hugo, W.B. Antimicrobial activity and action of silver. Prog. Med. Chem. 1994, 31, 351–370. [Google Scholar]
- Feng, Q.L.; Wu, J.; Chen, G.Q.; Cui, F.Z.; Kim, T.N.; Kim, J.O. A mechanistic study of the antibacterial effect of silver ions on Escherichia coli and Staphylococcus aureus. J. Biomed. Mater. Res. 2000, 52, 662–668. [Google Scholar] [CrossRef]
- Chaw, K.C.; Manimaran, M.; Tay, F.E. Role of silver ions in destabilization of intermolecular adhesion forces measured by atomic force microscopy in Staphylococcus epidermidis biofilms. Antimicrob. Agents Chemother. 2005, 49, 4853–4859. [Google Scholar] [CrossRef] [Green Version]
- Kalishwaralal, K.; BarathManiKanth, S.; Pandian, S.R.; Deepak, V.; Gurunathan, S. Silver nanoparticles impede the biofilm formation by Pseudomonas aeruginosa and Staphylococcus epidermidis. Colloids Surf. B Biointerfaces 2010, 79, 340–344. [Google Scholar] [CrossRef]
- Richter, K.; Facal, P.; Thomas, N.; Vandecandelaere, I.; Ramezanpour, M.; Cooksley, C.; Prestidge, C.A.; Coenye, T.; Wormald, P.J.; Vreugde, S. Taking the silver bullet colloidal silver particles for the topical treatment of biofilm-related infections. ACS Appl. Mater. Interfaces 2017, 9, 21631–21638. [Google Scholar] [CrossRef]
- Marambio-Jones, C.; Hoek, E.M.V. A review of the antibacterial effects of silver nanomaterials and potential implications for human health and the environment. J. Nanopart. Res. 2010, 12, 1531–1551. [Google Scholar] [CrossRef]
- Kim, J.S.; Kuk, E.; Yu, K.N.; Kim, J.H.; Park, S.J.; Lee, H.J.; Kim, S.H.; Park, Y.K.; Park, Y.H.; Hwang, C.Y.; et al. Antimicrobial effects of silver nanoparticles. Nanomedicine 2007, 3, 95–101. [Google Scholar] [CrossRef]
- Lara, H.H.; Garza-Treviño, E.N.; Ixtepan-Turrent, L.; Singh, D.K. Silver nanoparticles are broad-spectrum bactericidal and virucidal compounds. J. Nanobiotechnol. 2011, 9, 30. [Google Scholar] [CrossRef] [Green Version]
- Franci, G.; Falanga, A.; Galdiero, S.; Palomba, L.; Rai, M.; Morelli, G.; Galdiero, M. Silver nanoparticles as potential antibacterial agents. Molecules 2015, 20, 8856–8874. [Google Scholar] [CrossRef] [Green Version]
- Porel, S.; Ramakrishna, D.; Hariprasad, E.; Gupta Dutta, A.; Radhakrishnan, T. Polymer thin film with in situ synthesized silver nanoparticles as a potent reusable bactericide. Curr. Sci. 2011, 101, 927–934. [Google Scholar]
- Tran, Q.H.; Nguyen, V.Q.; Le, A.T. Silver nanoparticles: Synthesis, properties, toxicology, applications and perspectives. Adv. Nat. Sci. Nanosci. Nanotechnol. 2018, 9, 049501. [Google Scholar] [CrossRef]
- Luo, L.J.; Lin, T.Y.; Yao, C.H.; Kuo, P.Y.; Matsusaki, M.; Harroun, S.G.; Huang, C.C.; Lai, J.Y. Dual-functional gelatin-capped silver nanoparticles for antibacterial and antiangiogenic treatment of bacterial keratitis. J. Colloid Interfaces Sci. 2019, 536, 112–126. [Google Scholar] [CrossRef] [PubMed]
- Blanco, A.R.; Marino, A.; D’Arrigo, M.; Nostro, A. Efficacy of a colloidal silver-based topical solution on microbial biofilms. In Acta Ophthalmologica; Wiley: Hoboken, NJ, USA, 2018; Volume 96, p. 14. [Google Scholar]
- Blanco, A.R.; Sudano-Roccaro, A.; Spoto, G.C.; Nostro, A.; Rusciano, D. Epigallocatechin gallate inhibits biofilm formation by ocular staphylococcal isolates. Antimicrob. Agents Chemother. 2005, 49, 4339–4343. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Marino, A.; Blanco, A.R.; Ginestra, G.; Nostro, A.; Bisignano, G. Ex vivo efficacy of gemifloxacin in experimental keratitis induced by methicillin-resistant Staphylococcus aureus. Int. J. Antimicrob. Agents 2016, 48, 395–400. [Google Scholar] [CrossRef] [PubMed]
- Consoli, G.M.L.; Granata, G.; Picciotto, R.; Blanco, A.R.; Geraci, C.; Marino, A.; Nostro, A. Design, synthesis and antibacterial evaluation of a polycationic calix [4]arene derivative alone and in combination with antibiotics. MedChemComm 2017, 9, 160–164. [Google Scholar] [CrossRef]
- Blanco, A.R.; Nostro, A.; D’Angelo, V.; D’Arrigo, M.; Mazzone, M.G.; Marino, A. Efficacy of a fixed combination of tetracycline, chloramphenicol, and colistimethate sodium for treatment of Candida albicans keratitis. Investig. Ophthalmol. Vis. Sci. 2017, 58, 4292–4298. [Google Scholar] [CrossRef] [Green Version]
- Di Stefano, A.; D’Aurizio, E.; Trubiani, O.; Grande, R.; Di Campli, E.; Di Giulio, M.; Di Bartolomeo, S.; Sozio, P.; Iannitelli, A.; Nostro, A.; et al. Viscoelastic properties of Staphylococcus aureus and Staphylococcus epidermidis mono-microbial biofilms. Microb. Biotechnol. 2009, 2, 634–641. [Google Scholar] [CrossRef] [Green Version]
- Nostro, A.; Guerrini, A.; Marino, A.; Tacchini, M.; Di Giulio, M.; Grandini, A.; Akin, M.; Cellini, L.; Bisignano, G.; Saraçoğlu, H.T. In vitro activity of plant extracts against biofilm-producing food-related bacteria. Int. J. Food Microbiol. 2016, 238, 33–39. [Google Scholar] [CrossRef]
- Nostro, A.; Marino, A.; Ginestra, G.; Cellini, L.; Di Giulio, M.; Bisignano, G. Effects of adaptation to carvacrol on Staphylococcus aureus in the planktonic and biofilm phases. Biofouling 2017, 33, 470–480. [Google Scholar] [CrossRef]
- Scaffaro, R.; Lopresti, F.; D’Arrigo, M.; Marino, A.; Nostro, A. Efficacy of poly(lactic acid)/carvacrol electrospun membranes against Staphylococcus aureus and Candida albicans in single and mixed cultures. Appl. Microbiol. Biotechnol. 2018, 102, 4171–4181. [Google Scholar] [CrossRef]
- Nostro, A.; Marino, A.; Blanco, A.R.; Cellini, L.; Di Giulio, M.; Pizzimenti, F.; Roccaro, A.S.; Bisignano, G. In vitro activity of carvacrol against staphylococcal preformed biofilm by liquid and vapour contact. J. Med. Microbiol. 2009, 58, 791–797. [Google Scholar] [CrossRef]
- Azeredo, J.; Azevedo, N.F.; Briandet, R.; Cerca, N.; Coenye, T.; Costa, A.R.; Desvaux, M.; Di Bonaventura, G.; Hébraud, M.; Jaglic, Z.; et al. Critical review on biofilm methods. Crit. Rev. Microbiol. 2017, 43, 313–351. [Google Scholar] [CrossRef] [Green Version]
- Teweldemedhin, M.; Gebreyesus, H.; Atsbaha, A.H.; Asgedom, S.W.; Saravanan, M. Bacterial profile of ocular infections: A systematic review. BMC Ophthalmol. 2017, 17, 212. [Google Scholar] [CrossRef] [Green Version]
- Maneewattanapinyo, P.; Banlunara, W.; Thammacharoen, C.; Ekgasit, S.; Kaewamatawong, T. An evaluation of acute toxicity of colloidal silver nanoparticles. J. Vet. Med. Sci. 2011, 73, 1417–1423. [Google Scholar] [CrossRef] [Green Version]
- Nikaido, H.; Vaara, M. Molecular basis of bacterial outer membrane permeability. Microbiol. Rev. 1985, 1, 1–32. [Google Scholar] [CrossRef]
- Rai, M.K.; Deshmukh, S.D.; Ingle, A.P.; Gade, A.K. Silver nanoparticles: The powerful nanoweapon against multidrug-resistant bacteria. J. Appl. Microbiol. 2012, 112, 841–852. [Google Scholar] [CrossRef]
- Rai, M.; Yadav, A.; Gade, A. Silver nanoparticles as a new generation of antimicrobials. Biotechnol. Adv. 2009, 27, 76–83. [Google Scholar] [CrossRef]
- Sharifi-Rad, M.; Nazaruk, J.; Polito, L.; Morais-Braga, M.F.B.; Rocha, J.E.; Coutinho, H.D.M.; Salehi, B.; Tabanelli, G.; Montanari, C.; Del Mar Contreras, M.; et al. Matricaria genus as a source of antimicrobial agents: From farm to pharmacy and food applications. Microbiol. Res. 2018, 215, 76–88. [Google Scholar] [CrossRef]
- Singh, H.; Du, J.; Singh, P.; Yi, T.H. Ecofriendly synthesis of silver and gold nanoparticles by Euphrasia officinalis leaf extract and its biomedical applications. Artif. Cells Nanomed. Biotechnol. 2018, 46, 1163–1170. [Google Scholar] [CrossRef] [Green Version]
- Dogru, E.; Demirbas, A.; Altinsoy, B.; Duman, F.; Ocsoy, I. Formation of Matricaria chamomilla extract-incorporated Ag nanoparticles and size-dependent enhanced antimicrobial property. J. Photochem. Photobiol. B. 2017, 174, 78–83. [Google Scholar] [CrossRef]
- Deguchi, H.; Kitazawa, K.; Kayukawa, K.; Kondoh, E.; Fukumoto, A.; Yamasaki, T.; Kinoshita, S.; Sotozono, C. The trend of resistance to antibiotics for ocular infection of Staphylococcus aureus, coagulase-negative staphylococci, and Corynebacterium compared with 10-years previous: A retrospective observational study. PLoS ONE 2018, 13, e0203705. [Google Scholar] [CrossRef]
- Panáček, A.; Libor Kvítek, L.; Prucek, R.; Kolar, M.; Vecerova, R.; Pizúrova, N.; Sharma, V.K.; Nevecna, T.; Zboril, R. Silver colloid nanoparticles: Synthesis, characterization, and their antibacterial activity. J. Phys. Chem. 2006, 110, 16248–16253. [Google Scholar] [CrossRef] [PubMed]
- Shehabeldine, A.M.; Salem, S.S.; Ali, O.M.; Abd-Elsalam, K.A.; Elkady, F.M.; Hashem, A.H. Multifunctional silver nanoparticles based on chitosan: Antibacterial, antibiofilm, antifungal, antioxidant, and wound-healing activities. J. Fungi 2022, 8, 612. [Google Scholar] [CrossRef] [PubMed]
- Bispo, P.J.; Haas, W.; Gilmore, M.S. Biofilms in infections of the eye. Pathogens 2015, 4, 111–136. [Google Scholar] [CrossRef] [PubMed]
- Aslam, B.; Wang, W.; Arshad, M.I.; Khurshid, M.; Muzammil, S.; Rasool, M.H.; Nisar, M.A.; Alvi, R.F.; Aslam, M.A.; Qamar, M.U.; et al. Antibiotic resistance: A rundown of a global crisis. Infect. Drug Resist. 2018, 11, 1645–1658. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brahma, U.; Sharma, P.; Murthy, S.; Sharma, S.; Chakraborty, S.; Sundarapu Naga Appalaraju, S.N.; Vasundhra Bhandari, V. Decreased expression of femXAB genes and fnbp mediated biofilm pathways in OS-MRSA clinical isolates. Sci. Rep. 2019, 9, 16028. [Google Scholar] [CrossRef] [Green Version]
- Heidari, H.; Hadadi, M.; Sedigh Ebrahim-Saraie, H.; Mirzaei, A.; Taji, A.; Hosseini, S.R.; Motamedifar, M. Characterization of virulence factors, antimicrobial resistance patterns and biofilm formation of Pseudomonas aeruginosa and Staphylococcus spp. strains isolated from corneal infection. J. Fr. Ophthalmol. 2018, 41, 823–829. [Google Scholar] [CrossRef]
- Fabrega, J.; Renshaw, J.C.; Lead, J.R. Interactions of silver nanoparticles with Pseudomonas putida biofilms. Environ. Sci. Technol. 2009, 43, 9004–9009. [Google Scholar] [CrossRef]
- Gurunathan, S.; Han, J.W.; Kwon, D.N.; Kim, J.H. Enhanced antibacterial and anti-biofilm activities of silver nanoparticles against Gram-negative and Gram-positive bacteria. Nanoscale Res. Lett. 2014, 9, 373. [Google Scholar] [CrossRef] [Green Version]
- McCarthy, H.; Rudkin, J.K.; Black, N.S.; Gallagher, L.; O’Neill, E.; O’Gara, J.P. Methicillin resistance and the biofilm phenotype in Staphylococcus aureus. Front. Cell Infect. Microbiol. 2015, 5, 1. [Google Scholar] [CrossRef] [Green Version]


| Strains | Time (min) | |||
|---|---|---|---|---|
| 0 | 5 | 10 | 15 | |
| Log CFU/mL | ||||
| S. aureus 815 (MRSA) | 6.17 ± 0.31 | 4.25 ± 0.33 | 4.17 ± 0.35 | 3.30 ± 0.12 |
| S. aureus ATCC 6538 | 5.28 ± 0.24 | 3.48 ± 0.22 | 3.08 ± 0.15 | 2.52 ± 0.08 |
| S. epidermidis 813 | 6.06 ± 0.22 | 4.3 ± 0.18 | 4.0 ± 0.14 | 3.50 ± 0.10 |
| S. epidermidis ATCC 35984 | 5.05 ± 0.25 | 3.4 ± 0.15 | 2.9 ± 0.10 | 2.56 ± 0.12 |
| P. aeruginosa 1 (ofloxacin-resistant) | 8.0 ± 0.41 | 6.5 ± 0.40 | 5.5 ± 0.26 | 5.0 ± 0.30 |
| P. aeruginosa ATCC 9027 | 7.05 ± 0.35 | 5.1 ± 0.19 | 3.8 ± 0.08 | 2.70 ± 0.05 |
| C. albicans 4 | 4.25 ± 0.20 | 4.14 ± 0.25 | 4.0 ± 0.22 | 3.6 ± 0.15 |
| C. albicans ATCC 10231 | 4.0 ± 0.23 | 2.6 ± 0.23 | 2.42 ± 0.14 | 2.0 ± 0.11 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Blanco, A.R.; Marino, A.; D’Arrigo, M.; Nostro, A. Activity of Colloidal Silver Solution against Microorganisms Implicated in Ocular Infections. Coatings 2023, 13, 265. https://doi.org/10.3390/coatings13020265
Blanco AR, Marino A, D’Arrigo M, Nostro A. Activity of Colloidal Silver Solution against Microorganisms Implicated in Ocular Infections. Coatings. 2023; 13(2):265. https://doi.org/10.3390/coatings13020265
Chicago/Turabian StyleBlanco, Anna Rita, Andreana Marino, Manuela D’Arrigo, and Antonia Nostro. 2023. "Activity of Colloidal Silver Solution against Microorganisms Implicated in Ocular Infections" Coatings 13, no. 2: 265. https://doi.org/10.3390/coatings13020265






