Pullulan Films with PCMs: Recyclable Bio-Based Films with Thermal Management Functionality
Abstract
:1. Introduction
2. Experimental details
2.1. Materials
2.2. Methods
2.2.1. Preparation of the Solutions
2.2.2. Film Preparation
2.2.3. Recycling Process Applied for the Recovery of Pullulan and PCMs
2.3. Characterization
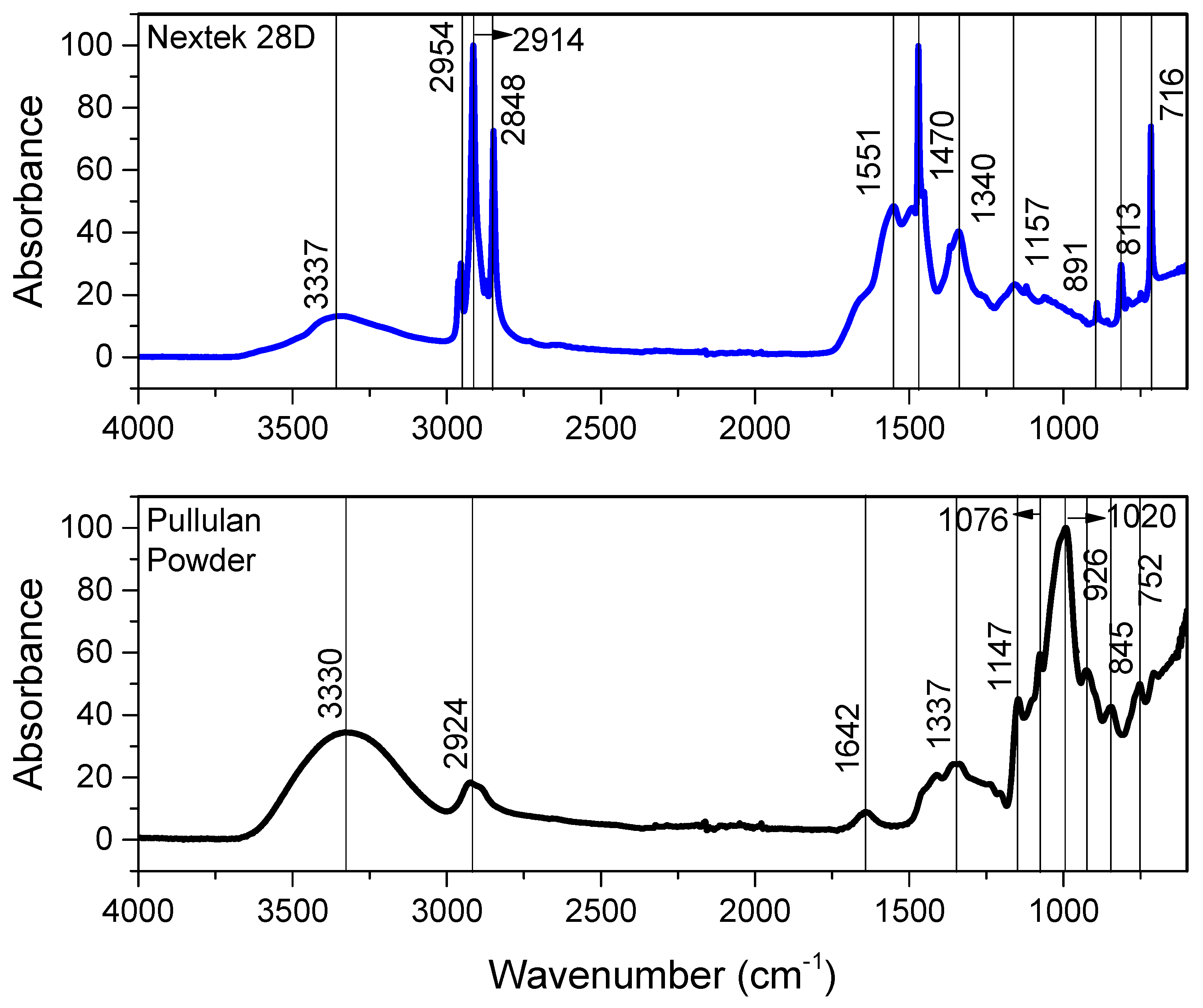
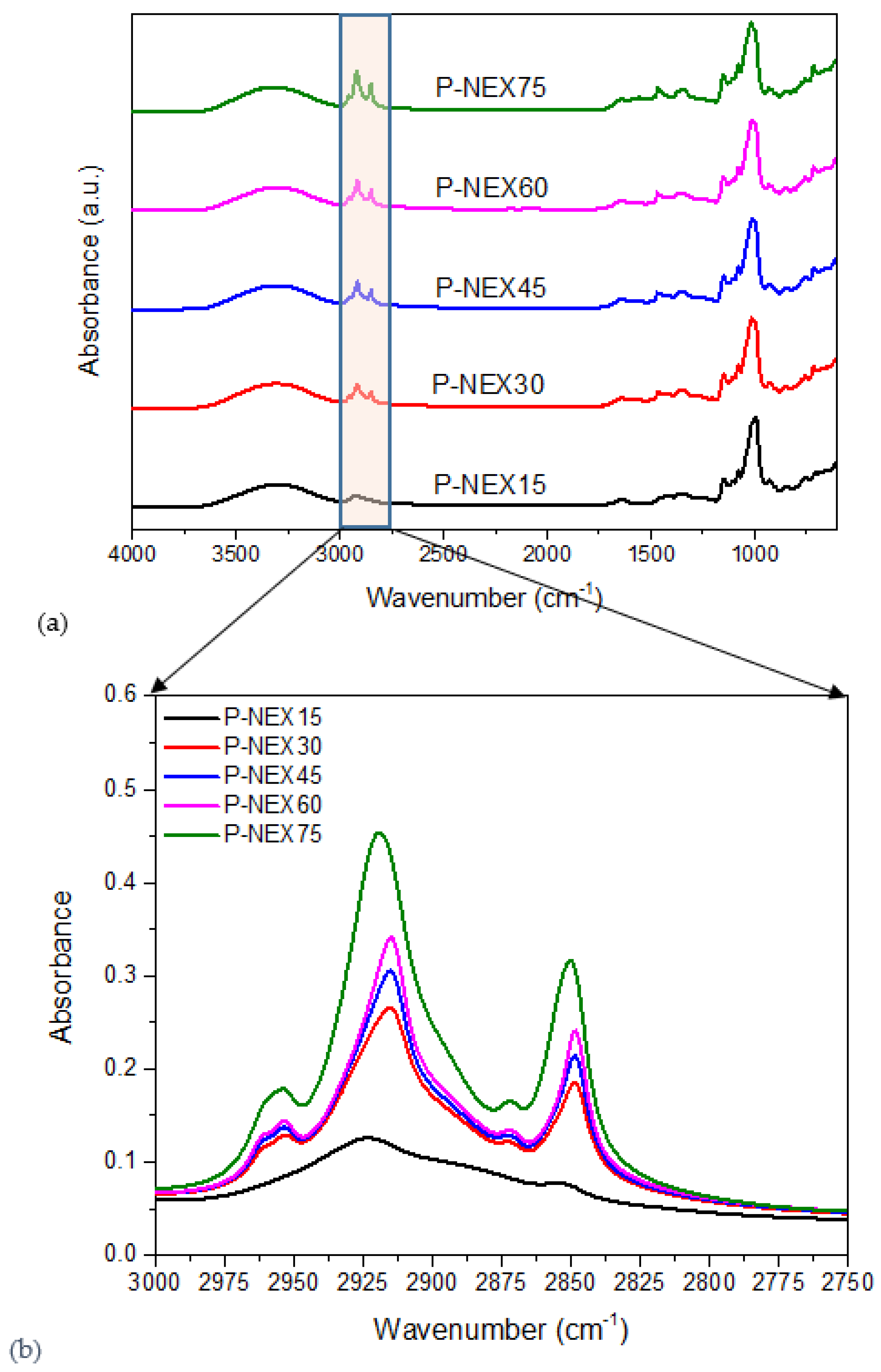
2.3.1. Fourier Transform Infrared Spectrometer (FTIR)
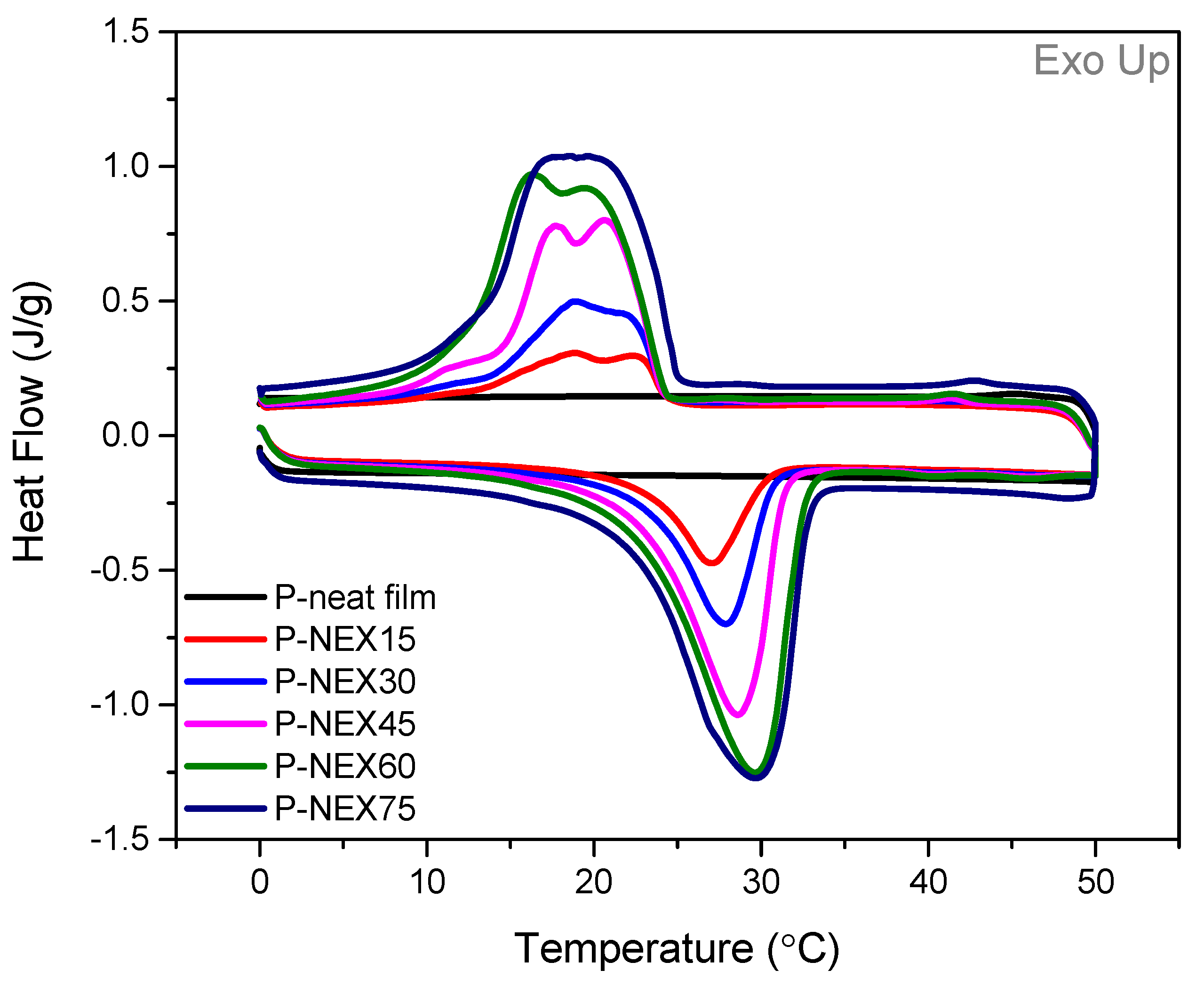
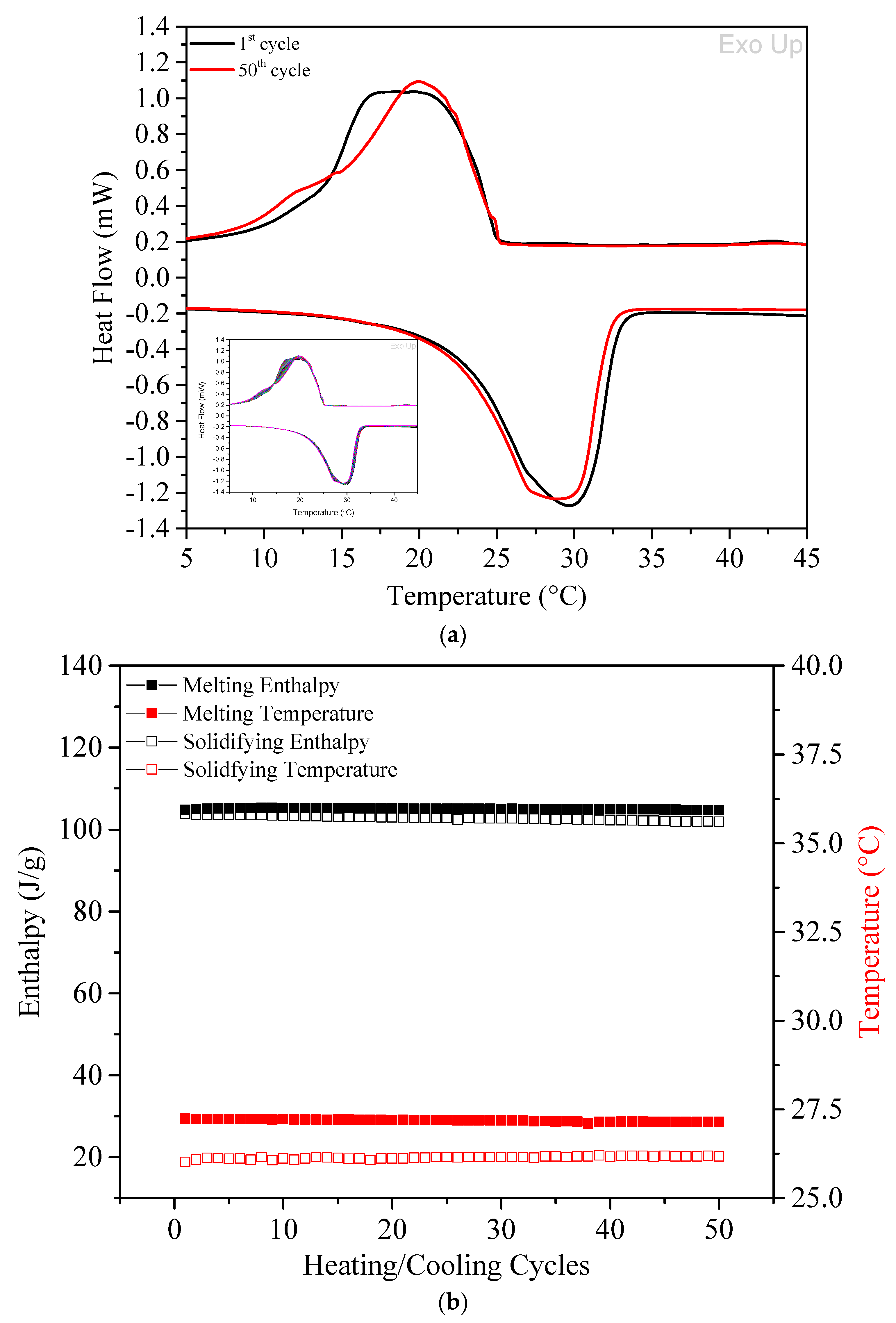
2.3.2. Differential Scanning Calorimetry (DSC)
2.3.3. Thermal Stability
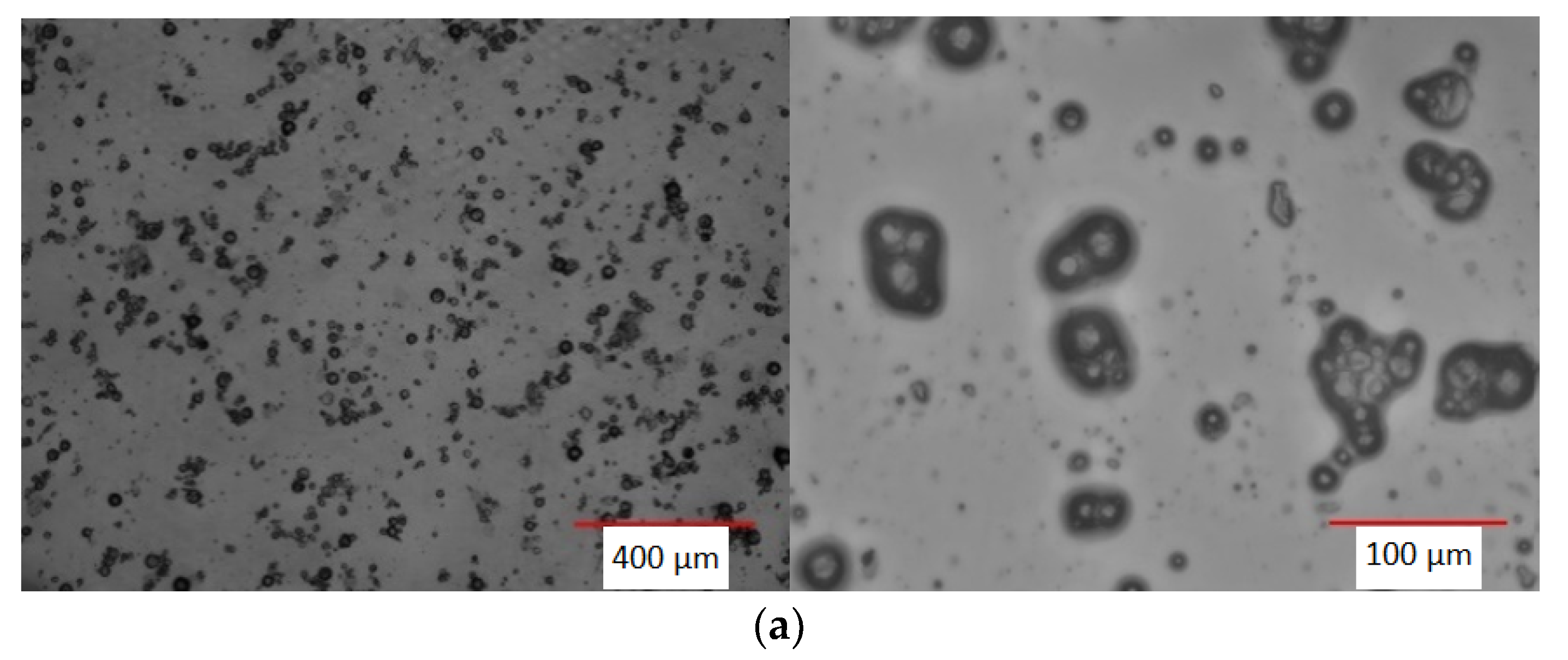
2.3.4. Optical Microscope (OM)
3. Results and Discussion
3.1. Pullulan-Based Thermoactive Films
3.1.1. Appearance of the Films Prepared
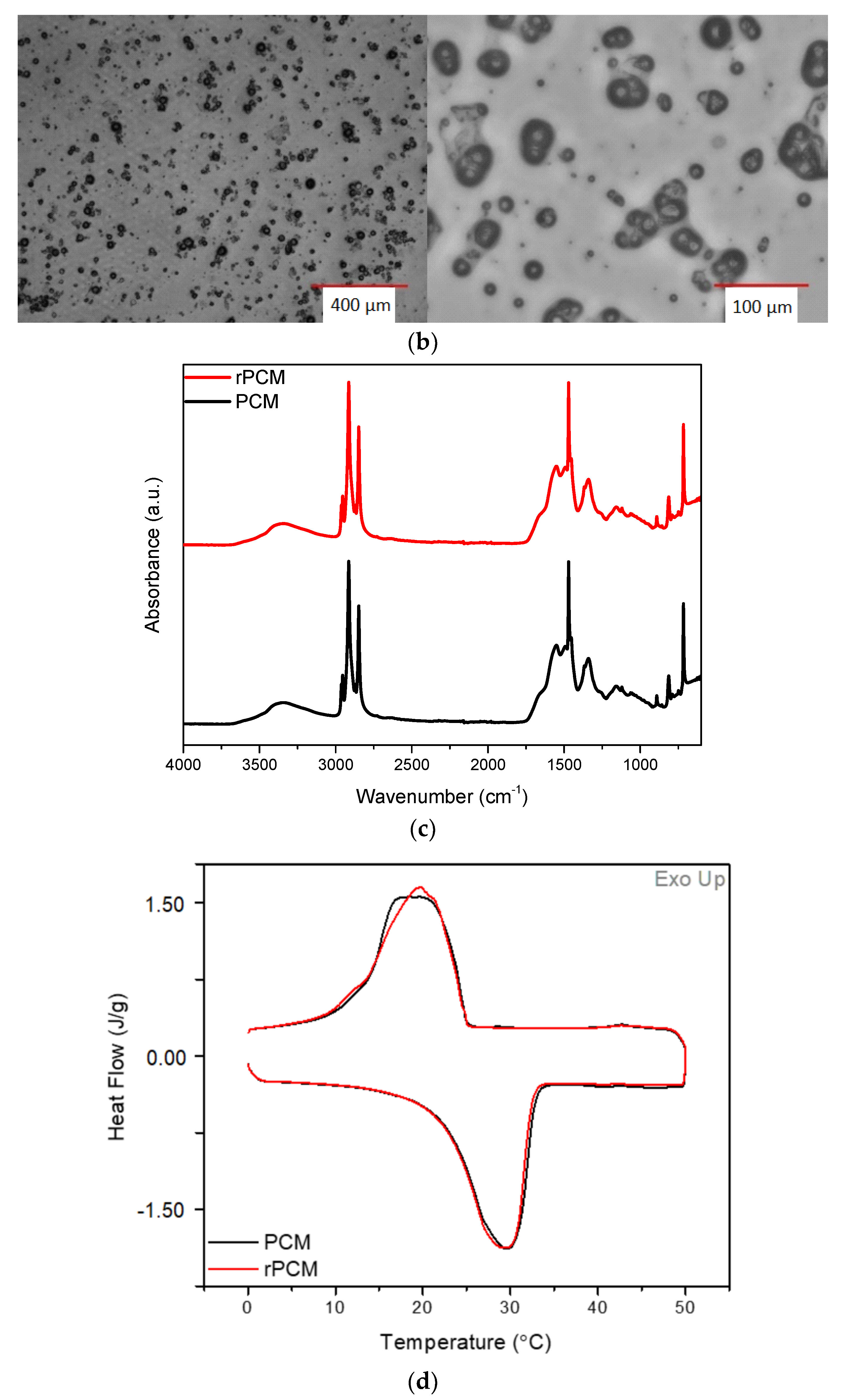
3.1.2. Fourier Transform Infrared Spectroscopy (FTIR)
3.1.3. Thermal Management Functionality
3.1.4. Stability Analysis—Repeated Heating & Cooling Cycles
3.2. Recovery of Pullulan and PCMs
4. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kraśniewska, K.; Pobiega, K.; Gniewosz, M. Pullulan-Biopolymer with Potential for Use as Food Packaging. Int. J. Food Eng. 2019, 15, 20190030. [Google Scholar] [CrossRef]
- Hassannia-Kolaee, M.; Khodaiyan, F.; Pourahmad, R.; Shahabi-Ghahfarrokhi, I. Development of Ecofriendly Bionanocomposite: Whey Protein Isolate/Pullulan Films with Nano-SiO2. Int. J. Biol. Macromol. 2016, 86, 139–144. [Google Scholar] [CrossRef]
- Shahabi-Ghahfarrokhi, I.; Khodaiyan, F.; Mousavi, M.; Yousefi, H. Preparation of UV-Protective Kefiran/Nano-ZnO Nanocomposites: Physical and Mechanical Properties. Int. J. Biol. Macromol. 2015, 72, 41–46. [Google Scholar] [CrossRef]
- Rhim, J.W.; Lee, S.B.; Hong, S.I. Preparation and Characterization of Agar/Clay Nanocomposite Films: The Effect of Clay Type. J. Food Sci. 2011, 76, N40–N48. [Google Scholar] [CrossRef] [PubMed]
- Trovatti, E.; Fernandes, S.C.M.; Rubatat, L.; Perez, D.d.S.; Freire, C.S.R.; Silvestre, A.J.D.; Neto, C.P. Pullulan-Nanofibrillated Cellulose Composite Films with Improved Thermal and Mechanical Properties. Compos. Sci. Technol. 2012, 72, 1556–1561. [Google Scholar] [CrossRef]
- Cozzolino, C.A.; Cerri, G.; Brundu, A.; Farris, S. Microfibrillated Cellulose (MFC): Pullulan Bionanocomposite Films. Cellulose 2014, 21, 4323–4335. [Google Scholar] [CrossRef]
- Silva, N.H.C.S.; Vilela, C.; Almeida, A.; Marrucho, I.M.; Freire, C.S.R. Pullulan-Based Nanocomposite Films for Functional Food Packaging: Exploiting Lysozyme Nanofibers as Antibacterial and Antioxidant Reinforcing Additives. Food Hydrocoll. 2018, 77, 921–930. [Google Scholar] [CrossRef]
- Introzzi, L.; Fuentes-Alventosa, J.J.; Cozzolino, C.A.; Trabattoni, S.; Tavazzi, S.; Bianchi, C.L.; Schiraldi, A.; Piergiovanni, L.; Farris, S. “Wetting Enhancer” Pullulan Coating for Antifog Packaging Applications. ACS Appl. Mater. Interfaces 2012, 4, 3692–3700. [Google Scholar] [CrossRef]
- Hassannia-Kolaee, M.; Khodaiyan, F.; Shahabi-Ghahfarrokhi, I. Modification of Functional Properties of Pullulan-Whey Protein Bionanocomposite Films with Nanoclay. J. Food Sci. Technol. 2016, 53, 1294–1302. [Google Scholar] [CrossRef]
- Pinto, R.J.B.; Almeida, A.; Fernandes, S.C.M.; Freire, C.S.R.; Silvestre, A.J.D.; Neto, C.P.; Trindade, T. Antifungal Activity of Transparent Nanocomposite Thin Films of Pullulan and Silver against Aspergillus Niger. Colloids Surf. B Biointerfaces 2013, 103, 143–148. [Google Scholar] [CrossRef]
- Liu, Y.; Liu, Y.; Han, K.; Cai, Y.; Ma, M.; Tong, Q.; Sheng, L. Effect of Nano-TiO2 on the Physical, Mechanical and Optical Properties of Pullulan Film. Carbohydr. Polym. 2019, 218, 95–102. [Google Scholar] [CrossRef] [PubMed]
- Ho, C.P.; Fan, J.; Newton, E.; Au, R. Improving Thermal Comfort in Apparel. In Improving Comfort in Clothing; Song, G., Ed.; Woodhead Publishing: Cambridge, UK, 2011; pp. 165–181. ISBN 978-1-84569-539-2. [Google Scholar]
- Das, A.; Alagirusamy, R. Introduction to Clothing Comfort. In Science in Clothing Comfort; Das, A., Alagirusamy, R., Eds.; Woodhead Publishing India: New Delhi, India, 2010; pp. 1–12. ISBN 978-1-84569-789-1. [Google Scholar]
- Erkan, G. Enhancing The Thermal Properties of Textiles With Phase Change Materials. Res. J. Text. Appar. 2004, 8, 57–64. [Google Scholar] [CrossRef]
- Mondal, S. Phase Change Materials for Smart Textiles—An Overview. Appl. Therm. Eng. 2008, 28, 1536–1550. [Google Scholar] [CrossRef]
- Sarier, N.; Onder, E. Organic Phase Change Materials and Their Textile Applications: An Overview. Thermochim. Acta 2012, 540, 7–60. [Google Scholar] [CrossRef]
- Alay, S.; Alkan, C.; Göde, F. Synthesis and Characterization of Poly(Methyl Methacrylate)/n-Hexadecane Microcapsules Using Different Cross-Linkers and Their Application to Some Fabrics. Thermochim. Acta 2011, 518, 1–8. [Google Scholar] [CrossRef]
- Nelson, G. Application of Microencapsulation in Textiles. Int. J. Pharm. 2002, 242, 55–62. [Google Scholar] [CrossRef] [PubMed]
- Sarier, N.; Onder, E. The Manufacture of Microencapsulated Phase Change Materials Suitable for the Design of Thermally Enhanced Fabrics. Thermochim. Acta 2007, 452, 149–160. [Google Scholar] [CrossRef]
- Zhang, W.; Hao, S.; Zhao, D.; Bai, G.; Zuo, X.; Yao, J. Preparation of PMMA/SiO2 PCM Microcapsules and Its Thermal Regulation Performance on Denim Fabric. Pigment Resin Technol. 2020, 49, 491–499. [Google Scholar] [CrossRef]
- Marani, A.; Nehdi, M.L. Integrating Phase Change Materials in Construction Materials: Critical Review. Constr. Build. Mater. 2019, 217, 36–49. [Google Scholar] [CrossRef]
- Mohaisen, K.O.; Hasan Zahir, M.; Maslehuddin, M.; Al-Dulaijan, S.U. Development of a Shape-Stabilized Phase Change Material Utilizing Natural and Industrial Byproducts for Thermal Energy Storage in Buildings. J. Energy Storage 2022, 50, 104205. [Google Scholar] [CrossRef]
- Liu, H.; Wang, X.; Wu, D.; Ji, S. Fabrication and Applications of Dual-Responsive Microencapsulated Phase Change Material with Enhanced Solar Energy-Storage and Solar Photocatalytic Effectiveness. Sol. Energy Mater. Sol. Cells 2019, 193, 184–197. [Google Scholar] [CrossRef]
- Choubineh, N.; Jannesari, H.; Kasaeian, A. Experimental Study of the Effect of Using Phase Change Materials on the Performance of an Air-Cooled Photovoltaic System. Renew. Sustain. Energy Rev. 2019, 101, 103–111. [Google Scholar] [CrossRef]
- Mondieig, D.; Rajabalee, F.; Laprie, A.; Oonk, H.A.J.; Calvet, T.; Cuevas-Diarte, M.A. Protection of Temperature Sensitive Biomedical Products Using Molecular Alloys as Phase Change Material. Transfus. Apher. Sci. 2003, 28, 143–148. [Google Scholar] [CrossRef] [PubMed]
- Kandasamy, R.; Wang, X.Q.; Mujumdar, A.S. Application of Phase Change Materials in Thermal Management of Electronics. Appl. Therm. Eng. 2007, 27, 2822–2832. [Google Scholar] [CrossRef]
- Singh, S.; Gaikwad, K.; Youn, S.L. Phase Change Materials for Advanced Cooling Packaging. Environ. Chem. Lett. 2018, 16, 845–859. [Google Scholar] [CrossRef]
- Gong, J.; Tang, W.; Xia, L.; Fu, Z.; Zhou, S.; Zhang, J.; Zhang, C.; Hua Ji, L.L.; Xu, W. Flexible and weavable 3D porous graphene/PPy/lignocellulose-based versatile fibrous wearables for thermal management and strain sensing. Chem. Eng. J. 2023, 452, 139338. [Google Scholar] [CrossRef]
- Xiang, B.; Zhang, R.; Zeng, X.; Yanlong, L.; Zhenyang, L. An Easy-to-Prepare Flexible Dual-Mode Fiber Membrane for Daytime Outdoor Thermal Management. Adv. Fiber Mater. 2022, 4, 1058–1068. [Google Scholar] [CrossRef]
- Pan, S.; Zhu, M. Nanoprocessed Silk Makes Skin Feel Cool. Adv. Fiber Mater. 2022, 4, 319–320. [Google Scholar] [CrossRef]
- Choudhury, A.R.; Saluja, P.; Prasad, G.S. Pullulan Production by an Osmotolerant Aureobasidium Pullulans RBF-4A3 Isolated from Flowers of Caesulia Axillaris. Carbohydr. Polym. 2011, 83, 1547–1552. [Google Scholar] [CrossRef]
- Terán Hilares, R.; Orsi, C.A.; Ahmed, M.A.; Marcelino, P.F.; Menegatti, C.R.; da Silva, S.S.; dos Santos, J.C. Low-Melanin Containing Pullulan Production from Sugarcane Bagasse Hydrolysate by Aureobasidium Pullulans in Fermentations Assisted by Light-Emitting Diode. Bioresour. Technol. 2017, 230, 76–81. [Google Scholar] [CrossRef]
- Hamidi, M.; Kennedy, J.F.; Khodaiyan, F.; Mousavi, Z.; Hosseini, S.S. Production Optimization, Characterization and Gene Expression of Pullulan from a New Strain of Aureobasidium Pullulans. Int. J. Biol. Macromol. 2019, 138, 725–735. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.M.; Wang, J.F.; Zha, X.Q.; Pan, L.H.; Zhang, H.L.; Luo, J.P. Structural Characterization and Immunomodulatory Activity of a New Polysaccharide from Jellyfish. Carbohydr. Polym. 2017, 159, 188–194. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Zhong, F.; Li, Y.; Shoemaker, C.F.; Xia, W. Preparation and Characterization of Pullulan-Chitosan and Pullulan-Carboxymethyl Chitosan Blended Films. Food Hydrocoll. 2013, 30, 82–91. [Google Scholar] [CrossRef]
- Niu, B.; Shao, P.; Chen, H.; Sun, P. Structural and Physiochemical Characterization of Novel Hydrophobic Packaging Films Based on Pullulan Derivatives for Fruits Preservation. Carbohydr. Polym. 2019, 208, 276–284. [Google Scholar] [CrossRef]
- Cheng, K.-C.; Demirci, A.; Catchmark, J.M. Effects of Plastic Composite Support and PH Profiles on Pullulan Production in a Biofilm Reactor. Appl. Microbiol. Biotechnol. 2010, 86, 853–861. [Google Scholar] [CrossRef] [PubMed]
- Trovatti, E.; Fernandes, S.C.M.; Rubatat, L.; Freire, C.S.R.; Silvestre, A.J.D.; Carlos, P.N. Sustainable Nanocomposite Films Based on Bacterial Cellulose and Pullulan. Cellulose 2012, 19, 729–737. [Google Scholar] [CrossRef]
- Kumar, V.; Mirzaei, A.; Bonyani, M.; Kim, K.-H.; Kim, H.W.; Kim, S.S. Advances in Electrospun Nanofiber Fabrication for Polyaniline (PANI)-Based Chemoresistive Sensors for Gaseous Ammonia. TRAC Trends Anal. Chem. 2020, 129, 115938. [Google Scholar] [CrossRef]
- Mirzaee, H.; Khodaiyan, F.; Kennedy, J.F.; Hosseini, S.S. Production, Optimization and Characterization of Pullulan from Sesame Seed Oil Cake as a New Substrate by Aureobasidium Pullulans. Carbohydr. Polym. Technol. Appl. 2020, 1, 100004. [Google Scholar] [CrossRef]
- Liang, R.; Hu, A.; Hatat-Fraile, M.; Zhou, N. Fundamentals on Adsorption, Membrane Filtration, and Advanced Oxidation Processes for Water Treatment. In Nanotechnology for Water Treatment and Purification; Hu, A., Apblett, A., Eds.; Springer: Cham, Switzerland, 2014; Volume 22, pp. 1–45. ISBN 978-3-319-06577-9. [Google Scholar] [CrossRef]
- Weiss, S.; Urdl, K.; Mayer, H.A.; Zikulnig-Rusch, E.M.; Kandelbauer, A. IR Spectroscopy: Suitable Method for Determination of Curing Degree and Crosslinking Type in Melamine–Formaldehyde Resins. J. Appl. Polym. Sci. 2019, 136, 47691. [Google Scholar] [CrossRef]
- Krupa, I.; Nógellová, Z.; Špitalský, Z.; Janigová, I.; Boh, B.; Sumiga, B.; Kleinová, A.; Karkri, M.; AlMaadeed, M.A. Phase Change Materials Based on High-Density Polyethylene Filled with Microencapsulated Paraffin Wax. Energy Convers. Manag. 2014, 87, 400–409. [Google Scholar] [CrossRef]
- Kizildag, N. Smart Composite Nanofiber Mats with Thermal Management Functionality. Sci. Rep. 2021, 11, 4256. [Google Scholar] [CrossRef] [PubMed]
- He, Y.; Li, H.; Luo, F.; Jin, Y.; Huang, B.; Qingrong, Q. Bio-based flexible phase change composite film with high thermal conductivity for thermal energy storage Composites Part A. Appl. Sci. Manuf. 2021, 151, 106638. [Google Scholar] [CrossRef]






| Properties | Nextek 28D |
|---|---|
| Appearance | White to slightly off-white color |
| Form | Dry powder (>97 solids) |
| Particle size (mean) | 15–30 micron |
| Free wax content | <2.5% |
| Melting point Heat of fusion | 28 °C (±2 °C) >155 J/g |
| Samples | PCM Content (wt.%) (Based on Film Weight) |
|---|---|
| P | - |
| P-NEX15 | 15 |
| P-NEX30 | 30 |
| P-NEX45 | 45 |
| P-NEX60 | 60 |
| P-NEX75 | 75 |
| Samples | Melting Temp. (°C) | Latent Heat of Melting (ΔHm) (J g−1) | Crystallization Temp. (°C) | Latent Heat of Crystallization (ΔHc) (J g−1) |
|---|---|---|---|---|
| neat Pullulan | - | - | - | - |
| P-NEX15 | 27.04 | 21.94 | 18.23/22.04 | 20.92 |
| P-NEX30 | 27.86 | 37.65 | 18.79/22.02 | 37.69 |
| P-NEX45 | 28.50 | 66.54 | 17.96/20.73 | 65.41 |
| P-NEX60 | 29.47 | 87.83 | 15.70/20.56 | 85.67 |
| P-NEX75 | 29.36 | 104.85 | 16.02/18.82 | 103.58 |
| Theoretical Value (ΔHm) (J g−1) | Experimental Value (ΔHm) (J g−1) | % | Theoretical Value (ΔHc) (J g−1) | Experimental Value (ΔHc) (J g−1) | % | |
|---|---|---|---|---|---|---|
| P-NEX15 | 25.99 | 21.94 | 84.4 | 25.58 | 20.92 | 81.8 |
| P-NEX30 | 51.98 | 37.65 | 72.4 | 51.15 | 37.69 | 73.7 |
| P-NEX45 | 77.97 | 66.54 | 85.3 | 76.73 | 65.41 | 84.8 |
| P-NEX60 | 104.00 | 87.83 | 84.5 | 102.30 | 85.46 | 83.5 |
| P-NEX75 | 129.95 | 104.85 | 80.7 | 127.88 | 103.58 | 81.0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kizildag, N. Pullulan Films with PCMs: Recyclable Bio-Based Films with Thermal Management Functionality. Coatings 2023, 13, 414. https://doi.org/10.3390/coatings13020414
Kizildag N. Pullulan Films with PCMs: Recyclable Bio-Based Films with Thermal Management Functionality. Coatings. 2023; 13(2):414. https://doi.org/10.3390/coatings13020414
Chicago/Turabian StyleKizildag, Nuray. 2023. "Pullulan Films with PCMs: Recyclable Bio-Based Films with Thermal Management Functionality" Coatings 13, no. 2: 414. https://doi.org/10.3390/coatings13020414
APA StyleKizildag, N. (2023). Pullulan Films with PCMs: Recyclable Bio-Based Films with Thermal Management Functionality. Coatings, 13(2), 414. https://doi.org/10.3390/coatings13020414





