A Ru-Doped VTi2.6O7.2 Anode with High Conductivity for Enhanced Sodium Storage
Abstract
:1. Introduction
2. Materials and Methods
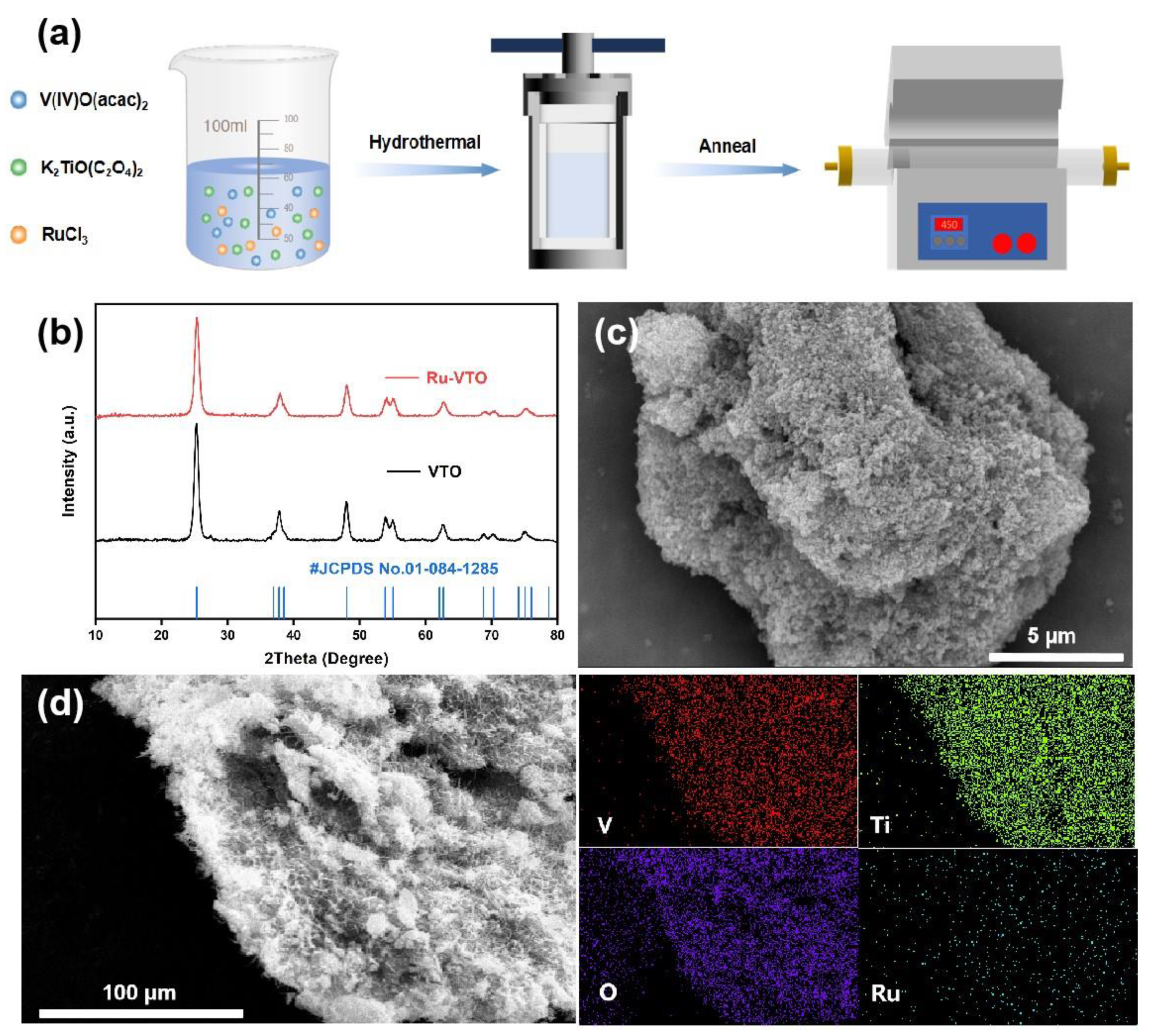
2.1. Sample Preparation
2.2. Electrochemical Measurements
2.3. Characterizations
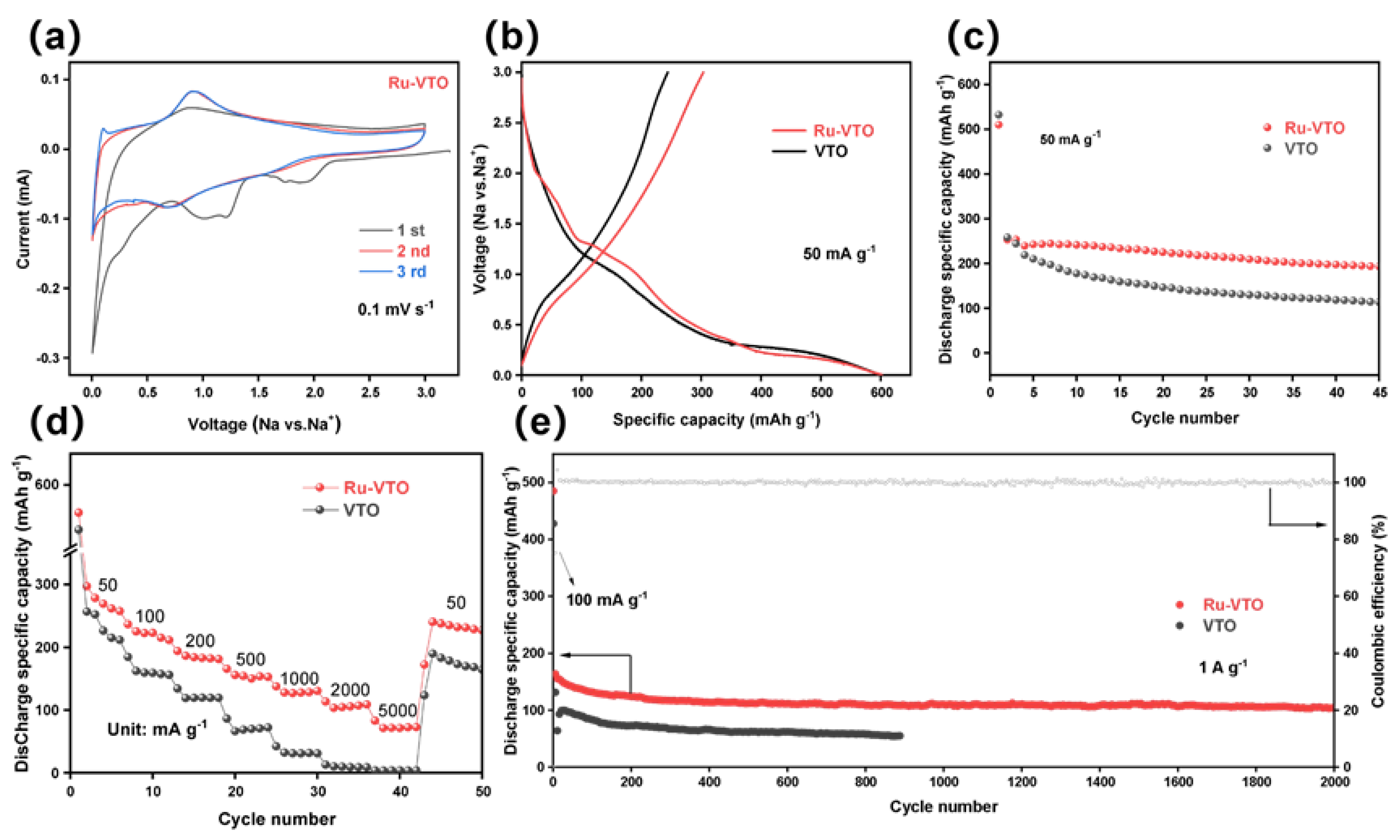
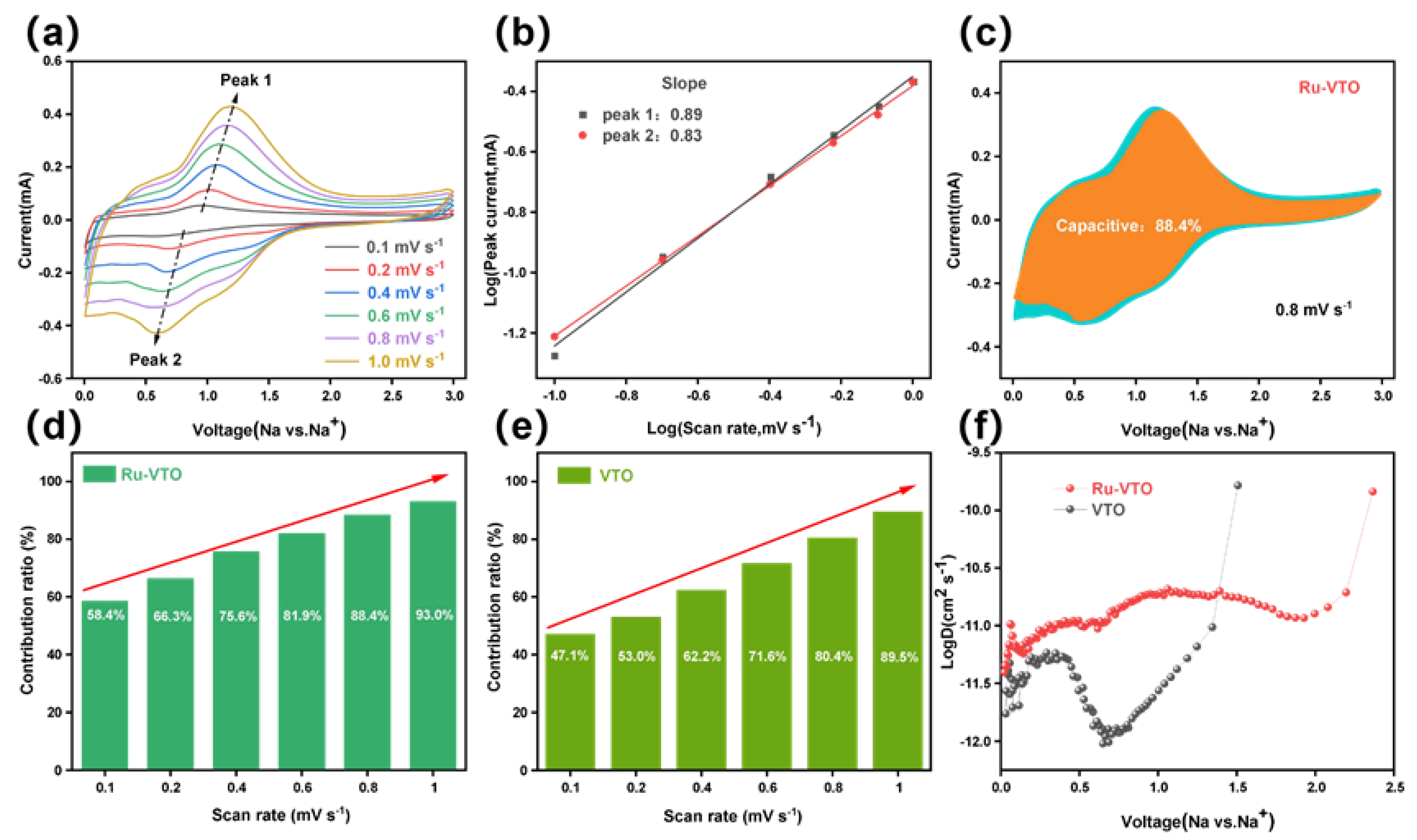
3. Results
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Chen, T.; Ouyang, B.; Fan, X.; Zhou, W.; Liu, W.; Liu, K. Oxide cathodes for sodium ion batteries: Designs, challenges, and perspectives. Carbon Energy 2022, 4, 170–199. [Google Scholar] [CrossRef]
- Hwang, J.Y.; Myung, S.T.; Sun, Y.K. Sodium-ion batteries: Present and future. Chem. Soc. Rev. 2017, 46, 3529–3614. [Google Scholar] [CrossRef] [Green Version]
- Duffner, F.; Kronemeyer, N.; Tübke, J.; Leker, J.; Winter, M.; Schmuch, R. Post-lithium-ion battery cell production and its compatibility with lithium-ion cell production infrastructure. Nat. Energy 2021, 6, 123–134. [Google Scholar] [CrossRef]
- Hezhen, Z.; Xuanpeng, W.; Kang, H.; Chen, Y.; Ruizhe, W.; Liming, W.; Liqiang, M. Enhanced lithium storage stability mechanism of ultra-high Nickel LiNi0.91Co0.06Al0.03O2@Ca3(PO4)2 cathode materials. J. Inorg. Mater. 2022, 37, 1030. [Google Scholar]
- Grey, C.P.; Hall, D.S. Prospects for lithium-ion batteries and beyond-a 2030 vision. Nat. Commun. 2020, 11, 6279. [Google Scholar] [CrossRef]
- Ma, X.; Qiao, F.; Qian, M.; Ye, Y.; Cao, X.; Wei, Y.; Li, N.; Sha, M.; Zi, Z.; Dai, J. Facile fabrication of flexible electrodes with poly(vinylidene fluoride)/Si3N4 composite separator prepared by electrospinning for sodium-ion batteries. Scr. Mater. 2021, 190, 153–157. [Google Scholar] [CrossRef]
- Nayak, P.K.; Yang, L.; Brehm, W.; Adelhelm, P. From lithium-ion to sodium-ion batteries: Advantages, challenges, and surprises. Angew. Chem. Int. Ed. 2018, 57, 102–120. [Google Scholar] [CrossRef]
- Trahey, L.; Brushett, F.R.; Balsara, N.P.; Ceder, G.; Cheng, L.; Chiang, Y.M.; Hahn, N.T.; Ingram, B.J.; Minteer, S.D.; Moore, J.S.; et al. Energy storage emerging: A perspective from the Joint Center for Energy Storage Research. Proc. Natl. Acad. Sci. USA 2020, 117, 12550–12557. [Google Scholar] [CrossRef]
- Dahbi, M.; Yabuuchi, N.; Kubota, K.; Tokiwa, K.; Komaba, S. Negative electrodes for Na-ion batteries. Phys. Chem. Chem. Phys. 2014, 16, 15007–15028. [Google Scholar] [CrossRef]
- He, H.; Sun, D.; Tang, Y.; Wang, H.; Shao, M. Understanding and improving the initial Coulombic efficiency of high-capacity anode materials for practical sodium-ion batteries. Energy Storage Mater. 2019, 23, 233–251. [Google Scholar] [CrossRef]
- Zhao, L.F.; Hu, Z.; Lai, W.H.; Tao, Y.; Peng, J.; Miao, Z.C.; Wang, Y.X.; Chou, S.L.; Liu, H.K.; Dou, S.X. Hard Carbon Anodes: Fundamental Understanding and Commercial Perspectives for Na-Ion Batteries beyond Li-ion and K-ion Counterparts. Adv. Energy Mater. 2020, 11, 2002704. [Google Scholar] [CrossRef]
- Sun, N.; Guan, Y.; Liu, Y.-T.; Zhu, Q.; Shen, J.; Liu, H.; Zhou, S.; Xu, B. Facile synthesis of free-standing, flexible hard carbon anode for high-performance sodium-ion batteries using graphene as a multi-functional binder. Carbon 2018, 137, 475–483. [Google Scholar] [CrossRef]
- Sun, Y.; Guo, S.; Zhou, H. Exploration of advanced electrode materials for rechargeable sodium-ion batteries. Adv. Energy Mater. 2018, 9, 1800212. [Google Scholar] [CrossRef]
- Tang, J.; Peng, X.; Lin, T.; Huang, X.; Luo, B.; Wang, L. Confining ultrafine tin monophosphide in Ti3C2Tx interlayers for rapid and stable sodium ion storage. eScience 2021, 1, 203–211. [Google Scholar] [CrossRef]
- Abubshait, H.A.; Alshahrani, T.; Alhashim, H.H.; Flemban, T.H.; Ali, G.; Mahmood, Q.; Laref, A.; Kattan, N.A. Dual coating strategy of CoS2@Co@C toward fast insertion/extraction anode material for sodium-ion batteries. Int. J. Energy Res. 2020, 45, 5283–5292. [Google Scholar] [CrossRef]
- Li, L.; Zheng, Y.; Zhang, S.; Yang, J.; Shao, Z.; Guo, Z. Recent progress on sodium-ion batteries: Potential high-performance anodes. Energy Environ. Sci. 2018, 11, 2310–2340. [Google Scholar] [CrossRef] [Green Version]
- Chen, Z.; Xu, L.; Chen, Q.; Hu, P.; Liu, Z.; Yu, Q.; Zhu, T.; Liu, H.; Hu, G.; Zhu, Z.; et al. Spray-pyrolysis-assisted synthesis of yolk@shell anatase with rich oxygen vacancies for efficient sodium storage. J. Mater. Chem. A 2019, 7, 6740–6746. [Google Scholar] [CrossRef]
- Liu, L.; Chen, X. Titanium dioxide nanomaterials: Self-structural modifications. Chem. Rev. 2014, 114, 9890–9918. [Google Scholar] [CrossRef] [PubMed]
- Nozik, A.J. Photoelectrolysis of water using semiconducting TiO2 crystals. Nature 1975, 257, 383–386. [Google Scholar] [CrossRef]
- Qiao, Y.; Hu, X.; Liu, Y.; Liang, G.; Croft, M.C.; Huang, Y. Surface modification of MoOxSy on porous TiO2 nanospheres as an anode material with highly reversible and ultra-fast lithium storage properties. J. Mater. Chem. A 2013, 1, 15128–15134. [Google Scholar] [CrossRef]
- Xia, T.; Zhang, W.; Murowchick, J.; Liu, G.; Chen, X. Built-in electric field-assisted surface-amorphized nanocrystals for high-rate lithium-ion battery. Nano Lett. 2013, 13, 5289–5296. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yue, L.; Zhao, H.; Wu, Z.; Liang, J.; Lu, S.; Chen, G.; Gao, S.; Zhong, B.; Guo, X.; Sun, X. Recent advances in electrospun one-dimensional carbon nanofiber structures/heterostructures as anode materials for sodium-ion batteries. J. Mater. Chem. A 2020, 8, 11493–11510. [Google Scholar] [CrossRef]
- Ni, Q.; Dong, R.; Bai, Y.; Wang, Z.; Ren, H.; Sean, S.; Wu, F.; Xu, H.; Wu, C. Superior sodium-storage behavior of flexible anatase TiO2 promoted by oxygen vacancies. Energy Storage Mater. 2020, 25, 903–911. [Google Scholar] [CrossRef]
- Rhee, O.; Lee, G.; Choi, J. Highly ordered TiO2 microcones with high rate performance for enhanced lithium-ion storage. ACS Appl. Mater. Interfaces 2016, 8, 14558–14563. [Google Scholar] [CrossRef]
- Yin, J.; Yang, H.; Kong, W.; Man, J.; Zhou, Z.; Feng, W.; Sun, J.; Wen, Z. Highly compacted TiO(2)/C microspheres via in-situ surface-confined intergrowth with ultra-long life for reversible Na-ion storage. J. Colloid Interface Sci. 2021, 582 (Pt B), 526–534. [Google Scholar] [CrossRef]
- Fang, X.; Ge, M.; Rong, J.; Zhou, C. Free-standing LiNi0.5Mn1.5O4/carbon nanofiber network film as lightweight and high-power cathode for lithium-ion batteries. ACS Nano 2014, 8, 4876–4882. [Google Scholar] [CrossRef]
- Wu, Z.; Han, X.; Zheng, J.; Wei, Y.; Qiao, R.; Shen, F.; Dai, J.; Hu, L.; Xu, K.; Lin, Y.; et al. Depolarized and fully active cathode based on Li(Ni0.5Co0.2Mn0.3)O2 embedded in carbon nanotube network for advanced batteries. Nano Lett. 2014, 14, 4700–4706. [Google Scholar] [CrossRef] [PubMed]
- Sheng, J.; Peng, C.; Yan, S.; Zhang, G.; Jiang, Y.; An, Q.; Wei, Q.; Ru, Q.; Mai, L. New anatase phase VTi2.6O7.2 ultrafine nanocrystals for high-performance rechargeable magnesium-based batteries. J. Mater. Chem. A 2018, 6, 13901–13907. [Google Scholar] [CrossRef]
- Song, W.; Ma, C.; Yan, S.; Shi, R.; Zhang, L.; Gao, T.; Li, B.; Chen, N.; Qiu, Z. An efficient Se-doping strategy to boost the sodium storage capacity of anatase TiO2 nanospheres. Scr. Mater. 2022, 215, 114705. [Google Scholar] [CrossRef]
- Wu, Y.; Liu, X.; Yang, Z.; Gu, L.; Yu, Y. Nitrogen-Doped Ordered Mesoporous Anatase TiO2 Nanofibers as Anode Materials for High-Performance Sodium-Ion Batteries. Small 2016, 12, 3522–3529. [Google Scholar] [CrossRef]
- Yan, D.; Yu, C.; Bai, Y.; Zhang, W.; Chen, T.; Hu, B.; Sun, Z.; Pan, L. Sn-doped TiO2 nanotubes as superior anode materials for sodium-ion batteries. Chem. Commun. 2015, 51, 8261–8264. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Sort, V.; Moudrakovski, I.; Feng, Y.; Aken, P.A.; Maier, J.; Yu, Y. Boosting sodium storage in TiF3/Carbon Core/Sheath nanofibers through an efficient mixed-conducting network. Adv. Energy Mater. 2019, 9, 1901470. [Google Scholar] [CrossRef]
- Sanad, M.; Rashad, M.M.; Powers, K. Enhancement of the electrochemical performance of hydrothermally prepared anatase nanoparticles for optimal use as high capacity anode materials in lithium ion batteries (LIBs). Appl. Phys. A 2015, 118, 665–674. [Google Scholar] [CrossRef]
- Peng, M.; Zhang, D.; Zheng, L.; Wang, X.; Lin, Y.; Xia, D.; Sun, Y.; Guo, G. Hierarchical Ru-doped sodium vanadium fluorophosphate hollow microspheres as a cathode of enhanced superior rate capability and ultralong stability for sodium-ion batteries. Nano Energy 2017, 31, 64–73. [Google Scholar] [CrossRef]
- Muniz, F.T.L.; Miranda, M.R.; Morilla dos Santos, C.; Sasaki, J.M. The Scherrer equation and the dynamical theory of X-ray diffraction. Acta Crystallogr. Sect. A Found. Adv. 2016, 72, 385–390. [Google Scholar] [CrossRef] [PubMed]
- Igarashi, H.; Tsuji, K.; Okuhara, T.; Misono, M. Effects of consecutive oxidation on the production of maleic anhydride in butane oxidation over four kinds of well-characterized vanadyl pyrophosphates. J. Phys. Chem. 1993, 97, 7065–7071. [Google Scholar] [CrossRef]
- Moser, T.; Schrader, G. Stability of model VPO catalysts for maleic anhydride synthesis. J. Catal. 1987, 104, 99–108. [Google Scholar] [CrossRef]
- Gonbeau, D.; Guimon, C.; Pfister-Guillouzo, G.; Levasseur, A.; Meunier, G.; Dormoy, R. XPS study of thin films of titanium oxysulfides. Surf. Sci. 1991, 254, 81–89. [Google Scholar] [CrossRef]
- Saied, S.; Sullivan, J.; Choudhury, T.; Pearce, C. A comparison of ion and fast atom beam reduction in TiO2. Vacuum 1988, 38, 917–922. [Google Scholar] [CrossRef]
- Huang, C.S.; Houalla, M.; Hercules, D.M.; Kibby, C.L.; Petrakis, L. Comparison of catalysts derived from the oxidation of ruthenium-thorium (Ru3Th7) with impregnated ruthenium/thoria catalysts. J. Phys. Chem. 1989, 93, 4540–4544. [Google Scholar] [CrossRef]
- Oh, S.M.; Hwang, J.Y.; Yoon, C.S.; Lu, J.; Amine, K.; Belharouak, I.; Sun, Y.K. High electrochemical performances of microsphere C-TiO(2) anode for sodium-ion battery. Appl. Mater. Interfaces 2014, 6, 11295–11301. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Zhou, M.; Wen, L.; Wang, C.; Zhao, H.; Mi, Y.; Liang, L.; Fu, Q.; Wu, M.; Lei, Y. Highly ordered three-dimensional Ni-TiO2 nanoarrays as sodium ion battery anodes. Chem. Mater. 2015, 27, 4274–4280. [Google Scholar] [CrossRef]
- Chen, J.; Ding, Z.; Wang, C.; Hou, H.; Zhang, Y.; Wang, C.; Zou, G.; Ji, X. Black anatase titania with ultrafast sodium-storage performances stimulated by oxygen vacancies. ACS Appl. Mater. Interfaces 2016, 8, 9142–9151. [Google Scholar] [CrossRef] [PubMed]
- He, H.; Gan, Q.; Wang, H.; Xu, G.-L.; Zhang, X.; Huang, D.; Fu, F.; Tang, Y.; Amine, K.; Shao, M. Structure-dependent performance of TiO2/C as anode material for Na-ion batteries. Nano Energy 2018, 44, 217–227. [Google Scholar] [CrossRef]
- McNulty, D.; Carroll, E.; O’Dwyer, C. Rutile TiO2 inverse opal anodes for Li-ion batteries with long cycle life, high-rate capability, and high structural stability. Adv. Energy Mater. 2017, 7, 1602291. [Google Scholar] [CrossRef]
- Zhang, Y.; Ding, Z.; Foster, C.W.; Banks, C.E.; Qiu, X.; Ji, X. Oxygen vacancies evoked blue TiO2(B) nanobelts with efficiency enhancement in sodium storage behaviors. Adv. Funct. Mater. 2017, 27, 1700856. [Google Scholar] [CrossRef]
- Augustyn, V.; Come, J.; Lowe, M.A.; Kim, J.W.; Taberna, P.L.; Tolbert, S.H.; Abruna, H.D.; Simon, P.; Dunn, B. High-rate electrochemical energy storage through Li+ intercalation pseudocapacitance. Nat. Mater. 2013, 12, 518–522. [Google Scholar] [CrossRef]
- Wu, L.; Bresser, D.; Buchholz, D.; Giffin, G.A.; Castro, C.R.; Ochel, A.; Passerini, S. Unfolding the mechanism of sodium insertion in anatase TiO2 nanoparticles. Adv. Energy Mater. 2015, 5, 1401142. [Google Scholar] [CrossRef]
- Zou, G.; Wang, C.; Hou, H.; Wang, C.; Qiu, X.; Ji, X. Controllable interlayer spacing of sulfur-doped graphitic carbon nanosheets for fast sodium-ion batteries. Small 2017, 13, 1700762. [Google Scholar] [CrossRef]
- Deiss, E. Spurious chemical diffusion coefficients of Li+ in electrode materials evaluated with GITT. Electrochim. Acta 2005, 50, 2927–2932. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, G.; Han, C.; Han, K.; Liu, J.; Chen, J.; Wang, H.; Zhang, L.; Wang, X. A Ru-Doped VTi2.6O7.2 Anode with High Conductivity for Enhanced Sodium Storage. Coatings 2023, 13, 490. https://doi.org/10.3390/coatings13030490
Zhang G, Han C, Han K, Liu J, Chen J, Wang H, Zhang L, Wang X. A Ru-Doped VTi2.6O7.2 Anode with High Conductivity for Enhanced Sodium Storage. Coatings. 2023; 13(3):490. https://doi.org/10.3390/coatings13030490
Chicago/Turabian StyleZhang, Guangwan, Chunhua Han, Kang Han, Jinshuai Liu, Jinghui Chen, Haokai Wang, Lei Zhang, and Xuanpeng Wang. 2023. "A Ru-Doped VTi2.6O7.2 Anode with High Conductivity for Enhanced Sodium Storage" Coatings 13, no. 3: 490. https://doi.org/10.3390/coatings13030490
APA StyleZhang, G., Han, C., Han, K., Liu, J., Chen, J., Wang, H., Zhang, L., & Wang, X. (2023). A Ru-Doped VTi2.6O7.2 Anode with High Conductivity for Enhanced Sodium Storage. Coatings, 13(3), 490. https://doi.org/10.3390/coatings13030490





