Comparison of Self-Assembled Monolayers Using 3-Aminopropyltrimethoxysilane and Decyltrimethoxysilane in Vapor Phase for Porous SiOCH Dielectrics
Abstract
1. Introduction
2. Experimental
2.1. Samples Preparation
2.2. Sample Characterization
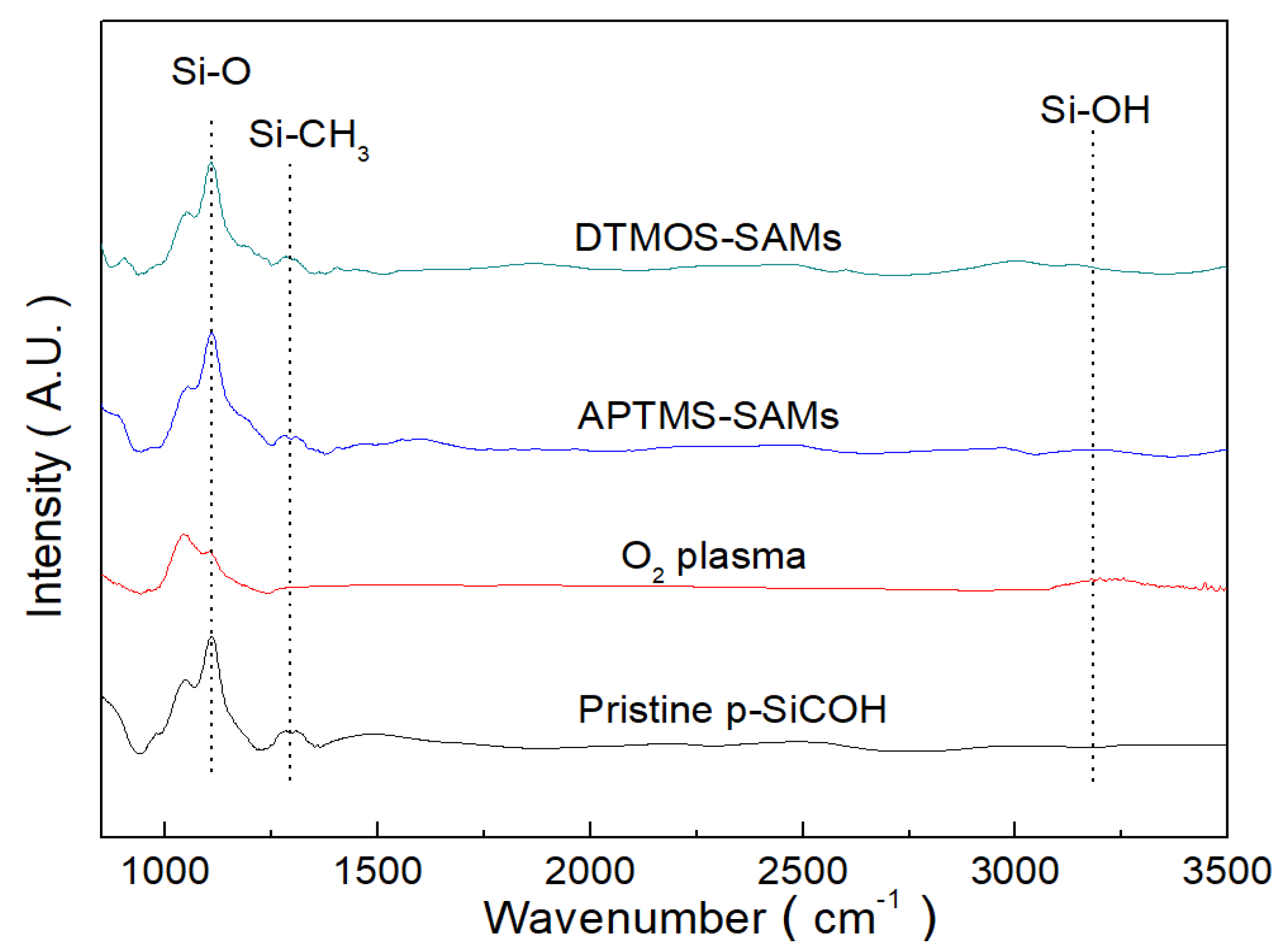
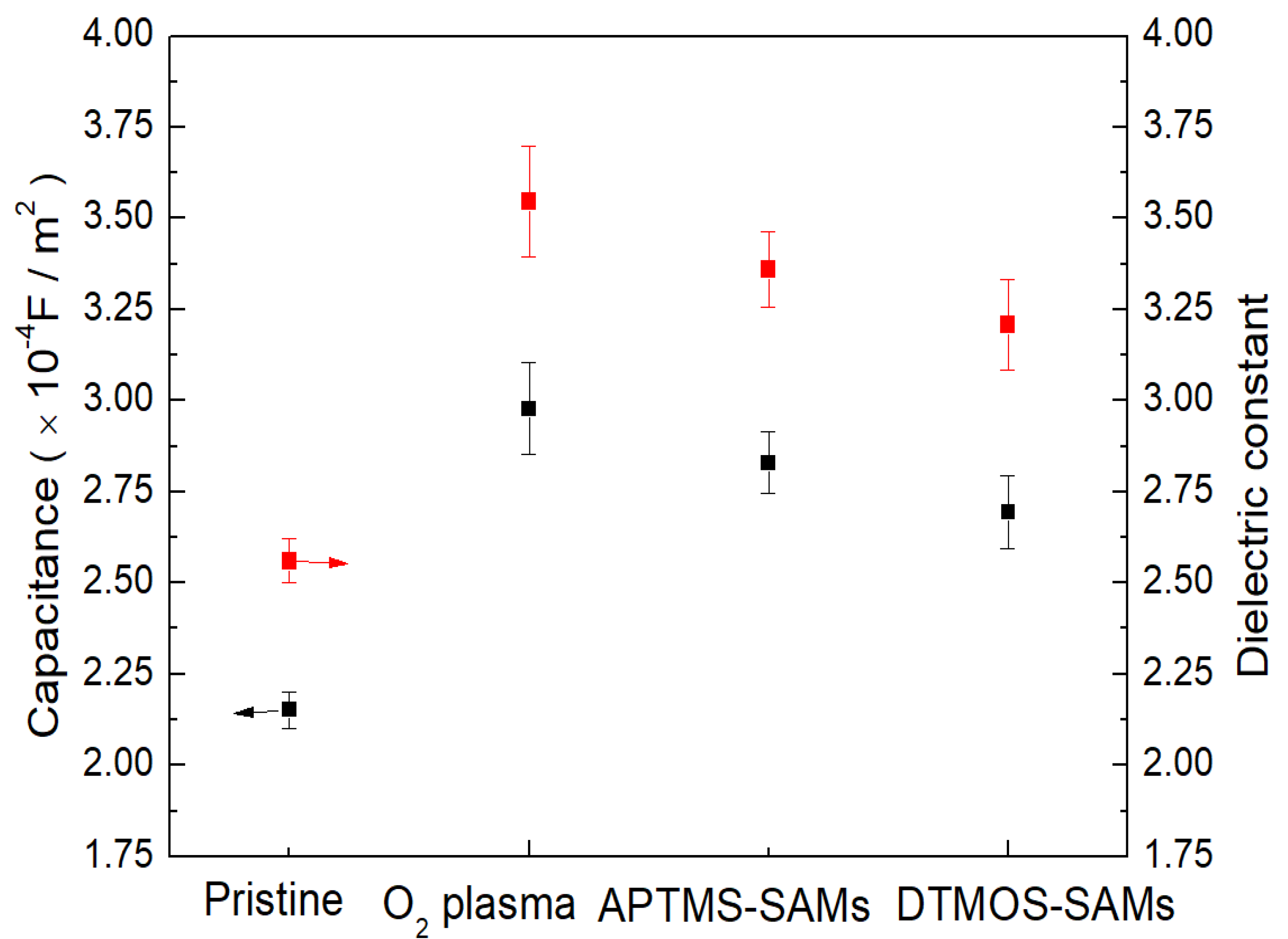
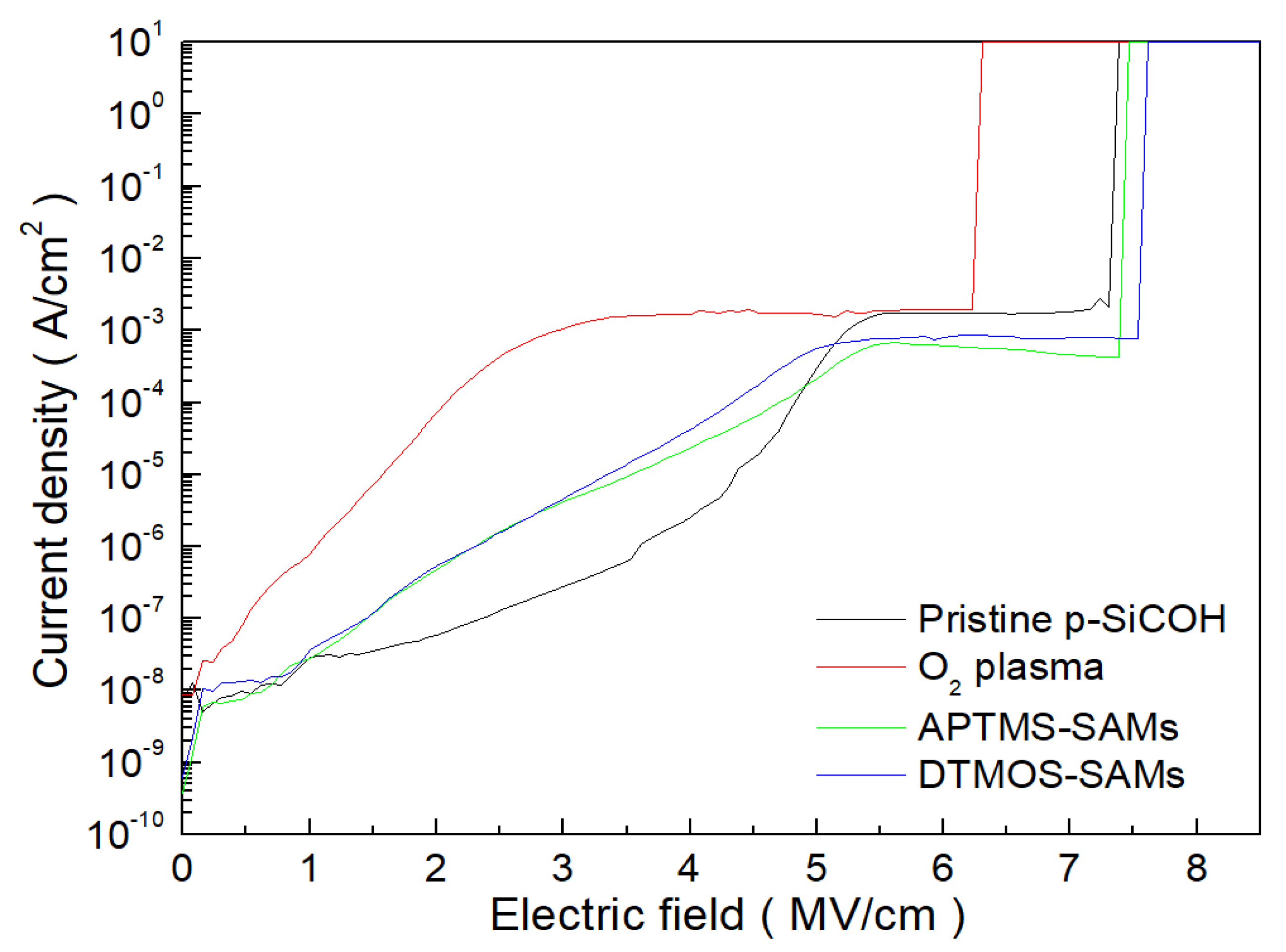
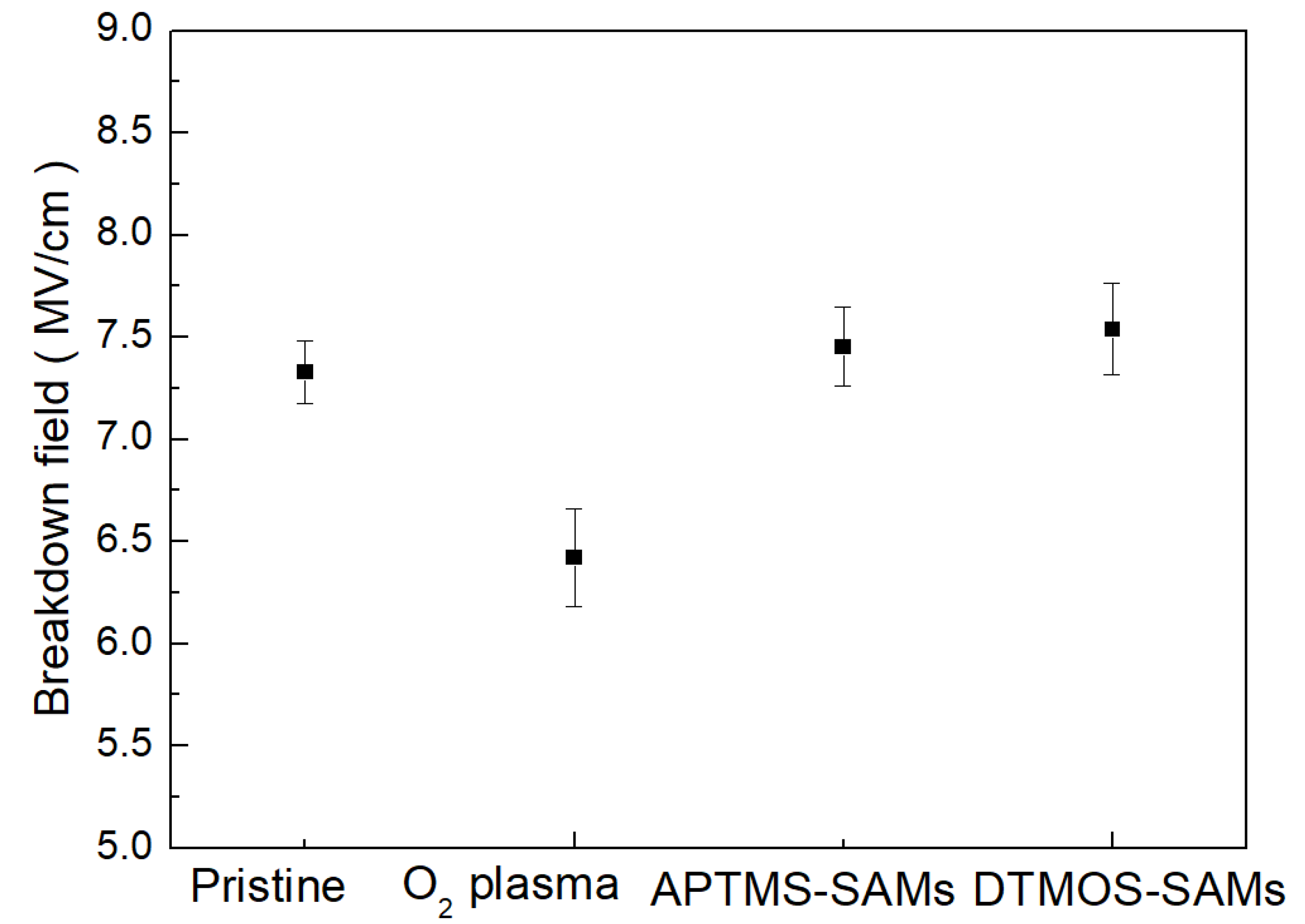
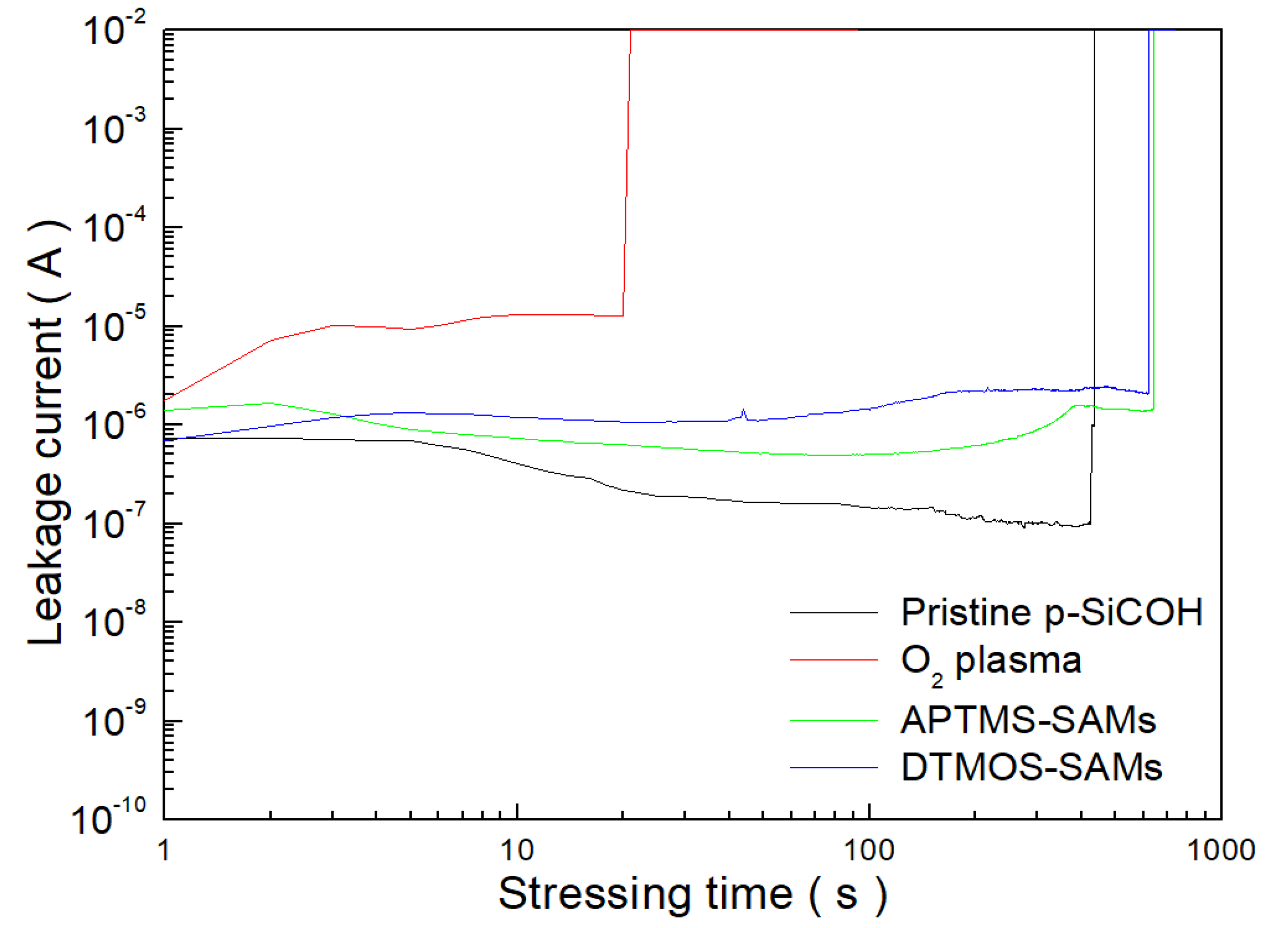
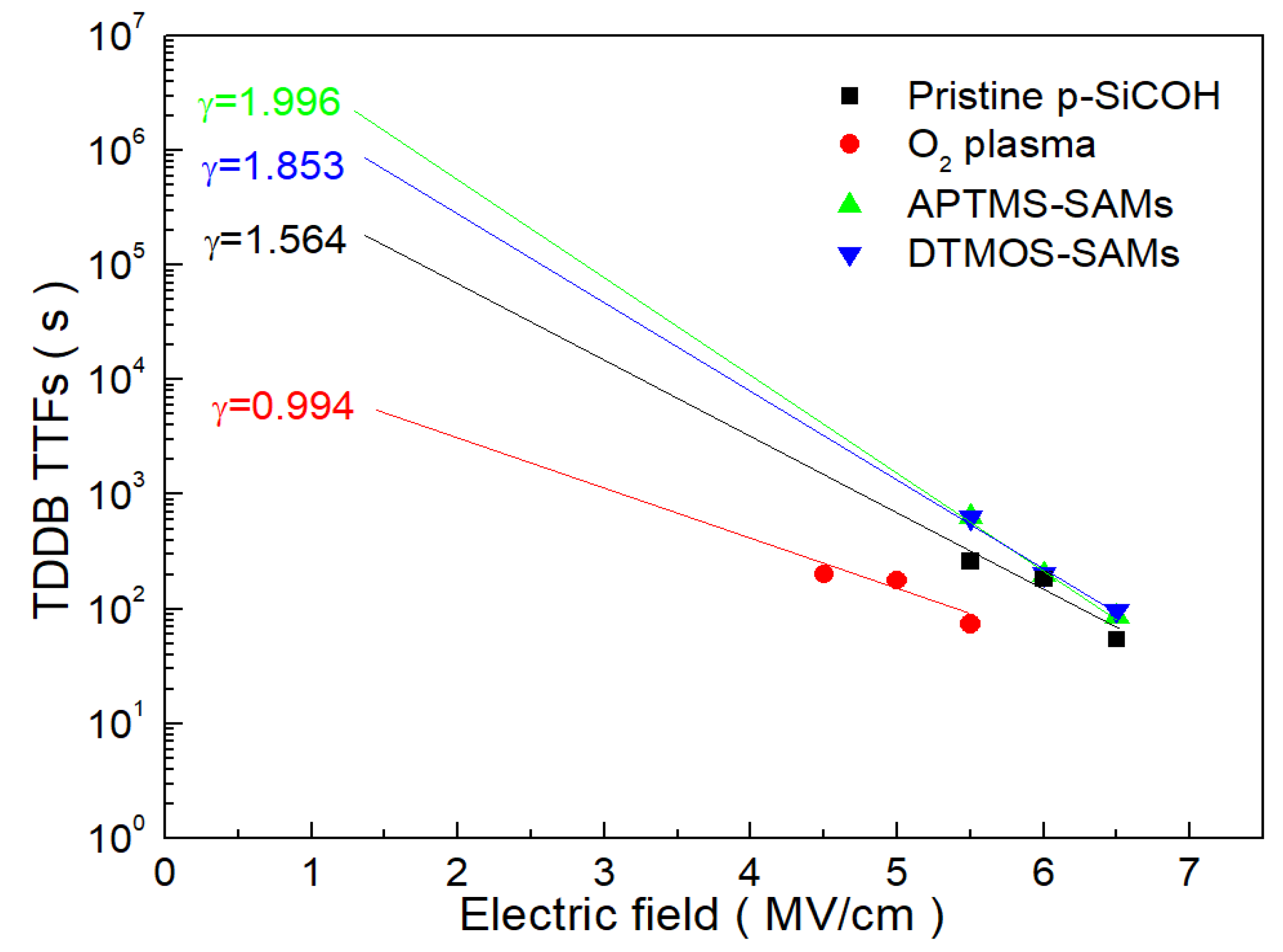
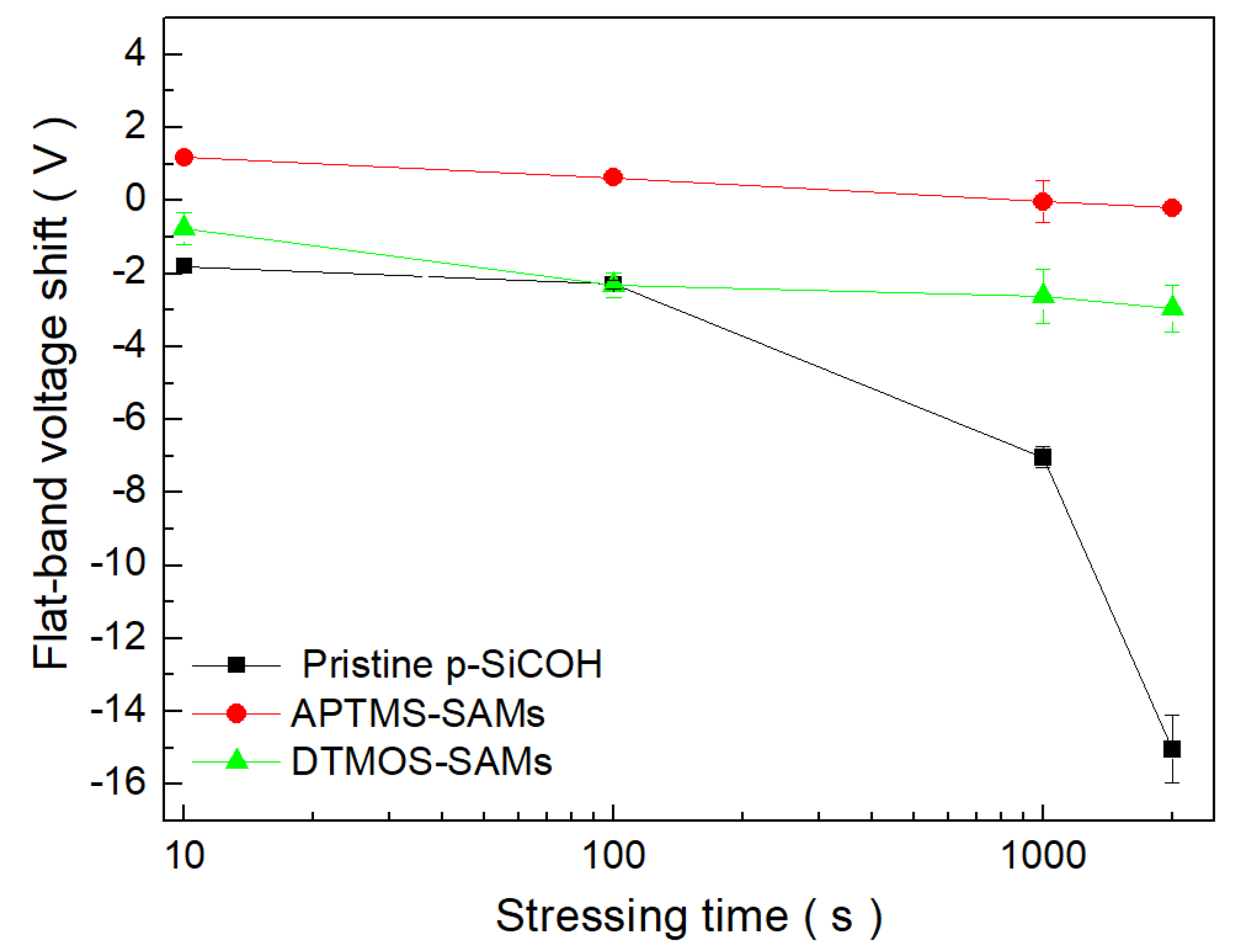
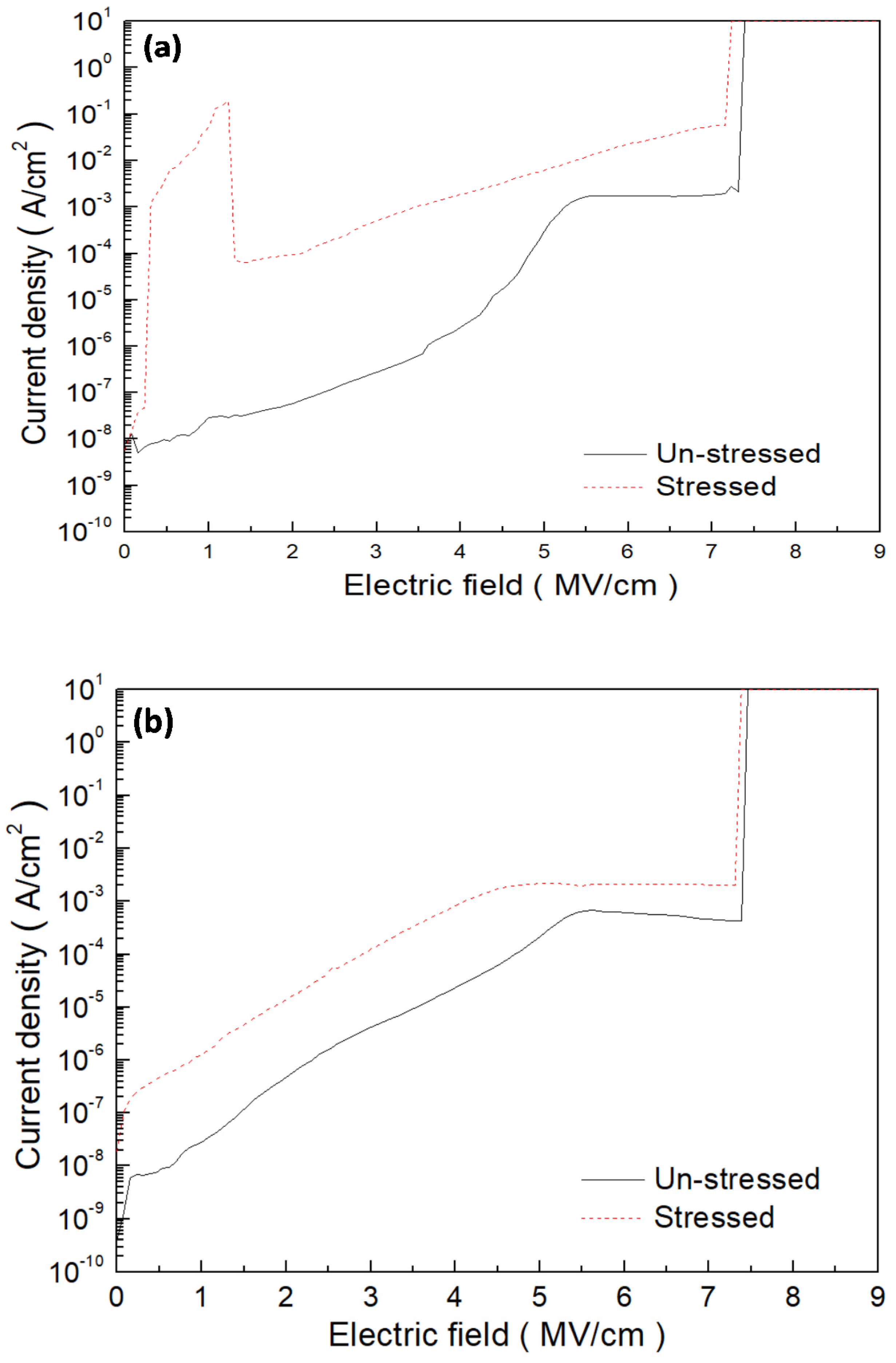
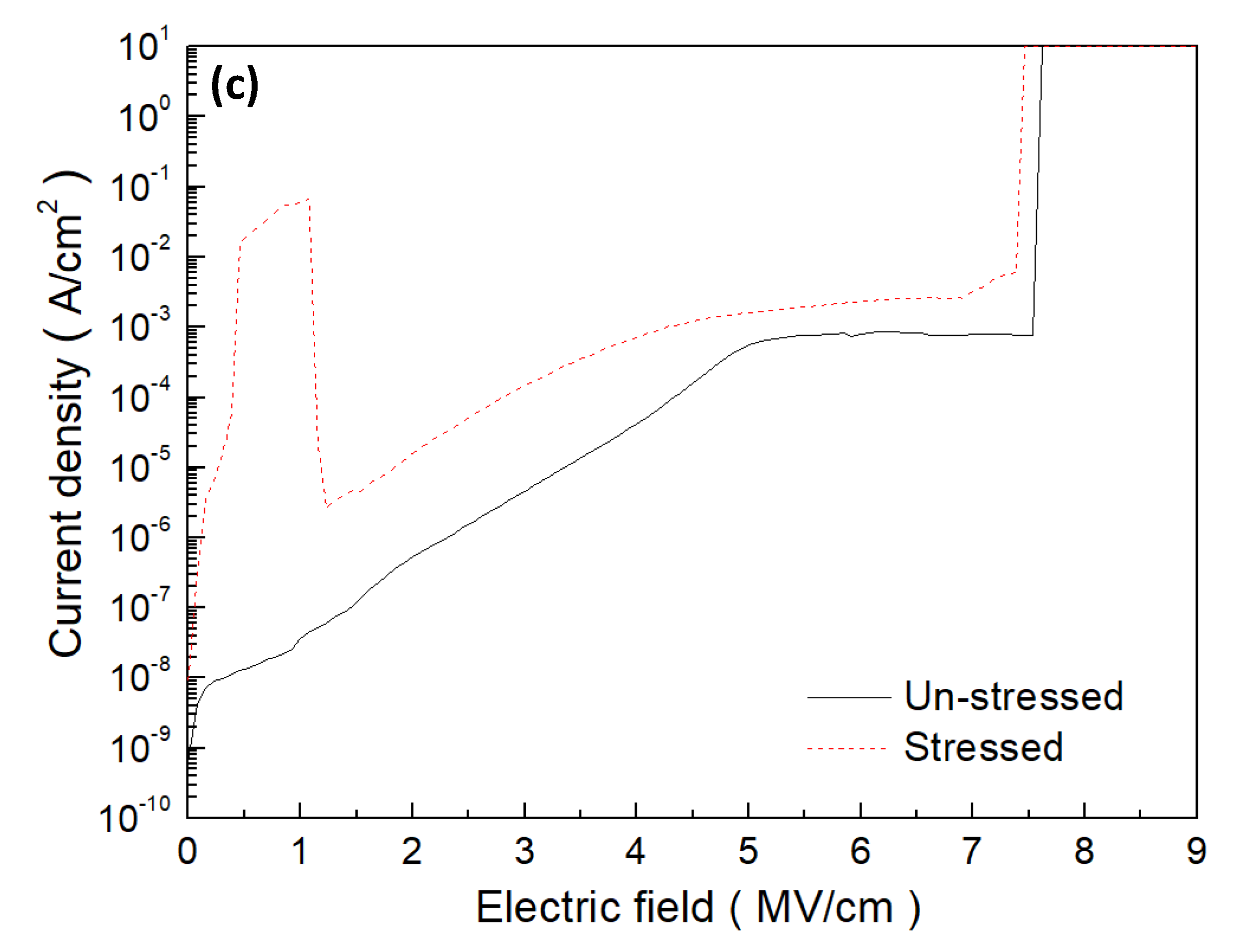
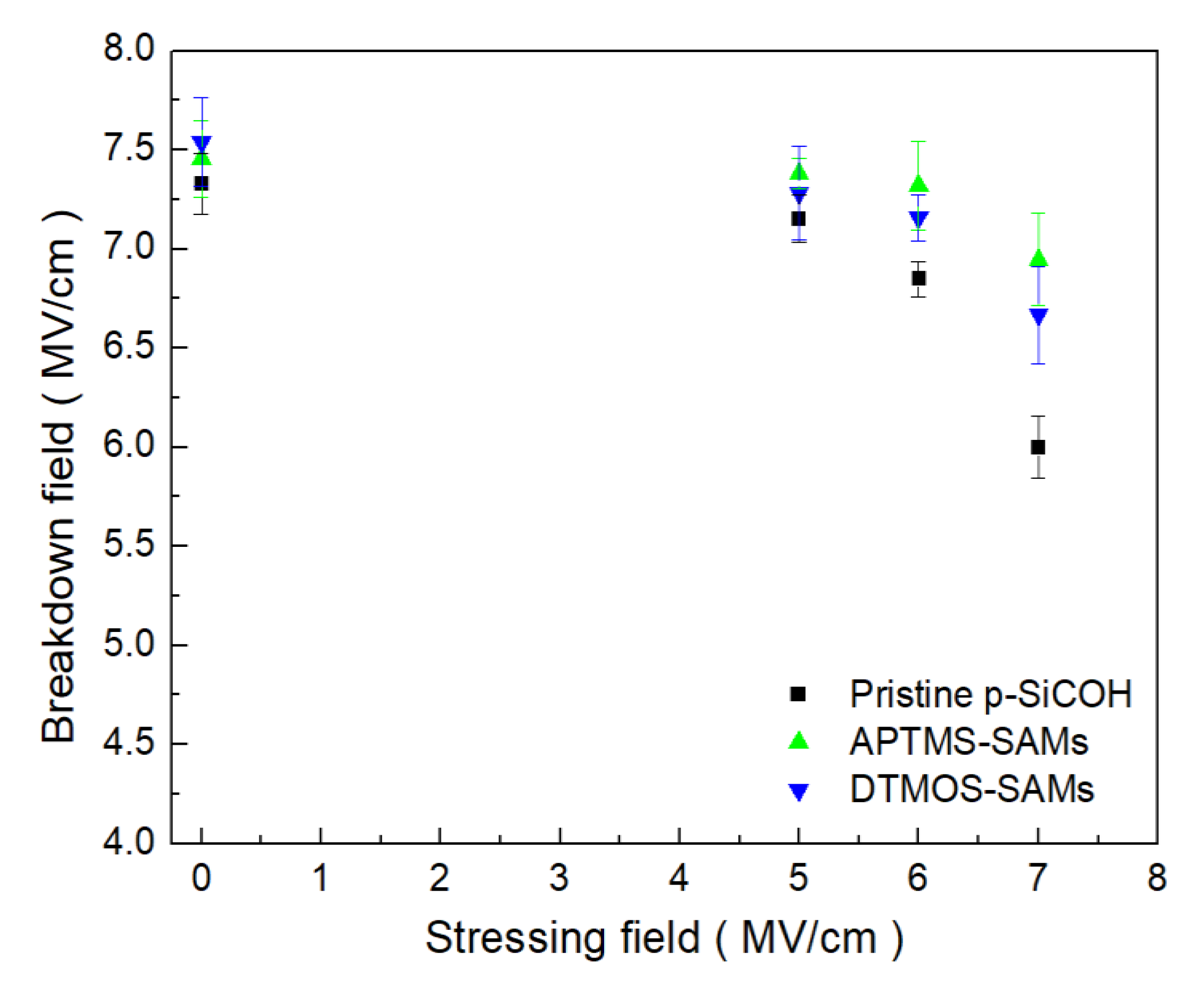
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Maex, K.; Baklanov, M.R.; Shamiryan, D.; Iacopi, F.; Brongersma, S.H.; Yanovitskaya, Z.S. Low dielectric constant materials for microelectronics. J. Appl. Phys. 2003, 93, 8793–8841. [Google Scholar] [CrossRef]
- Grill, A. Porous pSiCOH ultralow-k dielectrics for chip interconnects prepared by PECVD. Annu. Rev. Mater. Res. 2009, 39, 49–69. [Google Scholar] [CrossRef]
- Grill, A.; Gates, S.M.; Ryan, T.E.; Nguyen, S.V.; Priyadarshini, D. Progress in the development and understanding of advanced low k and ultralow k dielectrics for very large-scale integrated interconnects—State of the art. Appl. Phys. Rev. 2014, 1, 011306. [Google Scholar] [CrossRef]
- Grill, A. Plasma enhanced chemical vapor deposited SiCOH dielectrics: From low-k to extreme low-k interconnect materials. J. Appl. Phys. 2003, 93, 1785. [Google Scholar] [CrossRef]
- Cheng, Y.L.; Lin, B.H.; Huang, S.W. Effect of O2 Plasma Treatment on Physical, Electrical, and Reliability Characteristics of Low Dielectric Constant Materials. Thin Solid Films 2014, 572, 44–50. [Google Scholar] [CrossRef]
- Baklanov, M.R.; de Marneffe, J.-F.; Shamiryan, D.; Urbanowicz, A.M.; Shi, H.; Rakhimova, T.V.; Huang, H.; Ho, P.S. Plasma processing of low-k dielectrics. J. Appl. Phys. 2013, 113, 041101-1. [Google Scholar] [CrossRef]
- Shi, H.; Huang, H.; Bao, J.; Liu, J.; Ho, P.S. Roles of ions, photons, and radicals in inducing plasma damage to ultra low-k dielectrics. J. Vac. Sci. Technol. B 2012, 30, 011206-1. [Google Scholar] [CrossRef]
- Hosseini, M.; Koike, J. Amorphous CoTix as a liner/diffusion barrier material for advanced copper metallization. J. Alloys Compd. 2017, 721, 134–142. [Google Scholar] [CrossRef]
- Fang, K.L.; Tsui, B.Y. Metal drift induced electrical instability of porous low dielectric constant film. J. Appl. Phys. 2003, 93, 5546. [Google Scholar] [CrossRef]
- Chen, F.; Bravo, O.; Harmon, D.; Shinosky, M.; Aitken, J. Cu/low-k dielectric TDDB reliability issues for advanced CMOS technologies. Microelectron. Reliab. 2008, 48, 1375–1383. [Google Scholar] [CrossRef]
- Li, Z.; Tian, Y.; Teng, C.; Cao, H. Recent Advances in Barrier Layer of Cu Interconnects. Materials 2020, 48, 5049. [Google Scholar] [CrossRef] [PubMed]
- Hosseini, M.; Ando, D.; Sutou, Y.; Koike, J. Co and CoTix for contact plug and barrier layer in integrated circuits. Microelectron. Eng. 2018, 189, 78. [Google Scholar] [CrossRef]
- Caro, A.M.; Maes, G.; Borghs, G.; Whelan, C.M. Screening self-assembled monolayers as Cu diffusion barriers. Microelectron. Eng. 2008, 85, 2047–2050. [Google Scholar] [CrossRef]
- Brady-Boyd, A.; O’Connor, R.; Armini, S.; Selvaraju, V.; Hughes, G.; Bogan, J. On the use of (3-trimethoxysilylpropyl)diethylenetriamine self-assembled monolayers as seed layers for the growth of Mn based copper diffusion barrier layers. Appl. Surf. Sci. 2018, 427, 260–266. [Google Scholar] [CrossRef]
- Aldakov, D.; Bonnassieux, Y.; Geffroy, B.; Palacin, S. Selective electroless copper deposition on self-assembled dithiol monolayers. ACS Appl. Mater. Interfaces 2009, 1, 584–589. [Google Scholar] [CrossRef] [PubMed]
- Uedono, A.; Armini, S.; Zhang, Y.; Kakizaki, T.; Krause-Rehberg, R.; Anwand, W.; Wagner, A. Surface sealing using self-assembled monolayers and its effect on metal diffusion in porous low-K dielectrics studied using monoenergetic positron beams. Appl. Surf. Sci. 2016, 368, 272–276. [Google Scholar] [CrossRef]
- Sharma, S.; Kumar, M.; Rani, S.; Kumar, D. Deposition and characterization of 3-aminopropyltrimethoxysilane monolayer diffusion barrier for copper metallization. Metall. Mater. Trans. B 2014, 46, 928–932. [Google Scholar] [CrossRef]
- Ganesan, P.G.; Singh, A.P.; Ramanath, G. Diffusion barrier properties of carboxyland amine-terminated molecular nanolayers. Appl. Phys. Lett. 2004, 85, 579. [Google Scholar]
- Cheng, Y.L.; Lee, C.Y.; Peng, W.F.; Chen, G.S.; Fang, J.S. Comparison of Self-Assembled Monolayers on SiO2 and Porous SiOCH, Dielectrics by Decyltrimethoxysilane Vapor Treatment. Coatings 2022, 12, 926. [Google Scholar] [CrossRef]
- Cheng, Y.L.; Lee, C.Y.; Huang, J.W.; Chen, G.S.; Fang, J.S. Comparison of Various Low Dielectric Constant Materials. Thin Solid Films 2018, 660, 871–878. [Google Scholar]
- Cheng, Y.L.; Leon, K.W.; Huang, J.F.; Chang, W.Y.; Chang, Y.M.; Leu, J. Effect of moisture on electrical properties and reliability of low dielectric constant materials. Microelectron. Eng. 2014, 114, 12–16. [Google Scholar] [CrossRef]
- Vanstreels, K.; Ciofi, I.; Barbarin, Y.; Baklanov, M. Influence of porosity on dielectric breakdown of ultralow-k dielectrics. J. Vac. Sci. Technol. B 2013, 31, 050604. [Google Scholar] [CrossRef]
- Kao, K.C.; Chang, W.Y.; Chang, Y.M.; Leu, J.; Cheng, Y.L. Comprehensively Study of UV Curing Time Effect on Physical, Electrical, and Reliability Properties for Low Dielectric Constant Materials. J. Vac. Sci. Technol. A 2014, 32, 061514. [Google Scholar] [CrossRef]
- Armini, S.; Prado, J.L.; Krishtab, M.; Swerts, J.; Verdonck, P.; Meersschaut, J.; Conard, T.; Blauw, M.; Struyf, H.; Baklanov, M.R. Pore sealing of k 2.0 dielectrics assisted by self-assembled monolayers deposited from vapor phase. Microelectron. Eng. 2014, 120, 240–245. [Google Scholar] [CrossRef]
- Ishikawa, D.; Kobayashi, A.; Nakano, A.; Kimura, Y.; Matsushita, K.; Kobayashi, N.; Ditmer, G.; Kiermasz, A. Plasma-enhanced atomic layer deposition sealing property on extreme low-k film with k = 2.0 quantified by mass metrology. Jpn. J. Appl. Phys. 2013, 52, 05FG01. [Google Scholar] [CrossRef]
- He, M.; Lu, T.-M. Metal-Dielectric Interfaces in Gigascale Electronics: Thermal and Electrical Stability; Series in Materials Science; Springer: Berlin/Heidelberg, Germany, 2012; Volume 157. [Google Scholar]
- Mcpherson, J.W. Time dependent dielectric breakdown physics—Models revisited. Microelectron. Reliab. 2012, 52, 1753–1760. [Google Scholar] [CrossRef]
- Chen, F.; Shinosky, M.A. Electron fluence driven, Cu catalyzed, interface breakdown mechanism for BEOL low-k time dependent dielectric breakdown. Microelectron. Reliab. 2014, 54, 529–540. [Google Scholar] [CrossRef]
| SAMs Precursor | Molecular Formular | Structure | Molar Mass (g/mole) | Density (g/cm3) | Boiling Point at 760 mmHg (°C) | Projected Length (nm) | ||
|---|---|---|---|---|---|---|---|---|
| Head Group | Chain | Functional Group | ||||||
| APTMS | NH2(CH2)3Si(OCH3)3 | -Si(OCH3)3 | (CH2)3 | -NH2 | 179.29 | 1.02 | 194 | 1.45 |
| DTMOS | CH3((CH2)9)Si(OCH3)3 | -Si(OCH3)3 | (CH2)9 | -CH3 | 262.461 | 0.9 | 213.6 | 2.25 |
| SAMs | Thickness (nm) | Contact Angle (Degree) | Roughness (RMS; nm) |
|---|---|---|---|
| APTMS-SAMs | 1.52 ± 0.19 | 73.2 | 0.314 |
| DTMOS-SAMs | 3.72 ± 0.18 | 82.6 | 0.351 |
| Structure | Stud-Pull Tape Result |
|---|---|
| Cu/p-SiOCH/Si | 58/80 (72.5%) |
| Cu/APTMS-SAMs/p-SiOCH/Si | 0/80 (0%) |
| Cu/DTMOS-SAMs/p-SiOCH/Si | 0/80 (0%) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cheng, Y.-L.; Kao, J.; Zhang, H.-W.; Lee, C.-Y. Comparison of Self-Assembled Monolayers Using 3-Aminopropyltrimethoxysilane and Decyltrimethoxysilane in Vapor Phase for Porous SiOCH Dielectrics. Coatings 2023, 13, 507. https://doi.org/10.3390/coatings13030507
Cheng Y-L, Kao J, Zhang H-W, Lee C-Y. Comparison of Self-Assembled Monolayers Using 3-Aminopropyltrimethoxysilane and Decyltrimethoxysilane in Vapor Phase for Porous SiOCH Dielectrics. Coatings. 2023; 13(3):507. https://doi.org/10.3390/coatings13030507
Chicago/Turabian StyleCheng, Yi-Lung, Joe Kao, Hao-Wei Zhang, and Chih-Yen Lee. 2023. "Comparison of Self-Assembled Monolayers Using 3-Aminopropyltrimethoxysilane and Decyltrimethoxysilane in Vapor Phase for Porous SiOCH Dielectrics" Coatings 13, no. 3: 507. https://doi.org/10.3390/coatings13030507
APA StyleCheng, Y.-L., Kao, J., Zhang, H.-W., & Lee, C.-Y. (2023). Comparison of Self-Assembled Monolayers Using 3-Aminopropyltrimethoxysilane and Decyltrimethoxysilane in Vapor Phase for Porous SiOCH Dielectrics. Coatings, 13(3), 507. https://doi.org/10.3390/coatings13030507





