Photocatalytic Self-Cleaning PVDF Membrane Blended with MWCNT-ZnO Nanocomposites for RhB Removal
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Synthesis of MWCNT-ZnO
2.3. Membrane Preparation
2.4. Methods
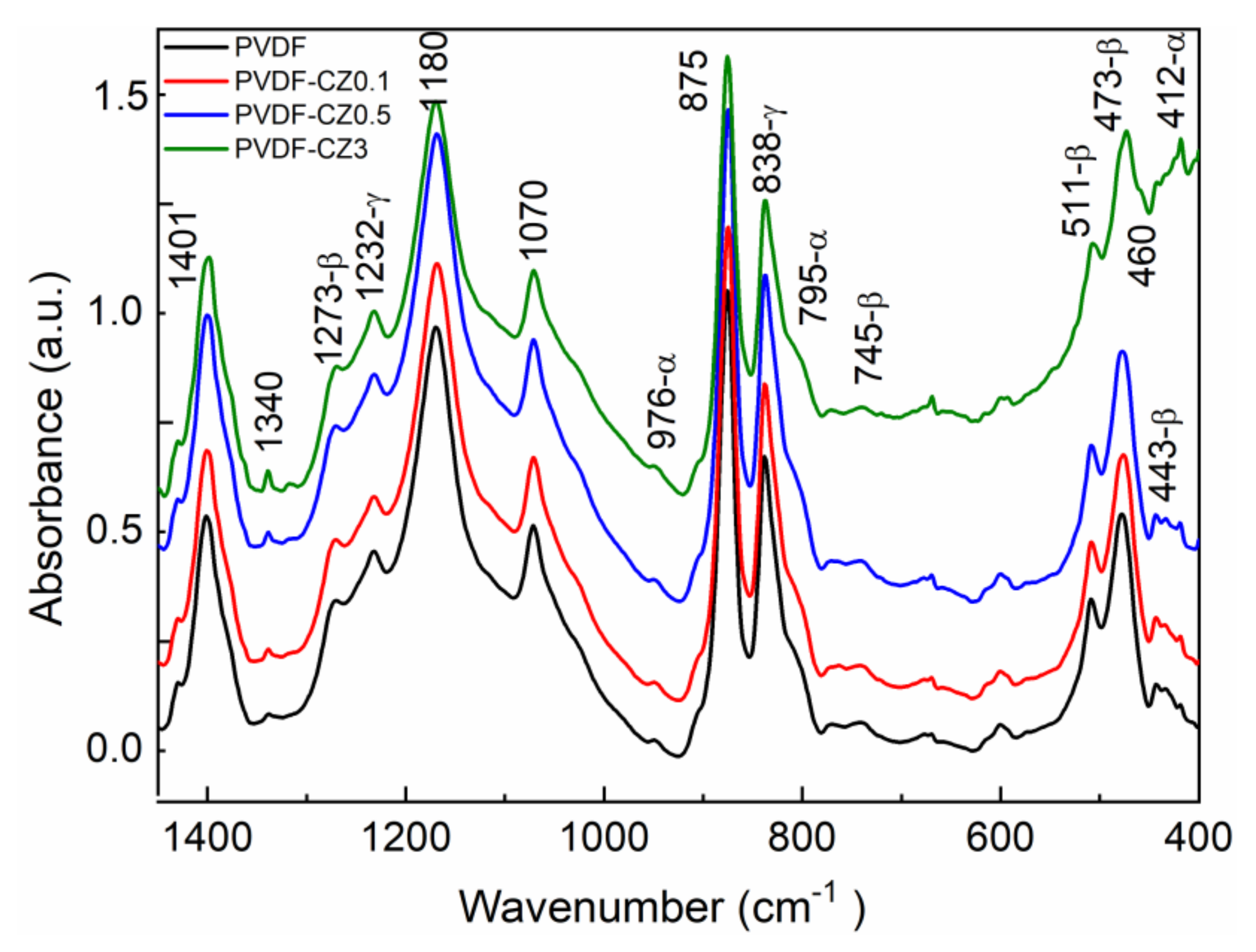
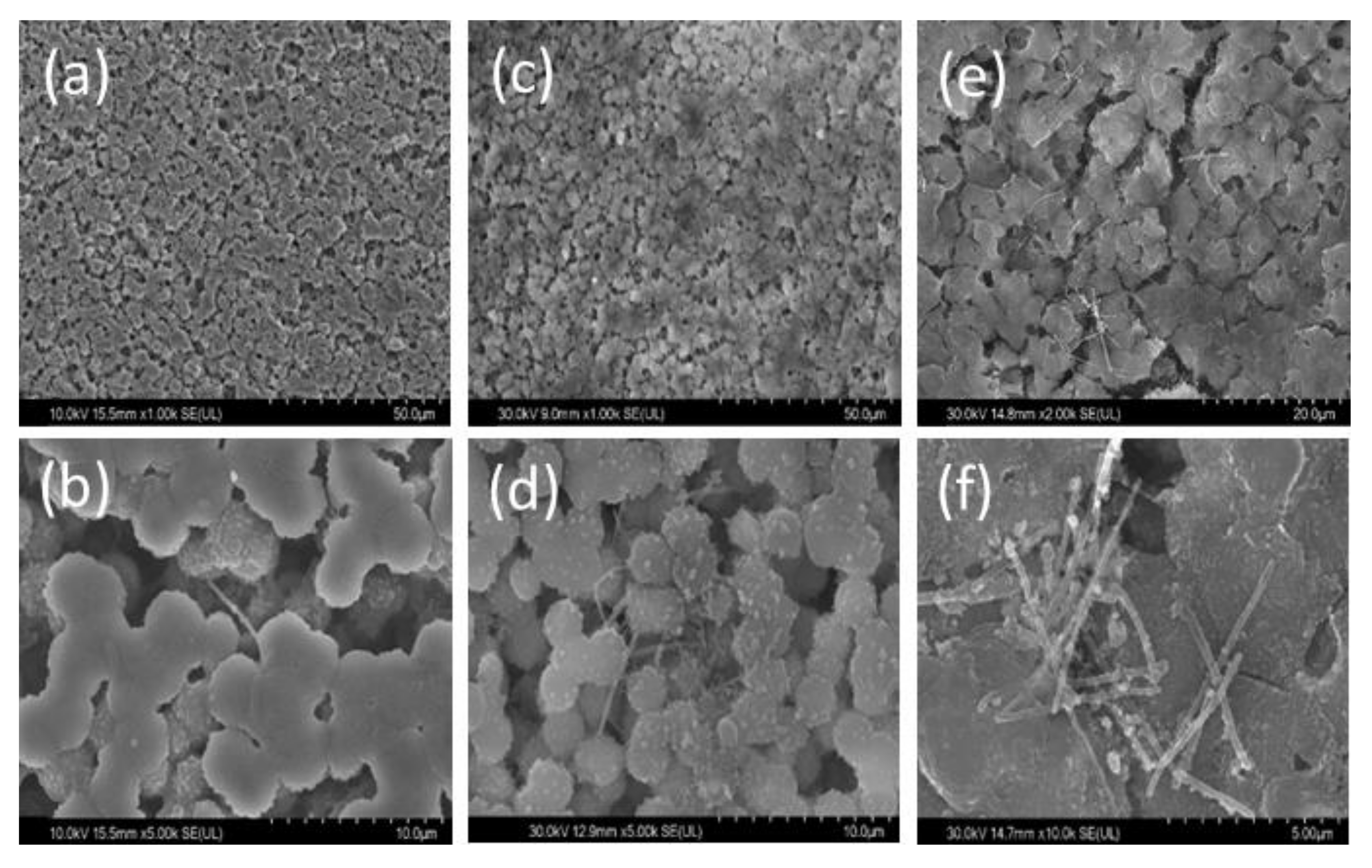
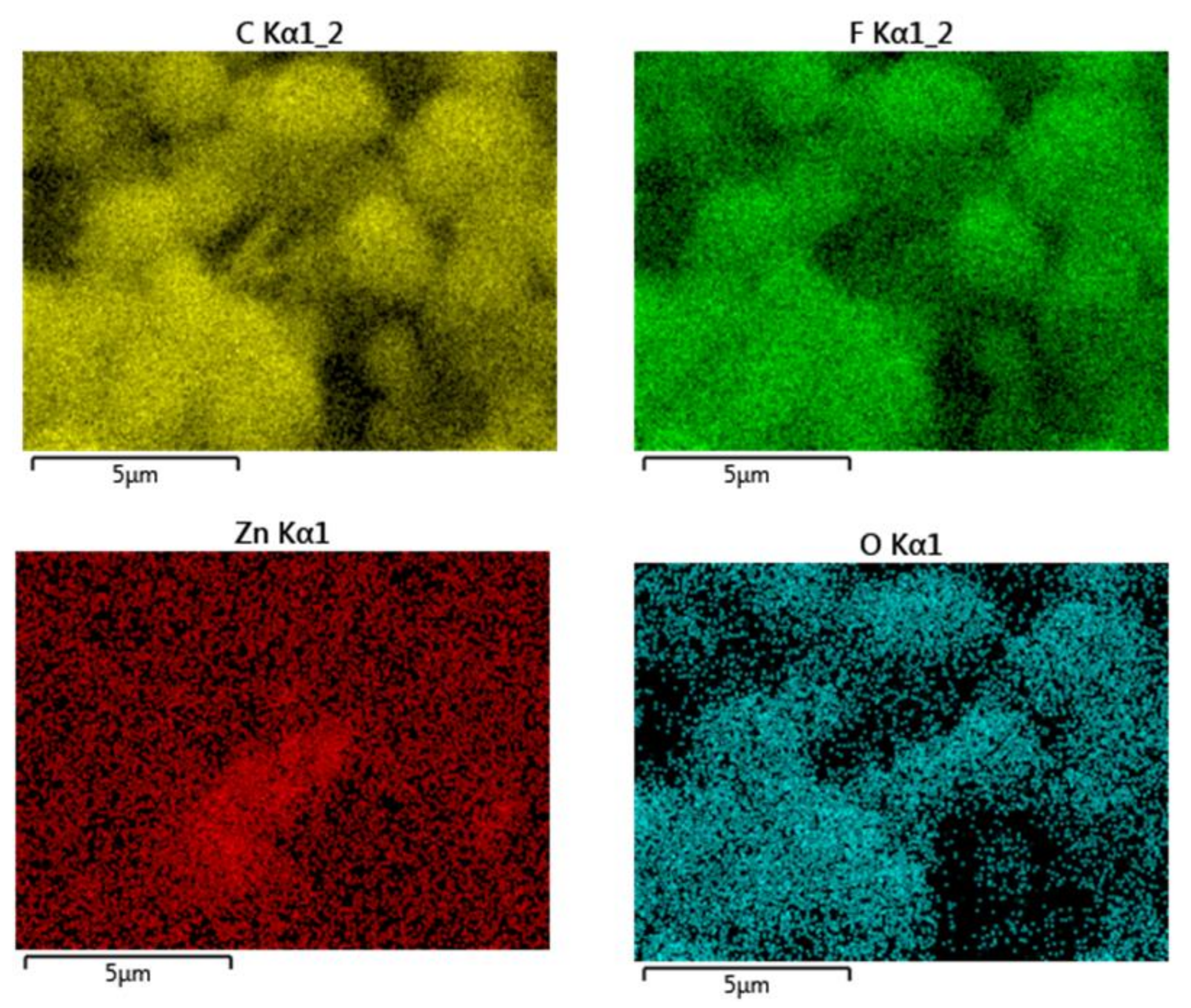
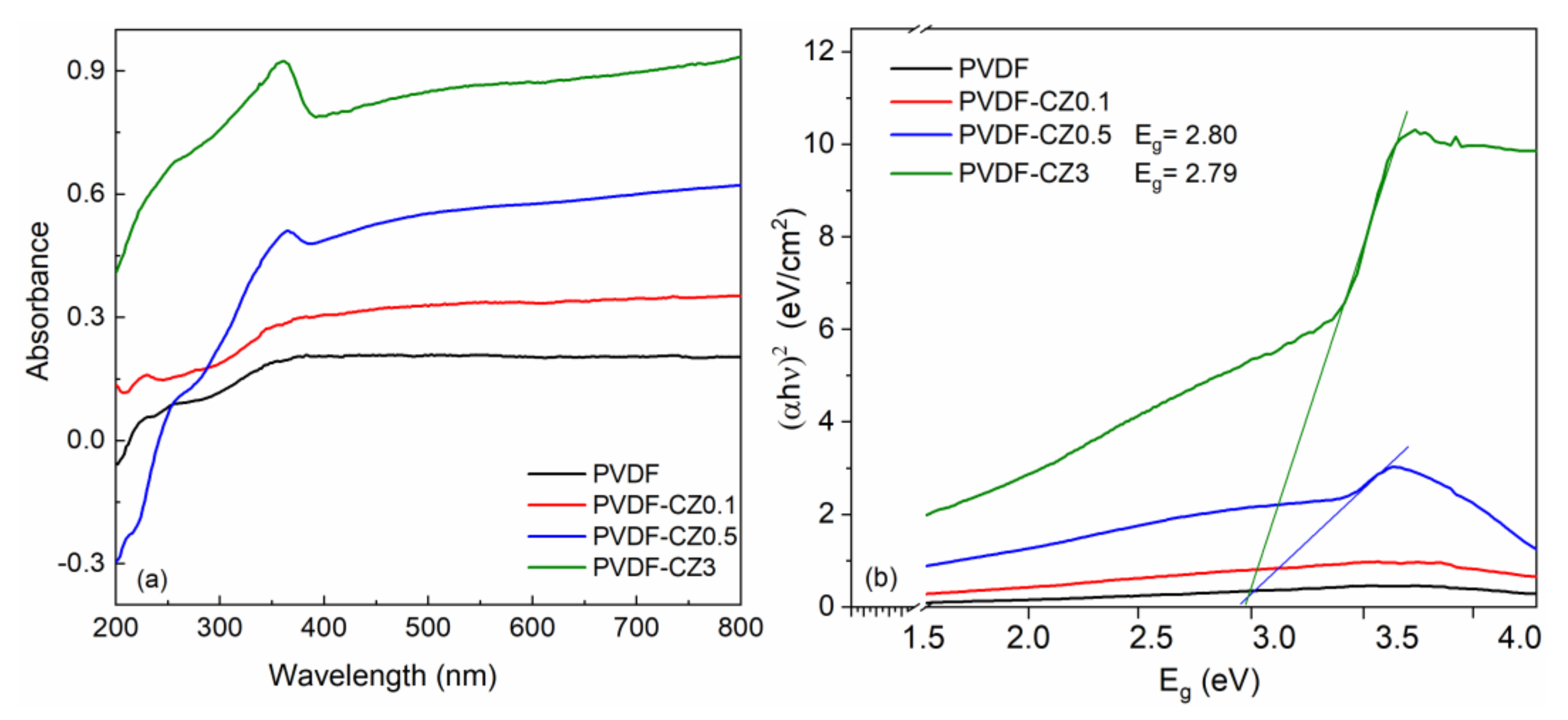
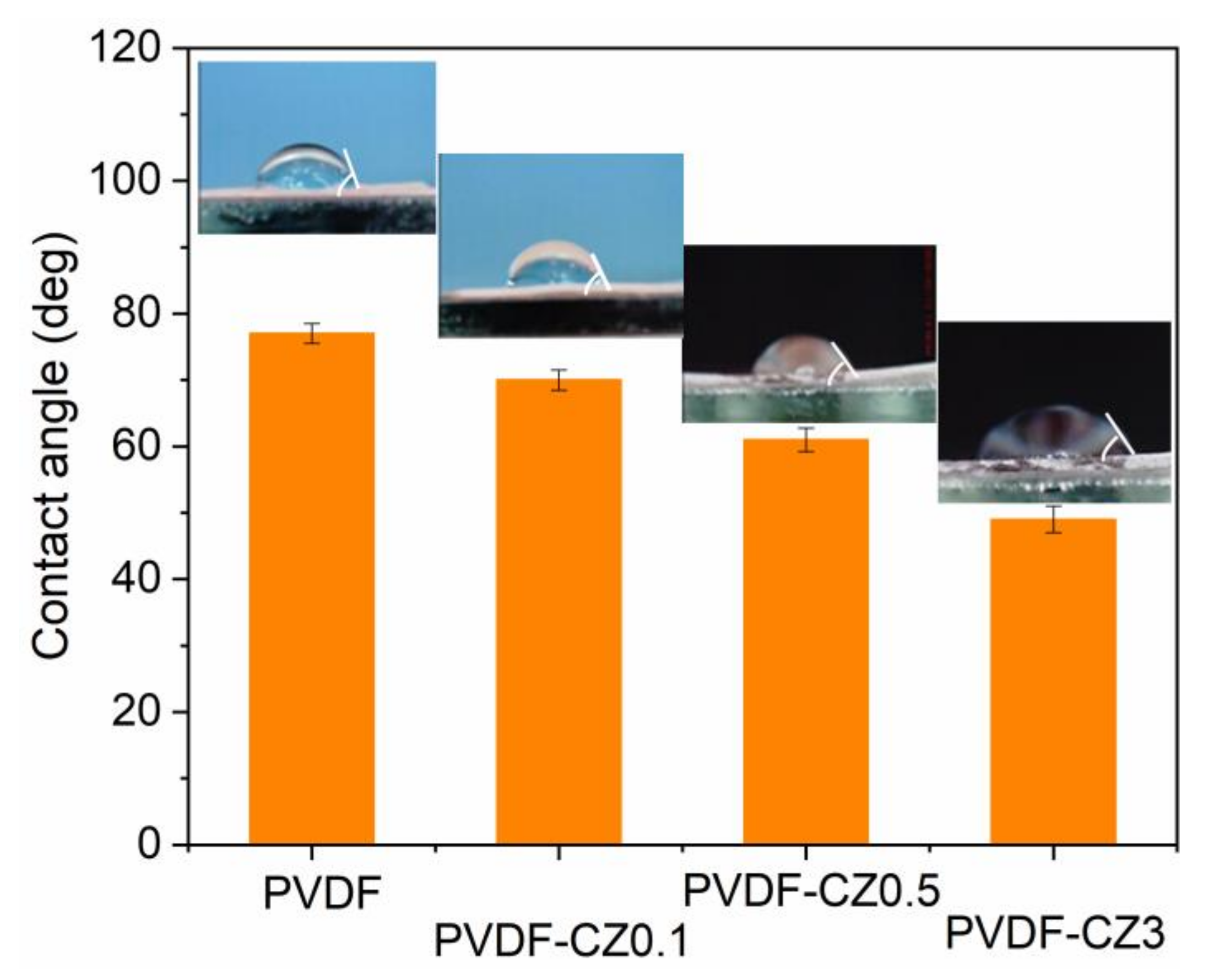
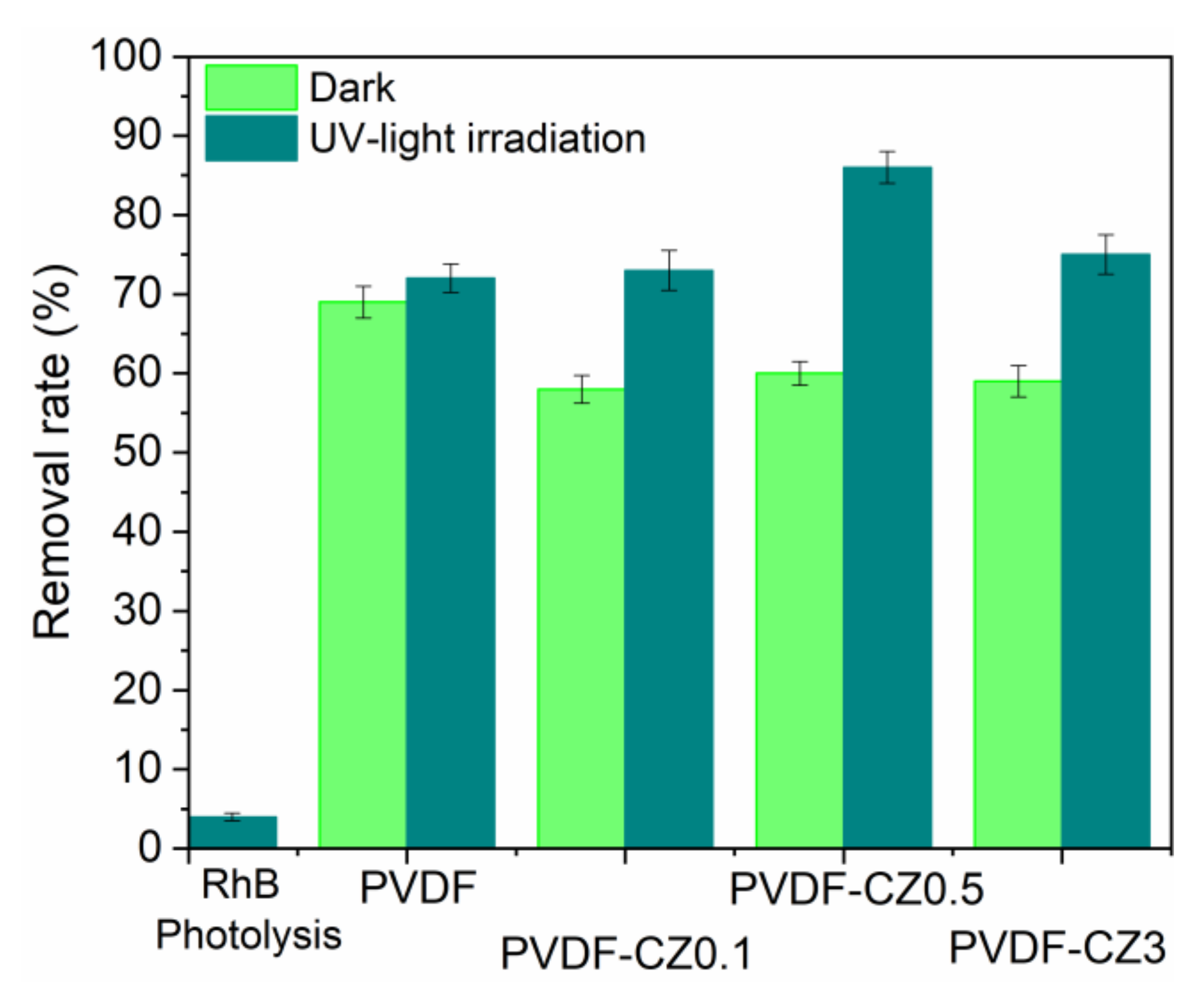
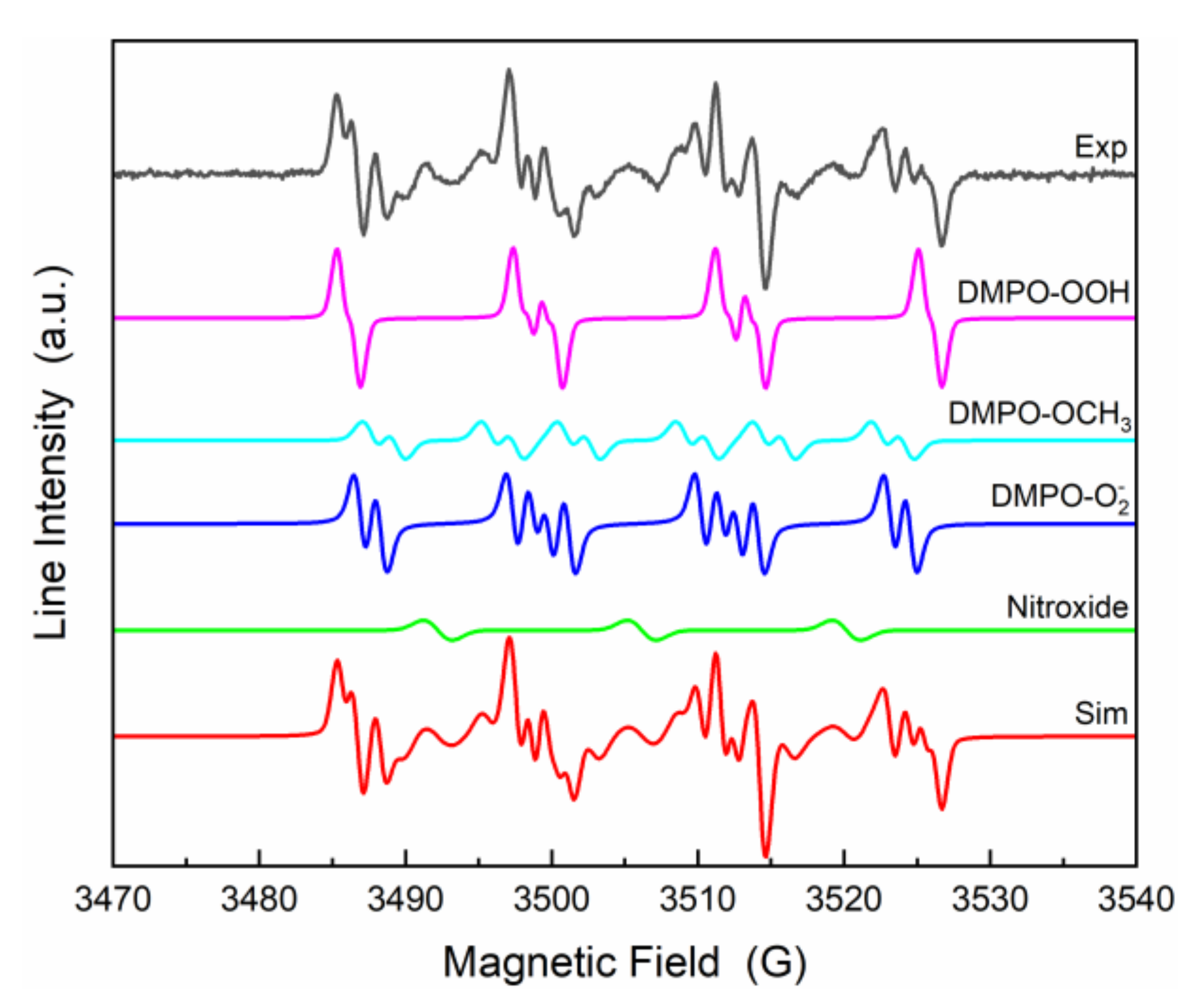
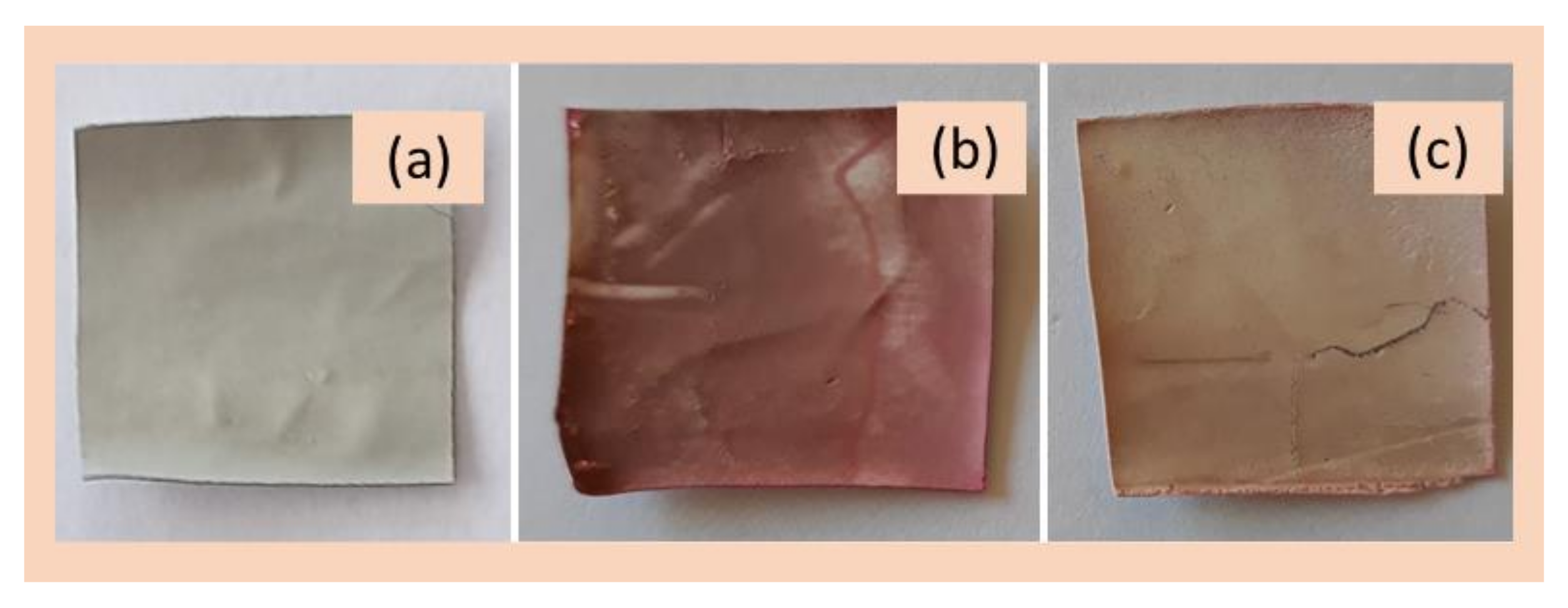
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sudarshan, S.; Bharti, V.S.; Harikrishnan, S.; Shukla, S.P.; Bhuvaneswari, G.R. Eco-toxicological effect of a commercial dye Rhodamine B on freshwater microalgae Chlorella vulgaris. Arch. Microbiol. 2022, 204, 658. [Google Scholar] [CrossRef]
- Wang, M.; Nie, X.; Tian, L.; Hu, J.; Yin, D.; Qiao, H.; Li, T.; Li, Y. Rhodamine B in spices determined by a sensitive UPLC-MS/MS method. Food Addit. Contam. Part B Surveill. 2019, 12, 59–64. [Google Scholar] [CrossRef] [PubMed]
- Bhattarai, N.; Chen, M.; Pérez, R.; Ravula, S.; Strongin, R.; McDonough, K.; Warner, I. Comparison of chemotherapeutic activities of rhodamine-based GUMBOS and NanoGUMBOS. Molecules 2020, 25, 3272. [Google Scholar] [CrossRef] [PubMed]
- Zakaria, N.A.; Hazwan Hussin, M.; Ahmad, A.L.; Leo, C.P.; Eong Poh, P.; Behzadian, K.; Akinwumi, I.I.; Moghayedi, A.; Diazsolano, J. Lignin modified PVDF membrane with antifouling properties for oil filtration. J. Water Process. Eng. 2021, 43, 102248–102256. [Google Scholar] [CrossRef]
- Wen, Y.; Yuan, J.; Ma, X.; Wang, S.; Liu, Y. Polymeric nanocomposite membranes for water treatment: A review. Environ. Chem. Lett. 2019, 17, 1539–1550. [Google Scholar] [CrossRef]
- Pendergast, M.; Hoek, E. A review of water treatment membrane nanotechnologies. Energy Environ. Sci. 2011, 4, 1946–1971. [Google Scholar] [CrossRef] [Green Version]
- Hudaib, B.; Abu-Zurayk, R.; Waleed, H.; Ibrahim, A.A. Fabrication of a Novel (PVDF/MWCNT/Polypyrrole) Antifouling High Flux Ultrafiltration Membrane for Crude Oil Wastewater Treatment. Membranes 2022, 12, 751. [Google Scholar] [CrossRef]
- Wu, L.; Huang, L.; Yao, Y.; Liu, Z.; Wang, T.; Yang, X.; Dong, C. Fabrication of polyvinylidene fluoride blending membrane coupling with microemulsion polymerization and their anti-fouling performance. Polymer 2020, 203, 122767–122772. [Google Scholar] [CrossRef]
- Vitola, G.; Mazzei, R.; Fontananova, E.; Giorno, L. PVDF membrane biofunctionalization by chemical grafting. J. Membr. Sci. 2015, 476, 483–489. [Google Scholar] [CrossRef]
- Popa, A.; Toloman, D.; Stefan, M.; Petran, A.; Macavei, S.; Ulinici, S.; Stan, M.; Barbu-Tudoran, L.; Vlassa, M.; Pana, O. Hybrid PVDF-P(L-DOPA)-ZnO membranes for dyes and antibiotics removal through simultaneous action of adsorption and photocatalysis processes. J. Environ. Chem. Eng. 2021, 9, 106812–106826. [Google Scholar] [CrossRef]
- Popa, A.; Toloman, D.; Stan, M.; Stefan, M.; Radu, T.; Vlad, G.; Ulinici, S.; Baisan, G.; Macavei, S.; Barbu-Tudoran, L.; et al. Tailoring the RhB removal rate by modifying the PVDF membrane surface through ZnO particles deposition. J. Inorg. Organomet. Polym. Mater. 2021, 31, 1642–1652. [Google Scholar] [CrossRef]
- Wang, W.; Sun, H. Effect of different forms of nano-ZnO on the properties of PVDF/ZnO hybrid membranes. J Appl. Polym. Sci. 2020, 137, e49070. [Google Scholar] [CrossRef]
- Teow, Y.H.; Ahmad, A.L.; Lim, J.K.; Ooi, B.S. Preparation and characterization of PVDF/TiO2 mixed matrix membrane via in situ colloidal precipitation method. Desalination 2012, 295, 61–69. [Google Scholar] [CrossRef]
- Wang, M.; Liu, G.; Yu, H.; Lee, S.H.; Wang, L.; Zheng, J.; Wang, T.; Yun, Y.; Lee, J.K. ZnO Nanorod Array Modified PVDF Membrane with Superhydrophobic Surface for Vacuum Membrane Distillation Application. ACS Appl. Mater. Interfaces 2018, 10, 13452–13461. [Google Scholar] [CrossRef] [PubMed]
- Gholami, S.; Llacuna, J.L.; Vatanpour, V.; Dehqan, A.; Paziresh, S.; Cortina, J.L. Impact of a new functionalization of multiwalled carbon nanotubes on antifouling and permeability of PVDF nanocomposite membranes for dye wastewater treatment. Chemosphere 2022, 294, 133699–133711. [Google Scholar] [CrossRef] [PubMed]
- Safarpour, M.; Khataee, A.; Vatanpour, V. Preparation of a Novel Polyvinylidene Fluoride (PVDF) Ultrafiltration Membrane Modified with Reduced Graphene Oxide/Titanium Dioxide (TiO2) Nanocomposite with Enhanced Hydrophilicity and Antifouling Properties. Ind. Eng. Chem. Res. 2014, 53, 13370–13382. [Google Scholar] [CrossRef]
- Martins, P.; Lopes, A.C.; Lanceros-Mendez, S. Electroactive phases of poly(vinylidene fluoride): Determination, processing and applications. Prog. Polym. Sci. 2014, 39, 683–695. [Google Scholar] [CrossRef]
- Zhang, R.; Yang, Z.; Hu, Z.; Zhao, C.; Zeng, K.; Yu, J.; Cai, L.; Chen, Z.; Jiang, J. Modification of PVDF membranes using BiOBr precursor in-situ deposition and tannic acid self-assembly for effectively removing organic pollutants. Appl. Surf. Sci. 2022, 599, 153888–153900. [Google Scholar] [CrossRef]
- Hudandini, M.; Puri, N.R.; Winardi, S.; Widiyastuti, W.; Shimada, M.; Kusdianto, K. Photocatalytic Activity of ZnO/Ag Nanoparticles Fabricated by a Spray Pyrolysis Method with Different O2:N2 Carrier Gas Ratios and Ag Contents. Catalysts 2022, 12, 1374. [Google Scholar] [CrossRef]
- Zhu, G.; Wang, H.; Yang, G.; Chen, L.; Guo, P.; Zhang, L. A facile synthesis of ZnO/CNT hierarchical microsphere composites with enhanced photocatalytic degradation of methylene blue. RSC Adv. 2015, 5, 72476–72481. [Google Scholar] [CrossRef]
- Prabhua, Y.T.; Sreedhara, B.; Palc, U. Achieving Enhanced Photocatalytic Activity of ZnO Supported on MWCNTs towards Degradation of Pollutants Under Visible Light. Mater. Today Proc. 2019, 8, 419–426. [Google Scholar]
- Saleh, T.A.; Gondal, M.A.; Drmosh, Q.A. Preparation of a MWCNT/ZnO nanocomposite and its photocatalytic activity for the removal of cyanide from water using a laser. Nanotechnology 2010, 21, 495705–495716. [Google Scholar] [CrossRef]
- Ge, F.F.; Tsou, C.H.; Youan, S.; De Guzman, M.R.; Zheng, C.Y.; Li, J.; Jia, C.F.; Cheng, B.Y.; Yang, P.C.; Gao, C. Barrier performance and biodegradability of antibacterial poly(butylene adipate-coterephthalate) nanocomposites reinforced with a new MWCNT-ZnO nanomaterial. Nanotechnology 2021, 32, 485706–485717. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Xu, M.; Feng, G.; Zhang, B.; Zhang, H.; Yang, C.; Yu, Q.; Wang, L.G.; Zhang, D. Visible-light responsive PVDF/carbon sphere@TiO2 membrane for dye scavenging and bacteria inactivation. Appl. Surf. Sci. 2022, 605, 154755–154766. [Google Scholar]
- Cai, X.; Lei, T.; Sun, D.; Lin, L. A critical analysis of the α, β and γ phases in poly(vinylidene fluoride) using FTIR. RSC Adv. 2017, 7, 15382–15389. [Google Scholar] [CrossRef] [Green Version]
- Pan, T.; Liu, J.; Deng, N.; Li, Z.; Wang, L.; Xia, Z.; Fan, J.; Liu, Y. ZnO Nanowires@PVDF nanofiber membrane with superhydrophobicity for enhanced anti-wetting and anti-scaling properties in membrane distillation. J. Membr. Sci. 2021, 621, 118877–118888. [Google Scholar] [CrossRef]
- Navarro Oliva, F.S.; Sahihi, M.; Lenglet, L.; Ospina, A.; Guenin, E.; Jaramillo-Botero, A.; Goddard, W.A.; Bedou, F. Nanoparticle size and surface chemistry effects on mechanical and physical properties of nano-reinforced polymers: The case of PVDF-Fe3O4 nano-composites. Polym. Test. 2023, 117, 107851–107863. [Google Scholar] [CrossRef]
- Barabaszov, K.C.; Holesova, S.; Plesník, L.; Kolska, Z.; Joszko, K.; Gzik-Zrosk, B. Hybrid Nanofillers Creating the Stable PVDF Nanocomposite Films and Their Effect on the Friction and Mechanical Properties. Polymers 2022, 14, 3831. [Google Scholar] [CrossRef]
- Apostolescu, N.; Tataru Farmus, R.E.; Harja, M.; Aurelia Vizitiu, M.; Cernatescu, C.; Cobzaru, C.; Apostolescu, G.A. Photocatalytic Removal of Antibiotics from Wastewater Using the CeO2/ZnO Heterojunction. Materials 2023, 16, 850. [Google Scholar] [CrossRef]
- Viana da Silva, A.F.; Cesca, K.; Ambrosi, A.; Zin, G.; Di Luccio, M.; Oliveira, J.V. An expedite facile method for modification of PVDF membranes with polydopamine and TiO2 to improve water permeation. Mater. Lett. 2022, 324, 132611. [Google Scholar] [CrossRef]
- Sudha, D.; Ranjith Kumar, E.; Shanjitha, S.; Munshi, A.M.; Al-Hazmi, G.A.A.; El-Metwaly, N.M.; Kirubavathy, S.J. Structural, optical, morphological and electrochemical properties of ZnO and graphene oxide blended ZnO nanocomposites. Ceram. Int. 2023, 49, 7284–7288. [Google Scholar] [CrossRef]
- Raub, A.; Mohd, A.; Yunas, J.; Mohamed, M.A.; Bais, B.; Hamzah, A.A.; Ridwan, J.; Kazmi, J.; Hassan, M.A. Synthesis and characterization of ZnO NRs with spray coated GO for enhanced photocatalytic activity. Ceram. Int. 2022, 48, 18238–18245. [Google Scholar] [CrossRef]
- Sagar, R.; Gaur, M.S.; Bhadoria, B.S. Investigation of TSDC and dielectric modulus of PVDF–BaZrO3 nanocomposites thin film. Vacuum 2018, 156, 375–383. [Google Scholar] [CrossRef]
- Shen, L.; Huang, Z.; Liu, Y.; Li, R.; Xu, Y.; Jakaj, G.; Lin, H. Polymeric Membranes Incorporated With ZnO Nanoparticles for Membrane Fouling Mitigation: A Brief Review. Front. Chem. 2020, 8, 224. [Google Scholar] [CrossRef] [PubMed]
- Silva, T.L.S.; Morales-Torres, S.; Figueiredo, J.L.; Silva, A.M.T. Multi-walled carbon nanotube/PVDF blended membranes with sponge- and finger-like pores for direct contact membrane distillation. Desalination 2015, 357, 233–245. [Google Scholar] [CrossRef]
- Lia, N.; Tian, Y.; Zhang, J.; Sun, Z.; Zhao, J.; Zhang, J.; Zuo, W. Precisely-controlled modification of PVDF membranes with 3D TiO2/ZnO nanolayer: Enhanced anti-fouling performance by changing hydrophilicity and photocatalysis under visible light irradiation. J. Membr. Sci. 2017, 528, 359–368. [Google Scholar] [CrossRef]
- Zhang, D.; Daia, F.; Zhang, P.; An, Z.; Zhao, Y.; Chen, L. The photodegradation of methylene blue in water with PVDF/GO/ZnO composite membrane. Mater. Sci. Eng. C 2019, 96, 684–692. [Google Scholar] [CrossRef]
- Hayashi, T.; Nakamura, K.; Suzuki, T.; Saito, N.; Murakami, Y. OH radical formation by the photocatalytic reduction reactions of H2O2 on the surface of plasmonic excited Au-TiO2 photocatalysts. Chem. Phys. Lett. 2020, 739, 136958. [Google Scholar] [CrossRef]
- Alyani, S.J.; Pirbazari, A.E.; Khalilsaraei, F.E.; Kolur, N.A.; Gilani, N. Growing Co-doped TiO2 nanosheets on reduced graphene oxide for efficient photocatalytic removal of tetracycline antibiotic from aqueous solution and modeling the process by artificial neural network. J. Alloy. Compd. 2019, 799, 169–182. [Google Scholar] [CrossRef]
- Stefan, M.; Leostean, C.; Popa, A.; Toloman, D.; Perhaita, I.; Cadis, A.; Macavei, S.; Pana, O. Highly stable MWCNT-CoFe2O4 photocatalyst. EGA-FTIR coupling as efficient tool to illustrate the formation mechanism. J. Alloy. Compd. 2022, 928, 167188. [Google Scholar] [CrossRef]
- Zhang, L.; Cheng, H.; Zong, R.; Zhu, Y. Photocorrosion suppression of ZnO nanoparticles via hybridization with graphite-like carbon and enhanced photocatalytic activity. J. Phys. Chem. C 2009, 113, 2368–2374. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Toloman, D.; Stefan, M.; Macavei, S.; Barbu-Tudoran, L.; Popa, A. Photocatalytic Self-Cleaning PVDF Membrane Blended with MWCNT-ZnO Nanocomposites for RhB Removal. Coatings 2023, 13, 594. https://doi.org/10.3390/coatings13030594
Toloman D, Stefan M, Macavei S, Barbu-Tudoran L, Popa A. Photocatalytic Self-Cleaning PVDF Membrane Blended with MWCNT-ZnO Nanocomposites for RhB Removal. Coatings. 2023; 13(3):594. https://doi.org/10.3390/coatings13030594
Chicago/Turabian StyleToloman, Dana, Maria Stefan, Sergiu Macavei, Lucian Barbu-Tudoran, and Adriana Popa. 2023. "Photocatalytic Self-Cleaning PVDF Membrane Blended with MWCNT-ZnO Nanocomposites for RhB Removal" Coatings 13, no. 3: 594. https://doi.org/10.3390/coatings13030594
APA StyleToloman, D., Stefan, M., Macavei, S., Barbu-Tudoran, L., & Popa, A. (2023). Photocatalytic Self-Cleaning PVDF Membrane Blended with MWCNT-ZnO Nanocomposites for RhB Removal. Coatings, 13(3), 594. https://doi.org/10.3390/coatings13030594






