Using Applied Electrochemistry to Obtain Nanoporous TiO2 Films on Ti6Al4V Implant Alloys and Their Preclinical In Vitro Characterization in Biological Solutions
Abstract
:1. Introduction
2. Materials and Methods
2.1. The Electrochemical Formation of the Nanoporous TiO2 Surface Layer on Ti6Al4V Grade 5 Alloy
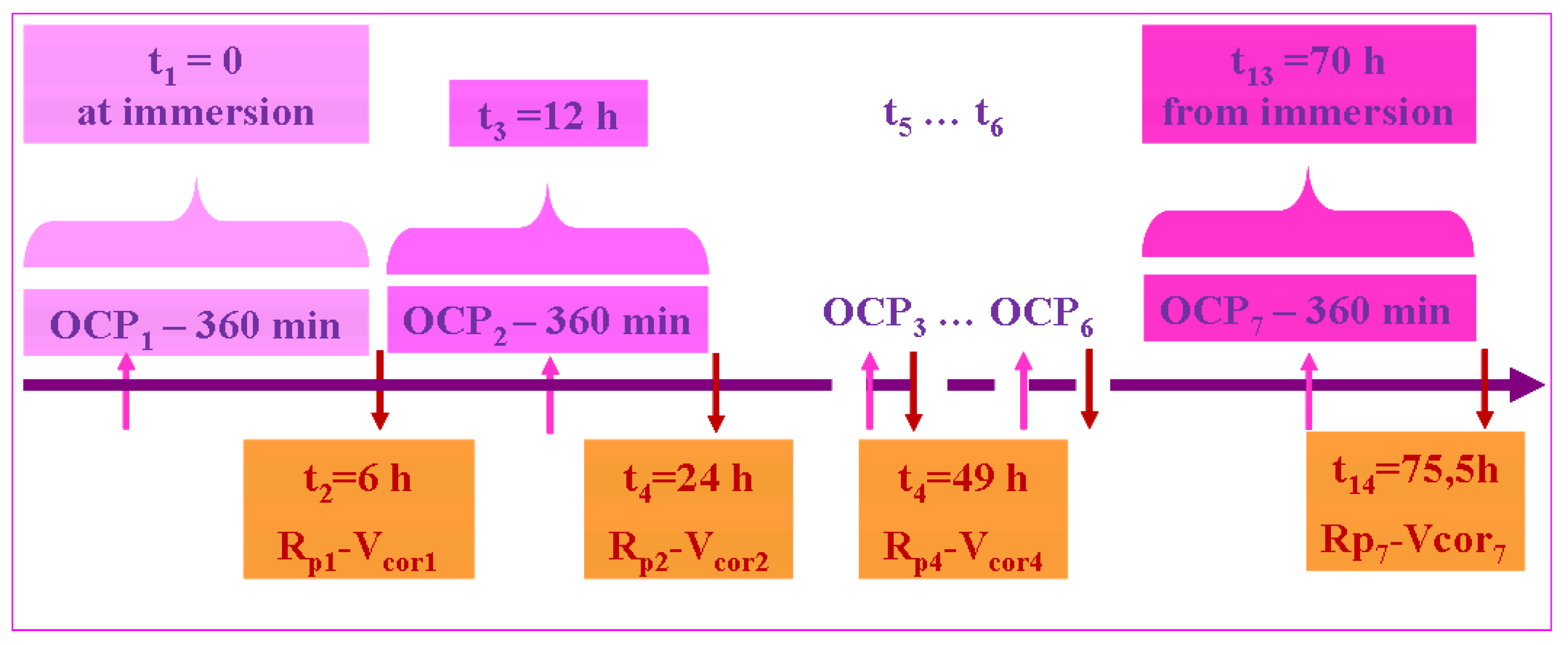
2.2. Evaluation of the Corrosion Resistance of the Untreated Ti6Al4V Grade 5 Alloy and the Treated Alloy with the Electrochemically Formed Nanoporous TiO2 Surface Film
- (1)
- At t1 = 0 (immersion): OCP1 lasting 360 min.
- (2)
- At 360 min: Rp1-Vcorr1, with a duration of 349 min.
- (3)
- At 709 min: OCP2, with a duration of 720 min (12 h).
- (4)
- At 1429 min: Rp2-Vcorr2, with a duration of 149 min.
- (5)
- At 1578 min: OCP3, with a duration of 720 min (12 h).
- (6)
- At 2298 min: Rp3-Vcorr3, with a duration of 149 min.
- (7)
- At 2447 min: OCP4, with a duration of 360 min (6 h).
- (8)
- At 2807 min: Rp4-Vcorr4, with a duration of 149 min.
- (9)
- At 2956 min: OCP5, with a duration of 360 min (6 h).
- (10)
- At 3316 min: Rp5-Vcorr5, with a duration of 149 min.
- (11)
- At 3465 min: OCP6, with a duration of 360 min (6 h).
- (12)
- At 3825 min: Rp6-Vcorr6, with a duration of 349 min.
- (13)
- At 4174 min: OCP7, with a duration of 360 min (6 h).
- (14)
- At 4534 min: Rp7-Vcorr7, with a duration of 349 min.
- (15)
- Total time: 4534 + 349 = 4883 min (81 h).
2.3. Characterization of the Untreated Grade 5 Ti6Al4V Alloy and the Treated Alloy with the Electrochemically Formed Nanoporous TiO2 Surface Film
3. Results
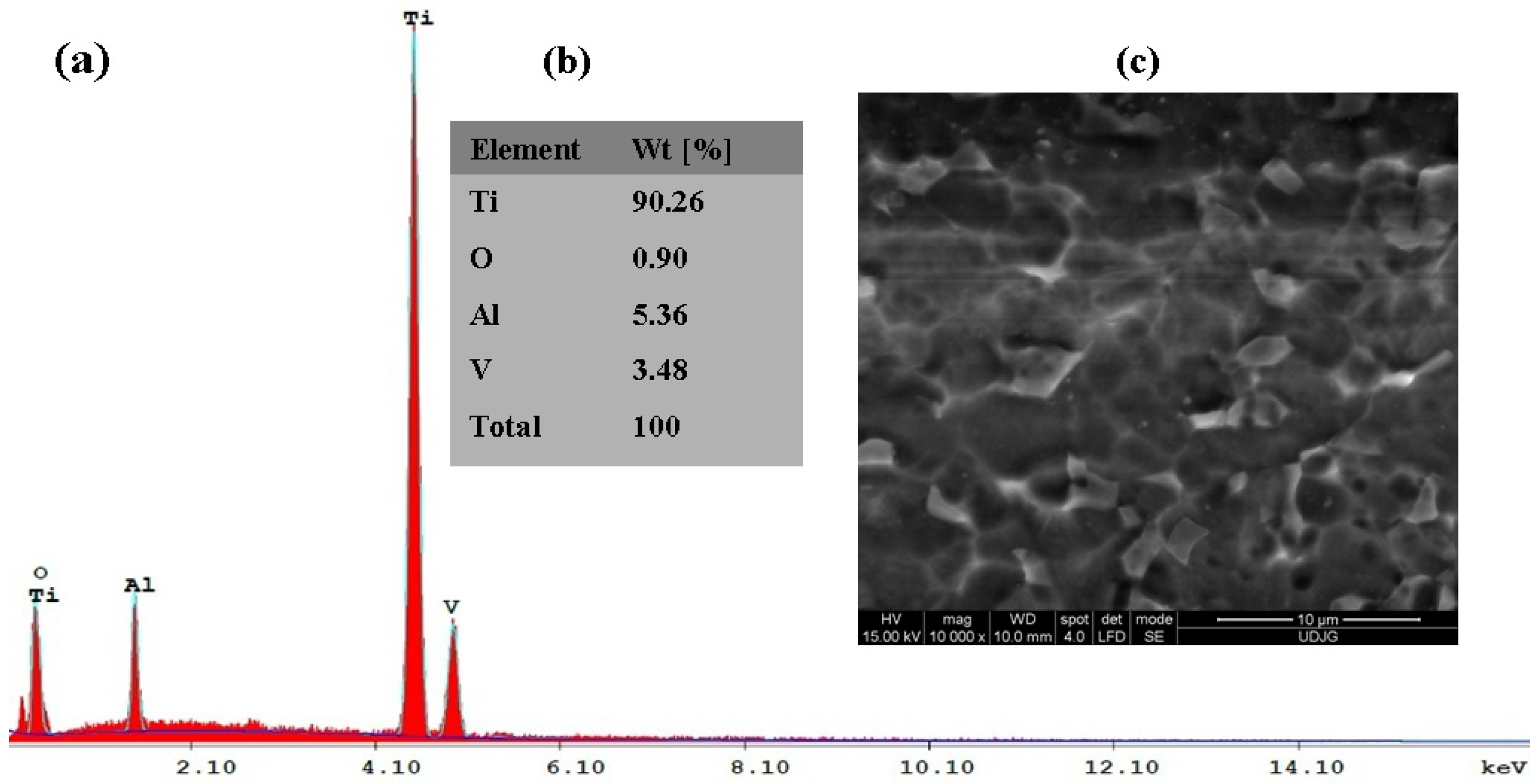
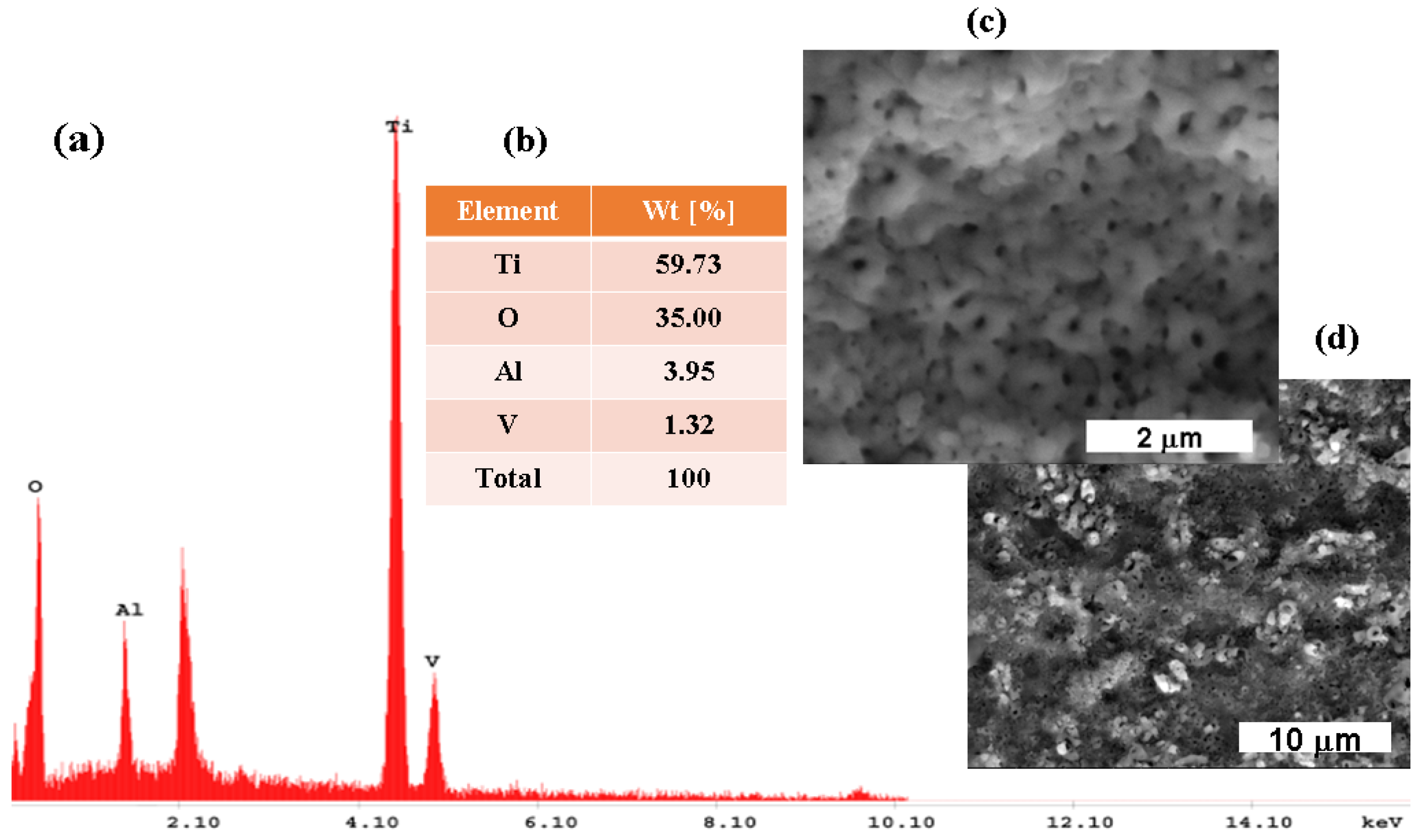
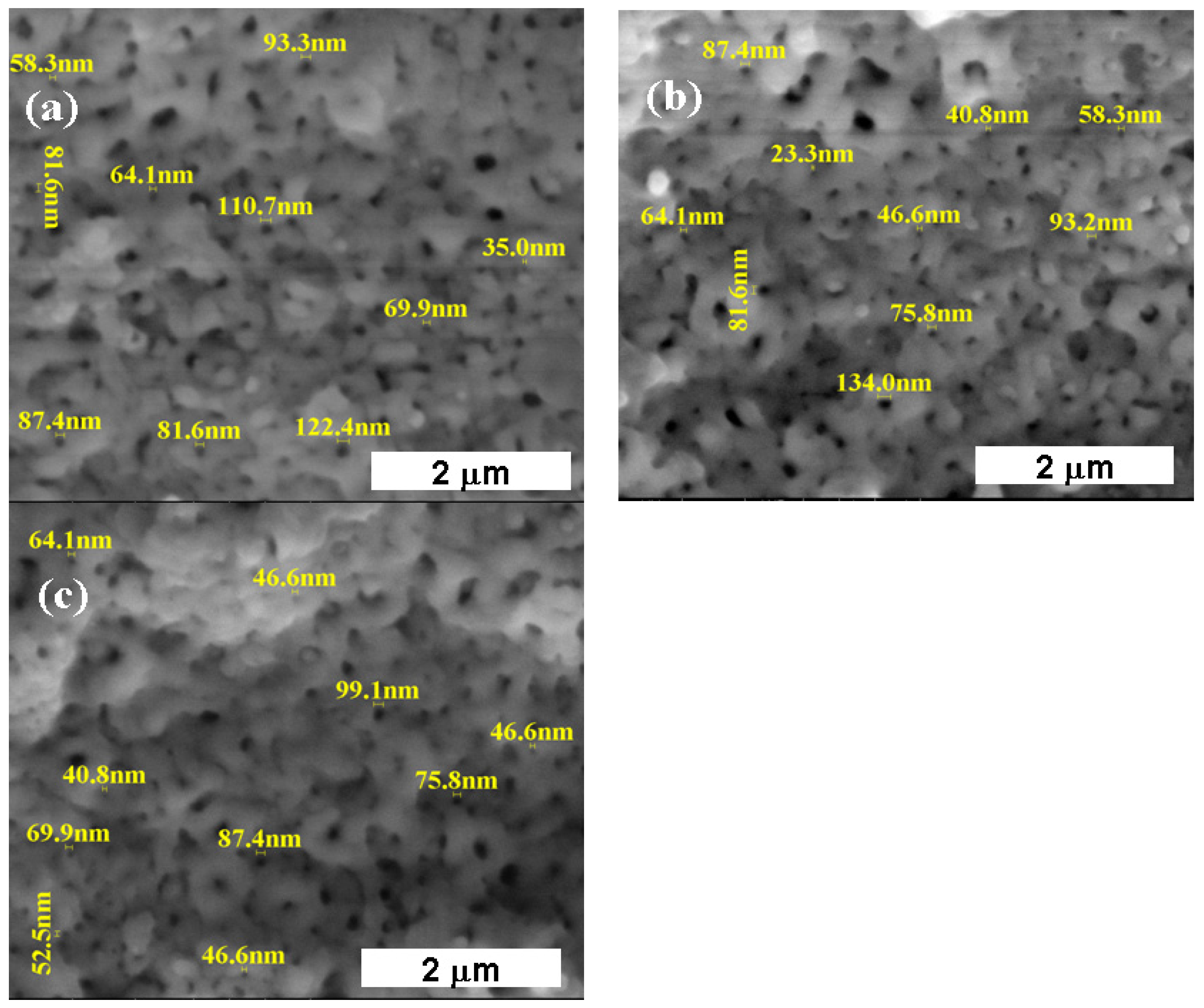
3.1. Morphological and Compositional Analysis of the Untreated Titanium Alloy Surfaces and Electrochemically Formed TiO2 Films through Electron Microscopy (SEM-EDX)
3.1.1. Untreated Ti6Al4V Grade 5 Alloy
3.1.2. Titanium Oxide Film Electrochemically Formed on the Surface of the Ti6Al4V Alloy
3.2. Evolution of the Open Circuit Potential (OCP) vs. Time in Hank’s Biological Solution for the Untreated Alloy and the Treated Alloy with the Electrochemically Formed Nanoporous TiO2 Surface Film
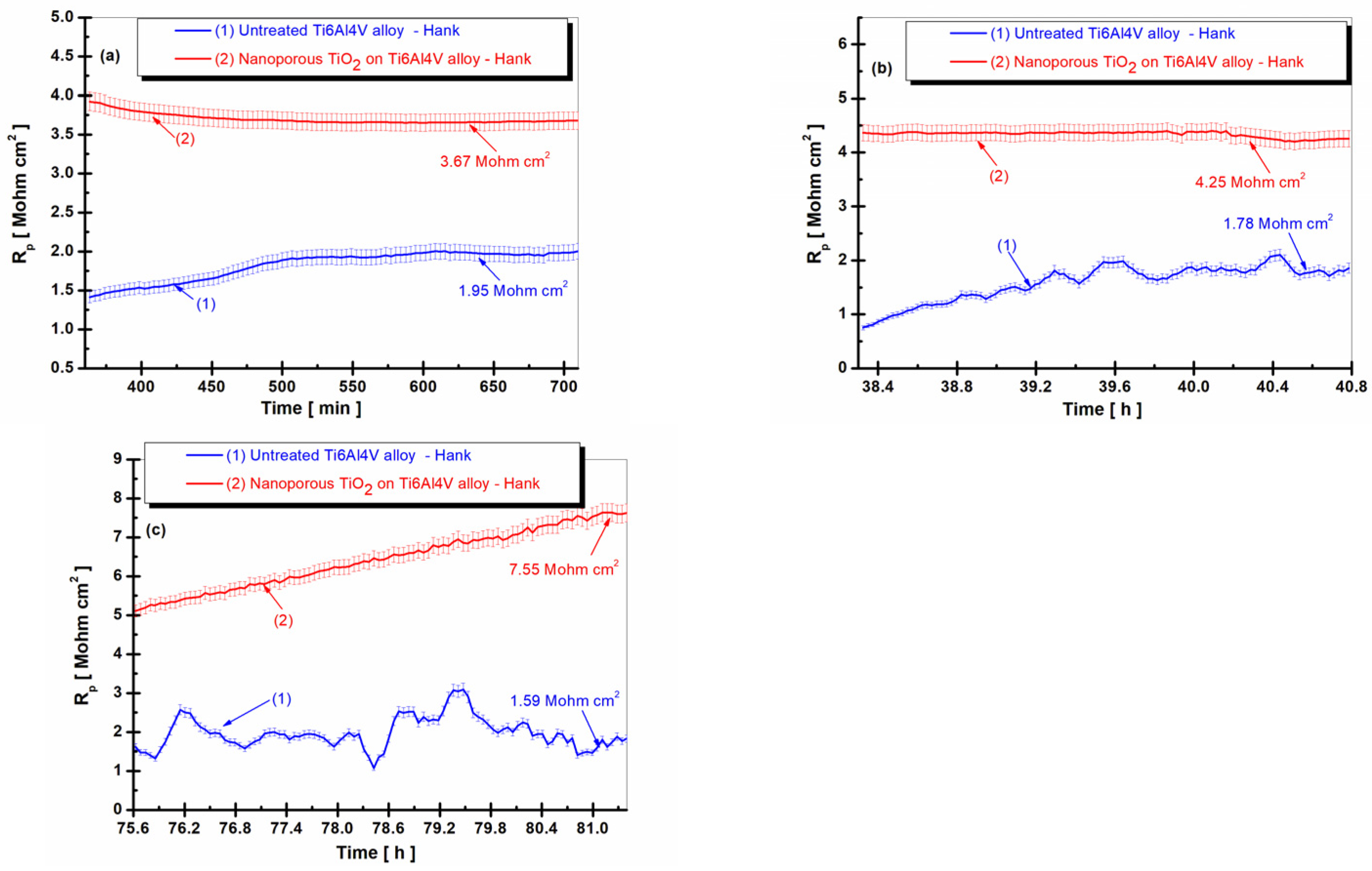
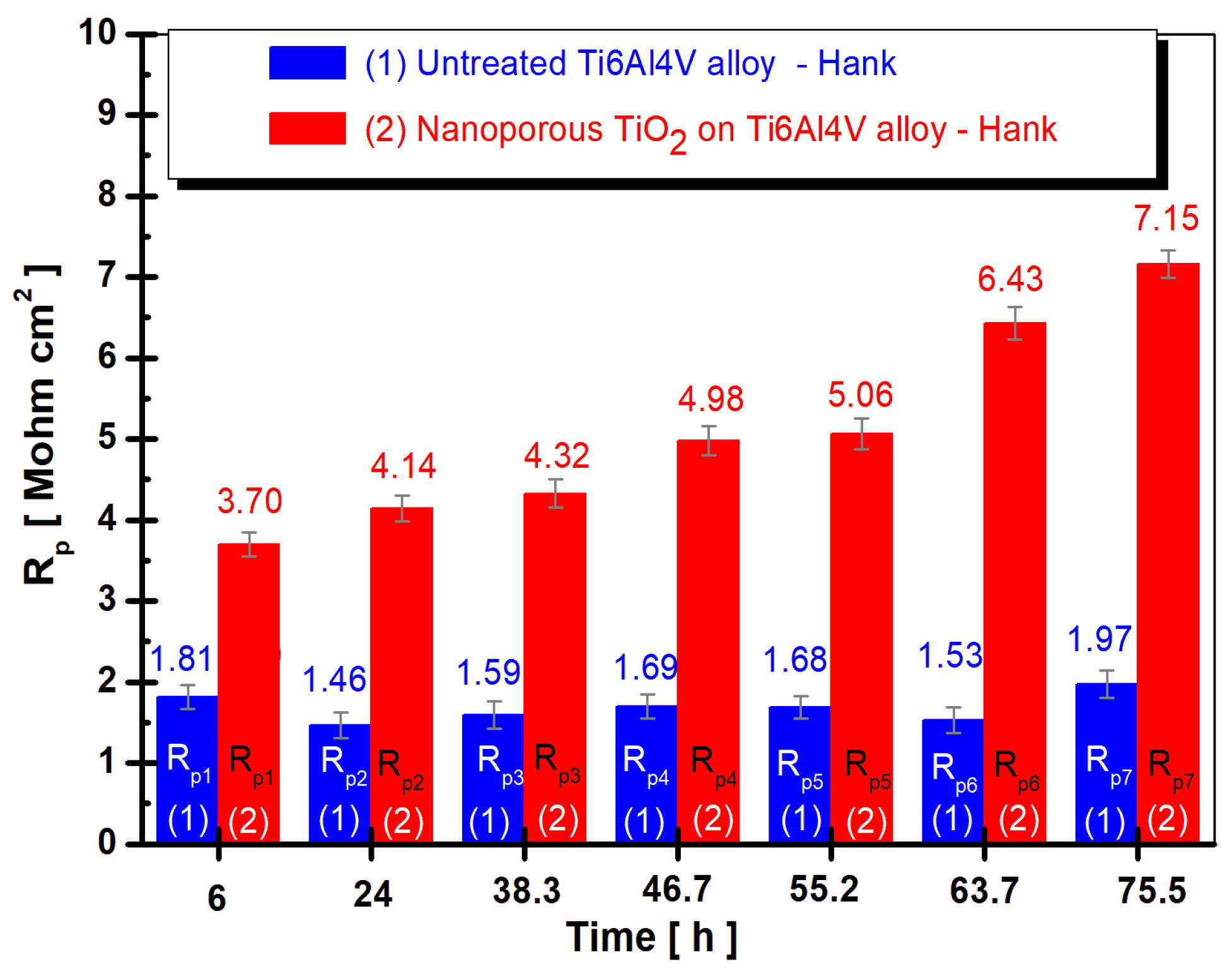
3.3. Evolution of the Polarization Resistance (Rp) vs. Time in Hank’s Biological Solution for the Untreated Alloy and the Treated Alloy with the Electrochemically Formed Nanoporous TiO2 Surface Film
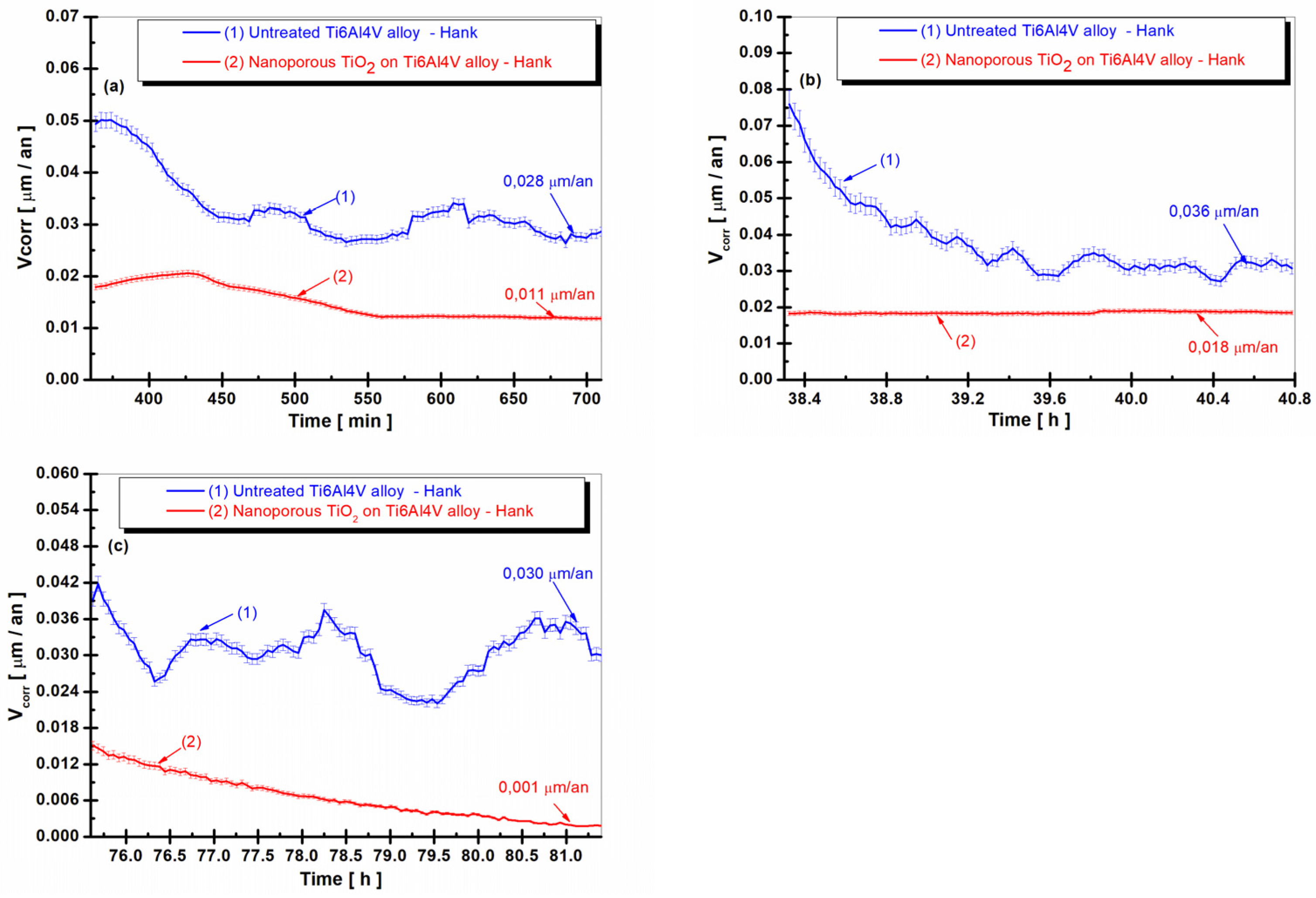
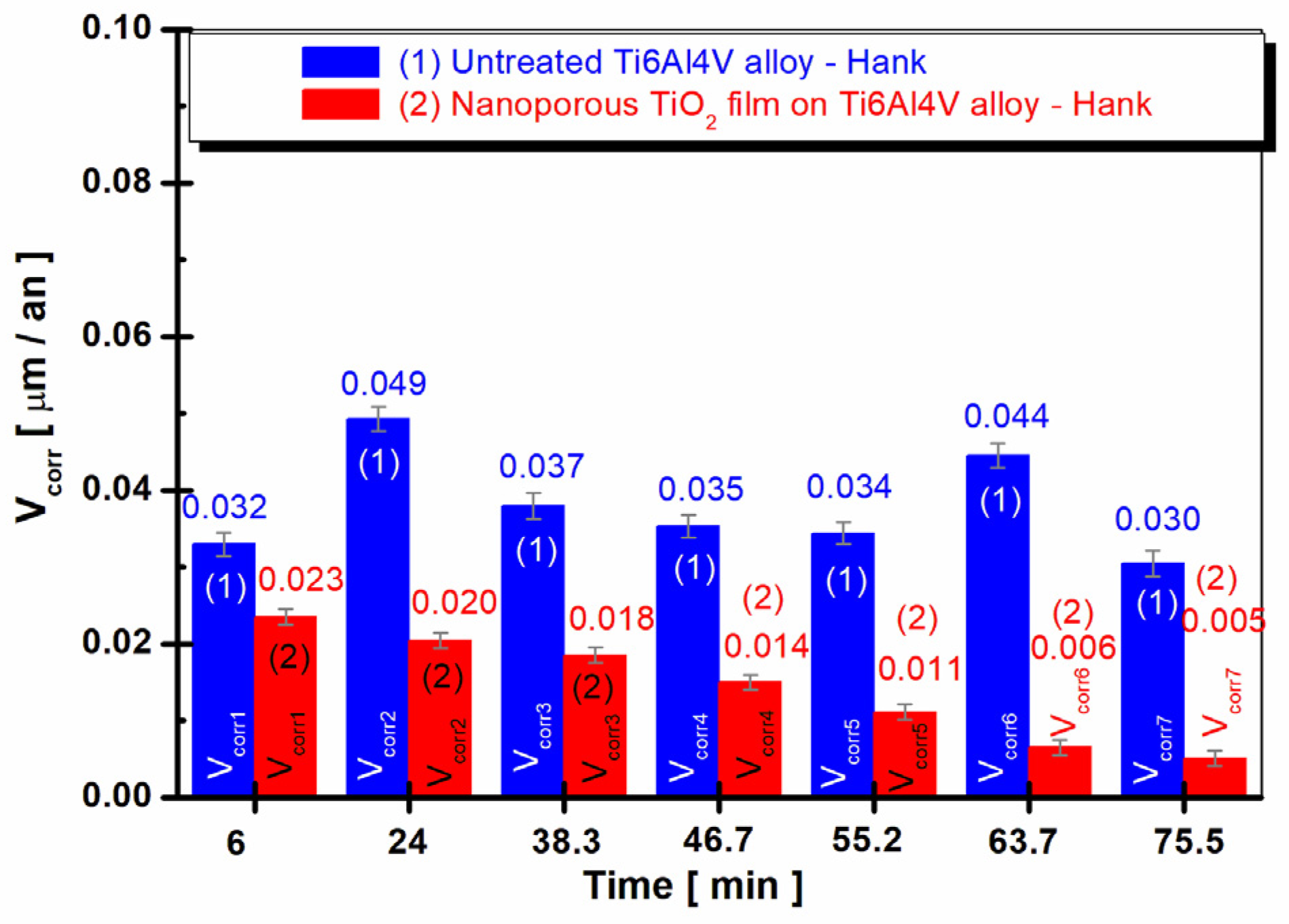
3.4. Time Evolution of the Corrosion Rate (Vcorr) in Hank’s Biological Solution of the Untreated Alloy and the Treated Alloy with the Electrochemically Formed Nanoporous TiO2 Surface Film
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Akhavan, O.; Ghaderi, E. Flash photo stimulation of human neural stem cells on graphene/TiO2 heterojunction for differentiation into neurons. Nanoscale 2013, 5, 10316–10326. [Google Scholar] [CrossRef]
- Chen, Q.; Thouas, G.A. Metallic implant biomaterials. Mater. Sci. Eng. R 2015, 87, 1–57. [Google Scholar] [CrossRef]
- Li, J.; Liu, X. Chemical surface modification of metallic biomaterials. In Surface Coating and Modification of Metallic Biomaterials; Woodhead Publishing: London, UK, 2015; pp. 159–183. [Google Scholar] [CrossRef]
- Verissimo, N.C.; Chung, S.; Webster, T.J. New nanoscale surface modifications of metallic biomaterials. In Surface Coating and Modification of Metallic Biomaterials; Woodhead Publishing: London, UK, 2015; pp. 249–273. [Google Scholar] [CrossRef]
- Jawed, S.F.; Rabadia, C.D.; Khan, M.A.; Khan, S.J. Effect of Alloying Elements on the Compressive Mechanical Properties of Biomedical Titanium Alloys: A Systematic Review. ACS Omega 2022, 7, 29526–29542. [Google Scholar] [CrossRef] [PubMed]
- Jimenez-Marcos, C.; Mirza-Rosca, J.C.; Baltatu, M.S.; Vizureanu, P. Experimental Research on New Developed Titanium Al-loys for Biomedical Applications. Bioengineering 2022, 9, 686. [Google Scholar] [CrossRef] [PubMed]
- Baltatu, M.S.; Chiriac-Moruzzi, C.; Vizureanu, P.; Tóth, L.; Novák, J. Effect of Heat Treatment on Some Titanium Alloys Used as Biomaterials. Appl. Sci. 2022, 12, 11241. [Google Scholar] [CrossRef]
- Bocchetta, P.; Chen, L.-Y.; Tardelli, J.D.C.; Reis, A.C.d.; Almeraya-Calderón, F.; Leo, P. Passive Layers and Corrosion Resis-tance of Biomedical Ti-6Al-4V and β-Ti Alloys. Coatings 2021, 11, 487. [Google Scholar] [CrossRef]
- Donachie, M.J. Titanium: A Technical Guide; ASM International: Almere, The Netherlands, 2000; ISBN 978-0-87170-686-7. [Google Scholar]
- Hanawa, T.; Asami, K.; Asaoka, K. Repassivation of titanium and surface oxide film regenerated in simulated bioliquid. J. Bio-Med. Mater. Res. 1998, 40, 530–538. [Google Scholar] [CrossRef]
- Brown, S.; Lemons, J. Medical Applications of Titanium and Its Alloys: The Material and Biological Issues; ASTM Special Series Publication: West Conshohocken, PA, USA, 1996; p. 1272. [Google Scholar]
- Neoh, K.G.; Hu, X.; Zheng, D.; Tang Kang, E. Balancing osteoblast functions and bacterial adhesion on functionalized titanium surfaces. Biomaterials 2012, 33, 2813–2822. [Google Scholar] [CrossRef]
- De Viteri, V.S.; Fuentes, E. Titanium and Titanium Alloys as Biomaterials. Tribol.-Fundam. Adv. 2013, 1, 154–181. [Google Scholar] [CrossRef] [Green Version]
- Williams, D. Titanium in Medicine: Material Science, Surface Science, Engineering, Biological Responses and Medical Applications; Brunette, D.M., Tengvall, P., Textor, M., Thompson, P., Eds.; Springer: Berlin/Heidelberg, Germany, 2001; pp. 13–24. [Google Scholar] [CrossRef]
- Adya, M.; Alam, M.; Ravindranath, T.; Mubeen, A.; Saluja, B. Corrosion in titanium dental implants: Literature review. J. Indian Prosthodont. Soc. 2005, 5, 126–131. [Google Scholar] [CrossRef]
- Chang, L.; Wang, Z.J.; Liu, Y.; Jia, Z.; Liang, S.-X. Additive Manufacturing of Titanium Alloys, Encyclopedia of Materials. Met. Alloy. 2022, 1, 256–274. [Google Scholar] [CrossRef]
- Asserghine, A.; Filotás, D.; Nagy, L.; Souto, R.M.; Nagy, G. Do titanium biomaterials get immediately and entirely repassivated? A perspective. NPJ Mater. Degrad. 2022, 6, 57. [Google Scholar] [CrossRef]
- Mohammed, M.T.; Khan, Z.A.; Siddiquee, A.N. Surface Modifications of Titanium Materials for developing Corro-sion Behavior in Human Body Environment: A Review. Procedia Mater. Sci. 2014, 6, 1610–1618. [Google Scholar] [CrossRef] [Green Version]
- Mardare, E.; Benea, L.; Bounegru, I. Electrochemical modifications of titanium and titanium alloys surface for bio-medical applications–A review. Ann. Dunarea Jos Univ. Galati. Fascicle IX. Metall. Mater. Sci. 2013, 36, 68–78. [Google Scholar]
- Hoque, M.E.; Showva, N.-N.; Ahmed, M.; Rashid, A.B.; Sadique, S.E.; El-Bialy, T.; Xu, H. Titanium and titanium alloys in dentistry: Current trends, recent developments, and future prospects. Heliyon 2022, 8, e11300. [Google Scholar] [CrossRef]
- Jáquez-Muñoz, J.M.; Gaona-Tiburcio, C.; Chacón-Nava, J.; Cabral-Miramontes, J.; Nieves-Mendoza, D.; Maldonado-Bandala, E.; Delgado, A.D.; Flores-De los Rios, J.P.; Bocchetta, P.; Almeraya-Calderón, F. Electrochemical Corrosion of Titanium and Titanium Alloys Anodized in H2SO4 and H3PO4 Solutions. Coatings 2022, 12, 325. [Google Scholar] [CrossRef]
- Prando, D.; Brenna, A.; Pedeferri, M.; Ormellese, M. Enhancement of pure titanium localized corrosion resistance by anodic oxidation. Mater. Corros. 2017, 69, 503–509. [Google Scholar] [CrossRef] [Green Version]
- Benea, L.; Celis, J.P. Reactivity of porous titanium oxide film and chitosan layer electrochemically formed on Ti-6Al-4V alloy in biological solution. Surf. Coat. Technol. 2018, 354, 145–152. [Google Scholar] [CrossRef]
- Benea, L.; Danaila, E.; Ponthiaux, P. Effect of titania anodic formation and hydroxyapatite electrodeposition on elec-trochemical behaviour of Ti–6Al–4V alloy under fretting conditions for biomedical applications. Corros. Sci. 2015, 91, 262–271. [Google Scholar] [CrossRef]
- Benea, L.; Simionescu-Bogatu, N.; Axente, E.R. Electrochemically obtained Al2O3 nanoporous layers with increased anticorrosive properties of aluminum alloy. J. Mater. Res. Technol. 2022, 17, 2636–2647. [Google Scholar] [CrossRef]
- Benea, L.; Mardare, E.; Mardare, M.; Celis, P. Preparation of titanium oxide and hydroxyapatite on Ti–6Al–4V alloy surface and electrochemical behavior in bio–simulated fluid solution. Corros. Sci. 2014, 80, 331–338. [Google Scholar] [CrossRef]
- Vasilescu, C.; Drob, P.; Vasilescu, E.; Demetrescu, I.; Ionita, D.; Prodana, M.; Drob, S.I. Characterisation and corrosion re-sistance of the electrodeposited hydroxyapatite and bovine serum albumin/hydroxyapatite films on Ti–6Al–4V–1Zr alloy sur-face. Corros. Sci. 2011, 53, 992–999. [Google Scholar] [CrossRef]
- Benea, L.; Ravoiu, A.; Celis, J.P. Anticorrosion performance of the electrochemically grown mixed porous oxide films on titanium alloy in biological solution. ACS Biomater. Sci. Eng. 2019, 5, 5925–5934. [Google Scholar] [CrossRef] [PubMed]
- Mazzarolo, A.; Curioni, M.; Vicenzo, A.; Skeldon, P.; Thompson, G.E. Anodic growth of titanium oxide: Electrochemical be-haviour and morphological evolution. Electrochim. Acta 2012, 75, 288–295. [Google Scholar] [CrossRef]
- Milošev, I.; Blejan, D.; Varvara, S.; Muresan, L.M. Effect of anodic oxidation on the corrosion behavior of Ti-based materials in simulated physiological solution. J. Appl. Electrochem. 2013, 43, 645–658. [Google Scholar] [CrossRef]
- Bayrak, Ö.; Ghahramanzadeh Asl, H.; Ak, A. Protein adsorption, cell viability and corrosion properties of Ti6Al4V alloy treated by plasma oxidation and anodic oxidation. Int. J. Miner. Metall. Mater. 2020, 27, 1269–1280. [Google Scholar] [CrossRef]
- Uttiya, S.; Contarino, D.; Prandi, S.; Carnasciali, M.M.; Gemme, G.; Mattera, L.; Rolandi, R.; Canepa, M.; Cavalleri, O. Anodic oxidation of titanium in sulphuric acid and phosphoric acid electrolytes. J. Mater. Sci. Nanotechnol. 2014, 1, S106. [Google Scholar] [CrossRef] [Green Version]
- Alves, A.C.; Wenger, F.; Ponthiaux, P.; Celis, J.P.; Pinto, A.M.; Rocha, L.A.; Fernandes, J.C.S. Corrosion mechanisms in tita-nium oxide-based films produced by anodic treatment. Electrochim. Acta 2017, 234, 16. [Google Scholar] [CrossRef]
- Torres, N.V.; Calderon, J.P.; Valencia, H.M.; Cecenes, R.L.; Cadena, I.R.; Bustos, E.S.; Valdés, C.I.R.; Rodriguez, J.G. Corrosion Resistance of a Plasma-Oxidized Ti6Al4V Alloy for Dental Applications. Coatings 2021, 11, 1136. [Google Scholar] [CrossRef]
- Lario, J.; Viera, M.; Vicente, Á.; Igual, A.; Amigó, V. Corrosion behaviour of Ti6Al4V ELI nanotubes for biomedical applications. J. Mater. Res. Technol. 2019, 8, 5548–5556. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Benea, L.; Ravoiu, A.; Neaga, V.; Axente, E.R. Using Applied Electrochemistry to Obtain Nanoporous TiO2 Films on Ti6Al4V Implant Alloys and Their Preclinical In Vitro Characterization in Biological Solutions. Coatings 2023, 13, 614. https://doi.org/10.3390/coatings13030614
Benea L, Ravoiu A, Neaga V, Axente ER. Using Applied Electrochemistry to Obtain Nanoporous TiO2 Films on Ti6Al4V Implant Alloys and Their Preclinical In Vitro Characterization in Biological Solutions. Coatings. 2023; 13(3):614. https://doi.org/10.3390/coatings13030614
Chicago/Turabian StyleBenea, Lidia, Anca Ravoiu, Veaceslav Neaga, and Elena Roxana Axente. 2023. "Using Applied Electrochemistry to Obtain Nanoporous TiO2 Films on Ti6Al4V Implant Alloys and Their Preclinical In Vitro Characterization in Biological Solutions" Coatings 13, no. 3: 614. https://doi.org/10.3390/coatings13030614






