Firefly Algorithm and Neural Network Employment for Dilution Analysis of Super Duplex Stainless Steel Clads over AISI 1020 Steel Using Gas Tungsten Arc Process
Abstract
1. Introduction
2. Design of Experiment
2.1. Base Metal and Filler Metal
2.2. Cladding
- Manufacturer: Tech Pro, Pvt. Ltd., Delhi, India;
- Supply voltage: 380/415/440 V;
- Cladding current range: 5 A–350 A (DC);
- Open circuit voltage: 80 V.
2.3. Preparation of Microhardness, Optical Microscopy, Pitting Corrosion, and Ferrite Percentage Evaluation Specimens
3. Testing
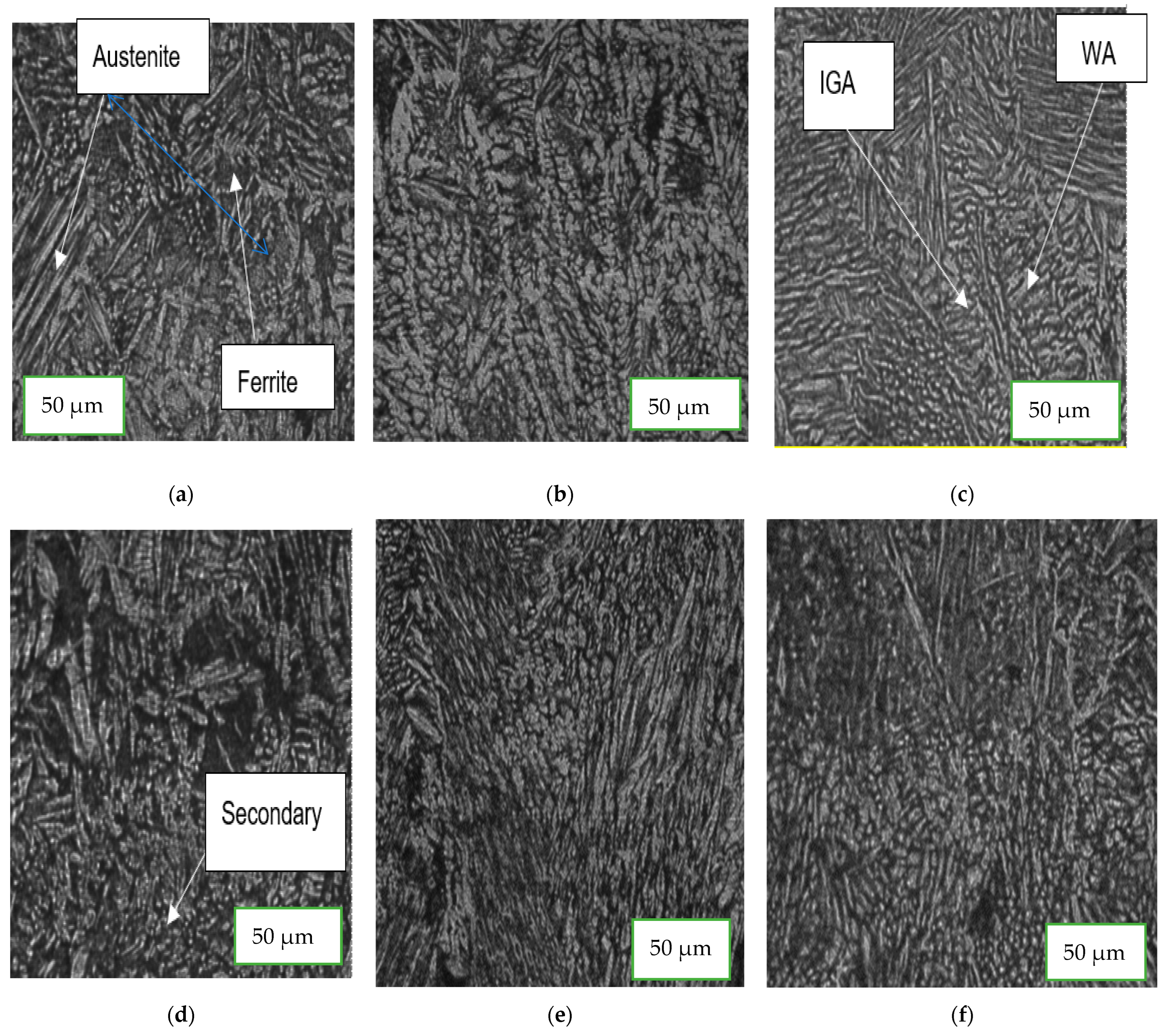
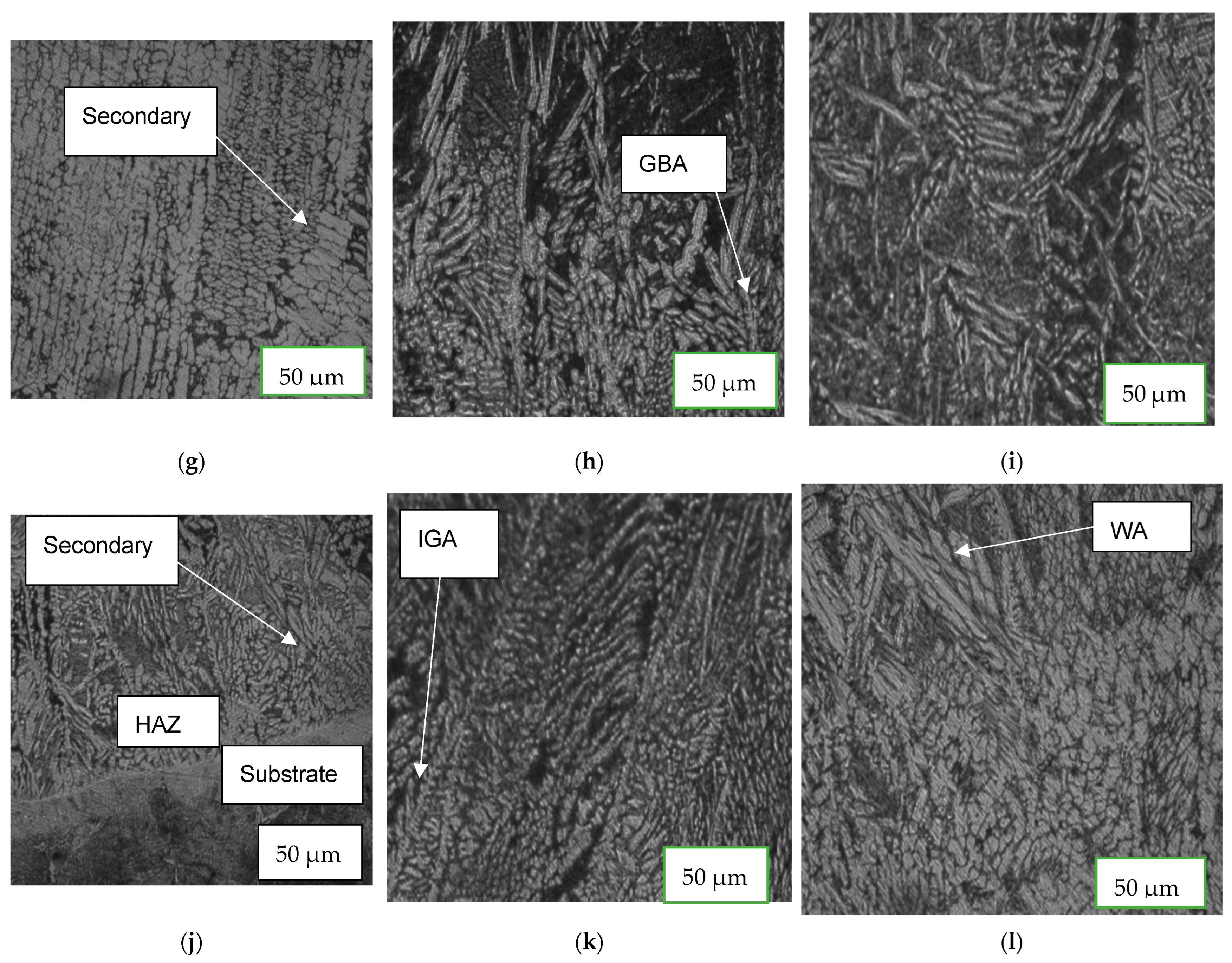
3.1. Microstructure Characterization
3.2. Micrograph Hardness Test
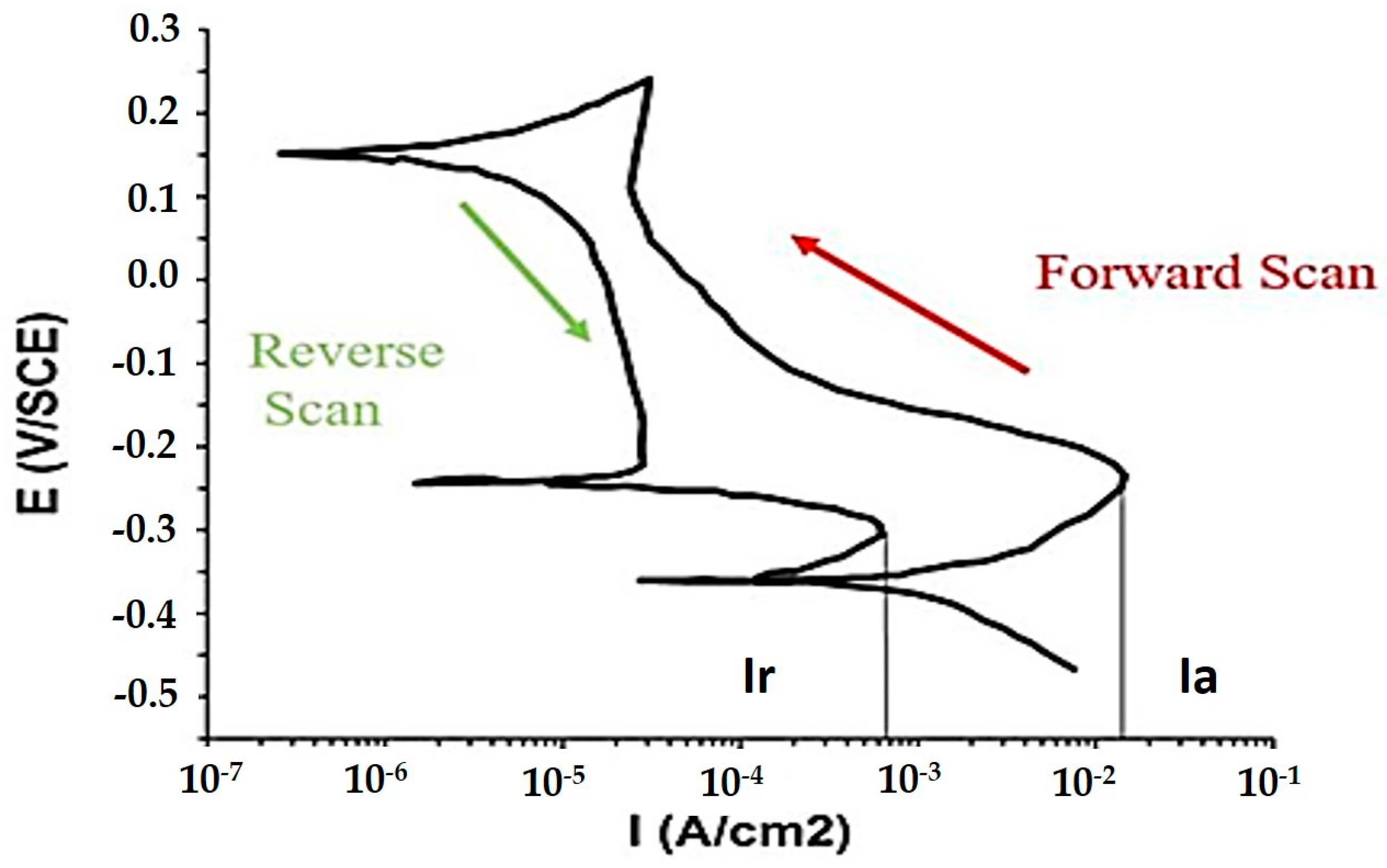
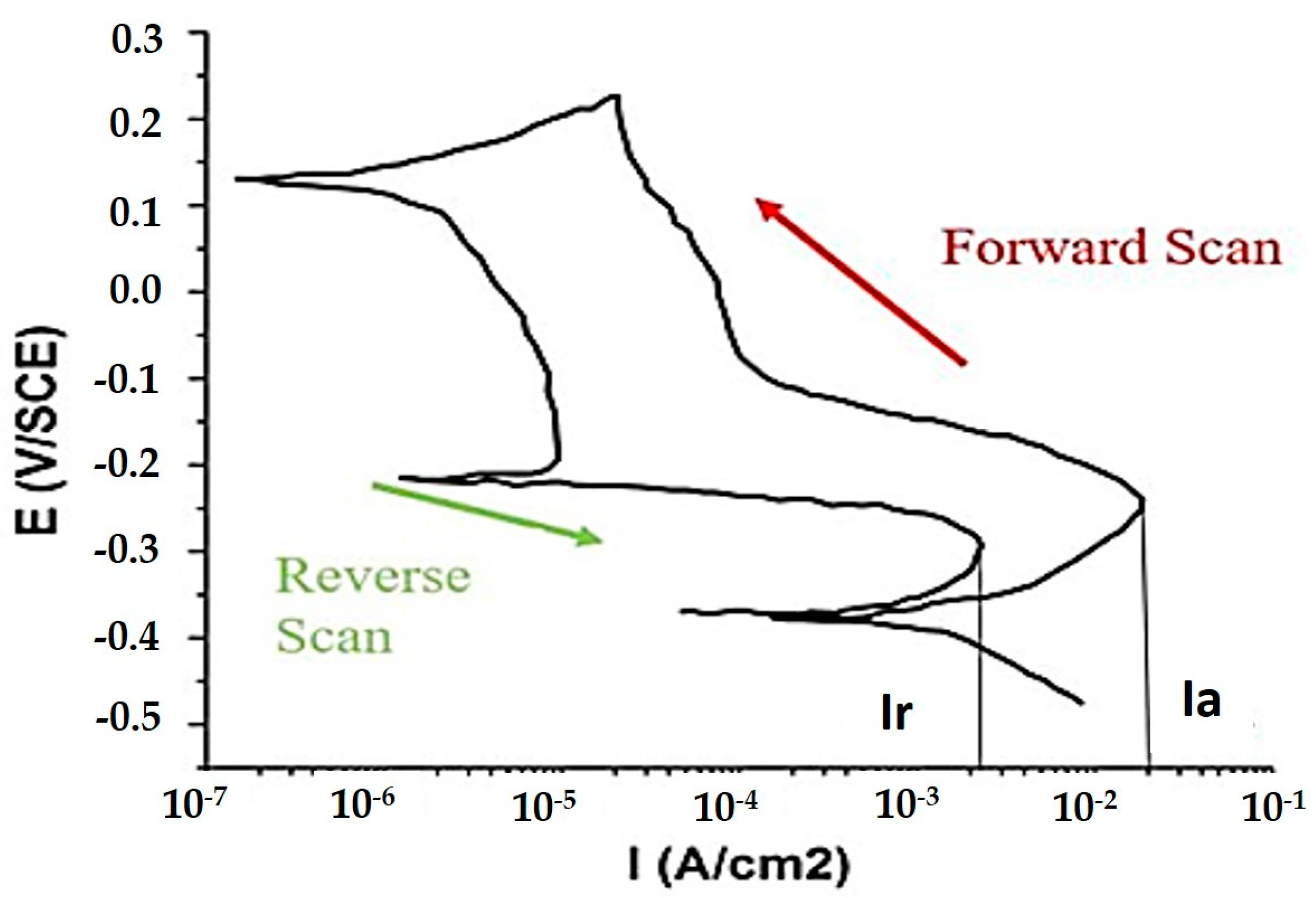
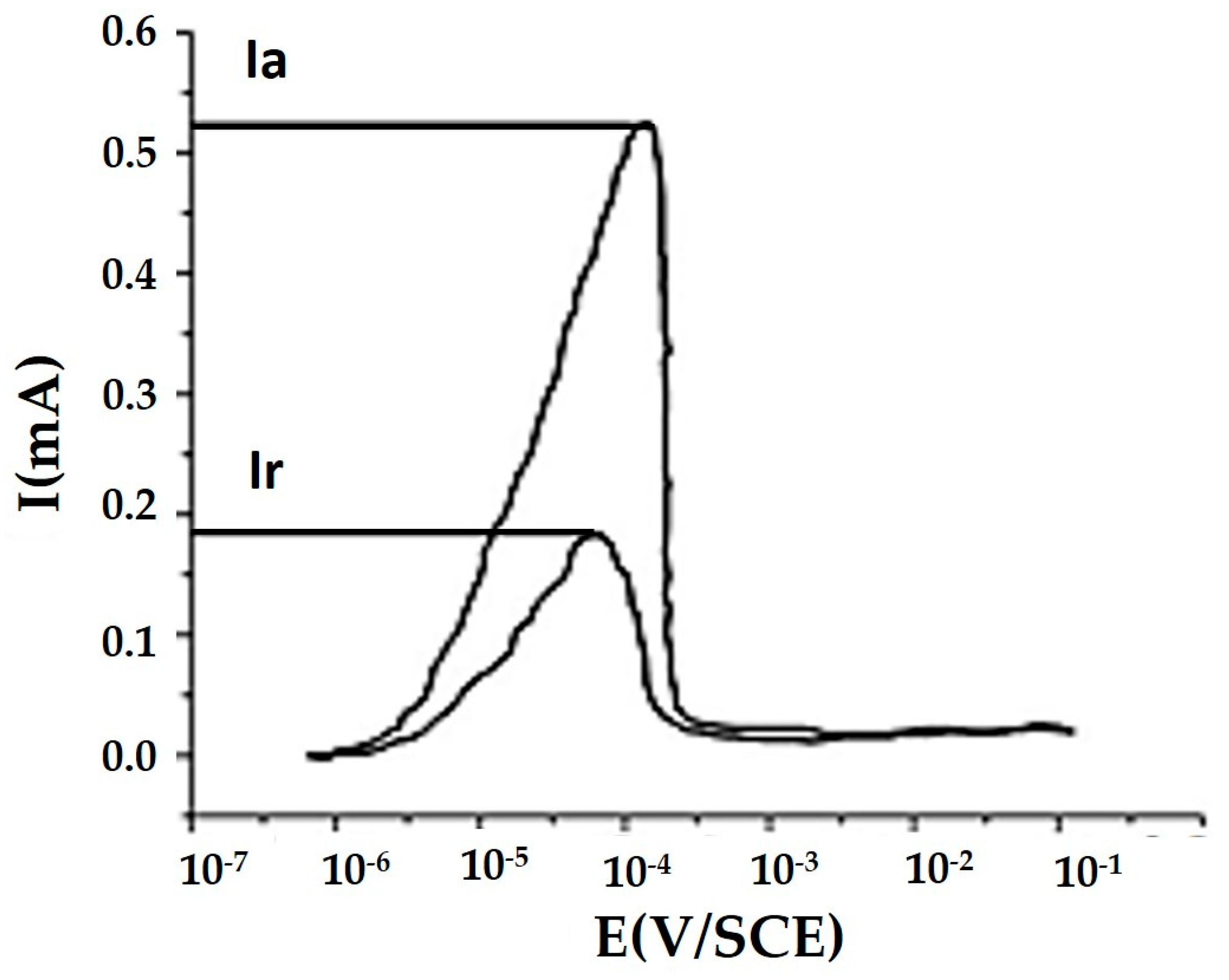
3.3. Double Loop Electrochemical Potentio-Kinetic Reactivation Tests (DL-EPR)
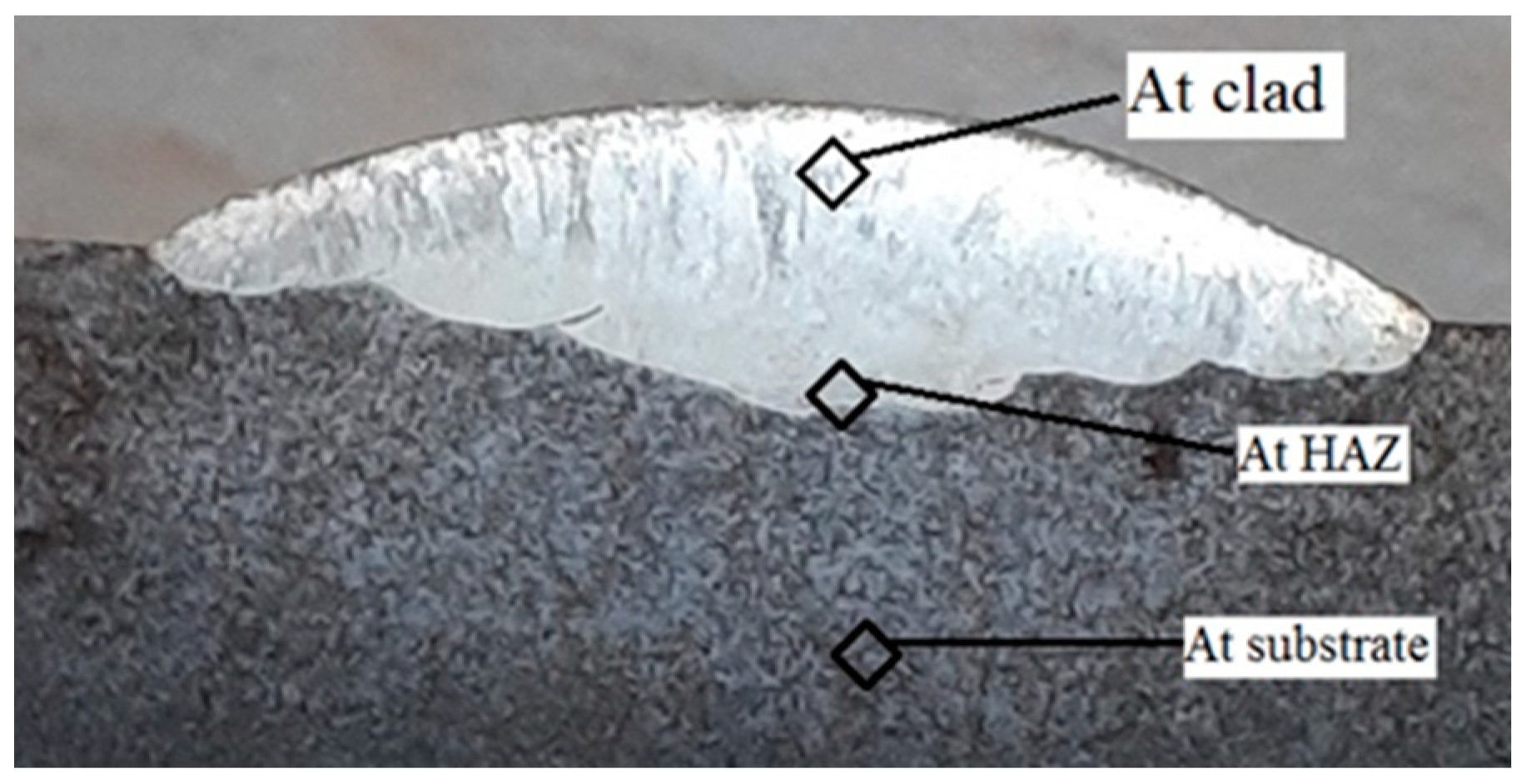
3.4. Dilution
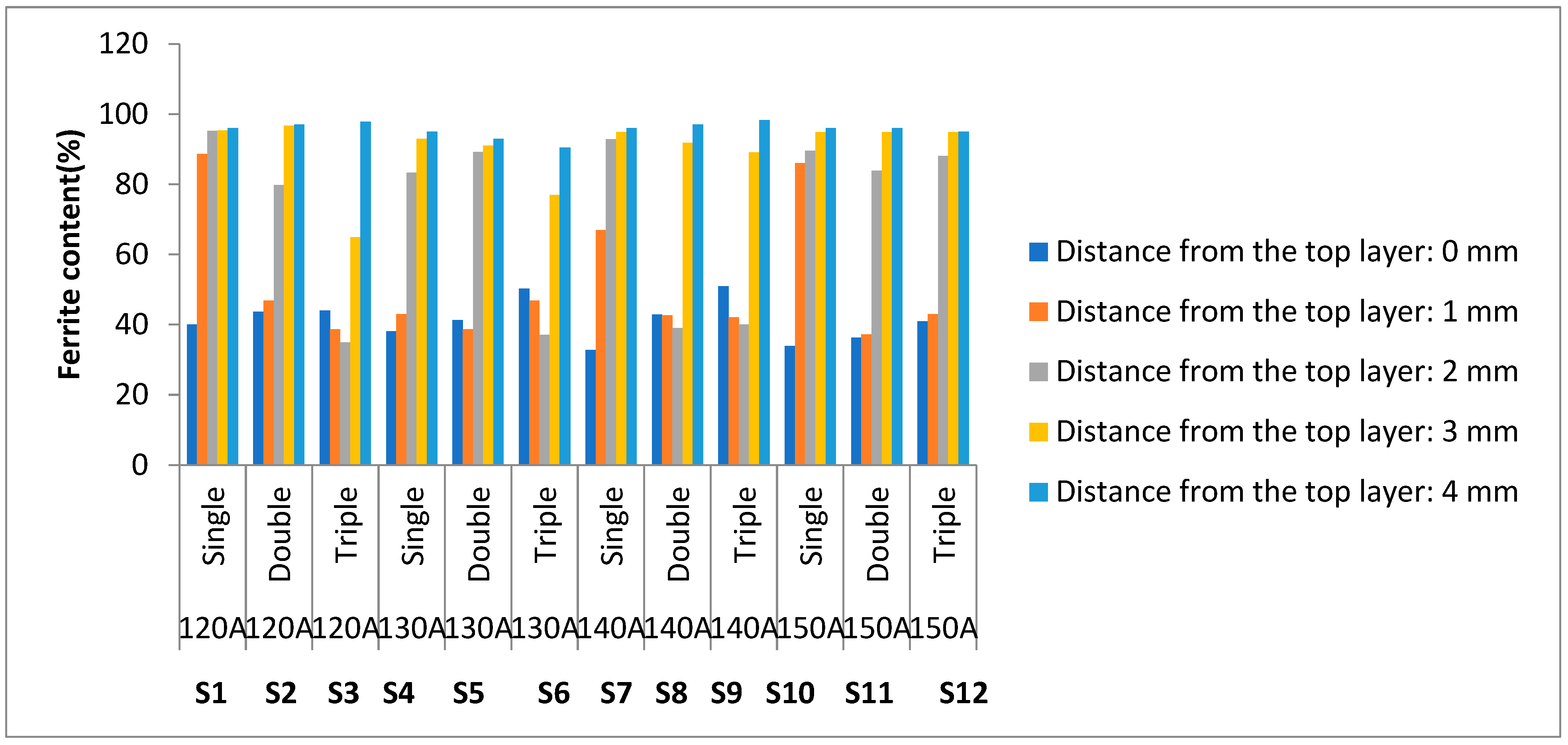
3.5. Ferrite Number
4. Result and Discussion
4.1. Pitting Corrosion Test (DLEPR)
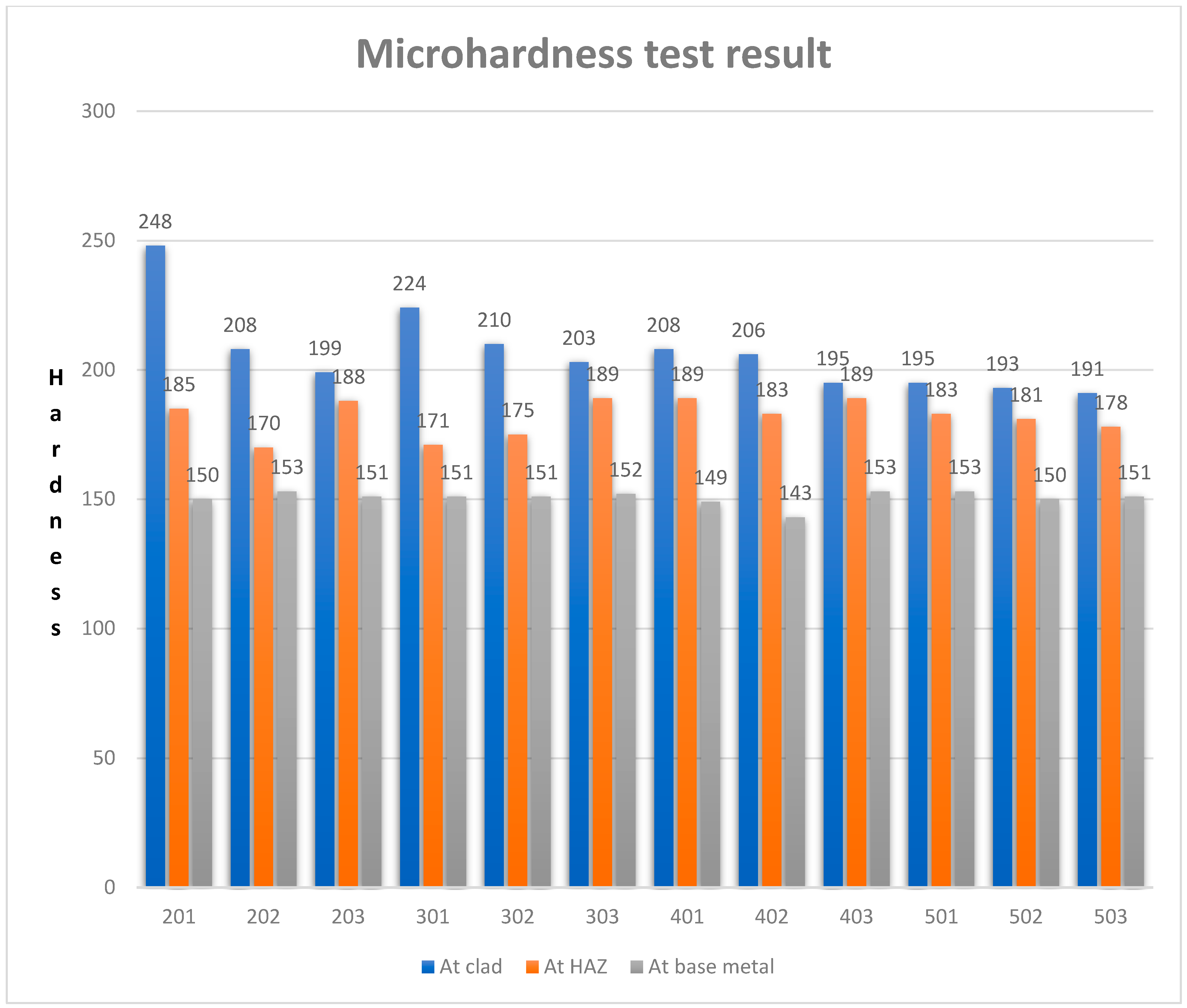
4.2. Microhardness
4.3. Microstructure Characterization
4.4. Ferrite Number
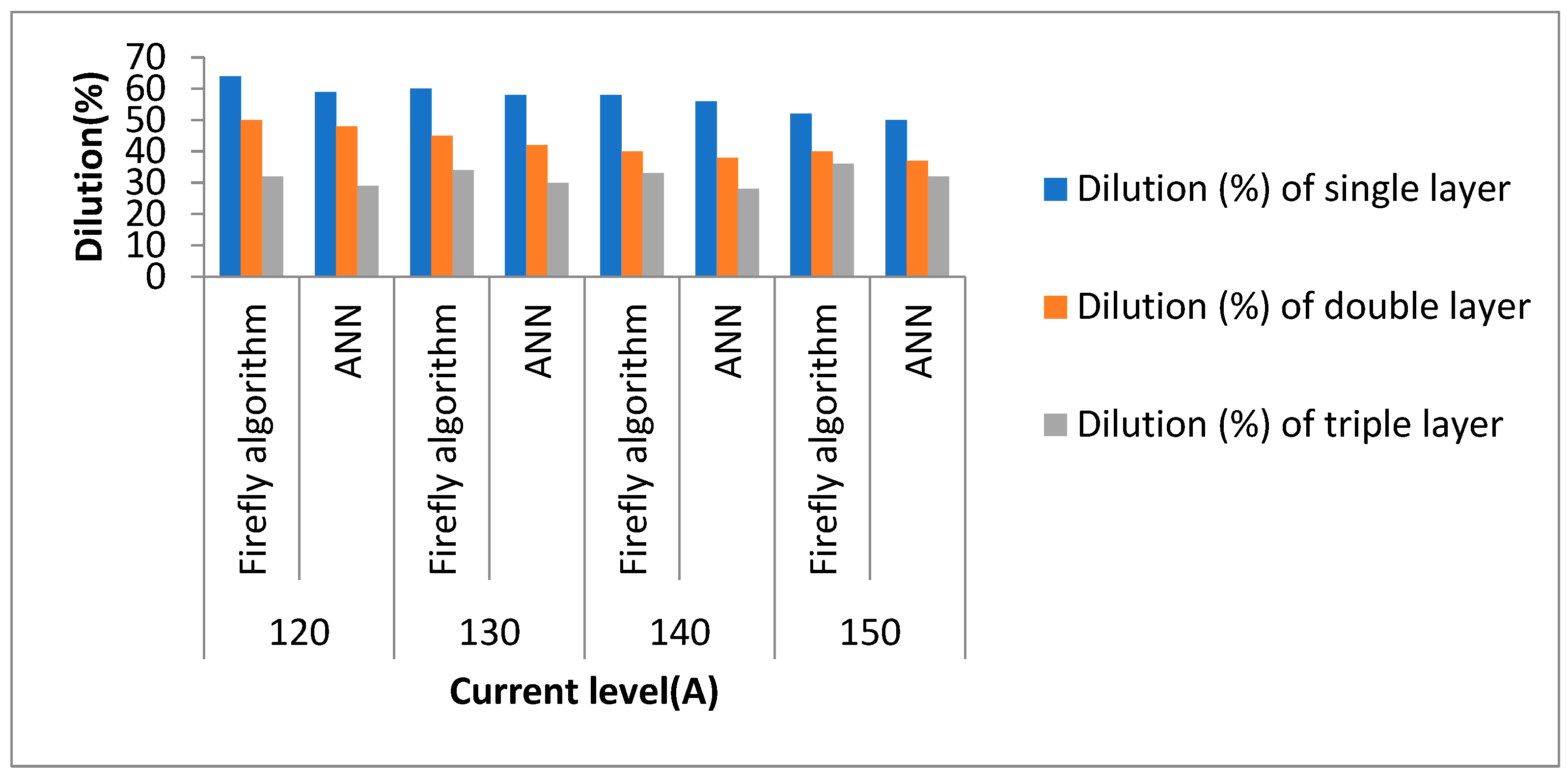
4.5. Dilution
4.6. Optimization of the Dilution Result
4.6.1. Regression Equation of Dilution
4.6.2. Effect of Current on Dilution
- The current value should be in the range of 120 A–150 A;
- Clad layers should be single, double, or triple;
- The response, i.e., dilution, should be minimum.
4.7. Artificial Neural Network (ANN) for the Optimization of the Dilution
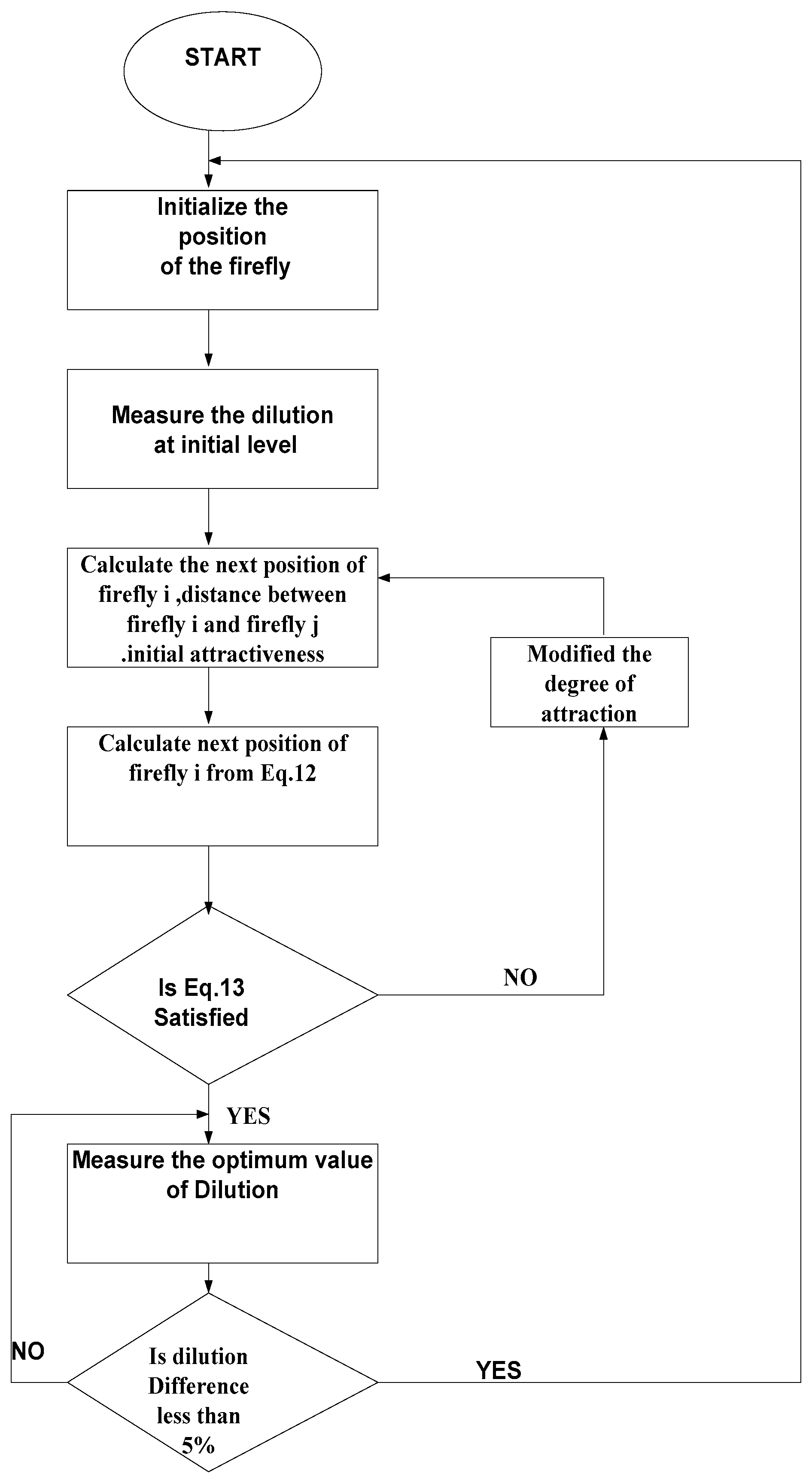
4.8. Design and Development of Firefly Algorithm (FA) for the Optimization of the Dilution
5. Advanced Study and Analysis of Multivariable System
5.1. Multivariable System Approach for Dilution Estimation
5.2. Three Principles of Experimental Setup
5.2.1. Randomization
5.2.2. Replication
5.2.3. Blocking
5.3. Estimation of Variance
6. Conclusions
- ∗
- The cladding of the super duplex stainless steel over mild steel improves the corrosive properties. The Ir/Ia% improves from 29% (mild steel) to 4.1% at the top and 11.9% at the intermediate layers;
- ∗
- The microhardness of the clad decreases with an increase in both the current level and the number of layers;
- ∗
- Microhardness varies between 191–248 at the clad, 170–189 at the HAZ, and 143–153 at the substrate for a 1 kgf load;
- ∗
- For a single-layered clad, on increasing the current level, the deposition rate of filler wire increases, resulting in an increase in the clad reinforcement compared to the penetration level, so the value of dilution decreases;
- ∗
- In the case of double layers of a clad, on increasing current level, dilution decreases due to the increment in clad width as well as reinforcement (simultaneous re-melting of the previously laid clad layer takes place in addition to the new layer deposit);
- ∗
- All triple layers possess almost the same dilution at different levels of current, but compared to a single layer and double layers, it was found to be minimum. Dilution reduces due to the re-melting of two successive layers in addition to the new layer deposit, which further reduces dilution;
- ∗
- Parametric optimization yields that a triple layer made at ~140 A shows minimum dilution (33.6%), optimum ferrite content (50.9%), optimum microhardness (195) maximum pitting corrosion/localized corrosion resistance, i.e., Ir/Ia% (4.1%).
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kahar, S.D. Review on electro-slag strip cladded weld overlays. Int. J. Adv. Eng. Res. Dev. 2016, 3, 1–8. [Google Scholar]
- Paschold, R.; Karlsson, L.; Gittos, M.F. Disbonding of austenitic weld overlays in hydroprocessing applications. ESAB Svetsaren 2007, 62, 10–15. [Google Scholar]
- Bhatt, R.B.; Kamat, H.S.; Ghosal, S.K.; De, P.K. Influence of nitrogen in the shielding gas on corrosion resistance of duplex stainless steel welds. ASM J. Mater. Eng. Perform. 1999, 8, 591–597. [Google Scholar] [CrossRef]
- Murugan, N.; Parmar, R.S. Mathematical models for bead geometry prediction in automatic stainless steel surfacing by MIG welding. Int. J. Join. Mater. 1995, 7, 71–80. [Google Scholar]
- de Lima-Neto, P.; Farias, J.P.; Herculano, L.F.G.; de Miranda, H.C.; Araújo, W.S.; Jorcin, J.B.; Pébère, N. Determination of the sensitized zone extension in welded AISI 304 stainless steel using non-destructive electrochemical techniques. Corros. Sci. 2008, 50, 1149–1155. [Google Scholar] [CrossRef]
- Paschold, R.; Karlsson, L. Electroslag strip cladding for corrosion resistance. ESAB Svetsaren 2001, 56, 62–67. [Google Scholar]
- Koch, G.H.; Brongers, M.P.; Thompson, N.G.; Virmani, Y.P.; Payer, J.H. Corrosion Cost and Preventive Strategies in the United States; National Academy of Sciences, USA (No. FHWA-RD-01-156); Federal Highway Administration: Washington, DC, USA, 2002.
- Singh, B.; Basak, M.; West, M.; Guha, I. Estimating the cost of corrosion in Indian industry. In Proceedings of the PETROTECH-2010, New Delhi, India, 31 October–3 November 2010; Available online: http://hdl.handle.net/20.500.11937/18048 (accessed on 4 January 2023).
- Parmar, R.S. Welding Process and Technology; Khanna Publisher: New Delhi, India, 2003. [Google Scholar]
- Srinath, K. Investigation on 410 L Stainless Steel Cladding of Mild Steel Valve Seat Rings by Plasma Transferred Arc Welding. Ph.D. Thesis, Anna University Chennai, Chennai, India, 2012. [Google Scholar]
- Liu, Y.; Ding, Y.; Yang, L.; Sun, R.; Zhang, T.B.; Yang, X. Research and progress of laser cladding on engineering alloys: A review. J. Manuf. Process. 2021, 66, 341–363. [Google Scholar] [CrossRef]
- Lucas, W. Arc surfacing and cladding processes to enhance performance in service and to repair worn components. Weld. Metal Fabr. 1994, 62, 55–61. [Google Scholar]
- Keskitalo, M.; Mäntyjärvi, K.; Sundqvist, J.; Powell, J.; Kaplan, A.F.H. Laser welding of duplex stainless steel with nitrogen as shielding gas. J. Mater. Process. Technol. 2015, 216, 381–384. [Google Scholar] [CrossRef]
- Aval, H.J.; Farzadi, A.; Serajzadeh, S.; Kokabi, A.H. Theoretical and experimental study of microstructures and weld pool geometry during GTAW of 304 stainless steel. Int. J. Adv. Manuf. Technol. 2009, 42, 1043–1051. [Google Scholar] [CrossRef]
- Arivazhagan, B.; Srinivasan, G.; Albert, S.; Bhaduri, A. A study on influence of heat input variation on microstructure of reduced activation ferritic martensitic steel weld metal produced by GTAW process. Fusion Eng. Des. 2011, 86, 192–197. [Google Scholar] [CrossRef]
- Elsawy, A.H. Characterization of the GTAW fusion line phases for super ferritic stainless steel weldments. J. Mater. Process. Technol. 2001, 118, 127–131. [Google Scholar] [CrossRef]
- Ghanty, P.; Paul, S.; Mukherjee, D.P.; Vasudevan, M.; Pal, N.R.; Bhaduri, A.K. Modelling weld bead geometry using neural networks for GTAW of austenitic stainless steel. Sci. Technol. Weld. Join. 2007, 12, 649–658. [Google Scholar] [CrossRef]
- Ogawa, T.; Aoki, S.; Sakamoto, T.; Zaizen, T. Weldability of Nitrogen-Containing Austenitic Stainless Steel: Part I. Chloride Pitting Corrosion Resistance. WELDING J. 1982, 61, 139–148. [Google Scholar]
- Baeslack, W.A., III; Duquette, D.J.; Savage, W.F. The effect of ferrite content on stress corrosion cracking in duplex stainless steel weld metals at room temperature. Corrosion 1979, 35, 45–54. [Google Scholar] [CrossRef]
- Saxena, A.; Kumar, R.; Singh, J.; Singh, G.K.; Kumar, V.S.; Pandey, J.P. An Invasive Weed Optimization for Sensor Less Control of Grid Integrated Wind Driven Doubly Fed Induction Generator. IEEE Access 2022, 10, 109082–109096. [Google Scholar] [CrossRef]
- Huang, Y.P.; Huang, M.Y.; Ye, C.E. A Fusion Firefly Algorithm with Simplified Propagation for Photovoltaic MPPT Under Partial Shading Conditions. IEEE Trans. Sustain. Energy 2020, 11, 2641–2652. [Google Scholar] [CrossRef]
- Torres, A.F.; Rocha, F.B.; Almeida, F.A.; Gomes, J.H.; Paiva, A.P.; Balestrassi, P.P. Multivariate Stochastic Optimization Approach Applied in a Flux-Cored Arc Welding Process. IEEE Access 2020, 8, 61267–61276. [Google Scholar] [CrossRef]





| Symbol | Name | Unit | Type | Levels | L1 | L2 | L3 | L4 |
|---|---|---|---|---|---|---|---|---|
| A (Numeric) | Current | A | Discrete | 4 | 120 | 130 | 140 | 150 |
| B (Categoric) | Layer | Nominal | 3 | Single | Double | Triple |
| S. No | Current | Layer |
|---|---|---|
| 1 | 120 | Single |
| 2 | 120 | Double |
| 3 | 120 | Triple |
| 4 | 130 | Single |
| 5 | 130 | Double |
| 6 | 130 | Triple |
| 7 | 140 | Single |
| 8 | 140 | Double |
| 9 | 140 | Triple |
| 10 | 150 | Single |
| 11 | 150 | Double |
| 12 | 150 | Triple |
| Material | Element (% wt) | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| C (%) | Si (%) | Mn (%) | P (%) | S (%) | Cr (%) | Ni (%) | Mo (%) | Cu (%) | |
| Substrate (AISI 1020) | 0.196 | 0.293 | 1.12 | 0.0041 | 0.011 | 0.128 | 0.034 | 0.027 | |
| Filler Wire (S32950) | 0.026 | 0.46 | 0.74 | 0.021 | 0.010 | 23.16 | 8.40 | 2.74 | 0.36 |
| Shielding Gas | Pure (99.99%) Argon |
|---|---|
| Shielding gas flow rate | 10 L·min−1 |
| Filler rod diameter | 2.4 mm |
| Non-consumable tungsten electrode | AWS EWTH-2 (98% W + 2% Th) |
| Filler wire diameter | 2.4 mm |
| Polarity | Electrode negative (EN) |
| Arc voltage (V) | 15 V |
| Heat input | 0.94 kJ·mm−1 |
| Material thickness | 12 mm |
| S.No. | Testing Zone | Ia | Ir | Ir/Ia (%) |
|---|---|---|---|---|
| 1 | Top clad layer | 1.56 × 10−2 (A/cm2) | 6.44 × 10−4 (A/cm2) | 4.1 |
| 2 | Intermediate clad layer | 1.89 × 10−2 (A/cm2) | 2.25 × 10−3 (A/cm2) | 11.9 |
| 3 | Base metal (AISI 1020 steel) | 0.54 mA | 0.16 mA | 29.6 |
| Term | Coefficient Estimate | DF | Standard Error | 95% CI Low | 95% CI High | VIF |
|---|---|---|---|---|---|---|
| Intercept | 44.65 | 1 | 1.18 | 39.58 | 49.71 | |
| A Current | −5.60 | 1 | 3.51 | −20.72 | 9.52 | 12.67 |
| B [1] | 11.31 | 1 | 1.67 | 4.15 | 18.48 | |
| B [2] | −2.29 | 1 | 1.67 | −9.46 | 4.87 | |
| AB [1] | −3.97 | 1 | 1.40 | −9.98 | 2.03 | |
| AB [2] | −0.2425 | 1 | 1.40 | −6.25 | 5.76 | |
| A2 | 0.4894 | 1 | 1.66 | −6.63 | 7.61 | 1.0000 |
| A2B [1] | −2.29 | 1 | 2.34 | −12.36 | 7.78 | |
| A2B [2] | 2.26 | 1 | 2.34 | −7.82 | 12.33 | |
| A3 | 2.15 | 1 | 3.70 | −13.77 | 18.08 | 12.67 |
| Number | Current | Layer | Dilution | Desirability | |
|---|---|---|---|---|---|
| 1 | 140.830 | Triple | 35.289 | 0.941 | Selected |
| 2 | 140.935 | Triple | 35.289 | 0.941 | |
| 3 | 140.456 | Triple | 35.291 | 0.941 | |
| 4 | 120.000 | Triple | 35.376 | 0.938 | |
| 5 | 144.246 | Double | 40.302 | 0.765 | |
| 6 | 144.061 | Double | 40.303 | 0.765 | |
| 7 | 150.000 | Single | 46.743 | 0.540 |
| S.No | Wold | Value | Wnew | Value |
|---|---|---|---|---|
| 1 | W1 | 7.8 | W1 | 8.5 |
| 2 | W2 | 8.2 | W2 | 9.4 |
| 3 | W3 | 9.1 | W3 | 9.6 |
| 4 | W4 | 8.9 | W4 | 9.7 |
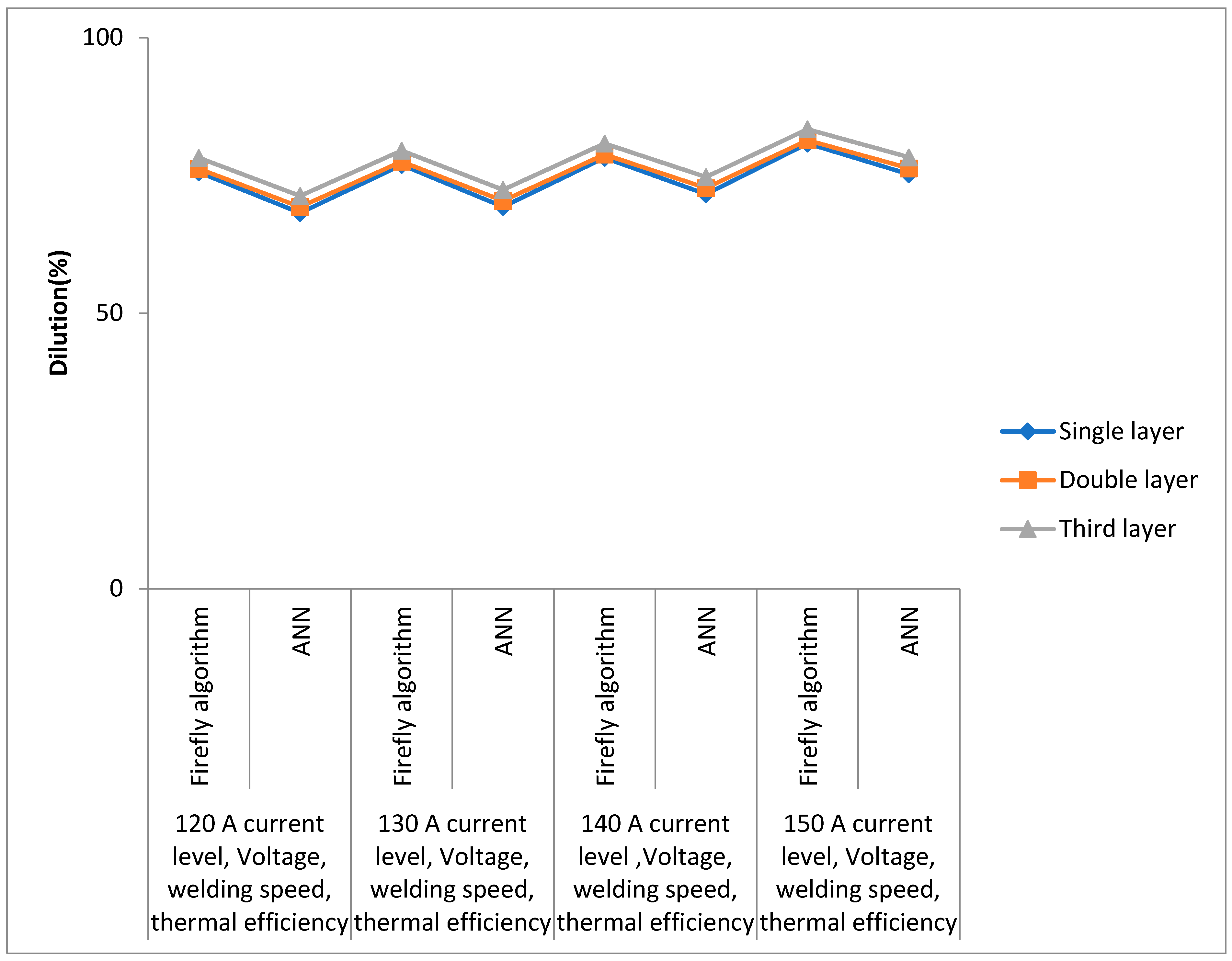
| Dilution (%) | 120 A Current Level, Voltage, Welding Speed, Thermal Efficiency | 130 A Current Level, Voltage, Welding Speed, Thermal Efficiency | 140 A Current Level, Voltage, Welding Speed, Thermal Efficiency | 150 A Current Level, Voltage, Welding Speed, Thermal Efficiency | ||||
|---|---|---|---|---|---|---|---|---|
| Firefly Algorithm | ANN | Firefly Algorithm | ANN | Firefly Algorithm | ANN | Firefly Algorithm | ANN | |
| Single layer | 75.6 | 68.2 | 76.9 | 69.3 | 78.2 | 71.6 | 80.8 | 75.2 |
| Double layer | 76.2 | 69.3 | 77.5 | 70.4 | 78.8 | 72.7 | 81.4 | 76.3 |
| Third layer | 78.2 | 71.3 | 79.5 | 72.4 | 80.8 | 74.7 | 83.4 | 78.3 |
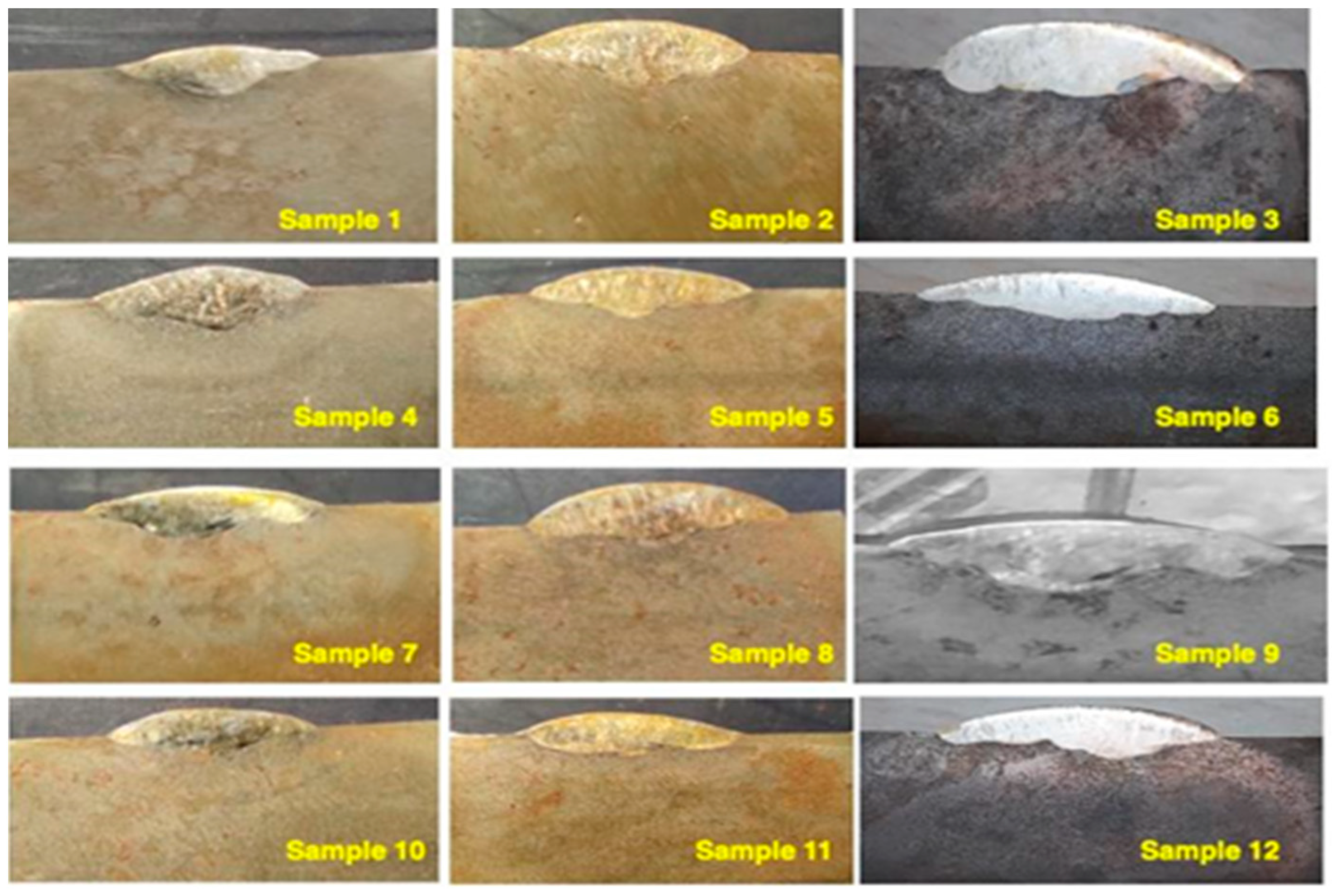
| Samples | Labeling |
|---|---|
| Sample 1 | A |
| Sample 2 | B [1] |
| Sample 3 | B [2] |
| Sample 4 | AB [1] |
| Sample 5 | AB [2] |
| Sample 6 | A2 |
| Sample 7 | A2B [1] |
| Sample 8 | A2B [2] |
| Sample 9 | A3 |
| Sample 10 | A |
| Sample 11 | B [1] |
| Sample 12 | B [2] |
| Events | Test 1 | Test 2 | Test 3 |
|---|---|---|---|
| 1 | Sample 1 | Sample 4 | Sample 10 |
| 2 | Sample 2 | Sample 5 | Sample 11 |
| 3 | Sample 3 | Sample 6 | Sample 9 |
| 4 | Sample 4 | Sample 7 | Sample 1 |
| 5 | Sample 5 | Sample 1 | Sample 2 |
| 6 | Sample 6 | Sample 2 | Sample 3 |
| 7 | Sample 7 | Sample 11 | Sample 4 |
| 8 | Sample 8 | Sample 12 | Sample 8 |
| 9 | Sample 9 | Sample 10 | Sample 12 |
| 10 | Sample 10 | Sample 9 | Sample 6 |
| 11 | Sample 11 | Sample 3 | Sample 7 |
| 12 | Sample 12 | Sample 8 | Sample 5 |
| Samples | |
|---|---|
| Sample 1 | 1.18 |
| Sample 2 | 3.51 |
| Sample 3 | 1.67 |
| Sample 4 | 1.67 |
| Sample 5 | 1.40 |
| Sample 6 | 1.40 |
| Sample 7 | 1.66 |
| Sample 8 | 2.34 |
| Sample 9 | 2.34 |
| Sample 10 | 3.70 |
| Sample 11 | 1.67 |
| Sample 12 | 1.67 |
| Samples | Variance (Var) |
|---|---|
| Sample 1 | 4.18 |
| Sample 2 | 36.96 |
| Sample 3 | 8.37 |
| Sample 4 | 8.37 |
| Sample 5 | 5.88 |
| Sample 6 | 5.88 |
| Sample 7 | 8.27 |
| Sample 8 | 16.43 |
| Sample 9 | 16.43 |
| Sample 10 | 41.07 |
| Sample 11 | 8.37 |
| Sample 12 | 8.37 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Majid, M.; Goel, L.; Saxena, A.; Srivastava, A.K.; Singh, G.K.; Verma, R.; Bhutto, J.K.; Hussein, H.S. Firefly Algorithm and Neural Network Employment for Dilution Analysis of Super Duplex Stainless Steel Clads over AISI 1020 Steel Using Gas Tungsten Arc Process. Coatings 2023, 13, 841. https://doi.org/10.3390/coatings13050841
Majid M, Goel L, Saxena A, Srivastava AK, Singh GK, Verma R, Bhutto JK, Hussein HS. Firefly Algorithm and Neural Network Employment for Dilution Analysis of Super Duplex Stainless Steel Clads over AISI 1020 Steel Using Gas Tungsten Arc Process. Coatings. 2023; 13(5):841. https://doi.org/10.3390/coatings13050841
Chicago/Turabian StyleMajid, Mohd., Love Goel, Abhinav Saxena, Ashish Kumar Srivastava, Gyanendra Kumar Singh, Rajesh Verma, Javed Khan Bhutto, and Hany S. Hussein. 2023. "Firefly Algorithm and Neural Network Employment for Dilution Analysis of Super Duplex Stainless Steel Clads over AISI 1020 Steel Using Gas Tungsten Arc Process" Coatings 13, no. 5: 841. https://doi.org/10.3390/coatings13050841
APA StyleMajid, M., Goel, L., Saxena, A., Srivastava, A. K., Singh, G. K., Verma, R., Bhutto, J. K., & Hussein, H. S. (2023). Firefly Algorithm and Neural Network Employment for Dilution Analysis of Super Duplex Stainless Steel Clads over AISI 1020 Steel Using Gas Tungsten Arc Process. Coatings, 13(5), 841. https://doi.org/10.3390/coatings13050841








