Preparation of Nanocellulose Whisker/Polyacrylamide/Xanthan Gum Double Network Conductive Hydrogels
Abstract
:1. Introduction
2. Experimental Section
2.1. Experimental Materials
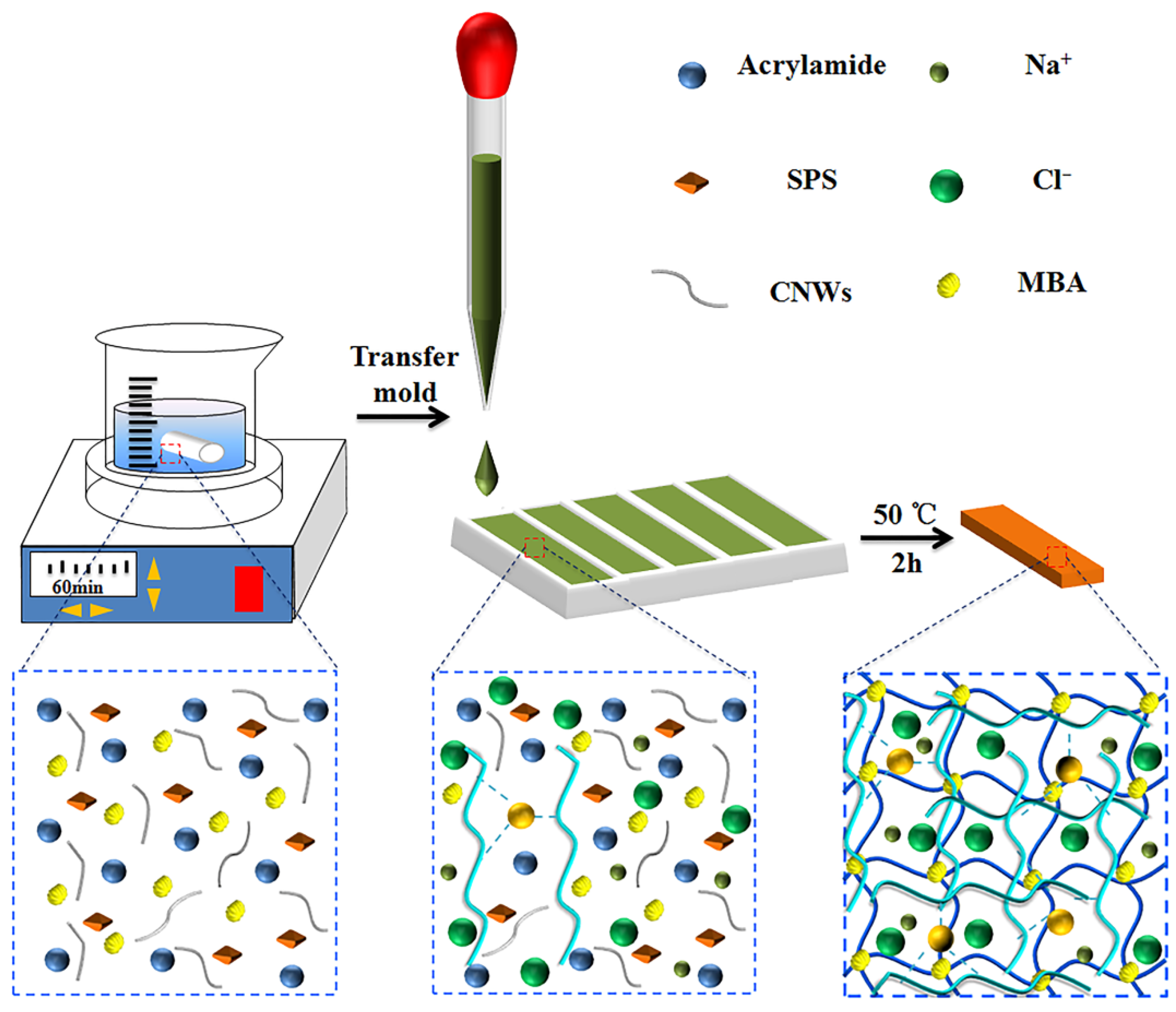
2.2. Preparation of PEC Hydrogels
2.3. Sensing Properties of PEC Composite Conductive Hydrogels
2.4. Swelling Properties of PEC Composite Conductive Hydrogels
2.5. Instrumentation and Characterization
3. Results and Discussion
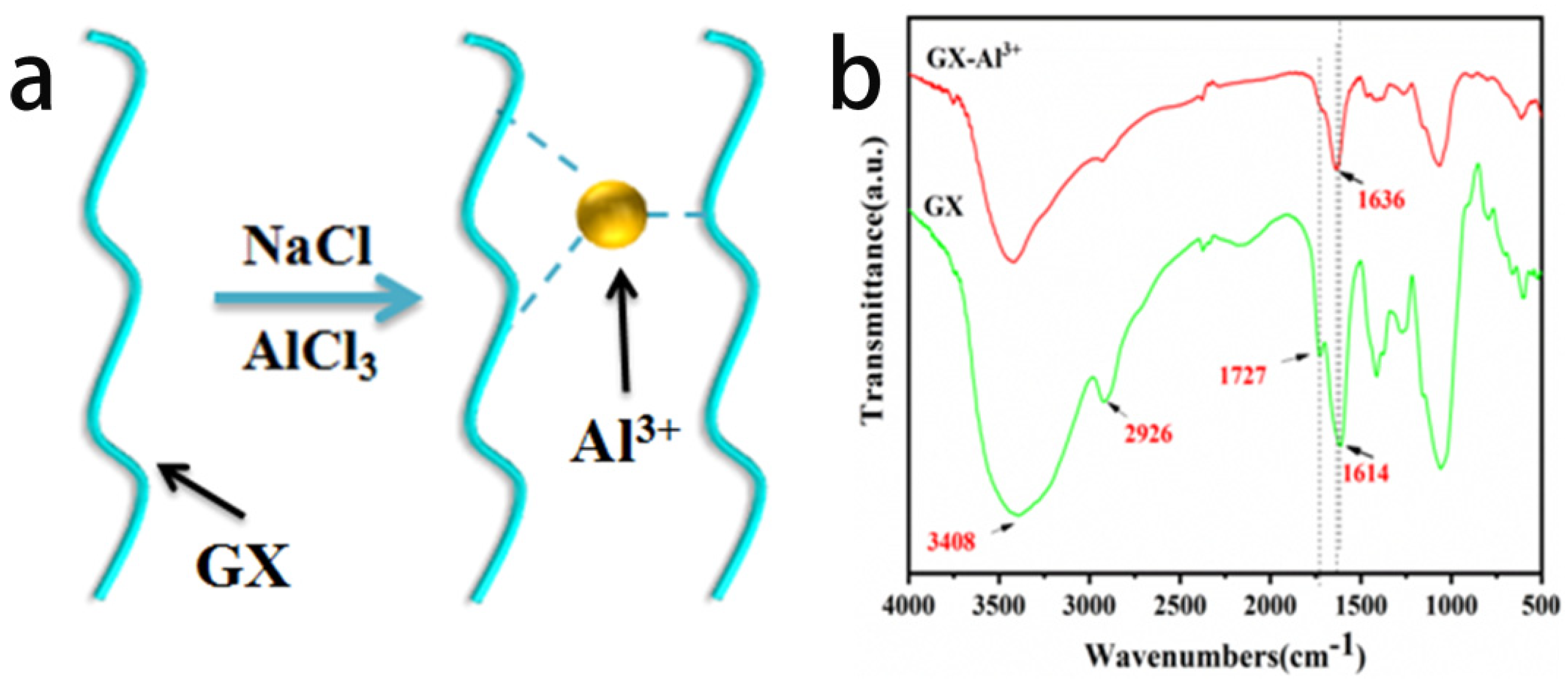
3.1. Formation of PEC Hydrogels
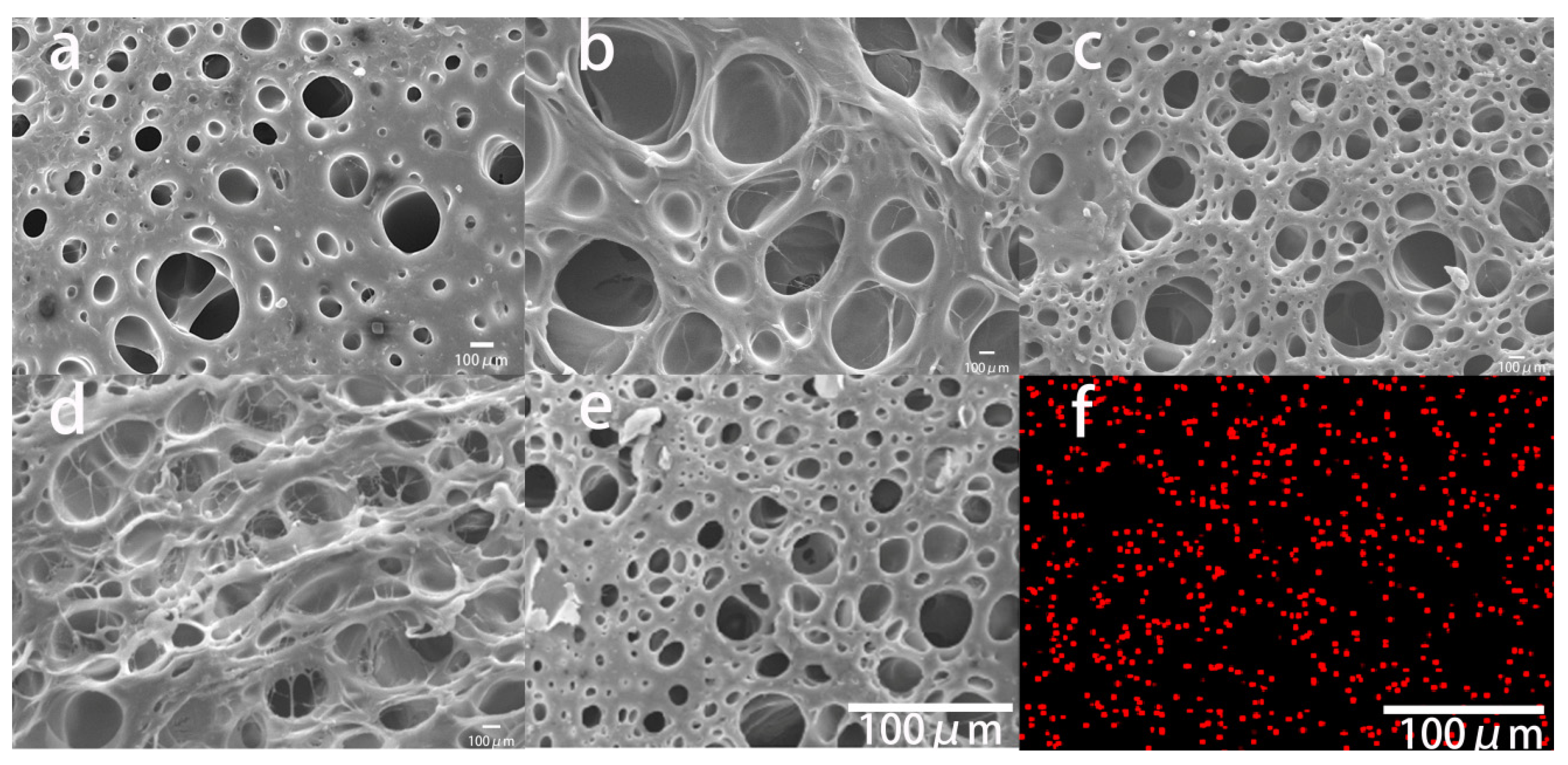
3.2. Microstructure of PEC Composite Conductive Hydrogels
3.3. TGA of PEC Composite Hydrogels
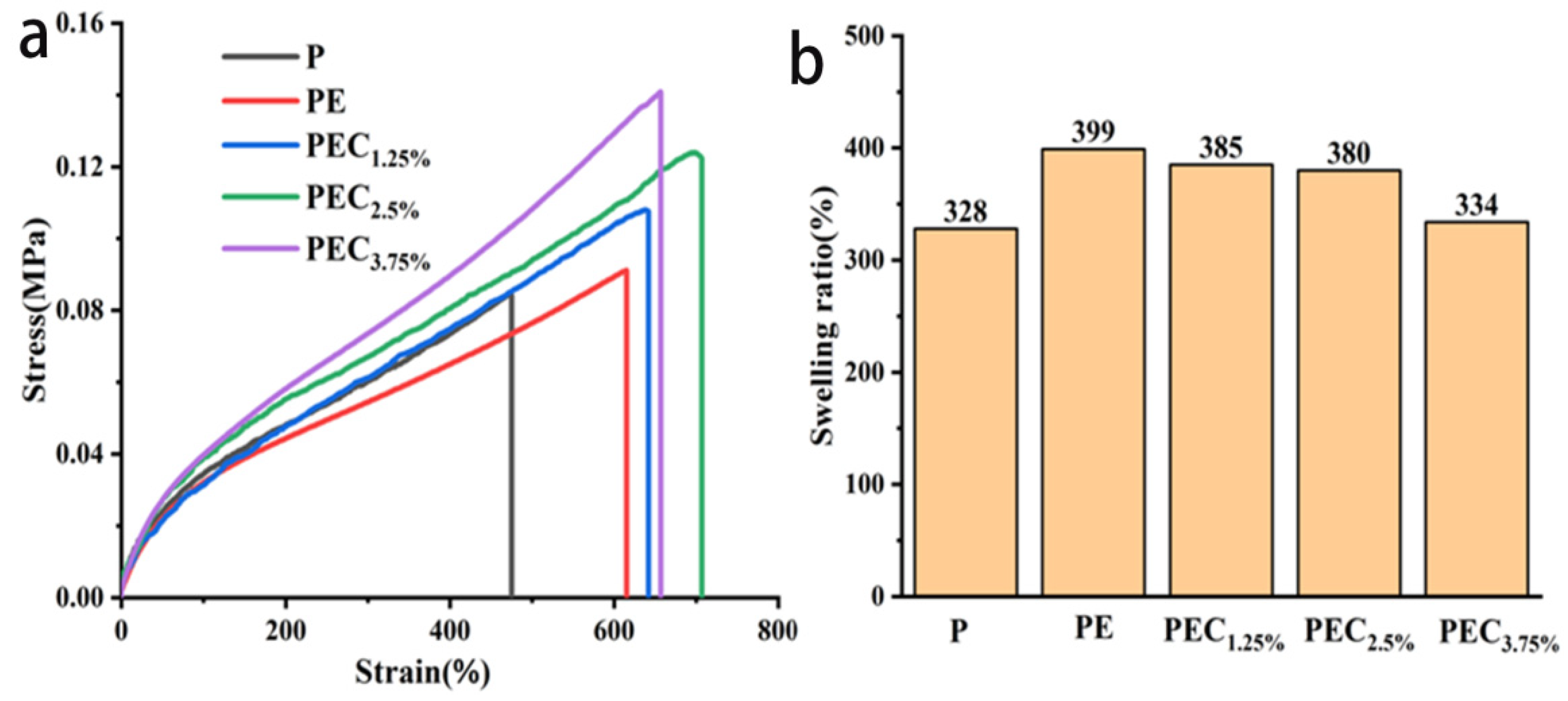
3.4. Mechanical and Swelling Properties of PEC Composite Hydrogels
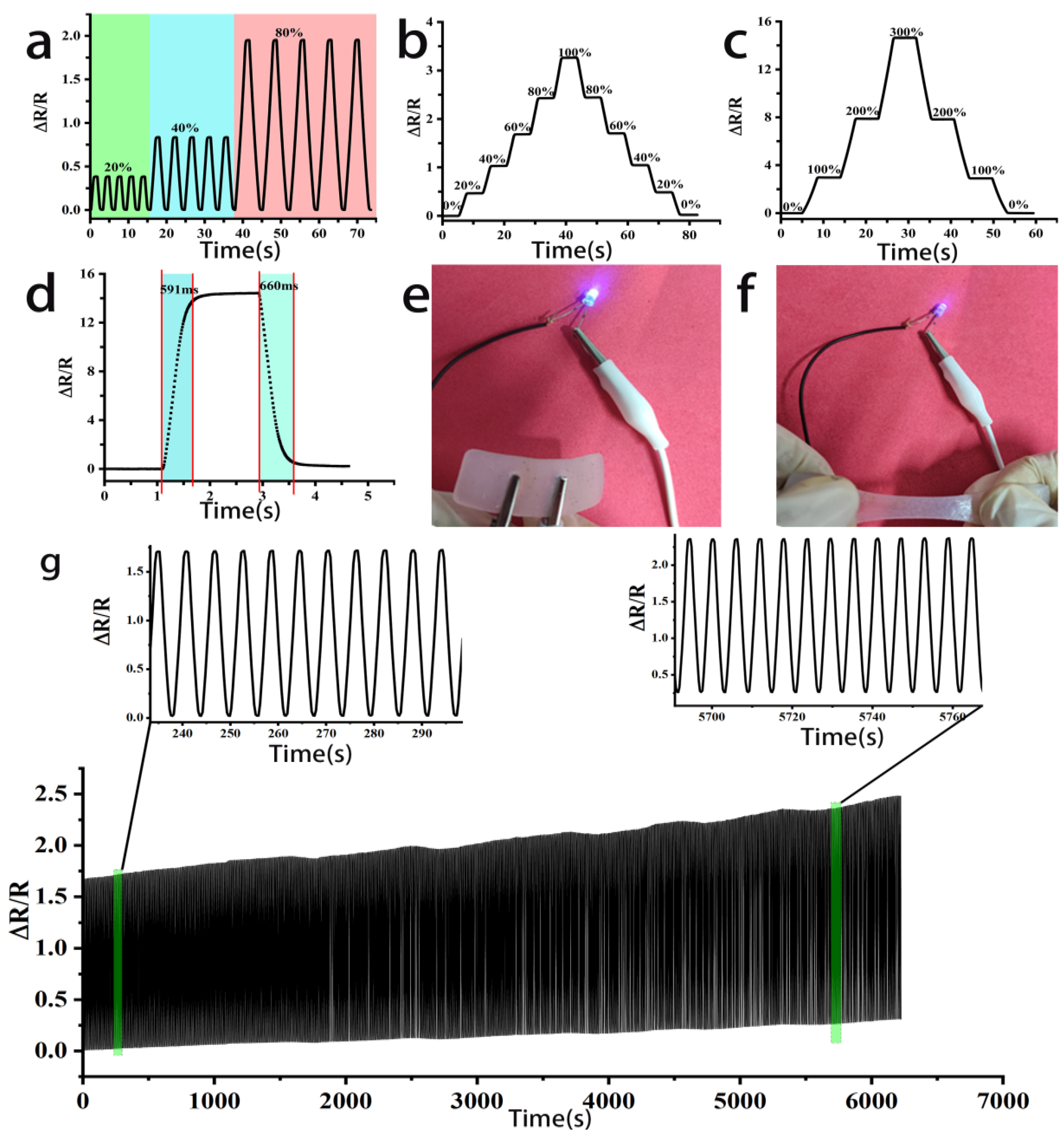
3.5. Conductive Properties of PEC Composite Conductive Hydrogels
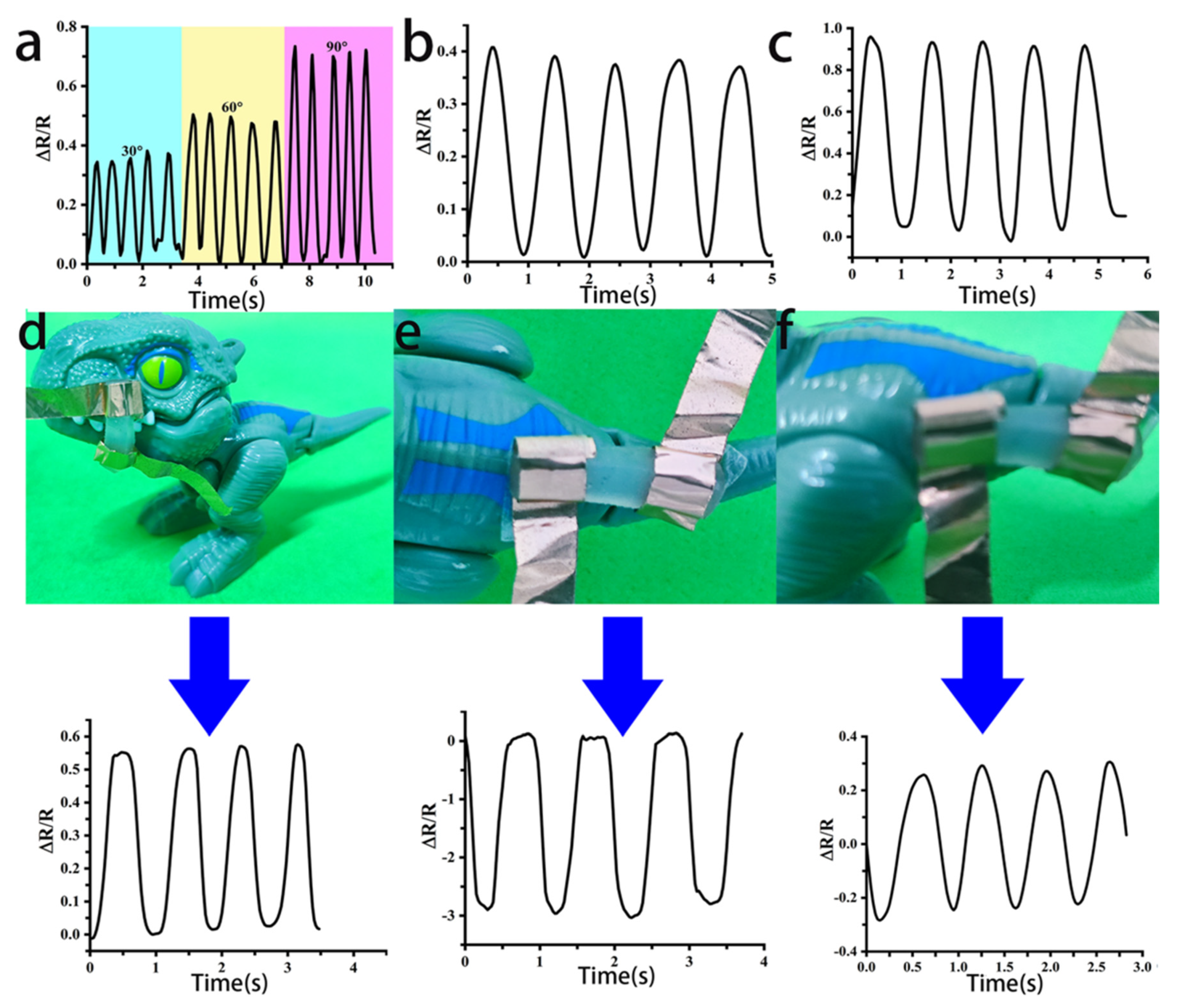
3.6. Applications in PEC Composite Conductive Hydrogel Strain Sensors
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zhang, Y.B.; Yu, J.K.; Ren, K.X.; Zuo, J.L.; Ding, J.X.; Chen, X.S. Thermosensitive Hydrogels as Scaffolds for Cartilage Tissue Engineering. Biomacromolecules 2019, 20, 1478–1492. [Google Scholar] [CrossRef] [PubMed]
- Ding, X.; Zhao, H.M.; Li, Y.Z.; Lee, A.L.; Li, Z.S.; Fu, M.J.; Li, C.N.; Yang, Y.Y.; Yuan, P.Y. Synthetic peptide hydrogels as 3D scaffolds for tissue engineering. Adv. Drug Deliver. Rev. 2020, 160, 78–104. [Google Scholar] [CrossRef] [PubMed]
- Cao, L.; Huang, S.Y.; Lai, F.L.; Fang, Z.M.; Cui, J.; Du, X.S.; Li, W.; Lin, Z.D.; Zhang, P.; Huang, Z.R. Sucrose in situ physically cross-linked of polyaniline and polyvinyl alcohol to prepare three-dimensional nanocomposite hydrogel with flexibility and high capacitance. Ionics 2021, 27, 3431–3441. [Google Scholar] [CrossRef]
- Zhang, W.; Wen, J.Y.; Ma, M.G.; Li, M.F.; Peng, F.; Bian, J. Anti-freezing, water-retaining, conductive, and strain-sensitive hemicellulose/polypyrrole composite hydrogels for flexible sensors. J. Mater. Res. Technol. 2021, 14, 555–566. [Google Scholar] [CrossRef]
- Bashir, S.; Hina, M.; Iqbal, J.; Jafer, R.; Ramesh, S.; Ramesh, K. Self-healable poly (N, N-dimethylacrylamide)/poly (3,4-ethylenedioxythiophene) polystyrene sulfonate composite hydrogel electrolytes for aqueous supercapacitors. J. Energy Storage 2022, 45, 103760. [Google Scholar] [CrossRef]
- Sun, Y.; Wang, S.; Du, X.; Du, Z.; Wang, H.; Cheng, X. Skin-conformal MXene-doped wearable sensors with self-adhesive, dual-mode sensing, and high sensitivity for human motions and wireless monitoring. J. Mater. Chem. B 2021, 9, 8667–8675. [Google Scholar] [CrossRef]
- Wei, Y.; Qian, Y.Y.; Zhu, P.H.; Xiang, L.J.; Lei, C.F.; Qiu, G.; Wang, C.Y.; Liu, Y.K.; Liu, Y.J.; Chen, G. Nanocellulose-templated carbon nanotube enhanced conductive organohydrogel for highly-sensitive strain and temperature sensors. Cellulose 2022, 29, 3829–3844. [Google Scholar] [CrossRef]
- Hao, F.; Maimaitiyiming, X. Fast 3D Printing with Chitosan/Polyvinyl alcohol/Graphene Oxide Multifunctional Hydrogel Ink that has UltraStretch Properity. Chemistryselect 2022, 7, e202200201. [Google Scholar] [CrossRef]
- Dai, R.; Zhou, H.; Huang, W.; Li, C.; Qin, C.; Liu, X.; Pan, Z. Conductive hydrogel-based electronics for intelligent sensing and smart controlling. J. Nanoelectron. Optoelectron. 2021, 16, 689–698. [Google Scholar] [CrossRef]
- Holtz, J.H.; Asher, S.A. Polymerized colloidal crystal hydrogel films as intelligent chemical sensing materials. Nature 1997, 389, 829–832. [Google Scholar] [CrossRef]
- Chen, Z.; Liu, J.; Chen, Y.; Zheng, X.; Liu, H.; Li, H. Multiple-Stimuli-Responsive and Cellulose Conductive Ionic Hydrogel for Smart Wearable Devices and Thermal Actuators. ACS Appl. Mater. Interfaces 2021, 13, 1353–1366. [Google Scholar] [CrossRef] [PubMed]
- Lin, F.; Qiu, Y.; Zheng, X.; Duanmu, Z.; Lu, Q.; Huang, B.; Tang, L.; Lu, B. One-pot mechanochemical assembly of lignocellulose nanofiber/graphite nanocomposites for wearable electronic devices. Chem. Eng. J. 2022, 437, 135286. [Google Scholar] [CrossRef]
- Xiao, G.; Wang, Y.; Zhang, H.; Zhu, Z.; Fu, S. Cellulose nanocrystal mediated fast self-healing and shape memory conductive hydrogel for wearable strain sensors. Int. J. Biol. Macromol. 2021, 170, 272–283. [Google Scholar] [CrossRef] [PubMed]
- Stricher, M.; Sarde, C.O.; Guenin, E.; Egles, C.; Delbecq, F. Cellulosic/Polyvinyl Alcohol Composite Hydrogel: Synthesis, Characterization and Applications in Tissue Engineering. Polymers 2021, 13, 3598. [Google Scholar] [CrossRef]
- Chen, X.Q.; Yan, H.Q.; Bao, C.L.; Zhu, Q.M.; Liu, Z.W.; Wen, Y.S.; Li, Z.Y.; Zhang, T.; Lin, Q. Fabrication and evaluation of homogeneous alginate/polyacrylamide-chitosan-gelatin composite hydrogel scaffolds based on the interpenetrating networks for tissue engineering. Polym. Eng. Sci. 2022, 62, 116–128. [Google Scholar] [CrossRef]
- Payo, I.; Polo, J.L.; López, B.; Serrano, D.; Rodríguez, A.M.; Antonia Herrero, M.; Martín-Pacheco, A.; Sánchez, I.; Vázquez, E. Signal conditioning circuit for gel strain sensors. Smart Mater. Struct. 2021, 31, 015020. [Google Scholar] [CrossRef]
- Ruano, G.; Iribarren, J.I.; Perez-Madrigal, M.M.; Torras, J.; Aleman, C. Electrical and Capacitive Response of Hydrogel Solid-Like Electrolytes for Supercapacitors. Polymers 2021, 13, 1337. [Google Scholar] [CrossRef]
- Liu, H.W.; Xu, D.H.; Hu, B.; Jiang, J.J.; Li, M.Y.; Zhao, D.; Zhai, W.T. Eco-friendly biogenic hydrogel for wearable skin-like iontronics. J. Mater. Chem. A 2021, 9, 4692–4699. [Google Scholar] [CrossRef]
- Zhang, B.; Zhang, X.; Wan, K.; Zhu, J.; Xu, J.; Zhang, C.; Liu, T. Dense Hydrogen-Bonding Network Boosts Ionic Conductive Hydrogels with Extremely High Toughness, Rapid Self-Recovery, and Autonomous Adhesion for Human-Motion Detection. Research 2021, 2021, 9761625. [Google Scholar] [CrossRef]
- Chen, Y.W.; Dai, S.P.; Zhu, H.; Hu, H.W.; Yuan, N.Y.; Ding, J.N. Self-healing hydrogel sensors with multiple shape memory properties for human motion monitoring. New J. Chem. 2021, 45, 314–320. [Google Scholar] [CrossRef]
- Xue, B.; Sheng, H.; Li, Y.; Li, L.; Di, W.; Xu, Z.; Ma, L.; Wang, X.; Jiang, H.; Qin, M.; et al. Stretchable and self-healable hydrogel artificial skin. Natl. Sci. Rev. 2022, 9, nwab147. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.Z.; Li, Z.; Liu, Z.Y.; Fu, J.K.; Shan, T.Y.; Yang, X.Y.; Lei, Q.Y.; Yang, Y.J.; Li, D.H. Flexible capacitive pressure sensors for wearable electronics. J. Mater. Chem. C 2022, 10, 1594–1605. [Google Scholar] [CrossRef]
- Miao, Y.; Xu, M.; Yu, J.; Zhang, L. Conductive cold-resistant and elastic hydrogel: A potential bionic skin for human-machine interaction control over artificial limbs. Sens. Actuators B Chem. 2021, 327, 128916. [Google Scholar] [CrossRef]
- Wei, Q.H.; Yang, R.B.; Sun, D.C.; Zhou, J.Y.; Li, M.Y.; Zhang, Y.F.; Wang, Y.E. Design and evaluation of sodium alginate/polyvinyl alcohol blend hydrogel for 3D bioprinting cartilage scaffold: Molecular dynamics simulation and experimental method. J. Mater. Res. Technol. 2022, 17, 66–78. [Google Scholar] [CrossRef]
- Jiao, Y.; Lu, K.Y.; Lu, Y.; Yue, Y.Y.; Xu, X.W.; Xiao, H.N.; Li, J.; Han, J.Q. Highly viscoelastic, stretchable, conductive, and self-healing strain sensors based on cellulose nanofiber-reinforced polyacrylic acid hydrogel. Cellulose 2021, 28, 4295–4311. [Google Scholar] [CrossRef]
- Jing, Z.; Xu, A.; Liang, Y.Q.; Zhang, Z.; Yu, C.; Hong, P.; Li, Y. Biodegradable Poly(acrylic acid-co-acrylamide)/Poly(vinyl alcohol) Double Network Hydrogels with Tunable Mechanics and High Self-healing Performance. Polymers 2019, 11, 952. [Google Scholar] [CrossRef]
- Kong, D.S.; El-Bahy, Z.M.; Algadi, H.; Li, T.; El-Bahy, S.M.; Nassan, M.A.; Li, J.R.; Faheim, A.A.; Li, A.; Xu, C.X.; et al. Highly sensitive strain sensors with wide operation range from strong MXene-composited polyvinyl alcohol/sodium carboxymethylcellulose double network hydrogel. Adv. Compos. Hybrid Mater. 2022, 5, 1976–1987. [Google Scholar] [CrossRef]
- Dai, X.; Long, Y.; Jiang, B.; Guo, W.; Sha, W.; Wang, J.; Cong, Z.; Chen, J.; Wang, B.; Hu, W. Ultra-antifreeze, ultra-stretchable, transparent, and conductive hydrogel for multi-functional flexible electronics as strain sensor and triboelectric nanogenerator. Nano Res. 2022, 15, 5461–5468. [Google Scholar] [CrossRef]
- Wu, Z.; Yang, X.; Wu, J. Conductive Hydrogel- and Organohydrogel-Based Stretchable Sensors. ACS Appl. Mater. Interfaces 2021, 13, 2128–2144. [Google Scholar] [CrossRef]
- Sun, X.Y.; Liang, Y.Z.; Ye, L.N.; Liang, H.Y. An extremely tough and ionic conductive natural-polymer-based double network hydrogel. J. Mater. Chem. B 2021, 9, 7751–7759. [Google Scholar] [CrossRef]
- Wan, C.C.; Zhang, L.Y.; Yong, K.T.; Li, J.; Wu, Y.Q. Recent progress in flexible nanocellulosic structures for wearable piezoresistive strain sensors. J. Mater. Chem. C 2021, 9, 11001–11029. [Google Scholar] [CrossRef]
- Qin, T.; Li, X.; Yang, A.; Wu, M.; Yu, L.; Zeng, H.; Han, L. Nanomaterials-enhanced, stretchable, self-healing, temperature-tolerant and adhesive tough organohydrogels with long-term durability as flexible sensors for intelligent motion-speech recognition. Chem. Eng. J. 2023, 461, 141905. [Google Scholar] [CrossRef]
- Pi, M.H.; Jiang, L.C.; Wang, Z.S.; Cui, W.; Shi, L.Y.; Ran, R. Robust and ultrasensitive hydrogel sensors enhanced by MXene/cellulose nanocrystals. J. Mater. Sci. 2021, 56, 8871–8886. [Google Scholar] [CrossRef]
- Cheng, D.Y.; Liao, I.H.; Yu, J.S.; Liao, Y.C. Highly compressible hydrogel reinforced with cellulose nanocrystals for ultrasound scanning via microwave-assisted synthesis. Cellulose 2022, 29, 9791–9802. [Google Scholar] [CrossRef]
- Wei, P.; Chen, W.W.; Song, Q.H.; Wu, Y.B.; Xu, Y.J. Superabsorbent hydrogels enhanced by quaternized tunicate cellulose nanocrystals with adjustable strength and swelling ratio. Cellulose 2021, 28, 3723–3732. [Google Scholar] [CrossRef]
- Lai, P.C.; Yu, S.S. Cationic Cellulose Nanocrystals-Based Nanocomposite Hydrogels: Achieving 3D Printable Capacitive Sensors with High Transparency and Mechanical Strength. Polymers 2021, 13, 688. [Google Scholar] [CrossRef]
- Hossen, M.J.; Sarkar, S.D.; Uddin, M.M.; Roy, C.K.; Azam, M.S. Mussel-Inspired Adhesive Nano-Filler for Strengthening Polyacrylamide Hydrogel. Chemistryselect 2020, 5, 8906–8914. [Google Scholar] [CrossRef]
- Debon, S.J.; Tester, R.F. In vitro binding of calcium, iron and zinc by non-starch polysaccharides. Food Chem. 2001, 73, 401–410. [Google Scholar] [CrossRef]
- Antonini, C.; Wu, T.; Zimmermann, T.; Kherbeche, A.; Thoraval, M.-J.; Nyström, G.; Geiger, T. Ultra-porous nanocellulose foams: A facile and scalable fabrication approach. Nanomaterials 2019, 9, 1142. [Google Scholar] [CrossRef]


| Code | NaCl (g) | GX (g) | AlCl3 (1 wt%) (mL) | CNWs (5 wt%) (g) | AAm (g) | SPS (mg) | MBA (mg) | Water (mL) |
|---|---|---|---|---|---|---|---|---|
| P | 0 | 0 | 0 | 0 | 8 | 80 | 4 | 32 |
| PE | 0.2 | 0.08 | 2 | 0 | 8 | 80 | 4 | 30 |
| PEC1.25% | 0.2 | 0.08 | 2 | 2 | 8 | 80 | 4 | 28 |
| PEC2.5% | 0.2 | 0.08 | 2 | 4 | 8 | 80 | 4 | 26 |
| PEC3.75% | 0.2 | 0.08 | 2 | 6 | 8 | 80 | 4 | 24 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Du, Z.; Wang, Y.; Li, X. Preparation of Nanocellulose Whisker/Polyacrylamide/Xanthan Gum Double Network Conductive Hydrogels. Coatings 2023, 13, 843. https://doi.org/10.3390/coatings13050843
Du Z, Wang Y, Li X. Preparation of Nanocellulose Whisker/Polyacrylamide/Xanthan Gum Double Network Conductive Hydrogels. Coatings. 2023; 13(5):843. https://doi.org/10.3390/coatings13050843
Chicago/Turabian StyleDu, Zhiwei, Yalei Wang, and Xiurong Li. 2023. "Preparation of Nanocellulose Whisker/Polyacrylamide/Xanthan Gum Double Network Conductive Hydrogels" Coatings 13, no. 5: 843. https://doi.org/10.3390/coatings13050843




