Atomic Layer Deposition of La2O3 Film with Precursor La(thd)3-DMEA
Abstract
1. Introduction
2. Experimental
2.1. Materials
2.2. Instrumentation
2.3. Synthesized of La(thd)3 (1)
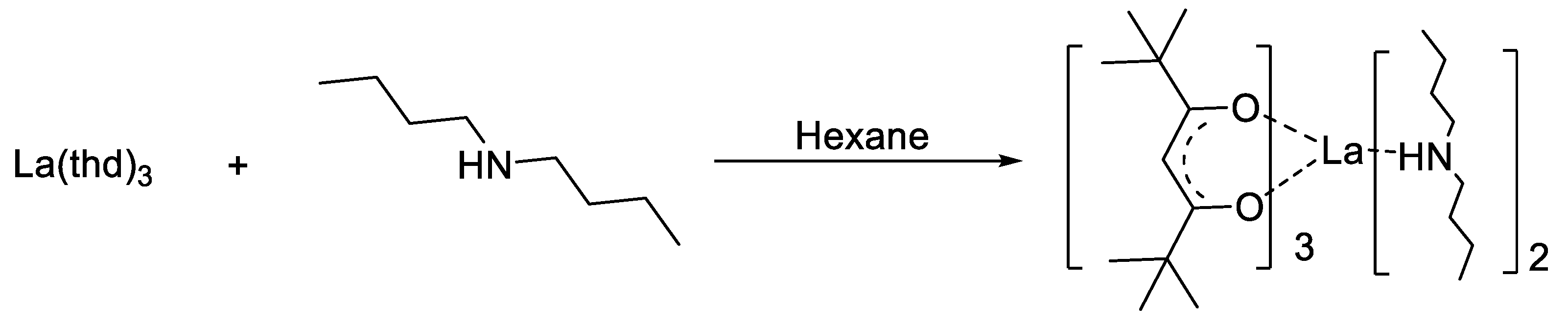
2.4. Synthesized of La(thd)3-DMEA (2)
2.5. Synthesized of La(thd)3-DBA (3)
2.6. Synthesized of La(thd)3-DPA (4)
2.7. Thermogravimetric Analysis
2.8. ALD of La2O3 Film Details
3. Result and Discussion
3.1. Crystal Structure Description
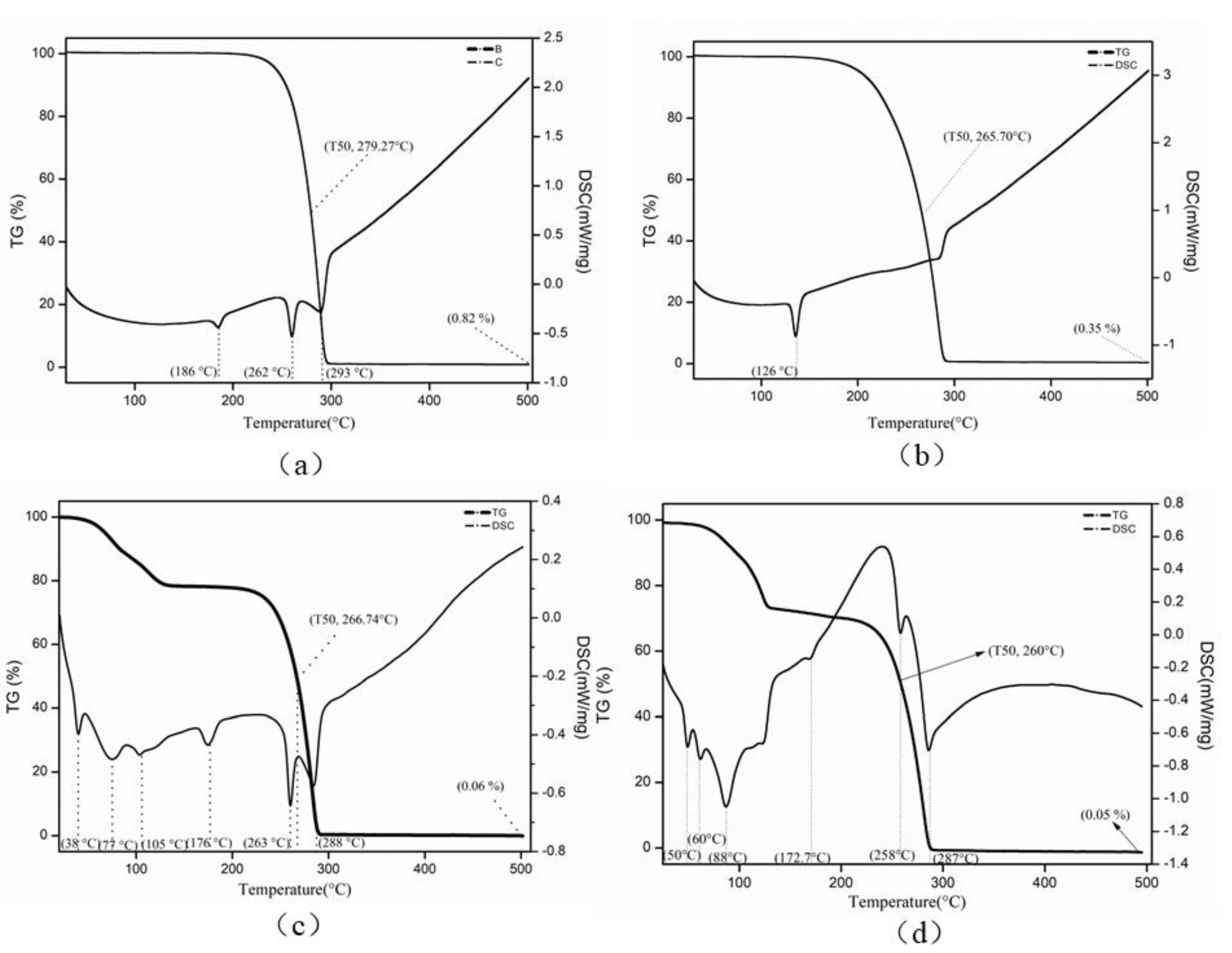
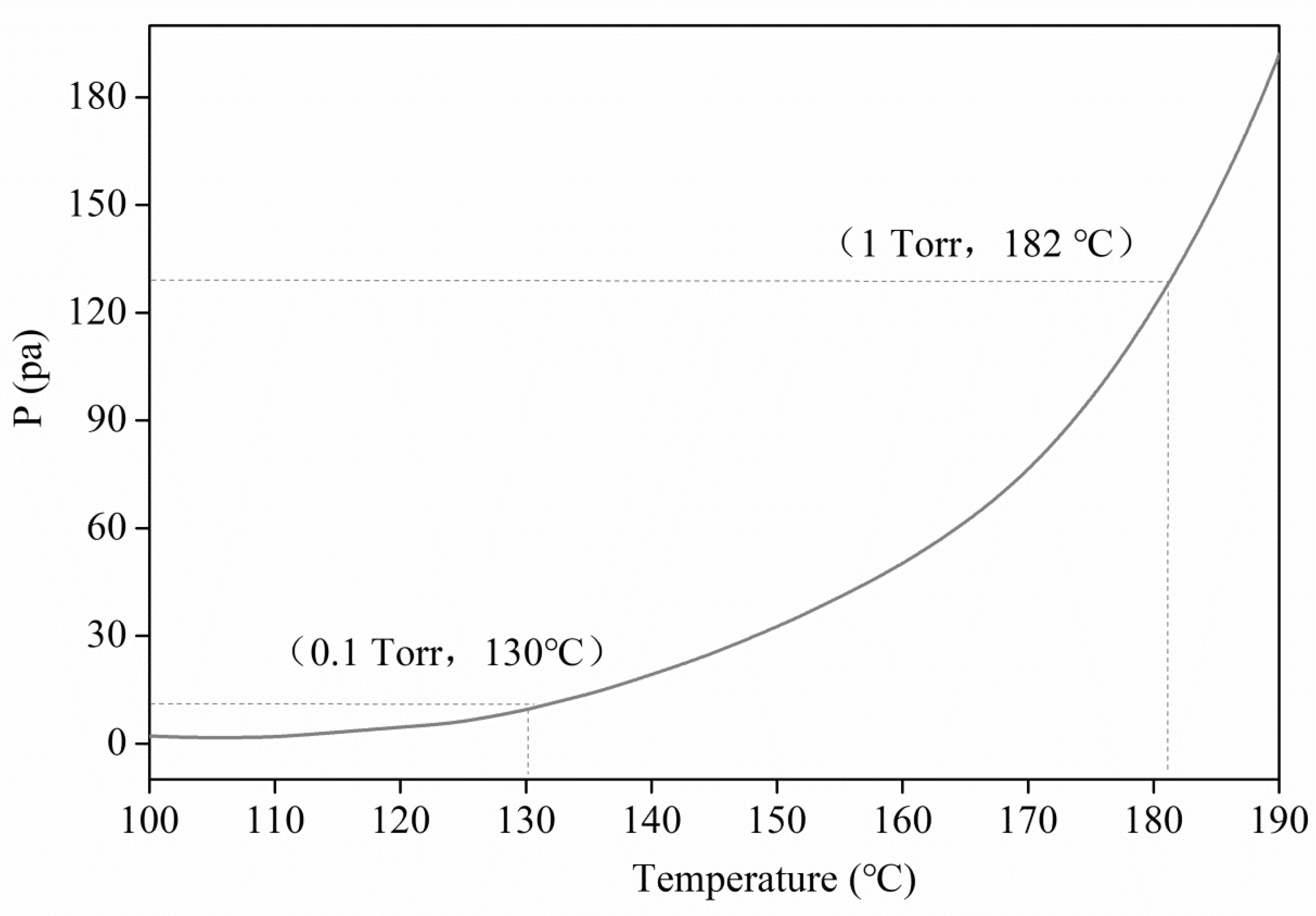
3.2. Thermal Behavior of the Lanthanum Precursor
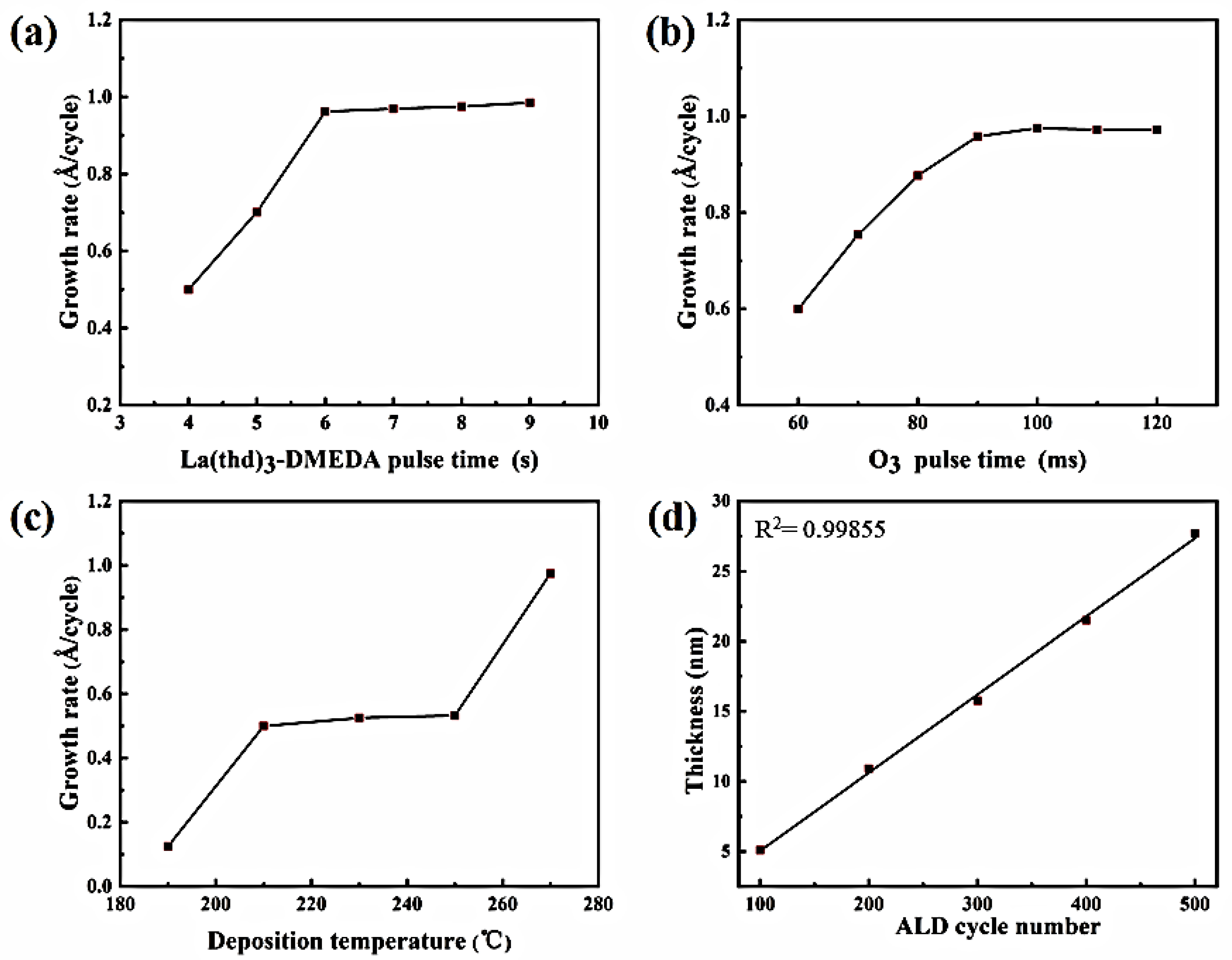
3.3. Growth Characteristics of La2O3 Deposition
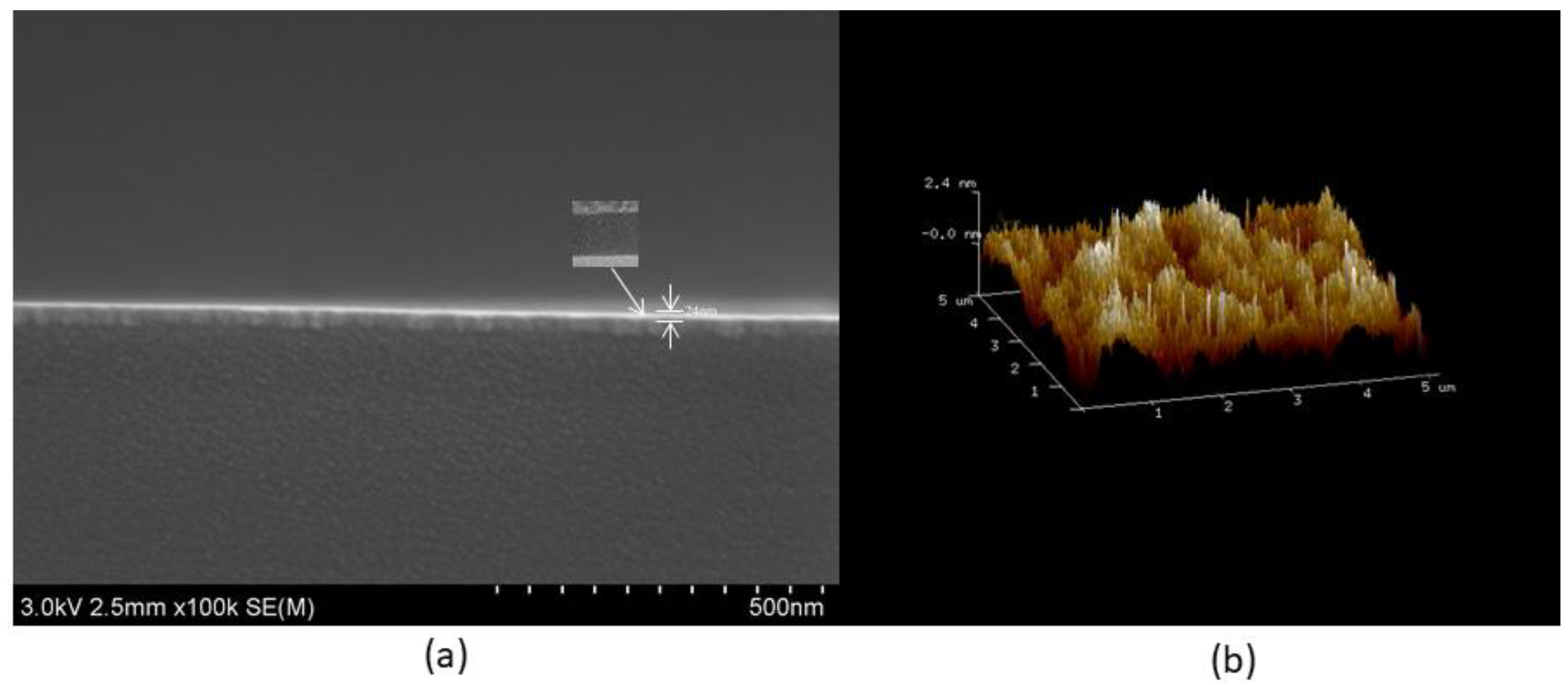
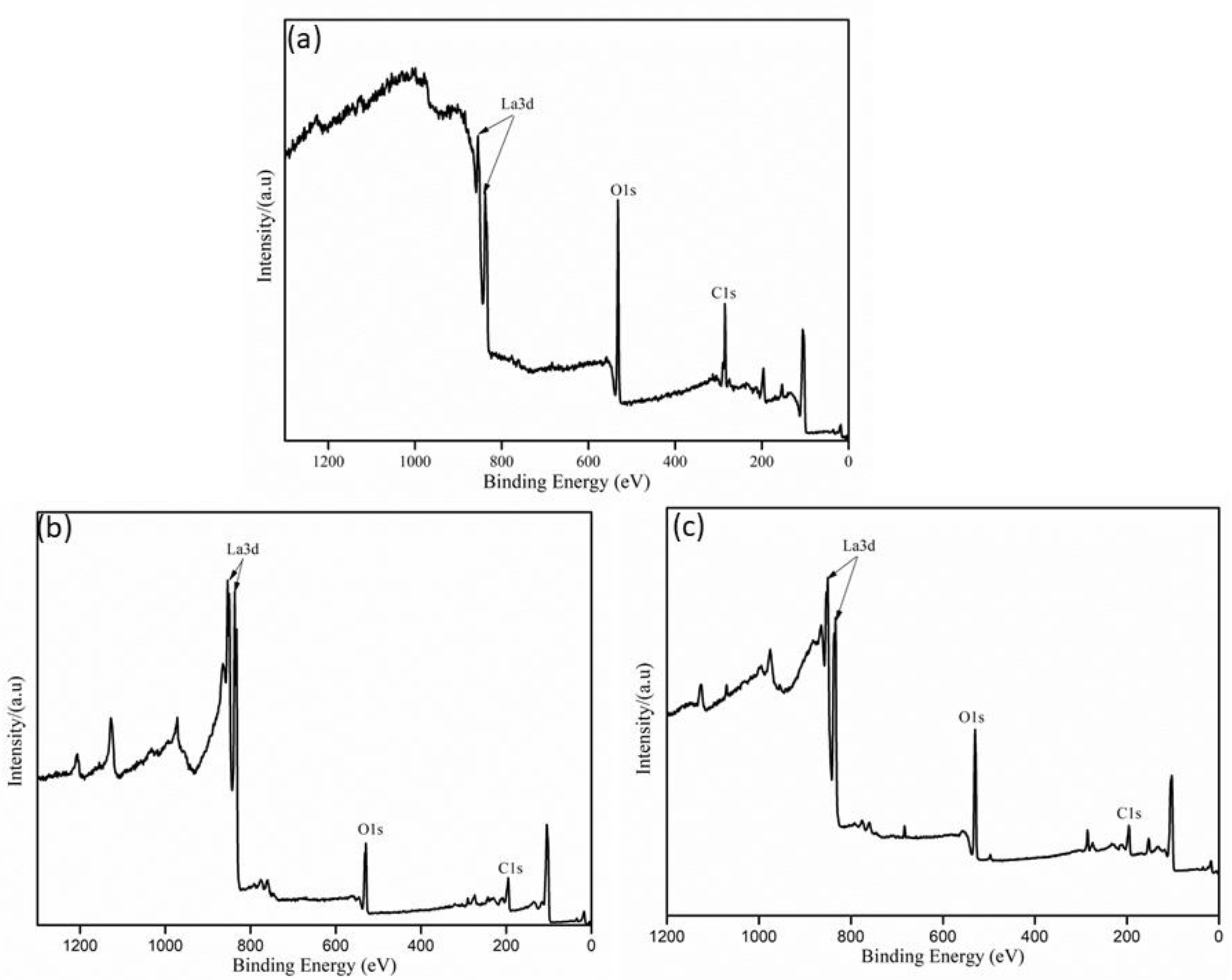
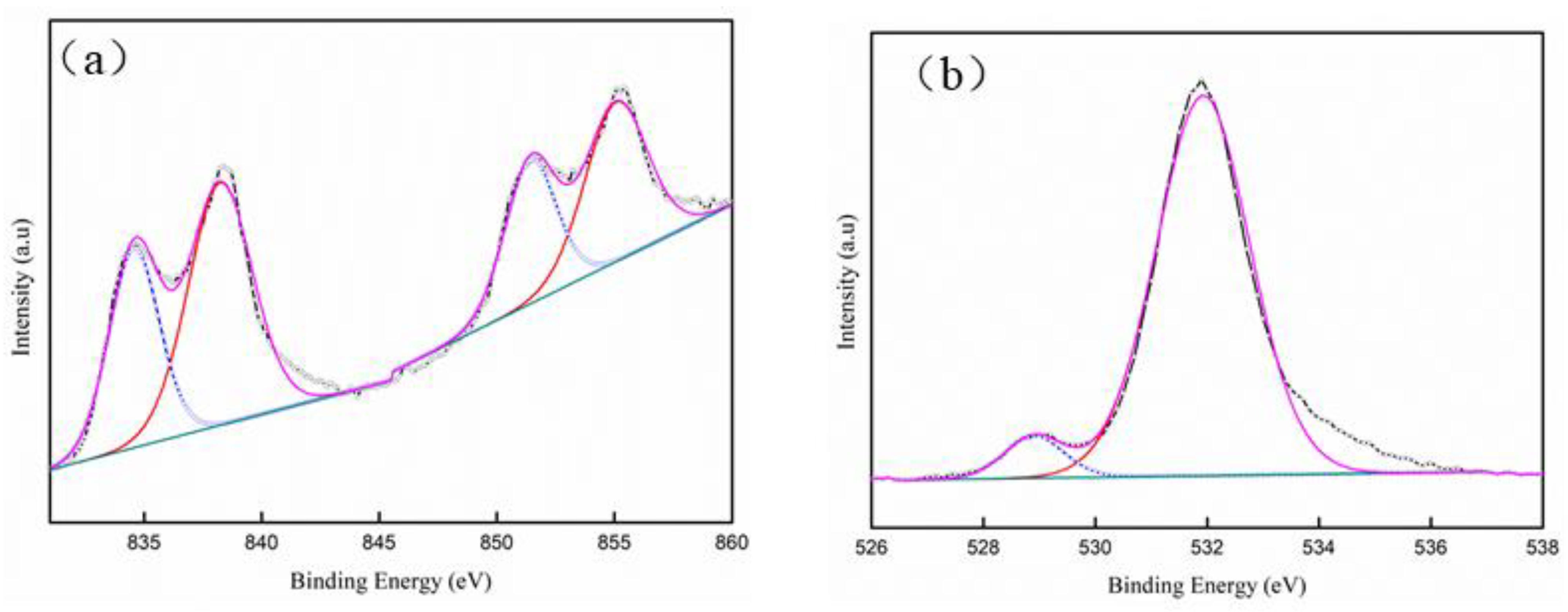
3.4. Characteristics of La2O3 Films
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kim, W.H.; Maeng, W.J.; Moon, K.J.; Myoung, J.M.; Kim, H. Growth characteristics and electrical properties of La2O3 gate oxides grown by thermal and plasma-enhanced atomic layer deposition. Thin Solid Films 2010, 519, 362–366. [Google Scholar] [CrossRef]
- Xiao, M.; Qiu, C.; Zhang, Z.; Peng, L.M. Atomic-layer-deposition growth of an ultrathin HfO2 film on graphene. ACS Appl. Mater. Interfaces 2017, 9, 34050–34056. [Google Scholar] [CrossRef]
- Dong, H.; Brennan, B.; Zhernokletov, D.; Kim, J.; Hinkle, C.L.; Wallace, R.M. In situ study of HfO2 atomic layer deposition on InP (100). Appl. Phys. Lett. 2013, 102, 171602-1–171602-4. [Google Scholar] [CrossRef]
- Egorov, K.V.; Lebedinskii, Y.Y.; Markeev, A.M.; Orlov, O.M. Full ALD Ta2O5-based stacks for resistive random access memory grown with in vacuo XPS monitoring. Appl. Surf. Sci. 2015, 356, 454–459. [Google Scholar] [CrossRef]
- Wang, X.; Liu, H.; Zhao, L.; Fei, C.; Feng, X.; Chen, S.; Wang, Y. Structural properties characterized by the film thickness and annealing temperature for La2O3 films grown by atomic layer deposition. Nanoscale Res. Lett. 2017, 12, 233. [Google Scholar] [CrossRef] [PubMed]
- Jinesh, K.B.; Lamy, Y.; Wolters, R.A.; Klootwijk, J.H.; Tois, E.; Roozeboom, F.; Besling, W.F.A. Silicon out-diffusion and aluminum in-diffusion in devices with atomic-layer deposited La2O3 thin films. Appl. Phys. Lett. 2008, 93, 192912. [Google Scholar] [CrossRef]
- Popovici, M.I.; Walke, A.M.; Bizindavyi, J.; Meersschaut, J.; Banerjee, K.; Potoms, G.; Kostantine, K.; Bosch, G.V.D.; Delhougne, R.; Kar, G.S.; et al. High-endurance ferroelectric (La, Y) and (La, Gd) Co-doped hafnium zirconate grown by atomic layer deposition. Acs. Appl. Electron. Mater. 2022, 4, 1823–1831. [Google Scholar] [CrossRef]
- Fan, J.; Liu, H.; Li, D.; Wang, S.; Duan, L.; Yu, X. Interfacial thermal stability and band alignment of La2O3/Al2O3 nanolaminates deposited by atomic layer deposition. J. Mater. Sci.-Mater. Electron. 2017, 28, 11253–11259. [Google Scholar] [CrossRef]
- Chen, L.; Yang, W.; Li, Y.; Sun, Q.Q.; Zhou, P.; Lu, H.L.; Zhang, D.W. Resistive switching properties of plasma enhanced-ALD La2O3 for novel nonvolatile memory application. J. Vac. Sci. Technol. A 2012, 30, 01A148-1–01A148-4. [Google Scholar] [CrossRef]
- Onn, T.M.; Gathmann, S.R.; Guo, S.; Solanki, S.P.S.; Walton, A.; Page, B.J.; Rojas, G.; Neurock, M.; Grabow, L.C.; Mkhoyan, K.A.; et al. Platinum Graphene Catalytic Condenser for Millisecond Programmable Metal Surfaces. J. Am. Chem. Soc. 2022, 144, 22113–22127. [Google Scholar] [CrossRef]
- Edelmann, F.T. Lanthanide amidinates and guanidinates in catalysis and materials science: A continuing success story. Chem. Soc. Rev. 2012, 41, 7657–7672. [Google Scholar] [CrossRef]
- Devi, A. ‘Old Chemistries’ for new applications: Perspectives for development of precursors for MOCVD and ALD applications. Coord. Chem. Rev. 2013, 257, 3332–3384. [Google Scholar] [CrossRef]
- Oh, I.K.; Kim, M.K.; Lee, J.S.; Lee, C.W.; Lansalot-Matras, C.; Noh, W.; Kim, H. The effect of La2O3-incorporation in HfO2 dielectrics on Ge substrate by atomic layer deposition. Appl. Surf. Sci. 2013, 287, 349–354. [Google Scholar] [CrossRef]
- Agrawal, K.S.; Barhate, V.N.; Patil, V.S.; Patil, L.S.; Mahajan, A.M. Plasma-enhanced atomic layer-deposited La2O3 ultra-thin films on Si and 6H-SiC: A comparative study. Appl. Phys. A-Mater. 2020, 126, 650. [Google Scholar] [CrossRef]
- Fei, C.; Liu, H.; Wang, X.; Fan, X. The influence of process parameters and pulse ratio of precursors on the characteristics of La1-xAlxO3 films deposited by atomic layer deposition. Nanoscale Res. Lett. 2015, 10, 180. [Google Scholar] [CrossRef] [PubMed]
- Kouda, M.; Suzuki, T.; Kakushima, K.; Ahmet, P.; Iwai, H.; Yasuda, T. Electrical properties of CeO2/La2O3 stacked gate dielectrics fabricated by chemical vapor deposition and atomic layer deposition. Jpn. J. Appl. Phys. 2012, 51, 121101-1–121101-5. [Google Scholar] [CrossRef]
- George, S.M. Atomic Layer Deposition: An Overview. Chem. Rev. 2010, 110, 111–131. [Google Scholar] [CrossRef]
- Mackus, A.J.M.; Bol, A.A.; Kessels, W.M.M. The use of atomic layer deposition in advanced nanopatterning. Nanoscale 2014, 6, 10941–10960. [Google Scholar] [CrossRef]
- Sawka, A. Metal-organic chemical vapour deposition of lanthana-doped ceria layers at low temperatures. Ceram. Int. 2021, 47, 5198–5208. [Google Scholar] [CrossRef]
- Chen, P.Y.; Hadamek, T.; Kwon, S.; Al-Quaiti, F.; Posadas, A.B.; Kim, M.J.; Ekerdt, J.G. Role of template layers for heteroepitaxial growth of lanthanum oxide on GaN(0001) via atomic layer deposition. J. Vac. Sci. Technol. A 2020, 38, 012403-1–012403-8. [Google Scholar] [CrossRef]
- Lou, X.; Gong, X.; Feng, J.; Gordon, R. Band offset analysis of ALD La2O3 on GaAs (111), (110) and (100) surfaces for epitaxial growth. ACS Appl. Mater. Inter. 2019, 11, 28515–28519. [Google Scholar] [CrossRef]
- Qian, C.; Zhang, X.; Li, J.; Xu, F.; Zhang, Y.; Shen, Q. Trisguanidinate lanthanide complexes: Syntheses, structures, and catalytic activity for mild amidation of aldehydes with amines. Organometallics 2009, 28, 3856–3862. [Google Scholar] [CrossRef]
- Endo, K.; Kato, K.; Takenaka, M.; Takagi, S. Electrical characteristic of atomic layer deposition La2O3/Si MOSFETs with ferroelectric-type hysteresis. Jpn. J. Appl. Phys. 2019, 58, SBBA05-1–SBBA05-5. [Google Scholar] [CrossRef]
- Suzuki, T.; Kouda, M.; Ahmet, P.; Iwai, H.; Kakushima, K.; Yasuda, T. La2O3 gate insulators prepared by atomic layer deposition: Optimal growth conditions and MgO/La2O3 stacks for improved metal-oxide-semiconductor characteristics. J. Vac. Sci. Technol. A 2012, 30, 01A148-1–01A148-4. [Google Scholar] [CrossRef]
- Mao, X.; Foucher, A.C.; Stach, E.A.; Gorte, R.J. Changes in Ni-NiO equilibrium due to LaFeO3 and the effect on dry reforming of CH4. J. Catal. 2020, 381, 561–569. [Google Scholar] [CrossRef]
- Eisentraut, K.J.; Sievers, R.E. Volatile Rare Earth Chelates. J. Am. Chem. Soc. 1965, 87, 5254–5256. [Google Scholar] [CrossRef]
- Stafford, N.A.; Katamreddy, R.; Guerin, L.; Feist, B.; Dussarrat, C.; Pallem, V.; Opila, R. Atomic layer deposition of rare-earth oxide thin films for high-k dielectric applications. ECS Trans. 2009, 19, 525–536. [Google Scholar] [CrossRef]
- Alexandra, M.Z. Lanthanide β-diketonate glyme complexes exhibiting unusual co-ordination modes. J. Chem. Soc. Dalton Trans. 1993, 15, 2379–2386. [Google Scholar]
- Neumayer, D.A.; Purtell, R.J.; Kane, W.F.; Grill, A. Preparation of (PB, LA)TIO3 films by metal organic chemical vapor deposition with new lanthanum precursors. Integr. Ferroelectr. 1997, 14, 85–93. [Google Scholar] [CrossRef]
- Kang, S.W.; Rhee, S.W. Deposition of La2O3 films by direct liquid injection metallorganic chemical vapor deposition. J. Electrochem. Soc. 2002, 149, C345–C348. [Google Scholar] [CrossRef]
- Nikolaeva, A.; Nygaard, R.; Martynova, I.; Tsymbarenko, D. Synthesis, structure and thermal behavior of volatile mononuclear mixed ligand complexes of rare-earth dipivaloylmethanates with diethylentriamine. Polyhedron 2020, 180, 114373. [Google Scholar] [CrossRef]
- Nemukhin, A.V.; Rogachev, A.Y.; Konyukhov, S.V.; Bochenkova, A.V.; Granovsky, A.A. QM/MM modeling of the structures and properties of the β-diketonate-based lanthanide complexes. Int. J. Quantum Chem. 2005, 104, 203–213. [Google Scholar] [CrossRef]
- Ahn, S.; Littlewood, P.; Liu, Y.; Marks, T.J.; Stair, P.C. Stabilizing supported Ni catalysts for dry reforming of methane by combined La doping and Al overcoating using atomic layer deposition. ACS Catal. 2022, 12, 10522–10530. [Google Scholar] [CrossRef]
- Lamagna, L.; Wiemer, C.; Perego, M.; Volkos, S.N.; Baldovino, S.; Tsoutsou, D.; Fanciulli, M. O3-based atomic layer deposition of hexagonal La2O3 films on Si(100) and Ge(100) substrates. Am. J. Phys. 2010, 108, 084108-1–084108-10. [Google Scholar] [CrossRef]
- Sheldrick, G.M. SHELXS-97, Program for Crystal Structure Solution; University of Göttingen: Göttingen, Germany, 1997. [Google Scholar]
- Sheldrick, G.M. SHELXL-97, Program for Crystal Structure Refinement; University of Göttingen: Göttingen, Germany, 1997. [Google Scholar]
- Wright, S.F.; Dollimore, D.; Dunn, J.G.; Alexander, K. Determination of the vapor pressure curves of adipic acid and triethanolamine using thermogravimetric analysis. Thermochim. Acta 2004, 421, 25–30. [Google Scholar] [CrossRef]
- Li, X.L.; Tsoutsou, D.; Scarel, G.; Wiemer, C.; Capelli, S.C.; Volkos, S.N.; Fanciulli, M. Chemical and structural properties of atomic layer deposited La2O3 films capped with a thin Al2O3 layer. J. Vac. Sci. Technol. A 2009, 27, L1–L7. [Google Scholar] [CrossRef]
- Du, L.; Wang, K.; Zhong, Y.; Liu, B.; Liu, X.; Ding, Y. A high growth rate process of ALD CeOx with amidinato-cerium [(N-iPr-AMD)3Ce] and O3 as precursors. J. Mater. Sci. 2020, 55, 5378–5389. [Google Scholar] [CrossRef]
- Bedoya, C.; Condorelli, G.G.; Finocchiaro, S.T.; Di Mauro, A.; Atanasio, D.; Fragala, I.L.; Carella, S. MOCVD of Lanthanum Oxides from La(tmhd)3 and La(tmod)3 Precursors: A Thermal and Kinetic Investigation. Chem. Vap. Depos. 2006, 12, 46–53. [Google Scholar] [CrossRef]
- Park, N.K.; Kang, D.K.; Kim, B.H.; Jo, S.J.; Ha, J.S. Electrical properties of La2O3 thin films grown on TiN/Si substrates via atomic layer deposition. Appl. Surf. Sci. 2006, 252, 8506–8509. [Google Scholar] [CrossRef]
- Barhate, V.; Agrawal, K.; Patil, V.; Patil, S.; Mahajan, A. Spectroscopic study of La2O3 thin films deposited by indigenously developed plasma-enhanced atomic layer deposition system. Int. J. Mod. Phys. B 2018, 32, 1840074-1–1840074-5. [Google Scholar] [CrossRef]
- Zhang, Y.; Du, L.; Liu, X.; Ding, Y. A high growth rate atomic layer deposition process for nickel oxide film preparation using a combination of nickel (II) diketonate–diamine and ozone. Appl. Surf. Sci. 2019, 481, 138–143. [Google Scholar] [CrossRef]
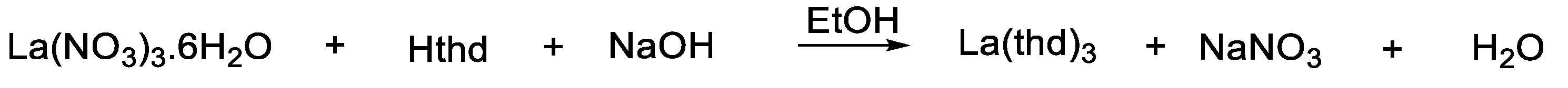




| Paremeters | Complex 2 | Complex 3 |
|---|---|---|
| Empirical formula | C37H69LaN2O6 | C39H93LaN2O6 |
| Temperature (K) | 150 | 150 |
| Crystal color | clear light colorless | Colorless |
| Formula weight | 776.85 | 945.16 |
| Crystal system | Monoclinic | Orthorhombic |
| Space group | P21/c | P212121 |
| a (Å) | 10.7711(6) | 16.7577(14) |
| b (Å) | 26.1684(14) | 17.4249(12) |
| c (Å) | 15.5595(10) | 18.9628(14) |
| α (°) | 90 | 90 |
| β (°) | 102.455(3) | 90 |
| γ (°) | 90 | 90 |
| Volume (Å3) | 4282.4(4) | 5537.2(7) |
| Z | 4 | 4 |
| F(000) | 1640 | 2024 |
| Refinement method | Full-matrix least-squares on F2 | Full-matrix least-squares on F2 |
| Reflections collection | 8440 | 7760 |
| Independent reflections | 7696 (Rint = 0.067) | 7201 (Rint = 0.062) |
| Range for data collection | 3.4–72.2 | 3.4–59.1 |
| h, k, l range | −13 ≤ h ≤ 11, −32 ≤ k ≤ 32, −19 ≤ l ≤ 19 | −18 ≤ h ≤ 18, −16 ≤ k ≤ 19, −21 ≤ l ≤ 21 |
| Goodness-of-fit on F2 | 1.106 | 1.07 |
| Final R indices [I > 2sigma(I2)] | R = 0.0552, wR2 = 0.1557 | R = 0.0486, wR2 = 0.1345 |
| R (all data) | R = 0.0584, wR2 = 0.1594 | R = 0.0520, wR2 = 0.1302 |
| Complex 2 | Complex 3 | ||
|---|---|---|---|
| Bond length | (Å) | Bond length | (Å) |
| O1—La1 | 2.448 (3) | La1—O6 | 2.441 (6) |
| La1—O4 | 2.433 (3) | La1—O1 | 2.443 (6) |
| La1—O5 | 2.442 (3) | La1—O3 | 2.451 (5) |
| La1—O3 | 2.450 (3) | La1—O4 | 2.459 (5) |
| La1—O2 | 2.468 (3) | La1—O2 | 2.463 (5) |
| La1—O6 | 2.487 (3) | La1—O5 | 2.502 (5) |
| La1—N2 | 2.756 (5) | La1—N2 | 2.742 (9) |
| La1—N1 | 2.786 (6) | La1—N1 | 2.783 (10) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhao, W.; Jiang, J.; Luo, Y.; Li, J.; Ding, Y. Atomic Layer Deposition of La2O3 Film with Precursor La(thd)3-DMEA. Coatings 2023, 13, 870. https://doi.org/10.3390/coatings13050870
Zhao W, Jiang J, Luo Y, Li J, Ding Y. Atomic Layer Deposition of La2O3 Film with Precursor La(thd)3-DMEA. Coatings. 2023; 13(5):870. https://doi.org/10.3390/coatings13050870
Chicago/Turabian StyleZhao, Wenyong, Jie Jiang, Yawen Luo, Jiahao Li, and Yuqiang Ding. 2023. "Atomic Layer Deposition of La2O3 Film with Precursor La(thd)3-DMEA" Coatings 13, no. 5: 870. https://doi.org/10.3390/coatings13050870
APA StyleZhao, W., Jiang, J., Luo, Y., Li, J., & Ding, Y. (2023). Atomic Layer Deposition of La2O3 Film with Precursor La(thd)3-DMEA. Coatings, 13(5), 870. https://doi.org/10.3390/coatings13050870






