Time-Dependent Passivation Performance of Plasma Sprayed FeCrMoCBY Amorphous Coating
Abstract
:1. Introduction
2. Experimental
2.1. Raw Materials and Coating Production
2.2. Microstructure Analysis
2.3. Electrochemical Measurements
3. Results and Discussion
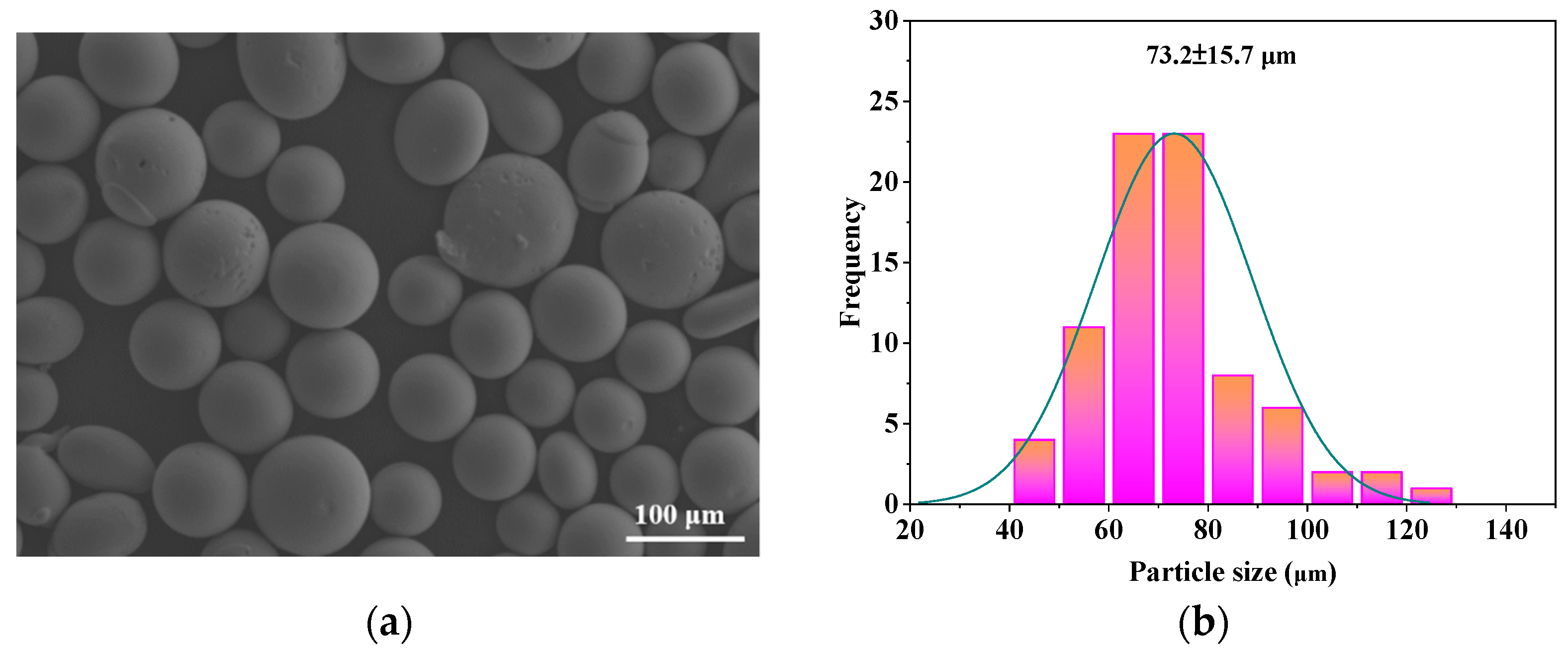
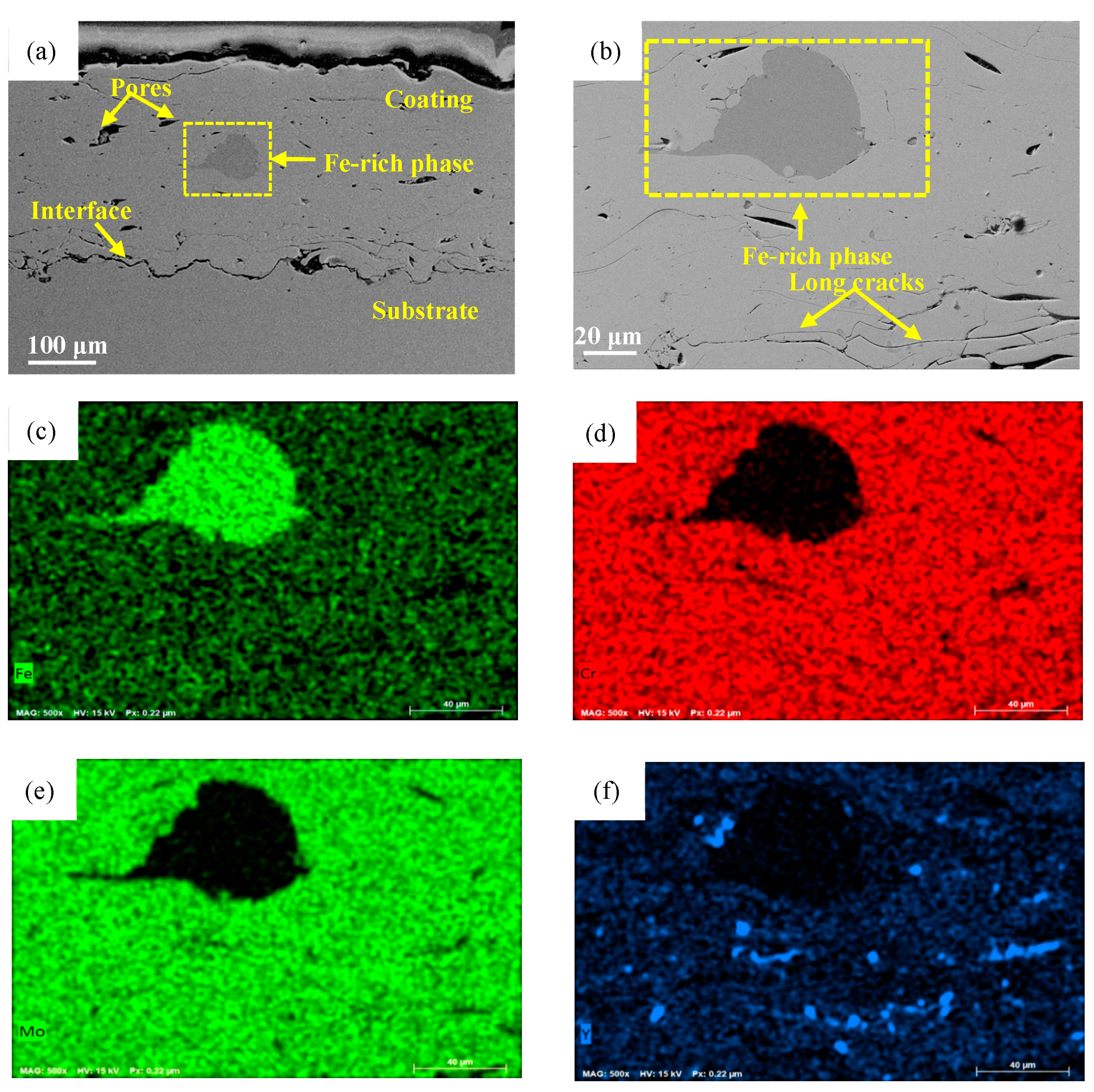
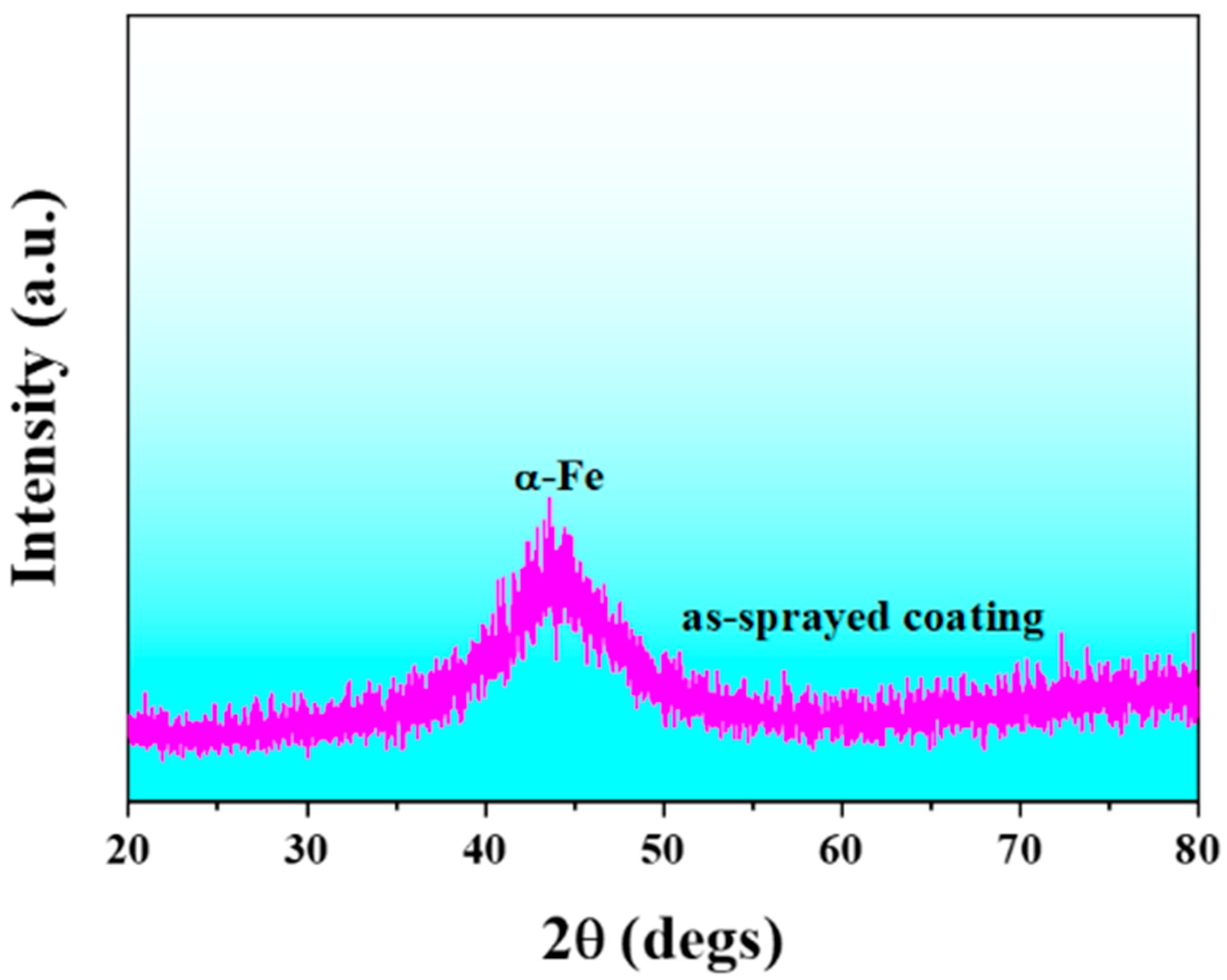
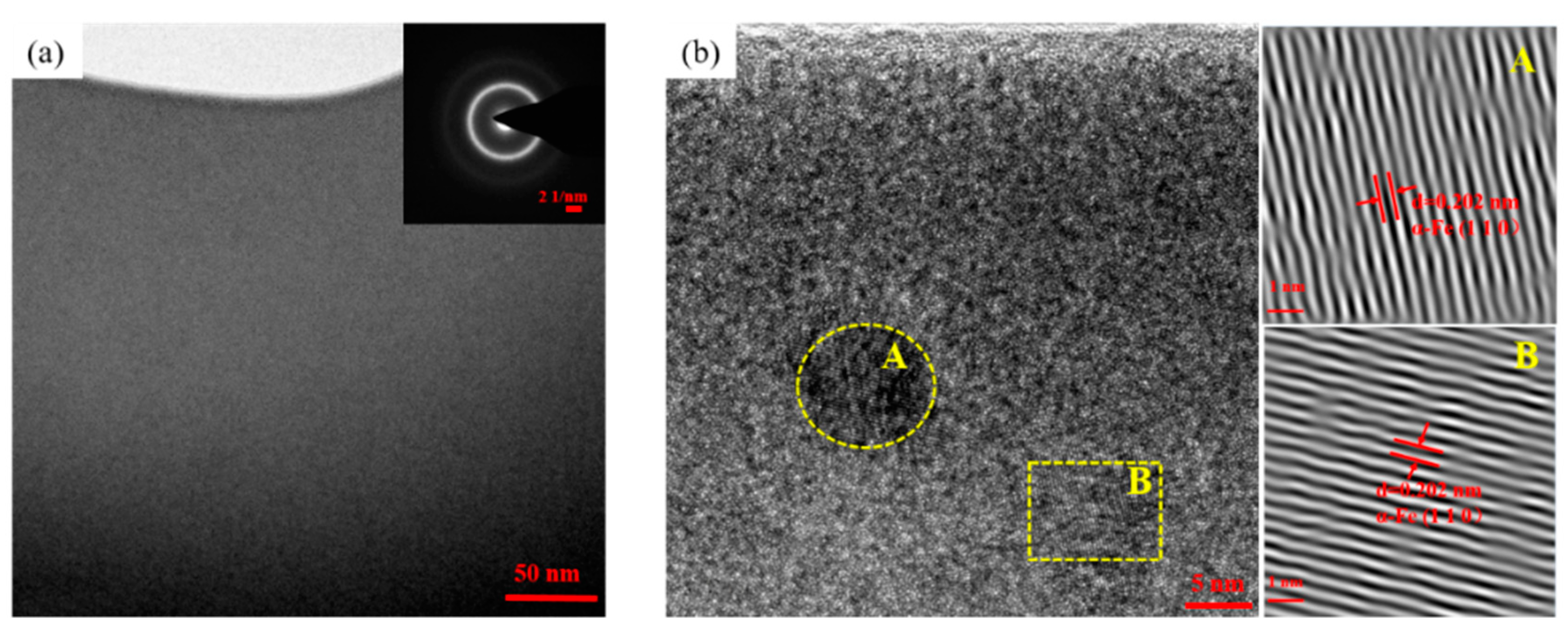
3.1. Microstructural Observations of FeCrMoCBY Amorphous Coating
3.2. Electrochemical Properties of FeCrMoCBY Amorphous Coating
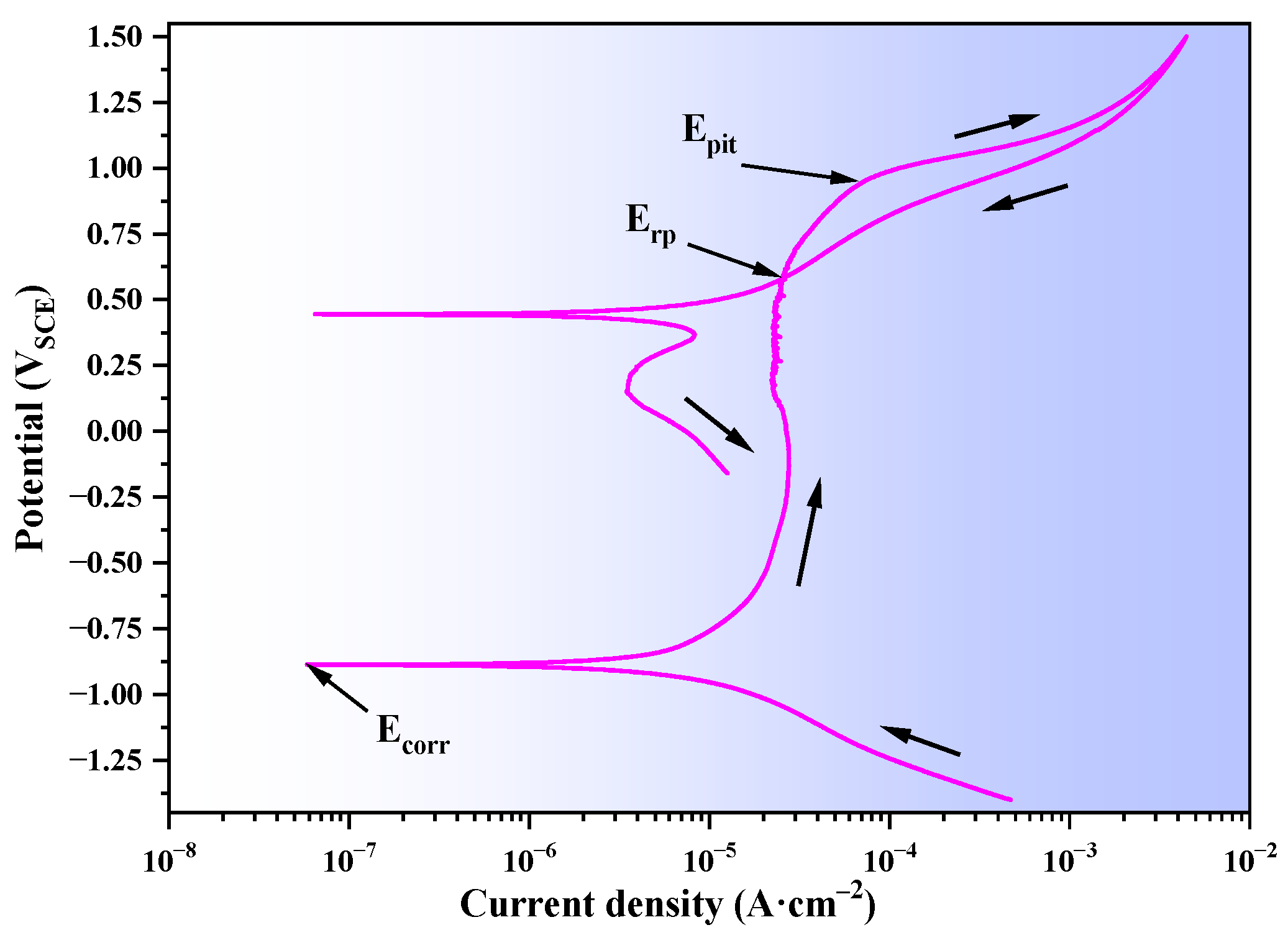
3.2.1. Cyclic Polarization Curve
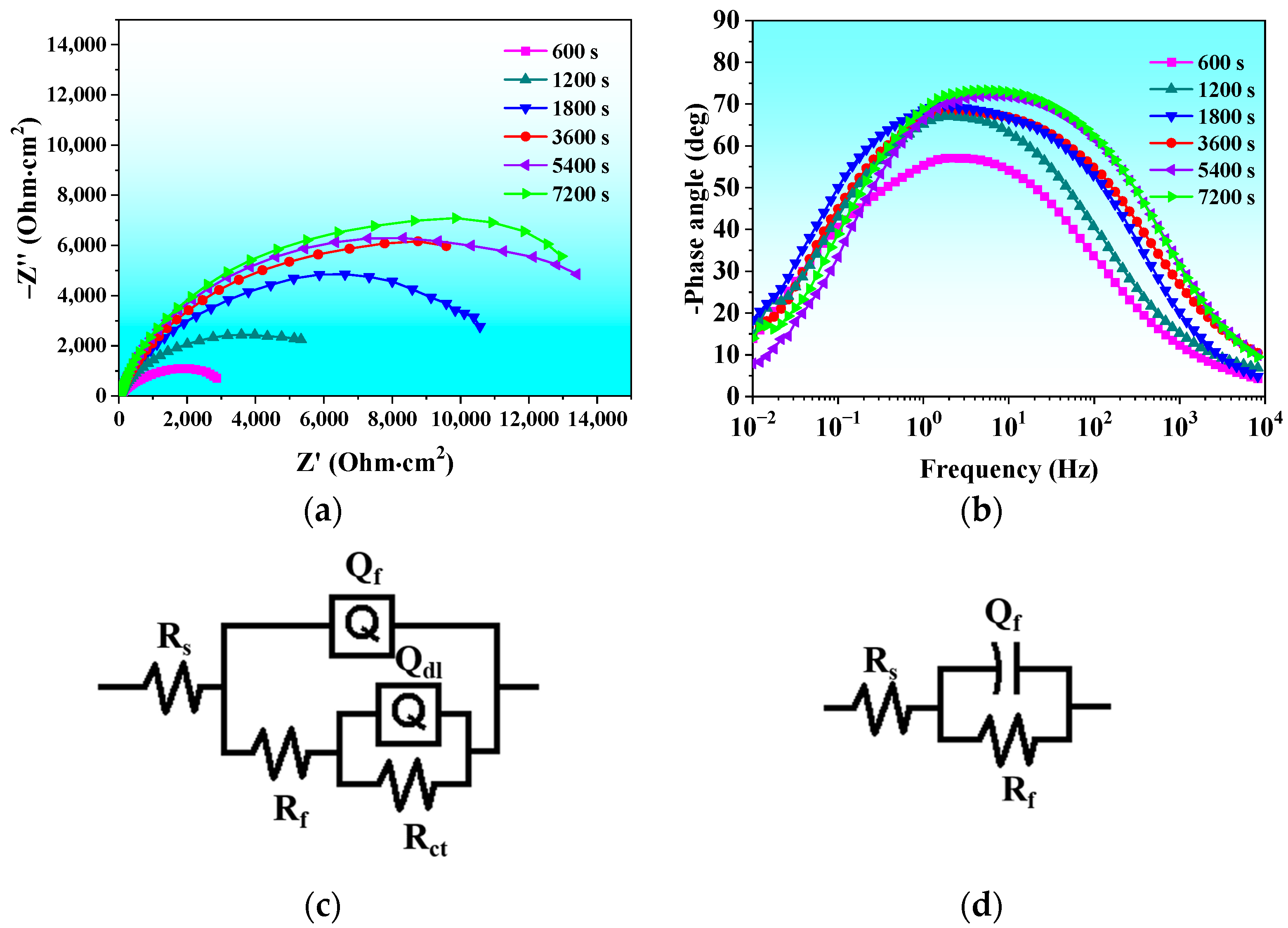
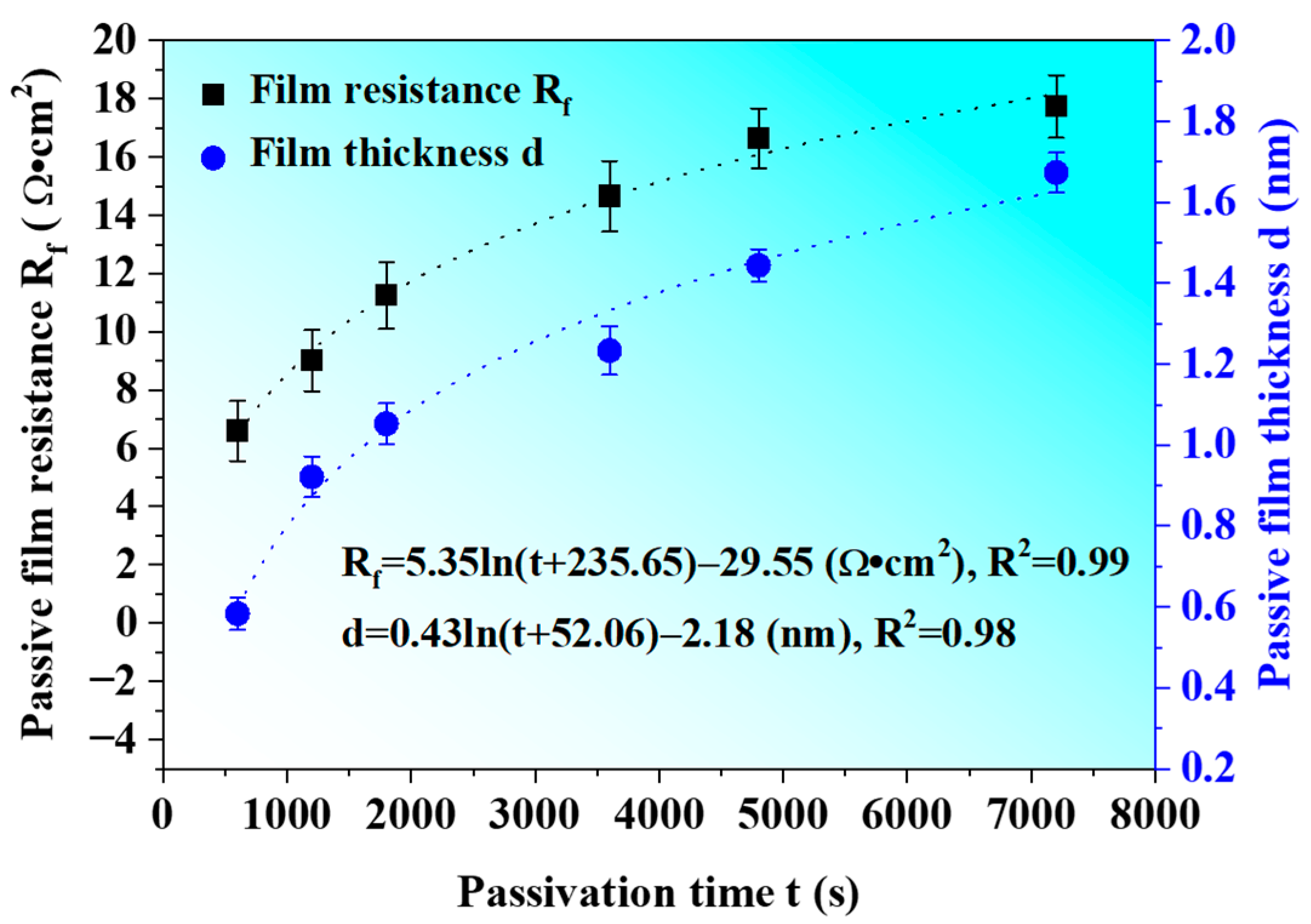
3.2.2. Electrochemical Impedance Spectra
3.2.3. Semiconducting Properties
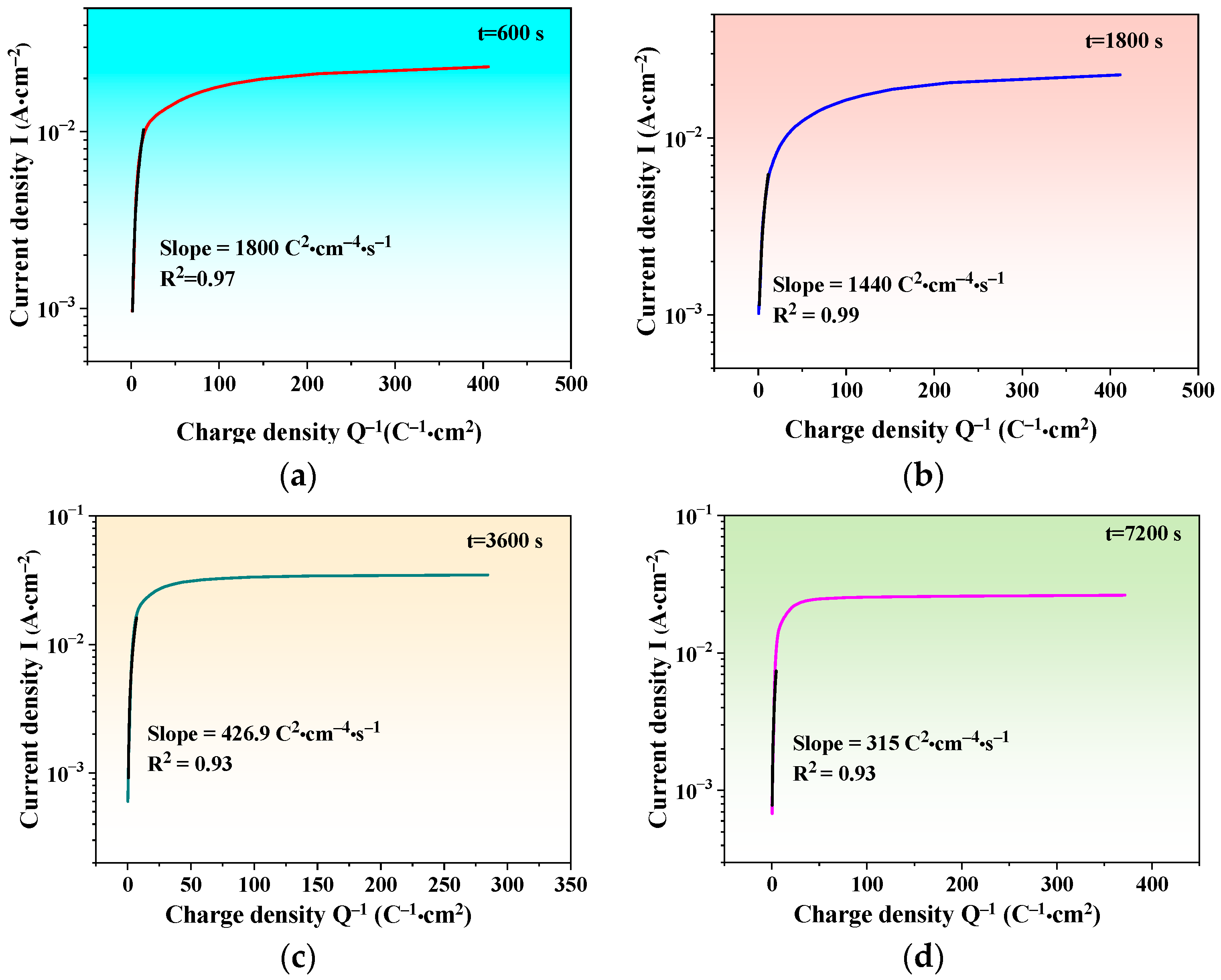
3.2.4. Self-Repairing Ability of Passive Film
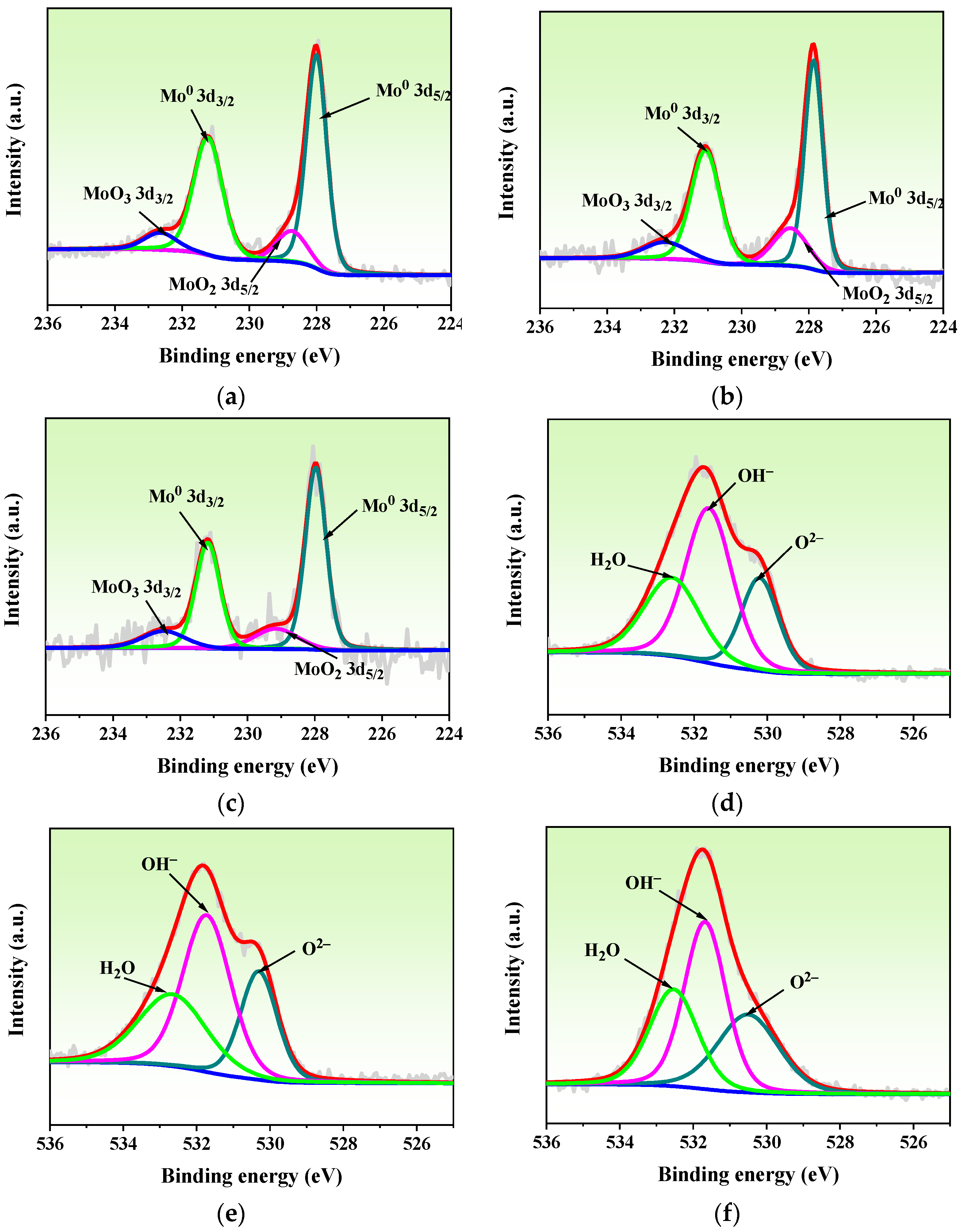
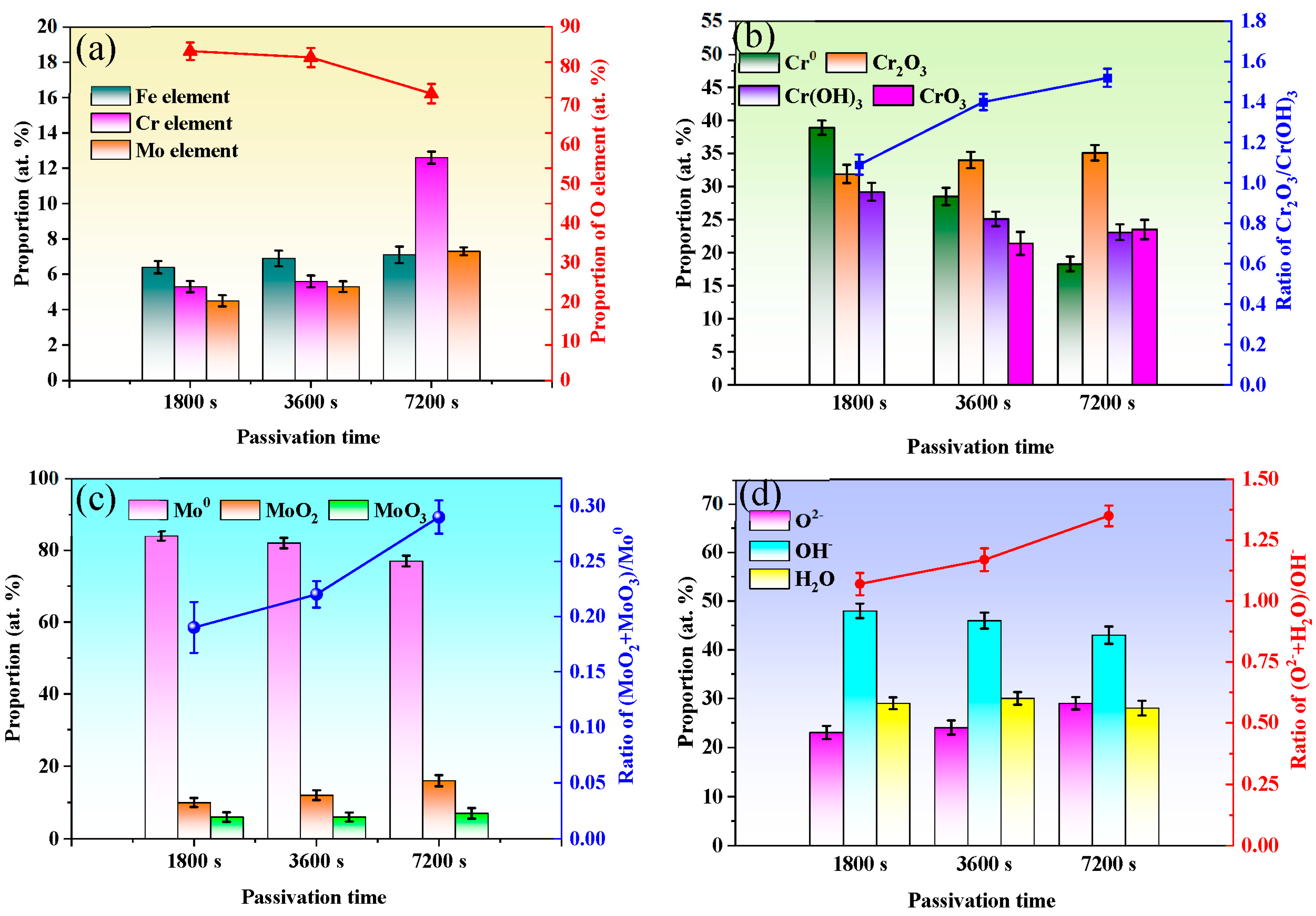
3.3. XPS Compositional Analysis
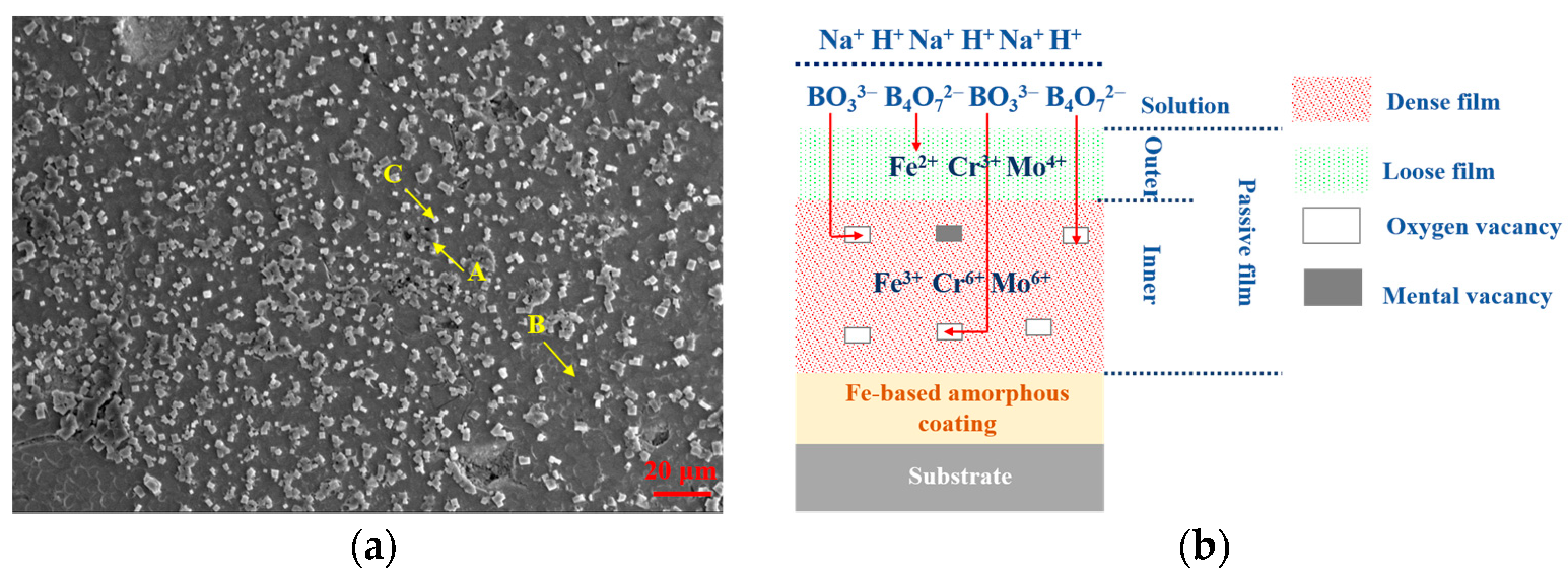
3.4. Growth Model of Passive Film
3.5. Passivation Mechanisms of Passive Film
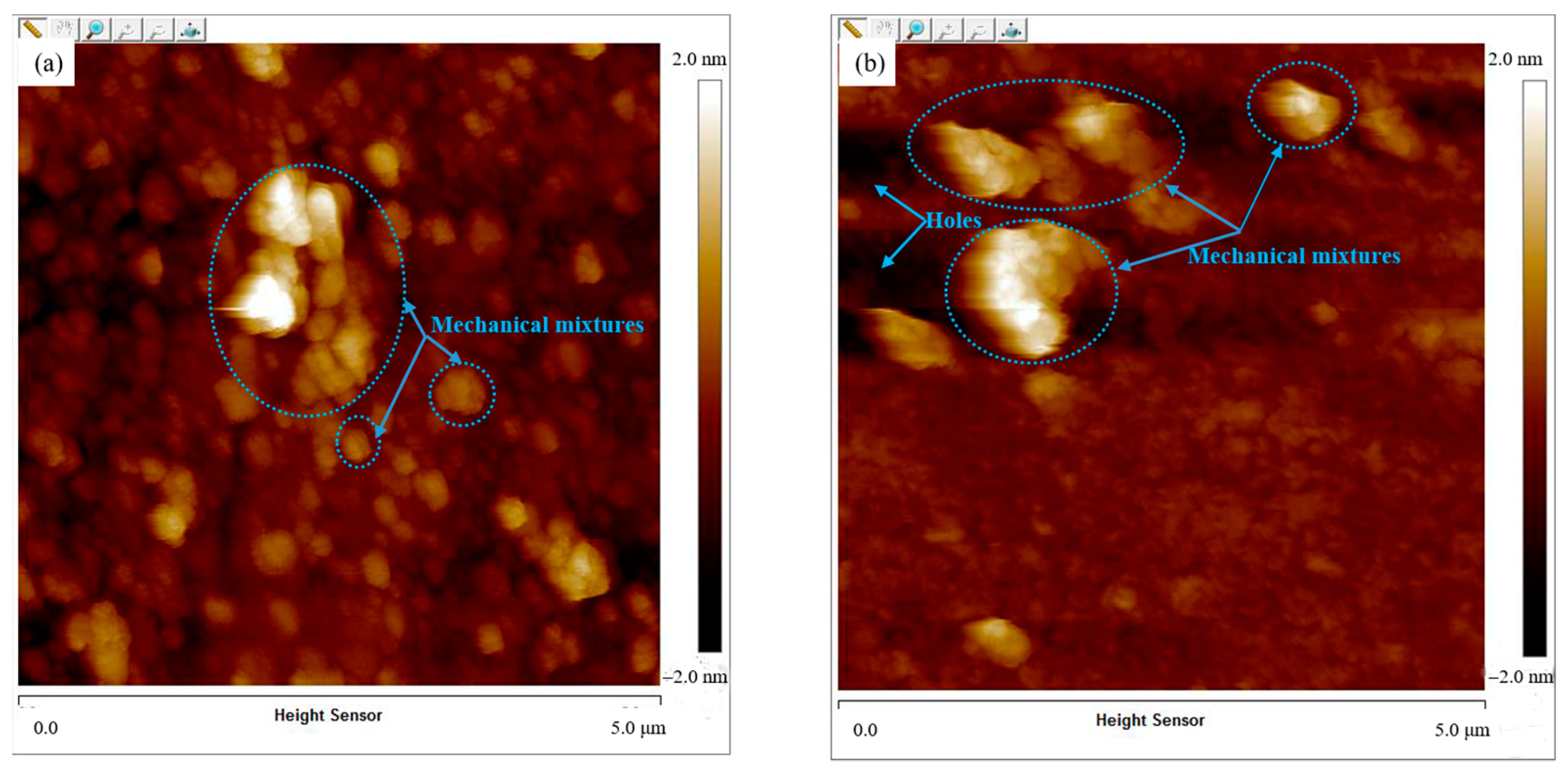
3.5.1. Mechanical Mixtures Adsorption process
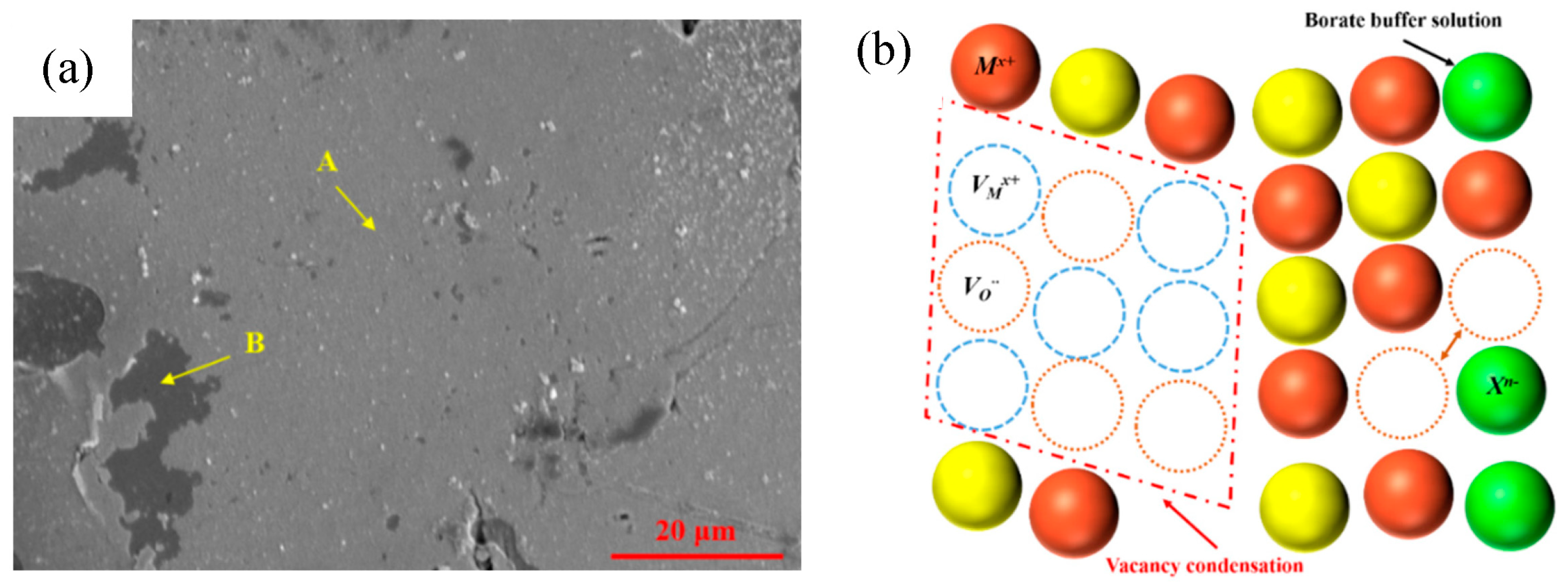
3.5.2. Cation Vacancy Condensation Process
3.5.3. AFM Topography of Film Surface
4. Conclusions
- (1)
- The resistance of Fe48Cr15Mo14C15B6Y2 amorphous coating in borate buffer solution to corrosive medium is significantly promoted with passivation time prolongation when verified by potentiostatic polarization testing.
- (2)
- Enhancement of self-repairing ability of passive film is attributed to a lower concentration of vacancies, more insoluble inner barrier oxides Cr2O3 and stable bounded water.
- (3)
- Based on the effective capacitance assumption, the association between the thickness of the passive film d and the passivation time t is as follows: (nm).
- (4)
- Mechanical mixtures are initially adsorbed on the coating surface along with volume expansion, stress initiation and passive film rupture. The cation vacancy condensation process will stabilize the growth trend of the passive oxides during the subsequent passivation process.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zhang, H.R.; Wang, S.L.; Yang, X.L.; Hao, S.L.; Chen, Y.H.; Li, H.X.; Pan, D. Interfacial characteristic and microstructure of Fe-based amorphous coating on magnesium alloy. Surf. Coat. Technol. 2021, 425, 127659. [Google Scholar] [CrossRef]
- Li, G.; Gan, Y.Y.; Liu, C.H.; Shi, Y.; Zhao, Y.C.; Kou, S.Z. Corrosion and Wear Resistance of Fe-Based Amorphous Coatings. Coatings 2020, 10, 73. [Google Scholar] [CrossRef]
- Farmer, J.C.; Choi, J.S.; Saw, C.K.; Rebak, R.H.; Day, S.D.; Lian, T.; Hailey, P.D.; Payer, J.H.; Branagan, D.J.; Aprigliano, L.F. Corrosion Resistance of Amorphous Fe49.7Cr17.7Mn1.9Mo7.4W1.6B15.2C3.8Si2.4 Coating: A New Criticality Control Material. Nucl. Technol. 2008, 161, 169–189. [Google Scholar] [CrossRef]
- Askandarani, A.; Hashmi, M.S.J.; Yilbas, B.S. Arc Spraying of Nanostructured Wires on Steel Surfaces: Influence of Coating Thickness on Microstructure and Fracture Toughness. Adv. Mater. Res. 2012, 445, 803–808. [Google Scholar] [CrossRef]
- Branagan, D.J.; Breitsameter, M.; Meacham, B.E.; Belashchenko, V. High-Performance Nanoscale Composite Coatings for Boiler Applications. J. Therm. Spray Technol. 2005, 14, 196–204. [Google Scholar] [CrossRef]
- Daroonparvar, M.; Yajid, M.A.M.; Yusof, N.M.; Bakhsheshi-Rad, H.R.; Hamzah, E. Microstructural characterisation of air plasma sprayed nanostructure ceramic coatings on Mg-1%Ca alloys (bonded by NiCoCrAlYTa alloy). Ceram. Int. 2016, 42, 357–371. [Google Scholar] [CrossRef]
- Heimann, R.B. The Nature of Plasma Spraying. Coatings 2023, 13, 622. [Google Scholar] [CrossRef]
- Sampath, S.; Herman, H. Rapid solidification and microstructure development during plasma spray deposition. J. Therm. Spray Technol. 1996, 5, 445–456. [Google Scholar] [CrossRef]
- Guo, R.Q.; Zhang, C.; Yang, Y.; Peng, Y.; Liu, L. Corrosion and wear resistance of a Fe-based amorphous coating in underground environment. Intermetallics 2012, 30, 94–99. [Google Scholar] [CrossRef]
- Koga, G.Y.; Junior, A.M.J.; Roche, V.; Nogueira, R.P.; Schulz, R.; Savoie, S.; Melle, A.K.; Loable, C.; Bolfarini, C.; Kiminami, C.S.; et al. Production and Corrosion Resistance of Thermally Sprayed Fe-Based Amorphous Coatings from Mechanically Milled Feedstock Powders. Metall. Mater. Trans. A 2018, 49, 4860–4870. [Google Scholar] [CrossRef]
- Baron, A.; Szewieczek, D.; Nawrat, G. Corrosion of amorphous and nanocrystalline Fe-based alloys and its influence on their magnetic behavior. Electrochim. Acta 2007, 52, 5690–5695. [Google Scholar] [CrossRef]
- Zhang, C.; Cha, K.C.; Wu, Y.; Liu, L. Pitting initiation in Fe-based amorphous coatings. Acta Mater. 2012, 60, 4152–4159. [Google Scholar] [CrossRef]
- Li, B.S.; Lin, A.; Wu, X.; Zhang, Y.M.; Gan, F.X. Electrodeposition and characterization of Fe-Cr-P amorphous alloys from trivalent chromium sulfate electrolyte. J. Alloys Compd. 2008, 453, 93–101. [Google Scholar] [CrossRef]
- Franco, V.; Conde, C.F.; Conde, A.; Kiss, L.F. Enhanced magnetocaloric response in Cr/Mo containing Nanoperm-type amorphous alloys. Appl. Phys. Lett. 2007, 90, 395–524. [Google Scholar] [CrossRef]
- Zhang, S.D.; Zhang, W.L.; Wang, S.G.; Gu, X.J.; Wang, J.Q. Characterisation of three-dimensional porosity in an Fe-based amorphous coating and its correlation with corrosion behavior. Corros. Sci. 2019, 93, 211–221. [Google Scholar] [CrossRef]
- Sadeghi, E.; Joshi, S. Chlorine-induced high-temperature corrosion and erosion-corrosion of HVAF and HVOF-sprayed amorphous Fe-based coatings. Surf. Coat. Technol. 2019, 371, 20–35. [Google Scholar] [CrossRef]
- Tsuchiya, H.; Fujimoto, S.; Shibata, T. Semiconductive behavior of passive films formed on Fe-Cr alloy. J. Electrochem. Soc. 2006, 16, 49–54. [Google Scholar] [CrossRef]
- Toledo-Matos, L.A.; Pech-Canul, M.A. Evolution of an iron passive film in a borate buffer solution (pH 8.4). J. Solid State Electrochem. 2011, 15, 1927–1934. [Google Scholar] [CrossRef]
- Kouisni, L.; Azzi, M.; Dalard, F.; Maximovitch, S. Phosphate coatings on magnesium alloy AM60: Part 2: Electrochemical behaviour in borate buffer solution. Surf. Coat. Technol. 2005, 192, 239–246. [Google Scholar]
- Hamadou, L.; Kadri, A.; Benbrahim, N. Characterisation of passive films formed on low carbon steel in borate buffer solution (pH 9.2) by electrochemical impedance spectroscopy. Appl. Surf. Sci. 2005, 252, 1510–1519. [Google Scholar] [CrossRef]
- Zhou, Z.; Wang, L.; Wang, F.C.; Zhang, H.F.; Liu, Y.B.; Xu, S.H. Formation and corrosion behavior of Fe-based amorphous metallic coatings by HVOF thermal spraying. Surf. Coat. Technol. 2009, 204, 563–570. [Google Scholar] [CrossRef]
- Guo, H.; Wu, N.C.; Zhang, Y.L.; Zhang, S.D.; Sun, W.H.; Wang, J.Q. Influence of coating thickness on the impact damage mode in Fe-based amorphous coatings. Surf. Coat. Technol. 2020, 390, 125650. [Google Scholar] [CrossRef]
- Movahedi, B.; Enayati, M.H.; Wong, C.C. Structural and Thermal Behavior of Fe-Cr-Mo-P-B-C-Si Amorphous and Nanocrystalline HVOF Coatings. J. Therm. Spray Technol. 2010, 19, 1093–1099. [Google Scholar] [CrossRef]
- Tillmann, W.; Khalil, O.; Baumann, I. Influence of Direct Splat-Affecting Parameters on the Splat-Type Distribution, Porosity, and Density of Segmentation Cracks in Plasma-Sprayed YSZ Coatings. J. Therm. Spray Technol. 2021, 30, 1015–1027. [Google Scholar] [CrossRef]
- Anupam, A.; Kottada, R.S.; Kashyap, S.; Meghwal, A.; Murty, B.S.; Berndt, C.C.; Ang, A.S.M. Understanding the Microstructural Evolution of High Entropy Alloy Coatings Manufactured by Atmospheric Plasma Spray Processing. Appl. Surf. Sci. 2020, 505, 144117. [Google Scholar] [CrossRef]
- Hoshiyama, Y.; Miyazaki, T.; Miyake, H. Zirconium carbide dispersed high Cr-Ni cast iron produced by plasma spraying. Surf. Coat. Technol. 2017, 228, S7–S10. [Google Scholar] [CrossRef]
- Vardelle, M.; Vardelle, A.; Leger, A.C.; Fauchais, P.; Gobin, D. Influence of particle parameters at impact on splat formation and solidification in plasma spraying processes. J. Therm. Spray Technol. 1995, 4, 50–58. [Google Scholar] [CrossRef]
- Shinoda, K.; Koseki, T.; Yoshida, T. Influence of impact parameters of zirconia droplets on splat formation and morphology in plasma spraying. J. Appl. Phys. 2006, 100, 074903. [Google Scholar] [CrossRef]
- Lin, T.J.; Sheu, H.H.; Lee, C.Y.; Lee, H.B. The study of mechanical properties and corrosion behavior of the Fe-based amorphous alloy coatings using high velocity oxygen fuel spraying. J. Alloys Compd. 2021, 867, 159132. [Google Scholar] [CrossRef]
- Zhou, S.F.; Yang, J.J.; Deng, C.; Jin, J.B.; Hu, L.X.; Lu, Y. Amorphous alloy reinforced Cu-based immiscible coatings by laser melting deposition: Separation mechanism and corrosion behavior. Mater. Des. 2023, 229, 111898. [Google Scholar] [CrossRef]
- Huang, B.; Zhang, C.; Zhang, G.; Liao, H.L. Wear and corrosion resistant performance of thermal-sprayed Fe-based amorphous coatings: A review. Surf. Coat. Technol. 2019, 377, 124896. [Google Scholar] [CrossRef]
- Inoue, A.; Kong, F.L.; Han, Y.; Zhu, S.L.; Churyumov, A.; Shalaan, E.; Al-Marzouki, F. Development and application of Fe-based soft magnetic bulk metallic glassy inductors. J. Alloys Compd. 2018, 731, 1303–1309. [Google Scholar] [CrossRef]
- Zhou, Z.; Wang, L.; He, D.Y.; Wang, F.C.; Liu, Y.B. Microstructure and Electrochemical Behavior of Fe-Based Amorphous Metallic Coatings Fabricated by Atmospheric Plasma Spraying. J. Therm. Spray Technol. 2011, 20, 344–350. [Google Scholar] [CrossRef]
- Wang, L.; Jiang, S.L.; Zheng, Y.G.; Ke, W.; Sun, W.H.; Wang, J.Q. Effect of porosity sealing treatments on the corrosion resistance of high-velocity oxy-fuel (HVOF)-sprayed Fe-based amorphous metallic coatings. Surf. Coat. Technol. 2011, 206, 1307–1318. [Google Scholar] [CrossRef]
- Li, Y.C.; Zhang, W.W.; Wang, Y.; Zhang, X.Y.; Sun, L.L. Effect of spray powder particle size on the bionic hydrophobic structures and corrosion performance of Fe-based amorphous metallic coatings. Surf. Coat. Technol. 2022, 437, 128377. [Google Scholar] [CrossRef]
- Gao, M.H.; Lu, W.Y.; Yang, B.J.; Zhang, S.D.; Wang, J.Q. High corrosion and wear resistance of Al-based amorphous metallic coating synthesized by HVAF spraying. J. Alloys Compd. 2018, 735, 1363–1373. [Google Scholar] [CrossRef]
- Kang, Y.H.; Chen, Y.M.; Wen, Y.X.; Wu, B.; Song, M.B. Effects of structural relaxation and crystallization on the corrosion resistance of an Fe-based amorphous coating. J. Non-Cryst. Solids. 2020, 550, 120378. [Google Scholar] [CrossRef]
- Wang, H.Z.; Cheng, Y.H.; Yang, J.Y.; Wang, Q.Q. Influence of laser remelting on organization, mechanical properties and corrosion resistance of Fe-based amorphous composite coating. Surf. Coat. Technol. 2021, 414, 127081. [Google Scholar] [CrossRef]
- Zhai, H.M.; Yuan, H.Y.; Li, W.S.; Zhang, X.J.; Li, X.S.; Cai, A.H. Corrosion resistance mechanisms of detonation sprayed Fe-based amorphous coating on AZ31B magnesium alloy. J. Non-Cryst. Solids 2022, 576, 121276. [Google Scholar] [CrossRef]
- Zhu, M.; Zhang, Q.; Yuan, Y.F.; Guo, S.Y.; Chen, Y.B. Study on the Microstructure and Alternating Current Corrosion Behavior of SAF2507 Super-Duplex Stainless Steel in 3.5% NaCl Solution. J. Mater. Eng. Perform. 2020, 29, 1366–1374. [Google Scholar] [CrossRef]
- Wang, M.Q.; Zhou, Z.H.; Wang, Q.J.; Wang, Z.H.; Zhang, X.; Liu, Y.Y. Role of passive film in dominating the electrochemical corrosion behavior of FeCrMoCBY amorphous coating. J. Alloys Compd. 2019, 811, 151962. [Google Scholar] [CrossRef]
- Wang, M.Q.; Zhou, Z.H.; Wang, Q.J.; Wu, L.T.; Zhang, X.; Wang, Z.H. Long term semiconducting and passive film properties of a novel dense FeCrMoCBY amorphous coating by atmospheric plasma spraying. Appl. Surf. Sci. 2019, 495, 143600. [Google Scholar] [CrossRef]
- Cheng, Y.F.; Luo, J.L. A comparison of the pitting susceptibility and semiconducting properties of the passive films on carbon steel in chromate and bicarbonate solutions. Appl. Surf. Sci. 2000, 167, 113–121. [Google Scholar] [CrossRef]
- Cheng, Y.F.; Luo, J.L. Electronic structure and pitting susceptibility of passive film on carbon steel. Electrochim. Acta 1999, 44, 2947–2957. [Google Scholar] [CrossRef]
- Lavigne, O.; Alemany-Dumont, C.; Normand, B.; Delichère, P.; Descamps, A. Cerium insertion in 316L passive film: Effect on conductivity and corrosion resistance performances of metallic bipolar plates for PEM fuel cell application. Surf. Coat. Technol. 2010, 205, 1870–1877. [Google Scholar] [CrossRef]
- Yamamoto, T.; Fushimi, K.; Seo, M.; Tsuri, S.; Adachi, T.; Habazaki, H. Depassivation-repassivation behavior of type-312L stainless steel in NaCl solution investigated by the micro-indentation. Corros. Sci. 2009, 51, 1545–1553. [Google Scholar] [CrossRef]
- Mao, F.X.; Yao, J.Z.; Zhou, Y.T.; Dong, C.F.; Kursten, B.; Macdonald, D.D. Determining the electric-field strength in a passive film via photo-induced electric fields. Corros. Sci. 2019, 154, 239–245. [Google Scholar] [CrossRef]
- Fukugaichi, S.; Tomosugi, Y.; Aono, H. Facile synthesis of hydrophilic layered double hydroxide film on aluminum plate. Inorg. Chem. Commun. 2022, 142, 109647. [Google Scholar] [CrossRef]
- Ju, P.F.; Zuo, Y.; Tang, T.M.; Zhao, X.H. The enhanced passivation of 316L stainless steel in a simulated fuel cell environment by surface plating with palladium. Corros. Sci. 2013, 66, 330–336. [Google Scholar] [CrossRef]
- König, U.; Schultze, J.W. The examination of the influence of a space-charge layer on the formation kinetics of thin passive films by Schottky-Mott analysis. Solid State Ionics 1992, 53, 255–264. [Google Scholar] [CrossRef]
- Zamani, P.; Valefi, Z.; Jafarzadeh, K. Comprehensive study on corrosion protection properties of Al2O3, Cr2O3 and Al2O3-Cr2O3 ceramic coatings deposited by plasma spraying on carbon steel. Ceram. Int. 2022, 48, 1574–1588. [Google Scholar] [CrossRef]
- Fernandez-Domene, R.M.; Blasco-Tamarit, E.; Garcia-Garcia, D.M.; Garcia-Anton, J. Passive and transpassive behaviour of Alloy 31 in a heavy brine LiBr solution. Electrochim. Acta 2013, 95, 1–11. [Google Scholar] [CrossRef]
- BenSalah, M.; Sabot, R.; Triki, E.; Dhouibi, L.; Refait, P.; Jeannin, M. Passivity of Sanicro28 (UNS N-08028) stainless steel in polluted phosphoric acid at different temperatures studied by electrochemical impedance spectroscopy and Mott-Schottky analysis. Corros. Sci. 2014, 86, 61–70. [Google Scholar] [CrossRef]
- Hirschorn, B.; Orazem, M.E.; Tribollet, B.; Vivier, V.; Frateur, I.; Musiani, M. Constant-Phase-Element Behavior Caused by Resistivity Distributions in Films I. Theory. J. Electrochem. Soc. 2010, 157, C452. [Google Scholar] [CrossRef]
- Benoit, M.; Bataillon, C.; Gwinner, B.; Miserque, F.; Orazem, M.E.; Sánchez-Sánchez, C.M.; Tribollet, B.; Vivier, V. Comparison of different methods for measuring the passive film thickness on metals. Electrochim. Acta 2016, 201, 340–347. [Google Scholar] [CrossRef]
- Fujimoto, S.; Kawachi, S.; Nishio, T.; Shibata, T. Impedance and photoelectrochemical properties of porous oxide film on Type304 stainless steel formed by square wave potential pulse polarization. J. Electroanal. Chem. 1999, 473, 265–271. [Google Scholar] [CrossRef]
- Liu, L.; Zhang, C. Fe-based amorphous coatings: Structures and properties. Thin Solid Films 2014, 561, 70–86. [Google Scholar] [CrossRef]
- Tian, W.P.; Yang, H.W.; Zhang, S.D. Synergistic Effect of Mo, W, Mn and Cr on the Passivation Behavior of a Fe-Based Amorphous Alloy Coating. Acta Metall. Sin. Engl. Lett. 2018, 31, 308–320. [Google Scholar] [CrossRef]
- Zhang, S.D.; Wu, J.; Qi, W.B.; Wang, J.Q. Effect of porosity defects on the long-term corrosion behaviour of Fe-based amorphous alloy coated mild steel. Corros. Sci. 2016, 110, 57–70. [Google Scholar] [CrossRef]
- Zhang, J.F.; Deng, C.M.; Song, J.B.; Deng, C.G.; Liu, M.; Dai, M.J. Electrochemical Corrosive Behaviors of Fe-Based Amorphous/ Nanocrystalline Coating on Stainless Steel Prepared by HVOF-Sprayed. Coatings 2019, 9, 226. [Google Scholar] [CrossRef]
- Narayanan, R.; Seshadri, S.K. Point defect model and corrosion of anodic oxide coatings on Ti-6Al-4V. Corros. Sci. 2008, 50, 1521–1529. [Google Scholar] [CrossRef]
- Zheng, S.J.; Cai, Z.B.; Pu, J.P.; Zeng, C.; Wang, L.P. Passivation behavior of VAlTiCrSi amorphous high-entropy alloy film with a high corrosion-resistance in artificial sea water. Appl. Surf. Sci. 2021, 542, 148520. [Google Scholar] [CrossRef]
- Park, K.J.; Ahn, S.J.; Kwon, H.S. Effects of solution temperature on the kinetic nature of passive film on Ni. Electrochim. Acta 2012, 56, 1662–1669. [Google Scholar] [CrossRef]
- Yang, H.; Wang, Y.Q.; Wang, H.; Jia, Q.X. Oxygen concentration and its effect on the leakage current in BiFeO3 thin films. Appl. Phys. Lett. 2010, 96, 1719. [Google Scholar] [CrossRef]
- Macdonald, D.D. The history of the point defect model for the passive state: A brief review of film growth aspects. Electrochim. Acta 2011, 56, 1761–1772. [Google Scholar] [CrossRef]
- Cao, D.; Ma, Y.F.; Shi, Z.N.; Xu, J.L.; Hu, X.W.; Wang, Z.W. Performance of protective oxide films on Fe-Ni alloy anodes in molten KF-AlF3-Al2O3 salts at 700 °C. Electrochim. Acta 2020, 347, 136275. [Google Scholar] [CrossRef]
- Ter-Ovanessian, B.; Alemany-Dumont, C.; Alemany-Dumont, C.; Normand, B. Electronic and transport properties of passive films grown on different Ni-Cr binary alloys in relation to the pitting susceptibility. Electrochim. Acta 2014, 133, 373–381. [Google Scholar] [CrossRef]
- Li, T.S.; Wu, J.; Frankel, G.S. Localized corrosion: Passive film breakdown vs. Pit growth stability, Part VI: Pit dissolution kinetics of different alloys and a model for pitting and repassivation potentials. Corros. Sci. 2021, 182, 109277. [Google Scholar] [CrossRef]



| Parameters | Input Values |
|---|---|
| Input power | 45.5 kW |
| Stand-off distance | 120 mm |
| Primary gas flow velocity | 49.6 L·min−1 |
| Secondary gas flow velocity | 24.6 L·min−1 |
| Carrier gas flow velocity | 7.5 L·min−1 |
| Powder delivery rate | 28 g·min−1 |
| Traversing speed | 140 mm·s−1 |
| Number of passes | 3 |
| Time (s) | Rs (Ω·cm2) | Qf (Ω−1·cm−2·sn) | n1 | Rf (kΩ·cm2) | Rct (kΩ·cm2) | Qdl (Ω−1 cm−2·sn) | n2 |
|---|---|---|---|---|---|---|---|
| 600 | 11.2 | 0.000469 | 0.77 | 6.56 | 1.275 | 0.0007157 | 0.71 |
| 1200 | 9.98 | 0.000231 | 0.79 | 9.01 | |||
| 1800 | 10.7 | 0.000157 | 0.81 | 11.25 | / | / | / |
| 3600 | 12.8 | 0.0000914 | 0.84 | 14.66 | / | / | / |
| 5400 | 12.6 | 0.0000466 | 0.88 | 16.64 | / | / | / |
| 7200 | 9.20 | 0.0000353 | 0.89 | 17.73 | / | / | / |
| Time (s) | 600 | 1200 | 1800 | 3600 | 5400 | 7200 |
|---|---|---|---|---|---|---|
| Nd (cm−3) | 5.04 × 1021 | 2.34 × 1021 | 1.86 × 1021 | 1.16 × 1021 | 9.36 × 1020 | 8.07 × 1020 |
| Ufb (VSCE) | −0.38 | −0.35 | −0.29 | −0.28 | −0.27 | −0.19 |
| Point | O | Fe | Cr | Mo | Na |
|---|---|---|---|---|---|
| A | 47.67 | 16.64 | 5.80 | 3.13 | 26.76 |
| B | 17.27 | 52.71 | 17.88 | 12.14 | / |
| C | 76.16 | / | / | / | 23.84 |
| Point | O | Fe | Cr | Mo |
|---|---|---|---|---|
| A | 5.67 | 57.78 | 21.86 | 14.70 |
| B | 27.87 | 45.28 | 15.57 | 11.27 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, M.; Zhou, Z.; Yi, Y.; Zhang, X. Time-Dependent Passivation Performance of Plasma Sprayed FeCrMoCBY Amorphous Coating. Coatings 2023, 13, 894. https://doi.org/10.3390/coatings13050894
Wang M, Zhou Z, Yi Y, Zhang X. Time-Dependent Passivation Performance of Plasma Sprayed FeCrMoCBY Amorphous Coating. Coatings. 2023; 13(5):894. https://doi.org/10.3390/coatings13050894
Chicago/Turabian StyleWang, Miqi, Zehua Zhou, Yu Yi, and Xin Zhang. 2023. "Time-Dependent Passivation Performance of Plasma Sprayed FeCrMoCBY Amorphous Coating" Coatings 13, no. 5: 894. https://doi.org/10.3390/coatings13050894






