Abstract
The construction of anticorrosion coatings containing antifouling agents is an effective way to ensure the long-term durability of marine steel infrastructures. In this work, an innovative hybrid coating was prepared by introducing biocide CuO nanoparticles in ordinary zinc coating to improve its protective ability for steel in aggressive salt water environments. The CuO nanoparticles were embedded inside the matrix of chitosan/alginate complexes to prevent spontaneous copper leaching during corrosive attacks. Two procedures were applied for the electrodeposition of hybrid/composite zinc-based coatings on low-carbon steel substrates (DC current): first—the co-electrodeposition of encapsulated CuO nanoparticles with zinc on a cathode (steel) electrode from a sulfate electrolyte with a relatively low pH value of about 4.5–5.0 and second—the encapsulated CuO nanoparticles were electrodeposited from aqueous solution as an intermediate layer between two zinc deposits. The particles size and stability of suspensions were evaluated using dynamic light scattering. Both hybrid coatings were compared in terms of surface morphology and hydrophilicity (SEM and AFM analysis, contact angle measurement) and corrosion resistance (potentiodynamic polarization curves, polarization resistance). The protective characteristics of the coatings were compared in a 3.5% NaCl solution and artificial sea water. The hybrid coating showed 2–4 times higher polarization resistance than the bare zinc coating during a 30 day immersion in artificial sea water, indicating that this coating has the necessary characteristics to be used in a marine environment.
1. Introduction
The corrosion and biofouling of metal structures in marine and coastal environments cause serious economic costs, safety and environmental problems. In marine environments, the corrosion is mainly induced by chloride ions [1,2]. The application of protective coatings on the metal surfaces plays an important role in limiting the corrosion. Coatings create safety barriers between the surrounding medium and the substrate. Zinc coatings are widely used for the protection of steel since the potential of zinc is more negative than that of the iron and offers sacrificial protection to the base metal during the corrosion process [3]. However, the contact of bare zinc coatings to aggressive media such as the marine environments leads to the formation of “white rust” [4]. One way to prolong their service life is by embedding different types of nanoparticles in the zinc matrix during the preparation of protective coatings. The incorporation of metal oxide particles, for example, has been found to enhance the protective parameters of the ordinary zinc layers due to the formation of mixed barrier layers that simultaneously contain corrosion products and incorporated particles [5,6,7,8,9,10]. In particular, the incorporation of CuO nanoparticles in the zinc matrix has recently been demonstrated to improve the anticorrosion performance of mild steel in salt solutions [5,7,10].
Marine environments are aggressive for metals, not only because of the high salinity, but also due to the presence of bacteria, fungi, algae, etc. which lead to biofouling and corrosion problems [11]. Traditionally, biocides are incorporated in antifouling coatings to prevent biofouling by killing potential marine organisms [12]. Copper oxide (CuO) particles exhibit excellent antifouling activity against marine microorganisms due to their capability of delivering sufficient amounts of the biocidal copper ions, mainly due to the reaction of copper with Cl− in the seawater [13]. Previous studies reported that the biocidal effect of copper oxide particles might also be due to the formation of reactive oxygen species able to destruct cells [14].
In aquatic systems, the dissolution of CuO nanoparticles depends on many factors such as ionic strength, pH, presence of dissolved organic matter, etc. [15]. In order to prevent the CuO nanoparticles from spontaneously leaching copper into the environment, an environmentally friendly chemical preparation based on chitosan and alginate complexation (using a cross-linking agent) has recently been proposed by Leonardi et al. [16,17].
Chitosan (CHI) is a naturally occurring cationic polysaccharide which is produced by partial deacetylation of chitin, while alginate (ALG) is a negatively charged polysaccharide, derived from brown algae [18]. The ionic interaction between CHI protonated amine groups and ALG carboxyl groups is mainly responsible for polyelectrolyte complexation, leading to the formation of polymeric shells on the CuO nanoparticles, although interaction via hydrogen bonding can also participate in this process [19]. CHI possesses antimicrobial and antifungal properties against a number of organisms because of its cationic nature, which makes it possible to interact with the negative charges of the cell membranes [20]. CHI and ALG have also been used as corrosion inhibitors due to the presence of nitrogen and oxygen atoms in their molecular structures. The adsorption of polymer molecules on the metal surfaces was mainly responsible for corrosion inhibition realized by both polymers. For example, the addition of CHI or ALG directly to a corrosion medium improves the protective properties of steel in a 1 M HCl solution significantly [21,22]. Sodium alginate has also been reported as a promising biopolymer for the protection of steel against corrosion in a 3.5% NaCl solution [23].
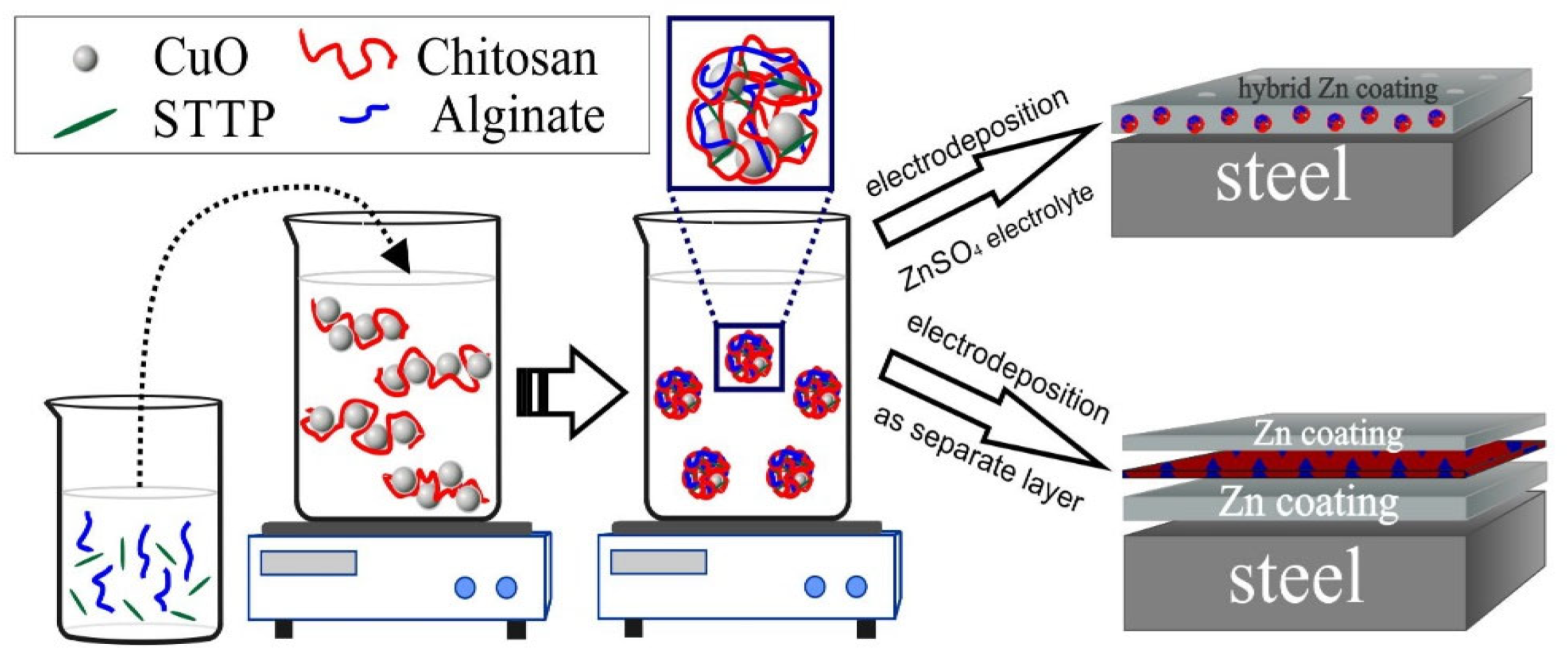
In the present work, two procedures for realizing of hybrid zinc coatings were applied based on the co-electrodeposition of zinc and CuO nanoparticles on low carbon steel (ZnH) or by the incorporation of CuO nanoparticles as an intermediate layer between two zinc layers, i.e., a multilayer hybrid system Zn/CuO/Zn (ZnS). Before electrodeposition, polymeric capsules containing CuO nanoparticles were prepared using CHI/ALG complexation in the presence of a crosslinking agent sodium tripolyphosphate (TPP) to prevent the spontaneous leaching of Cu2+ ions. The elaborated innovative hybrid coatings are expected to combine the biocide effect of CuO nanoparticles with the ability of zinc coatings to protect steel from destruction in salt-containing solution. In addition to the role of the biopolymer shell for slowing down the copper release during a corrosion attack, a positive effect of both polymers is also expected based on their antifouling and anticorrosion properties.
Two model corrosive media are selected to study the protective characteristics of the hybrid coatings—3.5% NaCl and artificial sea water (ASW). The encapsulation of the CuO nanoparticles with biopolymers is expected to slow down the copper release during corrosion attacks in order to prolong the coating activity against biofouling and corrosive agents.
2. Experimental Methods and Applied Materials
2.1. Procedure of CuO Encapsulation
Chitosan (CHI, Mw~50,000–190,000 kDa) with a deacetylation degree of 75%–85%, alginic acid sodium salt from brown algae (ALG, Mw~30,000–100,000 kDa), sodium tripolyphosphate (TPP), acetic acid and copper (II) oxide nanopowder (CuO, <50 nm particle size) are bought from Sigma Aldrich (Darmstadt, Germany).
The procedure for the preparation of encapsulated CuO nanoparticles is based on that described in previous works [16,17] with slight modifications. The concentrations of both polymers and the crosslinker are chosen as in [16] to assure optimal chitosan/tripolyphosphate and chitosan/alginate ratios (5:1 and 11:1, respectively) for the preparation of a stable suspension of encapsulated CuO nanoparticles. Briеfly, CHI (1 g/L) is dissolved in acetic acid (0.03 M) under stirring for 24 h at room temperature. CuO nanopowder (0.014 g) is added to 100 mL CHI solution (1 g/L) under ultrasound treatment in an ice bath for 15 min to obtain a well-dispersed suspension. Then, 20 mL of aqueous ALG solution (0.45 g/L) is mixed with 20 mL of TPP solution (1 g/L) and added to the CuO containing CHI suspension under magnetic stirring for 30 min (750 rpm) at room temperature. The pH of the final suspension is 4. The working concentration of the encapsulated CuO suspension is 0.1 g/L, which has been found optimal for obtaining a stable suspension, according to the above-described procedure [16].
2.2. Characterization of Encapsulated CuO Nanoparticles
The hydrodynamic diameters and zeta potentials of the CuO nanoparticles were investigated by dynamic light scattering (DLS) and laser Doppler velocimetry (Zetasizer Pro Red Label, Malvern Panalytical Ltd., Malvern, UK). A HeNe laser was the applied device while the intensity parameter was evaluated with a detector at 173°.
2.3. Electrodeposition of Hybrid Coating with Encapsulated CuO Nanoparticles
The obtaining of zinc and hybrid zinc coatings with CuO nanoparticles is performed on mild steel substrates (sizes 3 cm × 1 cm × 0.1 cm) from a slightly acidic sulfate electrolyte (pH 4.5–5.0) with a composition of 150 g/L ZnSO4·7H2O, 30 g/L NH4Cl and 30 g/L H3BO3 as well as two additives—wetting agent AZ1 (50 mL/L) and brightener AZ2 (mL/L).
The composition of the low-carbon steel is as follows (wt.%): C- 0.05–0.12; S ≤ 0.04; p ≤ 0.35; Mn—0.25–0.5; Cr ≤ 0.1; Si ≤ 0.03; Ni ≤ 0.3; Cu ≤ 0.3; As ≤ 0.08; Fe-balance.
The electrodeposition was carried out in an experimental cell with 600 mL electrolyte volume at ambient temperature and a cathodic current (DC current) density of 2 A/dm2 by using soluble zinc anodes. The duration of this step is 20 min. The concentration of the CuO nanoparticles used for the electrodeposition of the hybrid coating was 0.025 g/L. The thickness of the coatings was ~12 µm. After taking out the coatings from the electrolyte, they were exsiccated in air.
The second type of hybrid coating is prepared by three-step electrodeposition. First, a matt zinc layer (~8 µm thickness) was electrodeposited on the steel sample from the above-described sulfate electrolyte with the following conditions: cathodic current density of 2 A/dm2 and usage of zinc plates as anodes. Thereafter, the coating was washed with water and a new thin layer of polymeric encapsulated CuO nanoparticles from the previously prepared suspension was deposited at a cathodic current density of 0.025 A/dm2 and with an application of Pt-Ti (mesh) anodes. A final bright zinc coating (~4 µm thickness) was electrodeposited on the water rinsed CuO layer with the conditions of the initial zinc coating—Scheme 1.
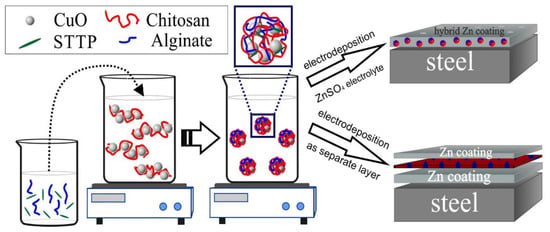
Scheme 1.
Encapsulation procedures and obtaining hybrid coating types.
2.4. Morphology of the Surface
The morphology of the surface and the distribution of CuO nanoparticles in the coatings are evaluated by the application of Scanning Electron Microscopy (INCA Energy 350 unit, Oxford, UK).
2.5. Corrosion and CVA Investigations
Newly developed coatings were characterized with electrochemical methods such as potentiodynamic (PDP) polarization curves and polarization resistance (Rp) measurements (using the Stern-Geary equation and a special device) concerning the corrosion properties of the coatings in two model media. The corrosion properties of the hybrid coatings were compared with the same parameters of the pure zinc. The experiments were realized by the application of PAR unit “VersaStat 4”. The reference electrode was a Saturated Calomel Electrode (SCE), and a platinum lamella was the counter one. The PDP curves ceased after a “naked eye” observation of the sample. The Rp measurements had a duration of 30 days. Cyclic Voltammetry (CVA) studies were carried out in the electrolytes for the electrodeposition of hybrid and ordinary zinc coatings in the potential area between −1.4 V and 0 V with a scan rate of 10 mV/s. In addition, CVA curves have been also carried out in electrolyte containing only CuO particles.
2.6. Atomic Force Microscopy (AFM) Investigations
AFM investigations were carried out on a NanoScope V system (Bruker Ltd., Bremen, Germany) working in tapping mode in air at ambient temperature. Silicon cantilevers (Tap 300Al-G, Budget Sensors, Innovative Solutions Ltd., Sofia, Bulgaria) with 30 nm thick aluminum coatings with high reflectivity were applied. The constant characteristic value of the cantilever force and the resonance frequency were in the range 40 N/m and 300 kHz, respectively, while the tip radius was less than 10 nm. The scanning rate was 1 Hz and the depictions were worked up in height mode with 512 × 512 pixels in JPEG format. All pictures obtained were additionally processed with a suitable software product—NanoScope (Bruker Inc., Birrica, MA, USA), which was also applied for section and roughness analysis.
2.7. Measurement and Evaluation of the Contact Angle
Contact angle evaluation was realized with water drops (~3 μL volume) and the application of an automatic goniometer/tensiometer (Model 290, Ramè—Hart Ltd., with DROP images Advanced v. software 2.4 (2012, Succasunna, NJ, USA) at ambient temperature. A Ramé-Hart automatic dispensing system was used to form and deposit the drops. The contact angles of 10 consecutive drops of 3 μL at randomly selected areas of the samples were evaluated.
2.8. Test Media and Reproducibility
Electrochemical-corrosion tests were maintained in two model media—3.5% NaCl solution (pH ~6.7) and artificial sea water (ASW)—according to ASTM D 665. These media were applied due to their practically equal ionic strength. The experimental results were summarized as an average from the data obtained with five samples per type, i.e., either zinc or hybrid zinc coatings tested in both model media. The measurement error was in the range of ±10%.
3. Results and Discussion
3.1. Characterization of Encapsulated CuO Nanoparticles
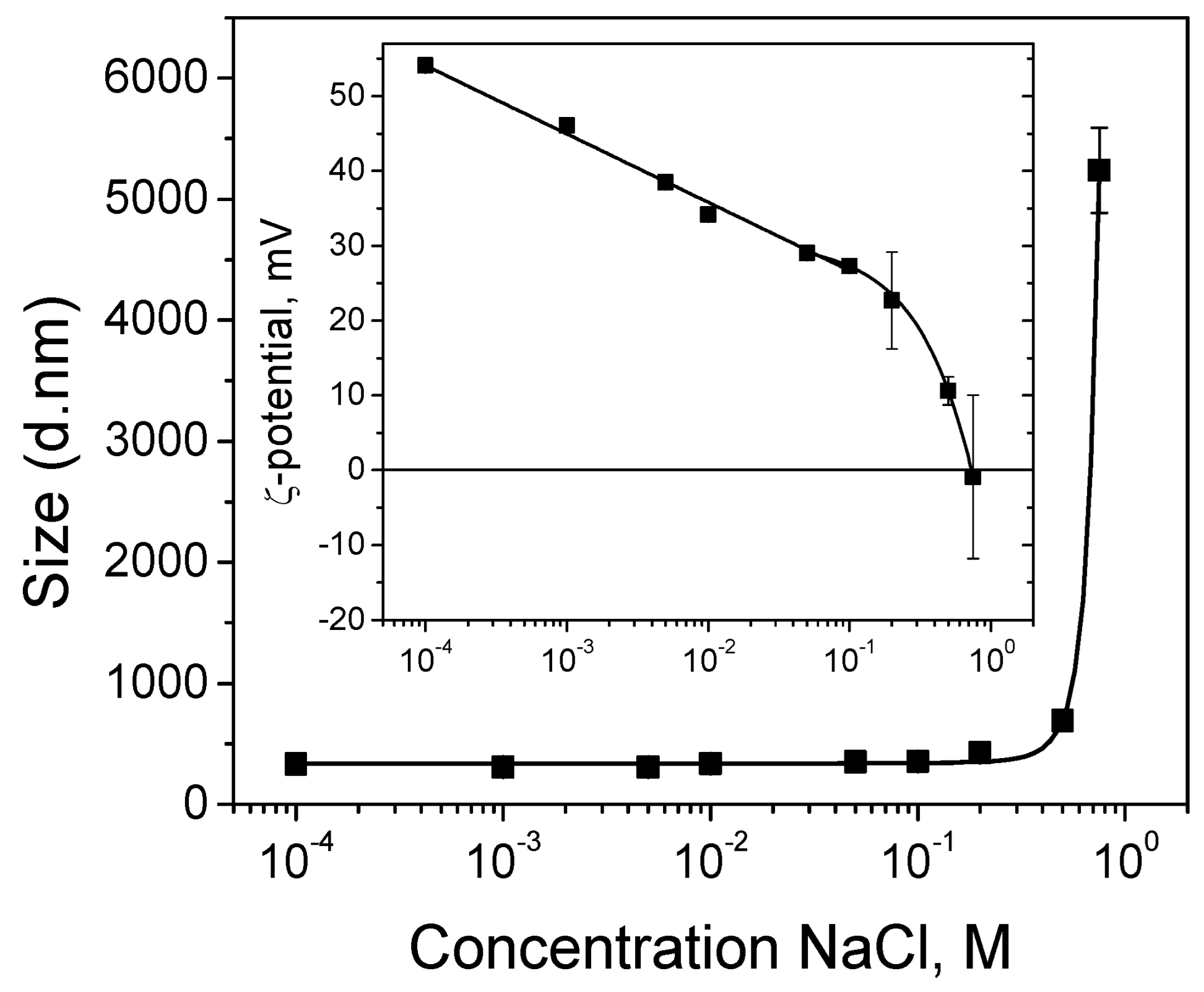
Figure 1 shows a mean diameter of the encapsulated CuO nanoparticles of about 335 nm, evaluated from the DLS measurements of their hydrodynamic diffusion coefficients in aqueous solution at pH 4. Similar results were obtained for particles prepared in closed conditions in [16]: a size of about 400 nm from transmission electron microscopy analysis and hydrodynamic diameter with a peak at 317 nm (with a standard deviation of 95 nm) obtained from the DLS experiment.
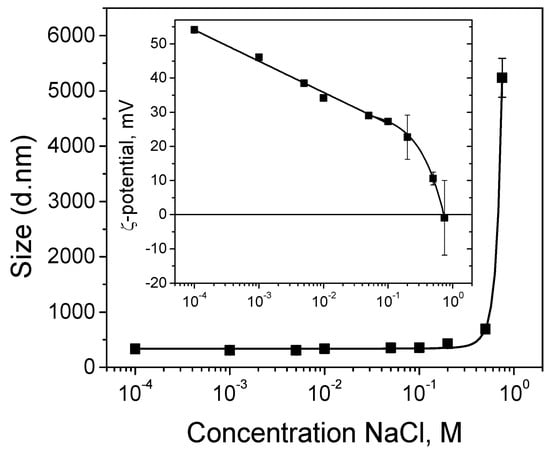
Figure 1.
Hydrodynamic diameters of encapsulated CuO nanoparticles in a suspension as a function of NaCl concentration. Inset: Zeta potentials of encapsulated nanoparticles at same conditions.
In order to prove the obtaining of stable suspension of encapsulated CuO nanoparticles, DLS measurements of zeta potentials are performed as well. The results show positively charged particles (zeta potential of about +54 mV), which means that they are suitable for electrodeposition on a cathode surface and the CuO suspension is electrostatically stabilized against aggregation (inset in Figure 1). It is well-known that particles with zeta potentials more positive than +30 mV (or more negative than −30 mV) are considered stable in suspensions [24].
The electrodeposition of metal and composite coatings is usually performed in electrolyte solutions of high ionic strength. In order to probe the suspension stability and integrity of the nanocapsules obtained through physical (electrostatic) interactions, DLS measurements are also performed in the presence of salt. Figure 1 demonstrates that until the content of the added NaCl reaches 0.75 M, the particle sizes stay almost constant. No measurable swelling of the capsules is observed, allowing us to consider them as an inert matrix that does not swell or disintegrate during the experiment. The rapid increase in the particles size at 0.75 M NaCl is attributed to agglomeration as a result of the drastic decrease in the particles surface charge. This is in accordance with the zeta potential measurements (inset in Figure 1). The results presented in the next section (Figure 2A) point to the incorporation of intact particles of slightly bigger sizes compared to the initial encapsulated CuO particles for the hybrid zinc coating obtained through the co-electrodeposition of zinc and CuO nanoparticles from sulfate electrolyte of about 1 M ionic strength. We could hypothesize, therefore, that unstable particle agglomerates sediment instead of reaching the working (cathode) electrode during the electrodeposition process.
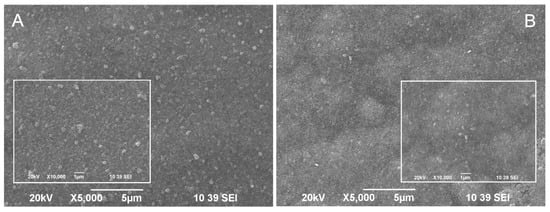
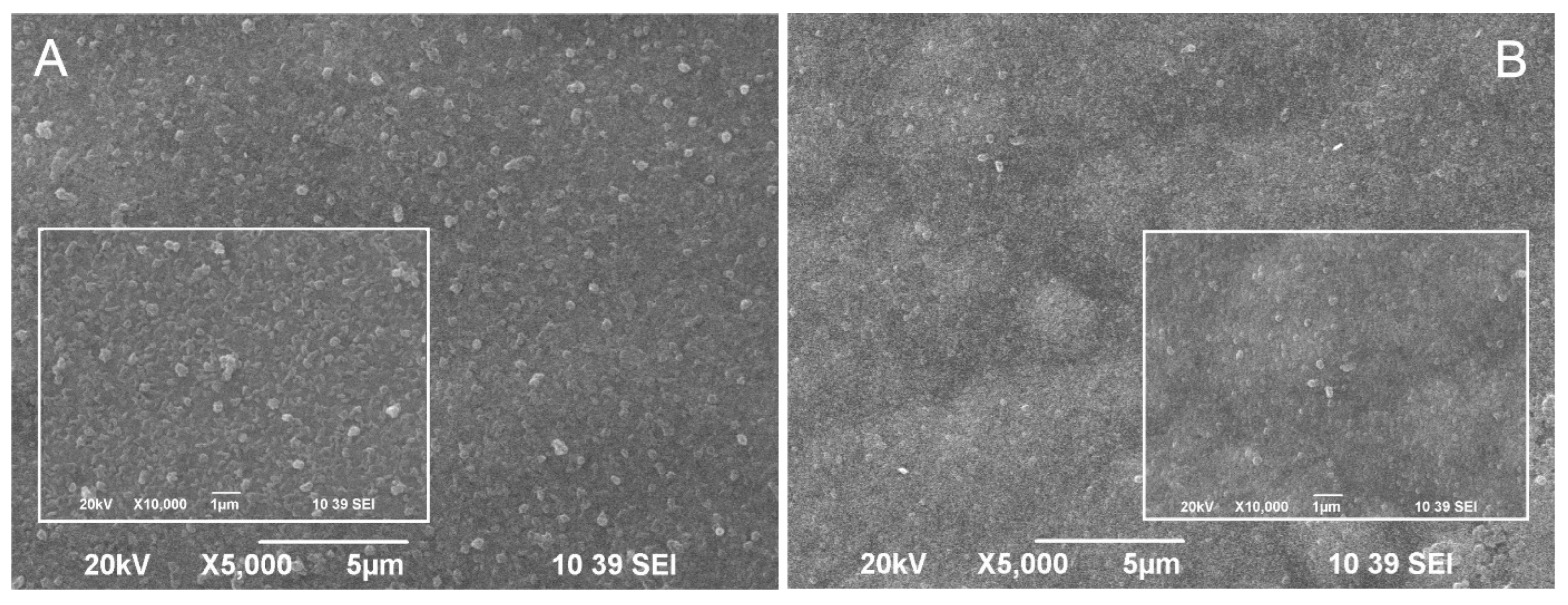
Figure 2.
SEM images of (A) hybrid coating obtained through co-electrodeposition of zinc and CuO nanoparticles (ZnH) and (B) hybrid coating with CuO nanoparticles embedded into the volume of the zinc as a separate layer (ZnS).
In order to incorporate smaller and well dispersed particles, the electrophoretic deposition of a separate (single) layer from CuO particles is realized at a lower salt concentration for the preparation of the second hybrid coating. In this particular case, the encapsulated CuO particles are deposited as an intermediate layer between two zinc deposits on steel substrate in the presence of 0.01 M NaCl. As shown in Figure 1, the particles size remains equal to that obtained in the absence of salt (335 nm), whereas the zeta potential value decreases to about +34 mV. This means that the electrophoretic deposition of the separate CuO layer is realized from suspension, stabilized against aggregation.
3.2. Surface Morphology
A SEM micrograph of the hybrid coating obtained through the co-electrodeposition of zinc and CuO nanoparticles from a zinc sulfate electrolyte is presented in Figure 2A. Figure 2B presents a SEM micrograph of the hybrid coating obtained by CuO incorporation as a separate layer into the structure of the zinc layer. It is obvious that CuO nanoparticles appear near or directly on the surface of both hybrid coating types. However, their surface morphology differs. Bright CuO particles can be registered on the surface of the first coating, slightly bigger than in suspension before co-electrodeposition with zinc (from DLS measurements). Smaller (vague) CuO nanoparticles are seen on the surface of the second coating type, most likely under a thin top zinc layer. The fact that many CuO particles are seen on the surface (or under a thin zinc layer) in both coatings can be attributed to particles detachment from the metal substrate during the process of electrodeposition. Earlier, an AFM study of the incorporation of particles in electrodeposited metal coatings revealed that the particles deposition strongly depends on their surface properties [25]. It was shown that hydrophilic particles were incorporated with difficulty into the metal matrix because of the presence of hydration layers around the particles, which prevented them from making “real” contact with the metal (electrode) surface. The proposed explanation in [25] is that during metal deposition, metal ions diffuse into the hydration layer, pushing up the particles.
As noted in [26], when steel structures are submerged in seawater, they are quickly colonized by marine microorganisms for the consequent formation of complex biofilm [27]. Controlling the biofilm formation is very important to prevent biofouling. Therefore, it can be concluded that the CuO nanoparticles disposal near the coatings surface offers a good possibility for the released Cu2+ ions to express their biocide effect on the early stages of biofouling.
3.3. Cyclic Voltammetry (CVA) Studies
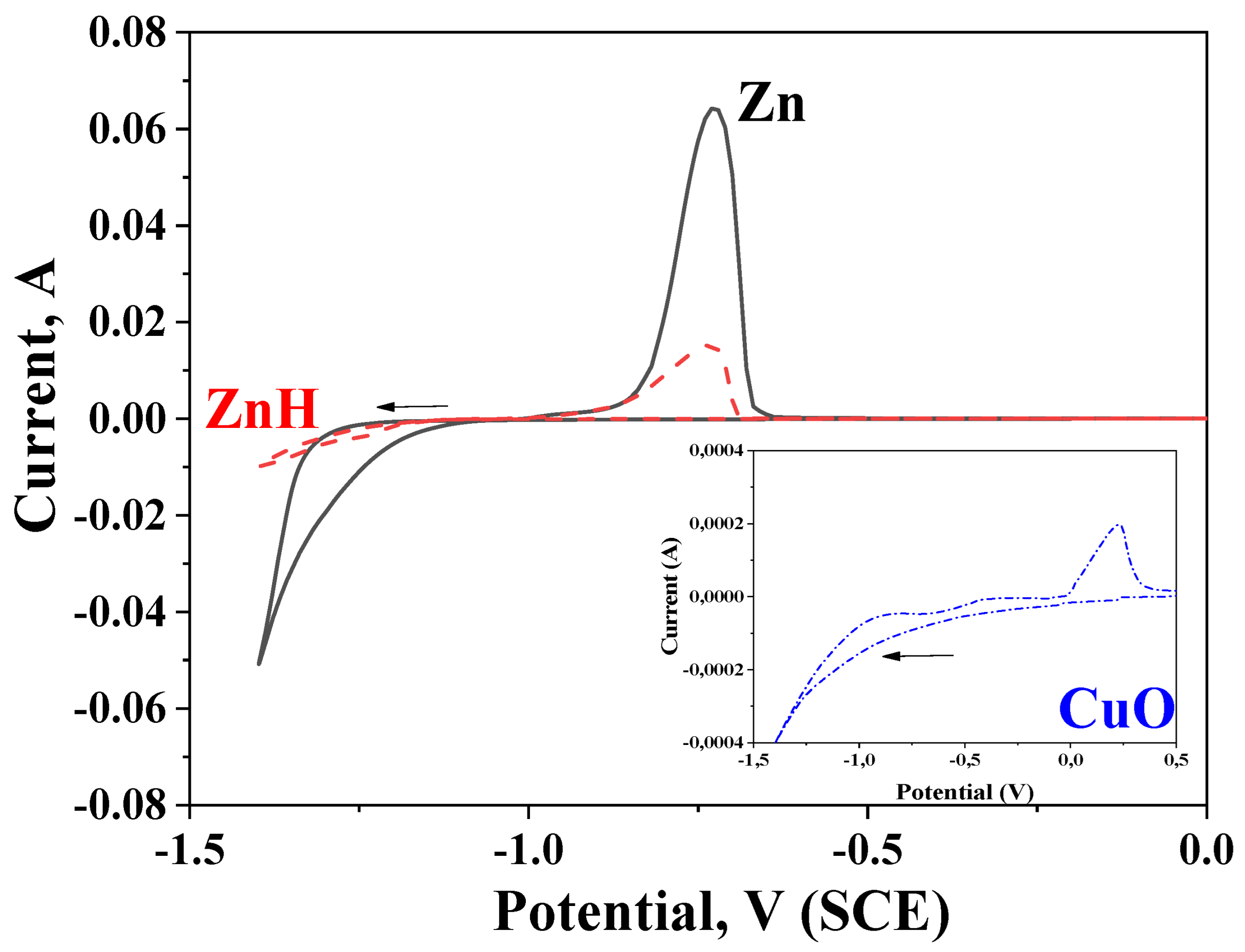
CVA investigations are presented in Figure 3. The tests are realized in the initial electrolytes used for obtaining of pure and hybrid zinc coatings, respectively. In addition, the results from electrodeposition and dissolution processes of only CuO nanoparticles are presented in the inset in Figure 3. As observed, the electrodeposition of the pure zinc coating is at a much higher current and begins at more negative potential values compared to the hybrid one, i.e., the deposition of the hybrid coating in the cathodic area is depolarized and is carried out at much lower cathodic current densities. In the anodic area of this curve, a peak occurs, the latter being characteristic for the anodic dissolution process, confirming the presence of ZnH.
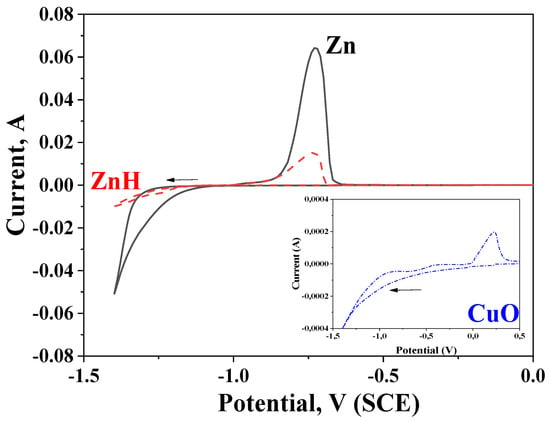
Figure 3.
Cyclic voltammetry measurements in solutions for obtaining hybrid or ordinary zinc coatings. Inset: CVA investigations in solution containing only CuO nanoparticles.
Obviously, the presence of chitosan/alginate modified CuO nanoparticles with a more positive potential compared to the zinc ions is the reason for this observation. In addition, the encapsulated CuO nanoparticles distinguish with greater sizes in comparison to the zinc ions and during electrodeposition on the steel substrate, they will physically hamper (partially) the electrodeposition of the free zinc ions. The Inset in Figure 3 demonstrates the deposition and dissolution processes in electrolyte, containing only chitosan/alginate modified CuO nanoparticles and proves their presence on the cathode even in the case when no other ions or charged particles are available.
3.4. Potentiodynamic Polarization (PDP) Curves
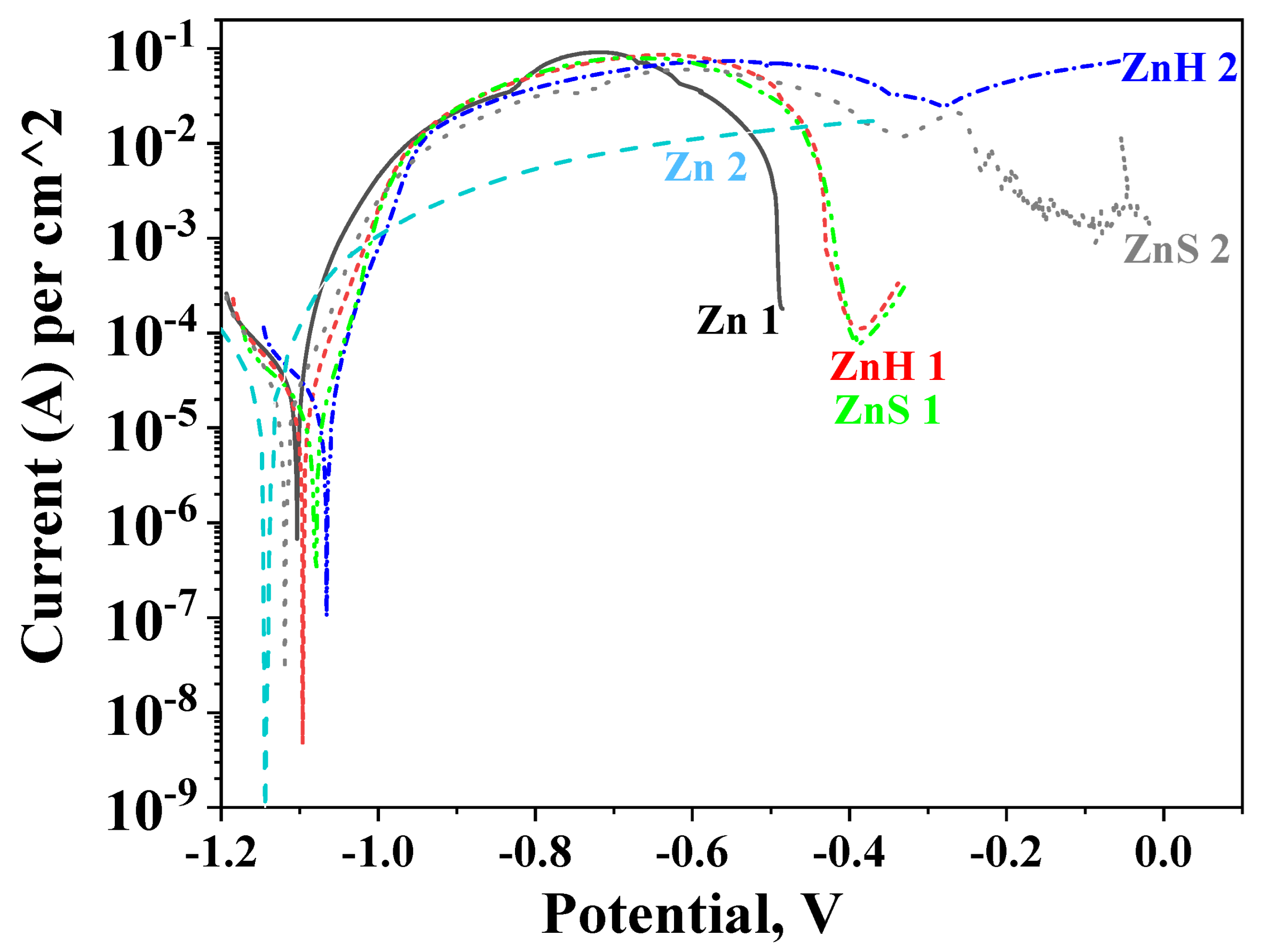
Potentiodynamic investigations of ordinary zinc and both hybrid coatings (ZnH or ZnS, respectively) in the model solution are presented in Figure 4. Some of the registered electrochemical parameters are shown in Table 1.
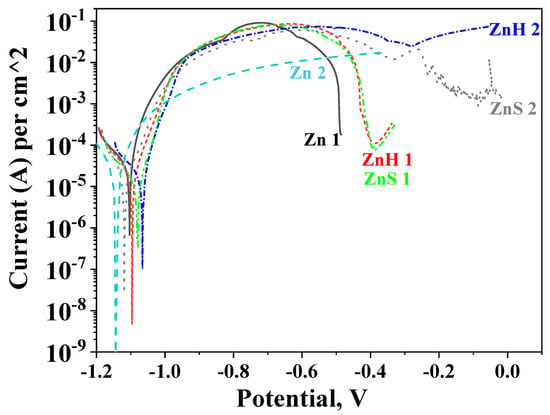
Figure 4.
Potentiodynamic polarization curves of ordinary zinc (Zn), hybrid coating obtained through co-electrodeposition of zinc and CuO nanoparticles (ZnH) and hybrid zinc coating with CuO nanoparticles deposited as a separate layer (ZnS) in 3.5% NaCl solution (1) and artificial sea water (2).

Table 1.
Electrochemical parameters received from potentiodynamic polarization investigations.
The corrosion potential of the ordinary zinc coating on steel in 3.5% NaCl solution can be found at more positive areas and its corrosion current is higher compared to the coating immersed in ASW (see Table 1). The shape of both curves differs—the anodic curve in 3.5% NaCl goes by a maximum at a potential of about −0.72 V while in ASW this curve increases up to the full dissolution of the coating in the potential zone of ~−0.4 V. The ordinary zinc in 3.5% NaCl solution practically dissolves at potential values of about −0.5 V. The main reason for the observed results seems to be the presence of corrosive products appearing during the investigation on the zinc coatings. In the case of 3.5% NaCl, the product of the corrosion is generally zinc hydroxide chloride Zn5(OH)8Cl2·H2O with a very low product of solubility [10]. This protects the low-carbon steel since the layer of zinc hydroxide chloride impedes to a certain degree the action of the aggressive chloride ions into the depth. Contrary to this, in ASW, many other ions present similar to Ca2+, Mg2+, Sr2+, K+, HCO3−, SO42−, Br−, F−. As a result, it could be expected that other corrosion products also will be available which distinguish with greater composition inhomogeneity.
Similarly, the hybrid coatings demonstrate some differences in both test media. In 3.5% NaCl, the anodic curve of ZnH goes by a maximum at about −0.73 V and then the anodic current decreases. This coating fully disappears at about −0.4 V. The ZnS sample demonstrates practically close anodic behavior and bares the steel substrate at close potential value. In ASW, the ZnH and ZnS coatings also show similar behavior—their curves pass through a maximum in the potential zone between −0.7 and −0.4 V. Thereafter, a weakly expressed “pseudo-passive” zone appears for the ZnS. Both coatings are fully dissolved at about 0 V. This result shows that the type of preliminary preparation and obtaining of ZnH and ZnS does not significantly affect the corrosion behavior in the individual media. One probable reason for this statement is the way of embedding the CuO nanoparticles in both coating types, which (according to the data from SEM) seems to be somewhat close.
Finally, it is obvious that the available encapsulated-with-CHI/ALG CuO nanoparticles positively influence the corrosion parameters of the ordinary zinc, ensuring longer anodic curves and lower corrosion current densities at conditions of external anodic polarization. In addition, ZnH and ZnS demonstrate longer anodic curves in ASW which can be assumed as a sign for their better stability and increased corrosion resistance. One possible reason for this behavior could be the greater aggressiveness of the chloride ions, in the presence of which the protective layer of corrosion products is destroyed faster compared to ASW under anodic polarization [7].
3.5. Polarization Resistance (Rp) Measurements
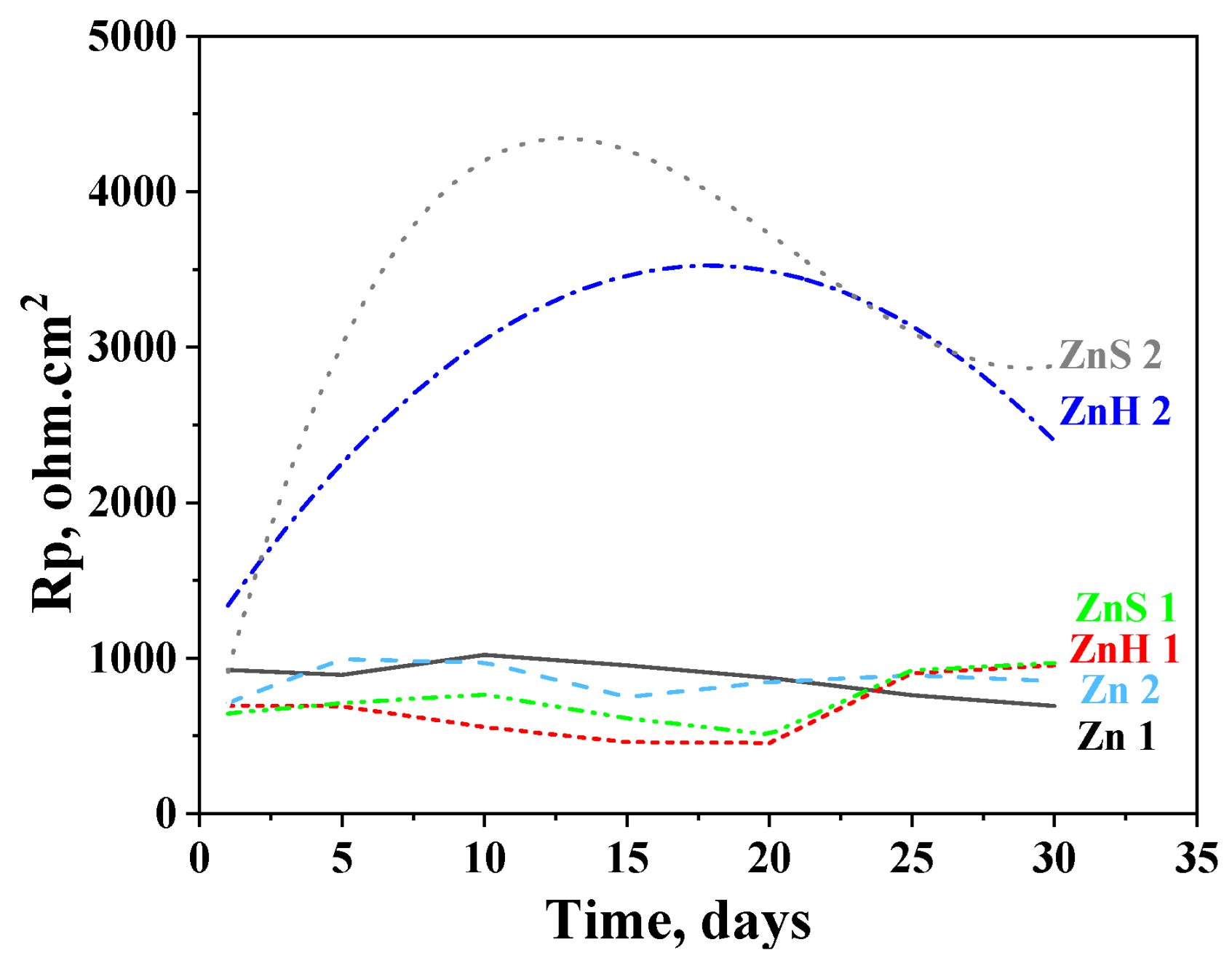
Polarization resistance results after a 30-day stay of the investigated objects in the test media are presented in Figure 5. It is well seen that a pure zinc sample shows close Rp values in 3.5% NaCl and ASW solutions during the test period of time (Zn 1 and Zn 2 curves, respectively). At the beginning of the test, their polarization resistance is ~750 Ω.cm2 (Zn 2) and ~900 Ω.cm2 (Zn 1) and at the end of the period, it is ~870 Ω.cm2 in ASW and ~700 Ω.cm2 in 3.5% NaCl solution.
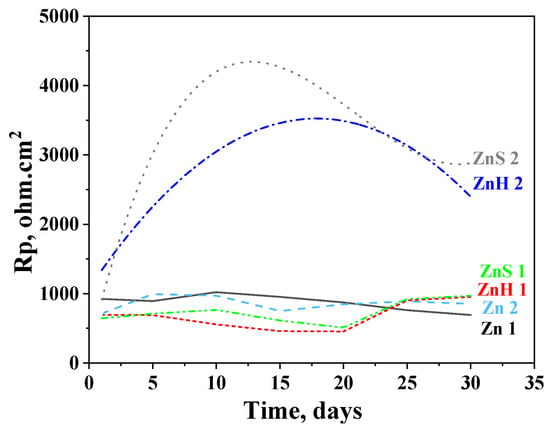
Figure 5.
Polarization resistance of ordinary zinc (Zn), hybrid coating obtained through co-electrodeposition of zinc and CuO nanoparticles (ZnH) and hybrid zinc coating with CuO nanoparticles deposited as a separate layer (ZnS) in 3.5% NaCl solution (1) and artificial sea water (2).
The experimental data for the hybrid zinc samples ZnH and ZnS in 3.5% NaCl demonstrate lower Rp values compared to the ordinary zinc (~500–600 ohm.cm2) during the immersion period until the 20th day but become somewhat higher at the end of the test—~950 ohm.cm2. This means that the presence of the CHI/ALG encapsulated CuO nanoparticles slightly positively influences their anticorrosion behavior in that medium.
The positive impact of the incorporated nanoparticles is expressed much stronger in ASW solution resulting in about 2–4 times higher Rp values. The course of the ZnS curve passes through a maximum after 12 days’ immersion (Rp ~4370 Ω.cm2); thereafter, it decreases until the 29th day which is followed by an increase at the end of the test (~2890 Ω.cm2). The Rp values of ZnH increase up to the 17th day (Rp ~3500 Ω.cm2) and then decrease, reaching 2420 Ω.cm2 at the last day of the investigation.
The experimental data obtained can be explicated with the presence of corrosion products in the model media as commented above. However, during the immersion test (“open-circuit” conditions), the forming and appearance of these compounds is realized slowly, contrary to the case of the external polarization (which is an accelerated test). In addition, the composition of the newly formed corrosion products differs in both media, leading to some differences in the corrosion behavior, which have been commented on and discussed in Point 3.4.
3.6. Atomic Force Microscopy (AFM) Images and Evaluation of the Contact Angle
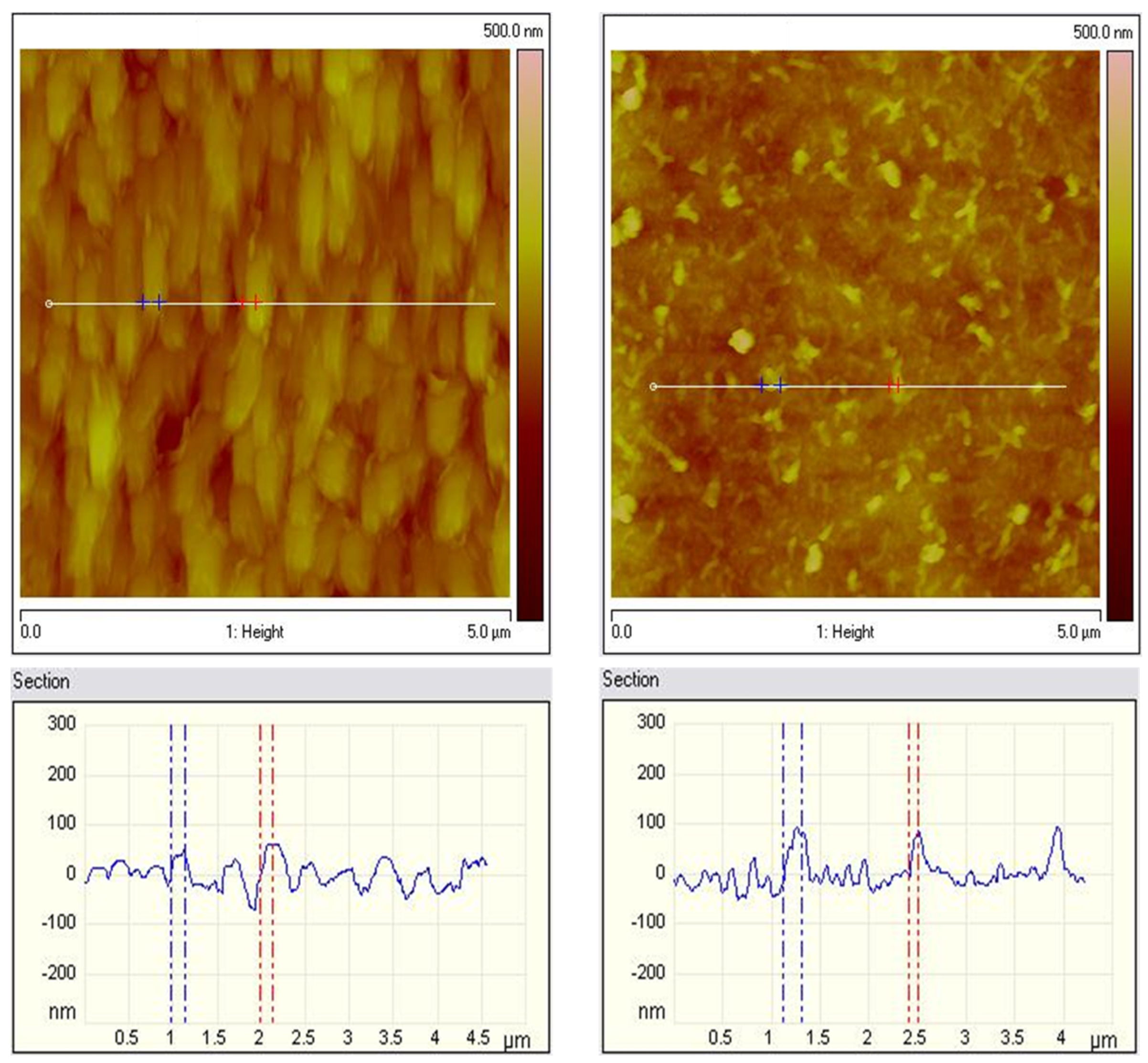
The AFM images of both hybrid coating types were compared in Figure 6. The roughness values for both types of coatings are close. The roughness of the ZnS sample with a scanning area of 5 × 5 μm2 (Figure 6 left) has a value of Ra = 34 nm and Rq = 43 nm, while the roughness of the ZnH sample (Figure 6 right) has a value of Ra = 31 nm and Rq = 39 nm, respectively. From the AFM measurements, it was found that for both types of samples, the roughness values are close, and the topography of the surface of the two samples is different, which is due to the inclusion of CuO in the system. Generally, AFM qualitatively matches the SEM studies.
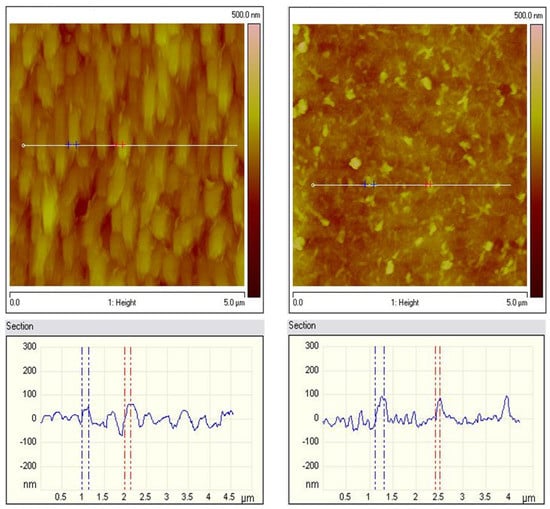
Figure 6.
AFM topography of ZnS (left) and ZnH (right) samples: 2D images and section analysis.
This could be partially a result of the different preparation procedures of the coatings. Individual small particles are observed for the ZnS sample, while predominantly homogeneous filamentous structures can be observed for the ZnH sample.
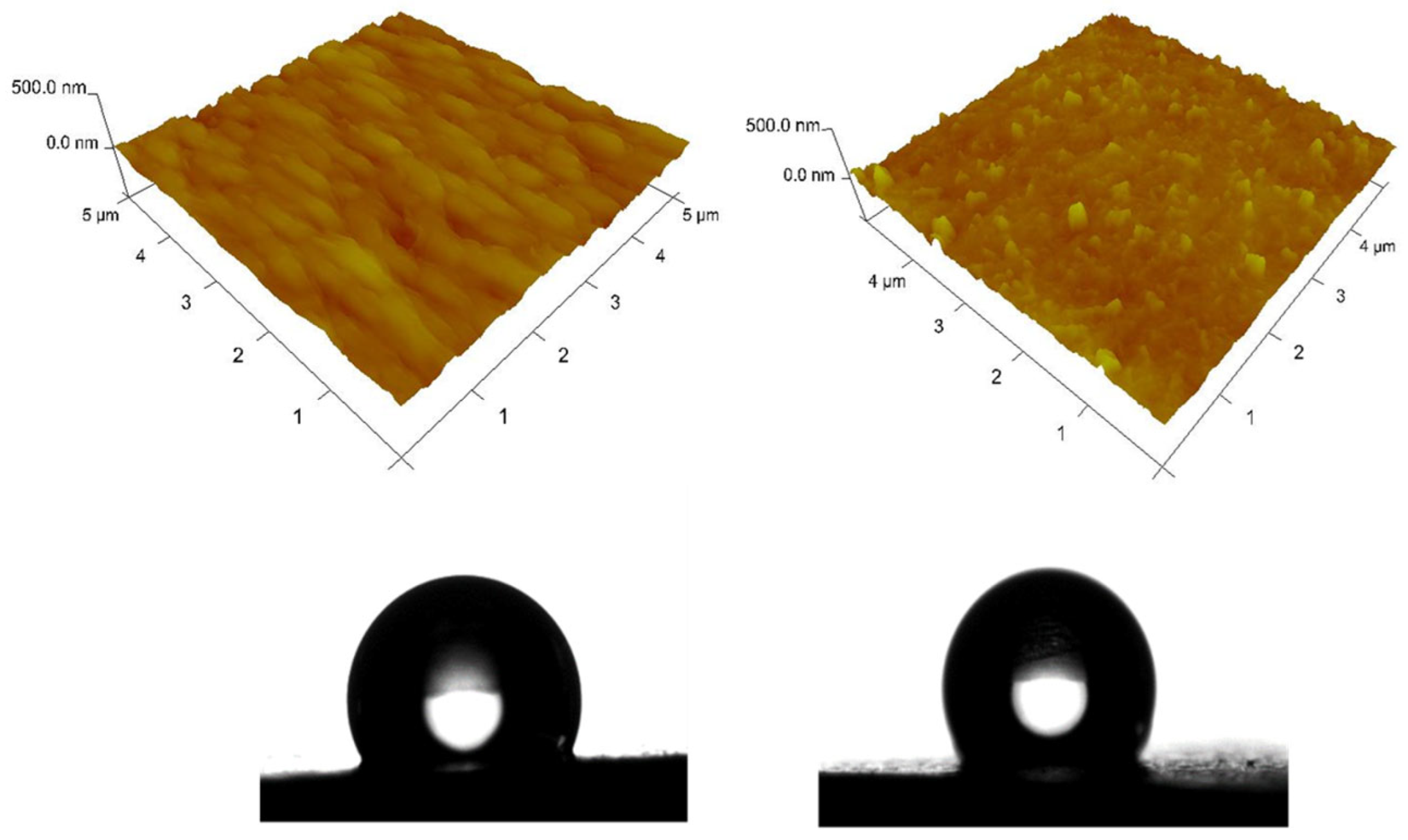
The wettability of the solid surfaces of both hybrid zinc coatings is determined through contact angle measurements (Figure 7). The water droplet contact angles of ZnS and ZnH samples are 116° and 121°, respectively, which means that the surface wettability of both coatings is unfavorable [28]. However, the surface of both hybrid samples is more hydrophobic collated to the surface of the pure zinc layer (94°) according to the results obtained in [7].
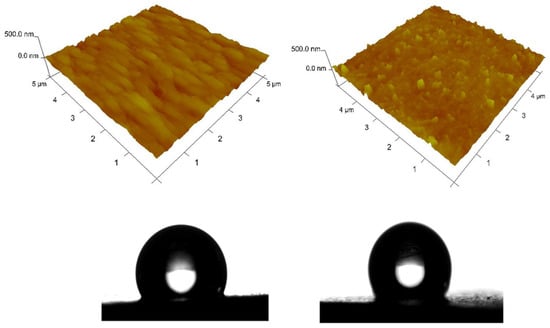
Figure 7.
AFM topography of ZnS (left) and ZnH (right) samples: 3D images and contact angle of the water drop.
4. Conclusions
- This study described the successful preparation of hybrid zinc coatings containing CHI/ALG-encapsulated CuO nanoparticles as biocide agents with a potential for low-carbon steel protection from corrosion and biofouling in the marine environment.
- Homogeneous hybrid zinc coatings were realized by the simultaneous or separate electrodeposition of encapsulated CuO nanoparticles with zinc on mild steel substrates.
- The hybrid zinc coatings provided corrosion protection of mild steel in a model medium of 3.5% NaCl and artificial sea water solution for investigation time period of 30 days. The preparation procedure of the coatings does not significantly affect their corrosion behavior.
- The protective properties of the hybrid coatings are better presented in ASW most probably because of the higher content of aggressive Cl− ions in the NaCl solution. Further investigations are needed regarding the antifouling performance of these coatings, showing potential for combined protective applications with low toxic environmental impact.
- It is planned to continue the current research by optimizing the procedures for obtaining hybrid coatings and conducting additional tests in selected environments.
Author Contributions
Conceptualization, T.R. and N.B. (Nikolai Boshkov); data curation, K.K. and N.B. (Nelly Boshkova); formal analysis, S.S., N.G., K.K. and N.B. (Nelly Boshkova); funding acquisition, N.B. (Nikolai Boshkov); investigation, K.K. and N.B. (Nelly Boshkova); methodology, S.S., N.G., K.K., N.B. (Nelly Boshkova) and T.R.; writing—review and editing, T.R. and N.B. (Nikolai Boshkov). All authors have read and agreed to the published version of the manuscript.
Funding
The authors express their gratitude to the project with the Fund “Scientific Investigations”, Bulgaria, KP-06-China/4 (KП-06-Kитaй/4), “Developing novel composite materials and their surface coatings for long-term anti-corrosion/biofouling applications” for the financial support and for the possibility to publish the obtained results.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Acknowledgments
The authors express their gratitude to the project with the Fund “Scientific Investigations”, Bulgaria, KP-06-China/4 (KП-06-Kитaй/4), “Developing novel composite materials and their surface coatings for long-term anti-corrosion/biofouling applications” for the financial support and for the possibility to publish the obtained results. The support of the European Regional Development Fund within the OP Science and Education for Smart Growth 2014–2020, Project CoE: National Centre for Mechatronics and Clean Technologies, No. BG05M2OP001-1.001-0008 is also acknowledged. Research equipment of the Distributed Research Infrastructure INFRAMAT, part of the Bulgarian National Roadmap for Research Infrastructures, supported by the Bulgarian Ministry of Education and Science was used in these investigations.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Popoola, P.A.I.; Malatji, N.; Fayomi, O.S. Fabrication and properties of zinc composite coatings for mitigation of corrosion in coastal and marine zone. In Applied Studies of Coastal and Marine Environments; InTech: London, UK, 2016; Volume 32, pp. 137–144. [Google Scholar]
- Maniam, K.K.; Paul, S. Corrosion performance of electrodeposited zinc and zinc-alloy coatings in marine environment. Corros. Mater. Degrad. 2021, 2, 163–189. [Google Scholar] [CrossRef]
- Ranganatha, S.; Venkatesha, T.V.; Vathsala, K.; Punith Kumar, M.K. Electrochemical studies on Zn/nano-CeO2 electrodeposited composite coatings. Surf. Coat. Technol. 2012, 208, 64–72. [Google Scholar] [CrossRef]
- De La Fuente, D.; Castano, J.G.; Morcillo, M. Long-term atmostpheric corrosion of zinc. Corros. Sci. 2007, 9, 1420–1436. [Google Scholar] [CrossRef]
- Deepa, K.; Arthoba Nayaka, Y. Synthesis of CuO micro and nanoparticles as composite additives for corrosion resistant Zn-composite coatings on mild steel. Inorg. Nano-Metal Chem. 2020, 50, 354–360. [Google Scholar] [CrossRef]
- Fayomi, O.S.I.; Popoola, A.P.I.; Loto, C.A. Tribomechanical investigation and anti-corrosion properties of Zn-TiO2 thin film composite coatings from electrolyte chloride bath. Intern. J. Electrochem. Sci. 2014, 9, 3885–3903. [Google Scholar]
- Boshkova, N.; Kamburova, K.; Radeva, T.; Simeonova, S.; Grozev, N.; Shipochka, M.; Boshkov, N. Comparative corrosion characterization of hybrid zinc coatings in Cl-contaning medium and artificial sea water. Coatings 2022, 12, 1798. [Google Scholar] [CrossRef]
- Deepa, K.; Venkatesha, T.V. Synthesis of CeO2 doped ZnO nanoparticles and their application in Zn-composite coating on mild steel. Arab. J. Chem. 2020, 13, 2309–2317. [Google Scholar]
- Kumar, C.M.P.; Chandrashekarappa, M.P.G.; Kulkarni, R.M.; Pimenov, D.Y.; Giasin, K. The effect of Zn and Zn–WO3 composites nano-coatings deposition on hardness and corrosion resistance in steel substrate. Materials 2021, 14, 2253. [Google Scholar] [CrossRef]
- Kamburova, K.; Boshkova, N.; Boshkov, N.; Radeva, T. Hybrid zinc coating with CuO nanocontainers containing corrosion inhibitor for combined protection of mild steel from corrosion and biofouling. Coatings 2022, 12, 1254. [Google Scholar] [CrossRef]
- Zhang, X.; Feng, Y.; Gao, D.; Ma, W.; Jin, C.; Jiang, X.; Lin, J.; Yang, F. Functionalization of cellulosic hydrogels with Cu2O@CuO nanospheres: Toward antifouling applications. Carbohydr. Polym. 2022, 282, 119136. [Google Scholar] [CrossRef] [PubMed]
- Gittens, J.E.; Smith, T.J.; Suleiman, R.; Akid, R. Current and emerging environmentally-friendly systems for fouling control in the marine environment. Biotechnol. Adv. 2013, 31, 1738–1753. [Google Scholar] [CrossRef]
- Adeleye, A.S.; Oranu, E.A.; Tao, M.; Keller, A.A. Release and detection of nanosized copper from a commercial antifouling paint. Water Res. 2016, 102, 374–382. [Google Scholar] [CrossRef] [PubMed]
- Raghavendra, G.M.; Jung, J.; Kim, D.; Seo, J. Chitosan-mediated synthesis of flowery-CuO, and its antibacterial and catalytic properties. Carbohydr. Polym. 2017, 172, 78–84. [Google Scholar] [CrossRef] [PubMed]
- Khan, R.; Inam, M.A.; Zam, S.Z.; Akram, M.; Shin, S.; Yeom, I.T. Coagulation and dissolution of CuO nanoparticles in the presence of dissolved organic matter under different pH values. Sustainability 2019, 11, 2825. [Google Scholar] [CrossRef]
- Leonardi, M.; Caruso, G.M.; Carroccio, S.C.; Boninelli, S.; Curcuruto, G.; Zimbone, M.; Allegra, M.; Torrisi, B.; Ferlito, F.; Miritello, M. Smart nanocomposites of chitosan/alginate nanoparticles loaded with copper oxide as alternative nanofertilizers. Environ. Sci. Nano 2021, 8, 174–187. [Google Scholar] [CrossRef]
- Goycoolea, F.M.; Lollo, G.; Remunan-Lopez, C.; Quaglia, F.; Alonso, M.J. Chitosan-Alginate Blended Nanoparticles as Carriers for the Transmucosal Delivery of Macromolecules. Biomacromolecules 2009, 10, 1736–1743. [Google Scholar] [CrossRef] [PubMed]
- Boddohi, S.; Moore, N.; Johnson, P.A.; Kipper, M.J. Polysaccharide-based polyelectrolyte complex nanoparticles from chitosan, heparin, and hyaluronan. Biomacromolecules 2009, 10, 1402–1411. [Google Scholar] [CrossRef]
- Unsoy, G.; Yalcin, S.; Khodadust, R.; Gunduz, G.; Gunduz, U. Synthesis, optimization and characterization of chitosan coated iron oxide nanoparticles produced for biomedical applications. J. Nanopart. Res. 2012, 14, 1–13. [Google Scholar] [CrossRef]
- Al-Naamani, L.; Dobretsov, S.; Dutta, J.; Grant Burgess, J. Chitosan-zinc oxide nanocomposite coatings for the prevention of marine biofouling. Chemosphere 2017, 168, 408–417. [Google Scholar] [CrossRef]
- Umoren, S.A.; Eduok, U.M. Application of carbohydrate polymers as corrosion inhibitors for metal substrates in different media: A review. Carbohydr. Polym. 2016, 140, 314–341. [Google Scholar] [CrossRef]
- Srivastava, M.; Srivastava, S.K.; Ji, N.G.; Prakash, R. Chitosan based new nanocomposites for corrosion protection of mild steel in aggressive chloride media. Intern. J. Biolog. Macromol. 2019, 140, 177–187. [Google Scholar] [CrossRef] [PubMed]
- Obot, I.B.; Ikenna, B.; Onyeachu, A.; Kumar, M. Sodium alginate: A promising biopolymer for corrosion protection of API X60 high strength carbon steel in saline medium. Carbohydr. Polym. 2017, 178, 200–208. [Google Scholar] [CrossRef]
- Meissner, T.; Oelschlagel, K.; Potthoff, A. Implications of the stability behavior of zinc oxide nanoparticles for toxicological studies. Int. Nano Lett. 2014, 4, 116–129. [Google Scholar] [CrossRef]
- Stappers, L.; Fransaer, J. AFM study of the incorporation of particles during electro-deposition. J. Electrochem. Soc. 2007, 154, D598–D611. [Google Scholar] [CrossRef]
- Wen, J.; Li, H. Thermal spray coatings for protection against microbiologically induced corrosion: Recent advances and future perspectives. J. Therm. Spray Technol. 2022, 31, 829–847. [Google Scholar] [CrossRef]
- Huggett, M.J.; Nedved, B.T.; Hadfield, M.G. Effects of initial surface wettability on biofilm formation and subsequent settlement of hydroides elegans. Biofouling 2009, 25, 387–399. [Google Scholar] [CrossRef] [PubMed]
- Adamson, A.W.; Gast, A.P. Physical Chemistry of Surfaces, 6th ed.; John Wiley & Sons: New York, NY, USA, 1997; pp. 347–380. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).