Capacitive Ferrosoferric Oxide as an Anode to Enhance the Electrical Energy Output and Storage of Microbial Fuel Cells
Abstract
:1. Introduction
2. Experimental Methods
2.1. Carbon Felt Film Pretreatment
2.2. Preparation of Carbon Felt Film/Ferric Oxide Electrode Material
2.3. Start-Up of Microbial Fuel Cells
2.4. Output Voltage of the Microbial Fuel Cell
- -
- Rex—Load resistance, unit: Ω
- -
- I—Current, unit: A
- -
- U— Output voltage, unit: V
2.5. Electrochemical Performance Test
2.6. Scanning Electron Microscopy-Energy-Dispersive Spectroscopy (SEM-EDS)
3. Results and Discussion
3.1. Physicochemical Characterization of the CF/Fe3O4 Electrode
3.2. Power Density Test and Polarization Curves of MFCs
3.3. Energy Storage Test of MFCs
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Qiao, Y.; Bao, S.; Li, C. Electrocatalysis in microbial fuel cells from electrode material to direct electrochemistry. Energy Environ. Sci. 2010, 3, 544–553. [Google Scholar]
- Ren, Z.; Ward, T.E.; Regan, J.M. Electricity Production from Cellulose in a Microbial Fuel Cell Using a Defined Binary Culture. Environ. Sci. Technol. 2007, 41, 4781–4786. [Google Scholar] [PubMed]
- Hidalgo, D.; Tommasi, T.; Bocchini, S.; Chiolerio, A.; Chiodoni, A.; Mazzarino, I.; Ruggeri, B. Surface modification of commercial carbon felt used as anode for Microbial Fuel Cells. Energy 2016, 99, 193–201. [Google Scholar]
- Cheng, S.; Logan, B.E. Ammonia treatment of carbon cloth anodes to enhance power generation of microbial fuel cells. Electrochem. Commun. 2007, 9, 492–496. [Google Scholar]
- Park, D.H.; Zeikus, J.G. Improved Fuel Cell and Electrode Designs for Producing Electricity from Microbial Degradation. Biotechnol. Bioeng. 2003, 81, 348–355. [Google Scholar]
- Kim, J.R.; Min, B.; Logan, B.E. Evaluation of procedures to acclimate a microbial fuel cell for electricity production. Appl. Microbiol. Biotechnol. 2005, 68, 23–30. [Google Scholar] [CrossRef] [PubMed]
- Thepsuparungsikul, N.; Ng, T.; Lefebvre, O.; Ng, H. Different types of carbon nanotubebased anodes to improve microbial fuel cell performance. Water Sci. Technol. 2014, 69, 1900–1910. [Google Scholar] [CrossRef] [PubMed]
- Qiao, Y.; Li, C.M.; Bao, S.; Bao, Q. Carbon nanotube/polyaniline composite as anode material for microbial fuel cells. J. Power Sources 2007, 170, 79–84. [Google Scholar]
- Lv, Z.; Xie, D.; Li, F.; Hu, Y.; Wei, C.; Feng, C. Microbial fuel cell as a biocapacitor by using pseudo-capacitive anode materials. J. Power Sources 2014, 246, 642–649. [Google Scholar]
- Zou, Y.; Pisciotta, J.; Baskakov, I.V. Nanostructured polypyrrole-coated anode for sun-powered microbial fuel cells. Bioelectrochemistry 2010, 79, 50–56. [Google Scholar] [PubMed]
- Peng, X.; Yu, H.; Wang, X.; Zhou, Q.; Zhang, S.; Geng, L.; Sun, J.; Cai, Z. Enhanced performance and capacitance behavior of anode by rolling Fe3O4 into activated carbon in microbial fuel cells. Bioresour. Technol. 2012, 121, 450–453. [Google Scholar] [CrossRef] [PubMed]
- Tang, J.; Yuan, Y.; Liu, T.; Zhou, S. High-capacity carbon-coated titanium dioxide core-shell nanoparticles modified three dimensional anodes for improved energy output in microbial fuel cells. J. Power Sources 2015, 274, 170–176. [Google Scholar] [CrossRef]
- Lv, Z.; Xie, D.; Yue, X.; Feng, C.; Wei, C. Ruthenium oxide-coated carbon felt electrode: A highly active anode for microbial fuel cell applications. J. Power Sources 2012, 210, 26–31. [Google Scholar] [CrossRef]
- Park, I.H.; Chrity, M.; Kim, P. Enhanced electrical contact of microbes using Fe3O4/CNT nanocomposite anode in mediator-less microbial fuel cell. Biosens. Bioelectron. 2014, 58, 75–80. [Google Scholar] [CrossRef] [PubMed]
- Fu, Y.; Xu, Q.; Zai, X. Low electrical potential anode modified with Fe/ferric oxide and its application in marine benthic microbial fuel cell with higher voltage and power output. Appl. Surf. Sci. 2014, 289, 472–477. [Google Scholar] [CrossRef]
- Ji, J.; Jia, Y.; Wu, W. A layer-by-layer self-assembled Fe2O3 nanorod-based composite multilayer film on ITO anode in microbial fuel cell. Colloids Surf. A Physicochem. Eng. Asp. 2011, 390, 56–61. [Google Scholar] [CrossRef]
- Pandit, S.; Khilari, S.; Roy, S. Improvement of power generation using Shewanella putrefaciens mediated bioanode in a single chambered microbial fuel cell: Effect of different anodic operating conditions. Bioresour. Technol. 2014, 66, 451–457. [Google Scholar] [CrossRef]
- Leonard, M.; Tender, S.A.; Ethan, G. The first demonstration of a microbial fuel cell as a viable power supply: Powering a meteorological buoy. J. Power Sources 2008, 179, 571–575. [Google Scholar]
- Deeke, A.; Sleutels, T.H.J.A.; Hamelers, H.V.M.; Buisman, C.J.N. Capacitive Bioanodes Enable Renewable Energy Storage in Microbial Fuel Cells. Environ. Sci. Technol. 2012, 46, 3554–3560. [Google Scholar] [CrossRef] [PubMed]
- Deeke, A.; Sleutels, T.H.; Heijne, A.; Hamelers, H.V.; Buisman, C.J. Influence of the thickness of the capacitive layer on the performance of bioanodes in Microbial Fuel Cells. J. Power Sources 2013, 243, 611–616. [Google Scholar] [CrossRef]
- Wang, Y.; Li, B.; Zeng, L.; Cui, D.; Xiang, X.; Li, W. Polyaniline/Mesoporous Tungsten Trioxide Composite as Anode Electrocatalyst for High-Performance Microbial Fuel Cells. Biosens. Bioelectron. 2013, 41, 582–588. [Google Scholar] [CrossRef]
- Liang, P.; Zhang, C.; Jiang, Y.; Bian, Y.; Zhang, H.; Sun, X.; Yang, X.; Zhang, X.; Huang, X. Performance enhancement of microbial fuel cell by applying transient-state regulation. Appl. Energy 2017, 185, 582–588. [Google Scholar] [CrossRef]
- Wang, Y.; Chen, Y.; Wen, Q. Microbial fuel cells: Enhancement with a polyaniline/carbon felt capacitive bioanode and reduction of Cr (VI) using the intermittent operation. Environ. Chem. Lett. 2018, 16, 319–326. [Google Scholar] [CrossRef]
- Lai, B.; Tang, X.; Li, H.; Du, Z.; Liu, X.; Zhang, Q. Power production enhancement with a polyaniline modified anode in microbial fuel cells. Biosens. Bioelectron. 2011, 28, 373–377. [Google Scholar] [CrossRef]
- Wang, P.; Li, H.; Du, Z. Polyaniline Synthesis by cyclic voltammetry for anodic modification in microbial fuel cells. Int. J. Electrochem. Sci. 2014, 9, 2038–2046. [Google Scholar]
- Hou, J.; Liu, Z.; Li, Y. Polyaniline modified stainless steel fiber felt for highperformance microbial fuel cell anodes. J. Clean Energy Technol. 2015, 3, 165–169. [Google Scholar] [CrossRef]
- Qi, R.; Guo, J.; Liu, Y.; Zhang, R.; Gan, Z. Effects of salt content on secondary structure of protein in sodium alginate/antarctic krill protein composite system and characterization of fiber properties. Dye. Pigment. 2019, 171, 107686. [Google Scholar] [CrossRef]
- Huang, H.B.; Zeng, X.P.; Li, W.; Wang, H.; Wang, Q.; Yang, Y. Reinforced conducting hydrogels prepared from the in situ polymerization of aniline in an aqueous solution of sodium alginate. J. Mater. Chem. A 2014, 2, 16516–16522. [Google Scholar] [CrossRef]
- Li, Y.; Zhao, X.; Xu, Q.; Zhang, Q.; Chen, D. Facile preparation and enhanced capacitance of the polyaniline/sodium alginate nanofiber network for supercapacitors. Langmuir 2011, 27, 6458–6463. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Zheng, H.; Lin, C.; Zheng, J.; Chen, Y.; Wen, Q.; Wang, S.; Xu, H.; Qi, L. Development of a 3D porous sponge as a bioanode coated with polyaniline/sodium alginate/nitrogen-doped carbon nanotube composites for high-performance microbial fuel cells. J. Appl. Electrochem. 2020, 50, 621–630. [Google Scholar] [CrossRef]
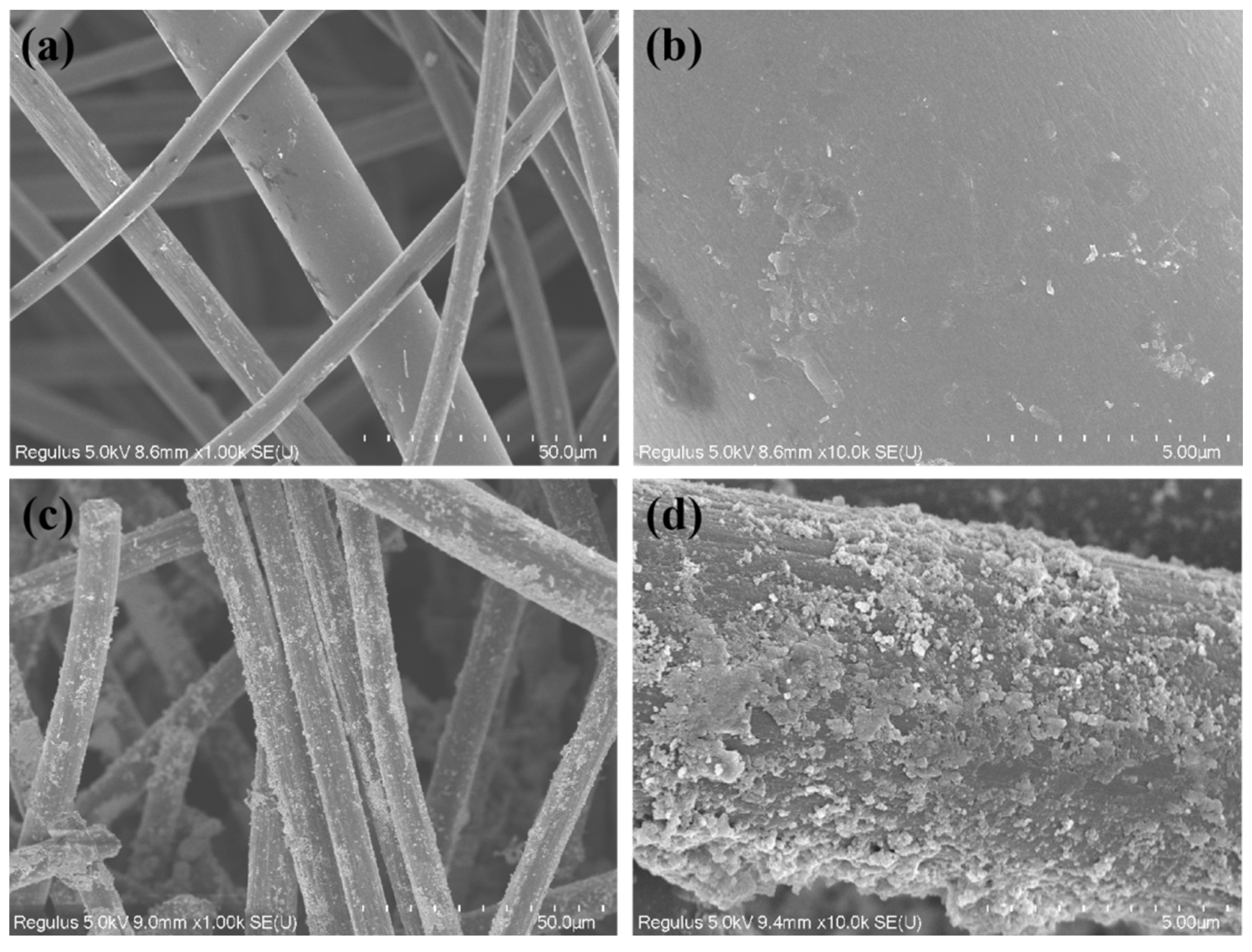
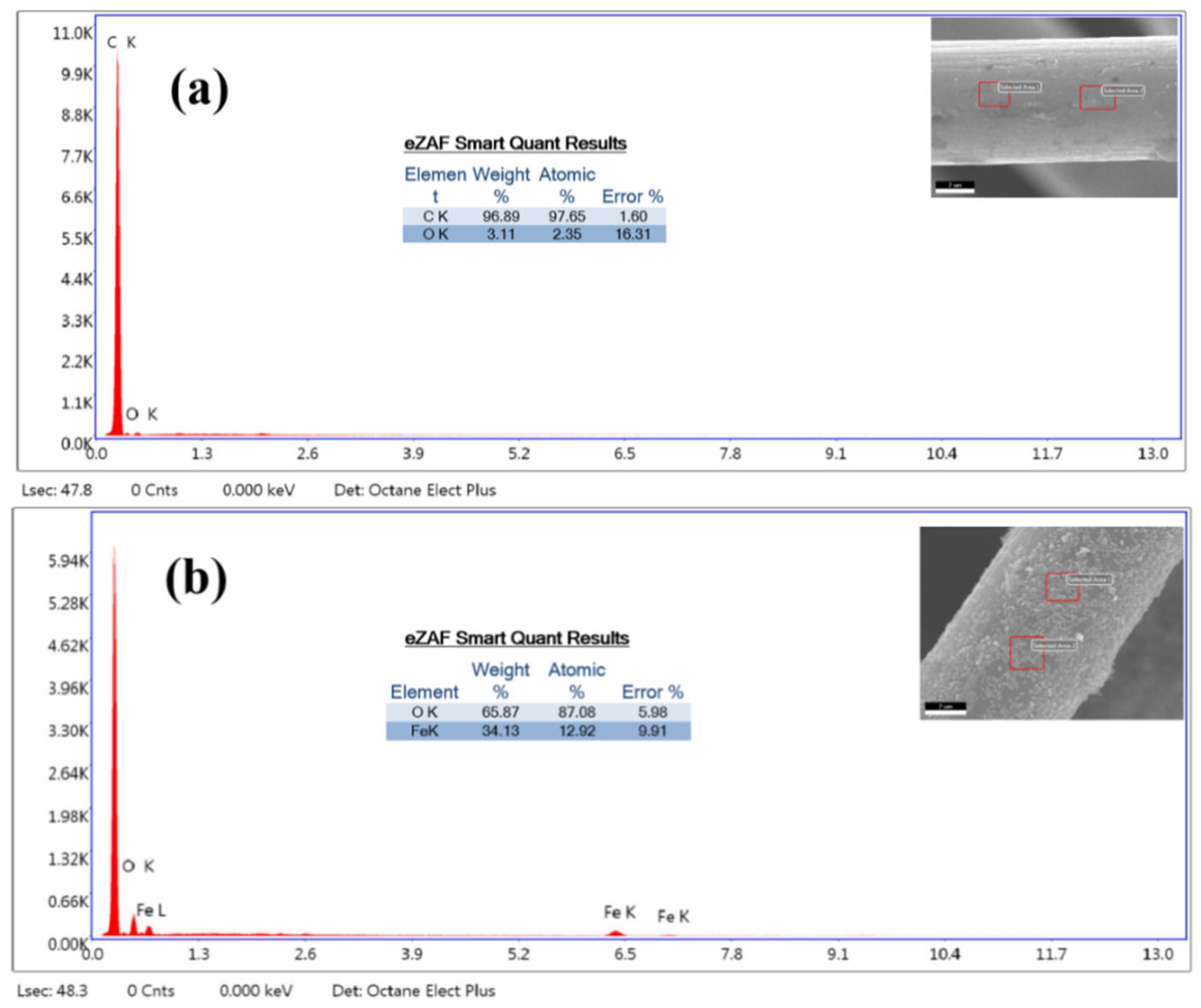
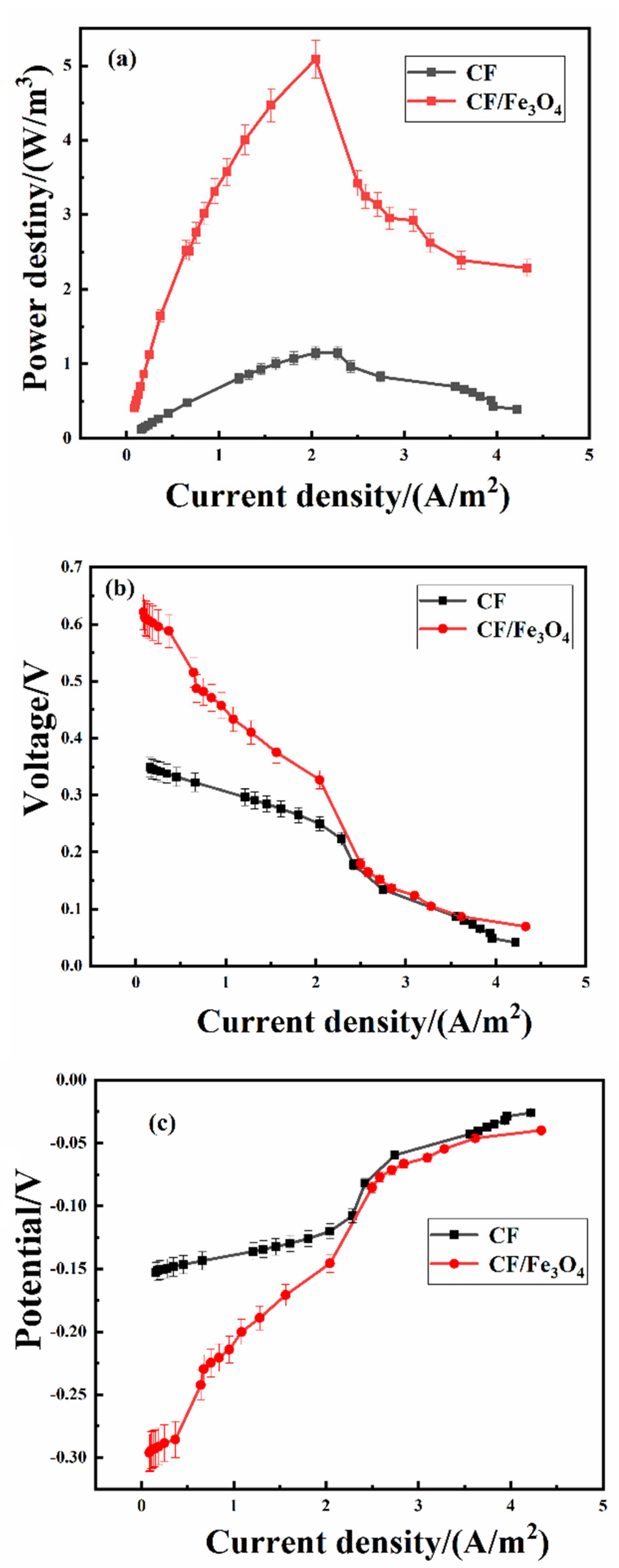

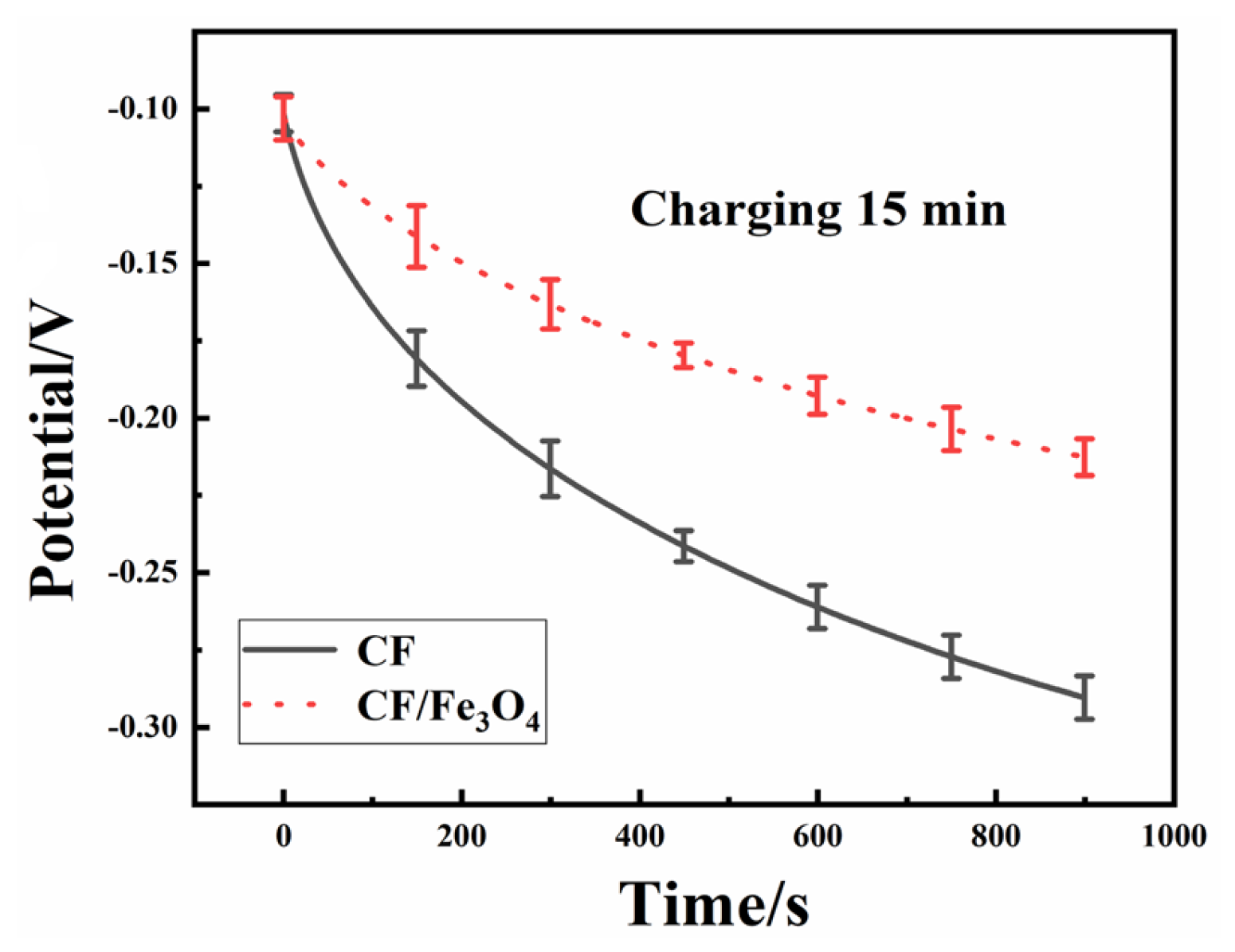
| Anodes | Parameters | C15/D15 | C30/D30 | C45/D45 | C60/D60 |
|---|---|---|---|---|---|
| CF anode | ih (A/m2) | 8.72 | 8.37 | 10.75 | 10.16 |
| is (A/m2) | 0.42 | 0.42 | 0.42 | 0.41 | |
| Qs (C/m2) | 28.76 | 44.39 | 51.73 | 94.12 | |
| Qt (C/m2) | 406.76 | 800.39 | 1185.73 | 1570.12 | |
| CF/Fe3O4 anode | ih (A/m2) | 20.97 | 21.69 | 24.43 | 22.82 |
| is (A/m2) | 1.11 | 1.06 | 1.15 | 1.15 | |
| Qs (C/m2) | 91.07 | 124.93 | 208.85 | 242.90 | |
| Qt (C/m2) | 1090.07 | 2032.93 | 3313.85 | 4382.90 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Y.; Wang, J.; Dong, J.; Tian, Y. Capacitive Ferrosoferric Oxide as an Anode to Enhance the Electrical Energy Output and Storage of Microbial Fuel Cells. Coatings 2023, 13, 901. https://doi.org/10.3390/coatings13050901
Wang Y, Wang J, Dong J, Tian Y. Capacitive Ferrosoferric Oxide as an Anode to Enhance the Electrical Energy Output and Storage of Microbial Fuel Cells. Coatings. 2023; 13(5):901. https://doi.org/10.3390/coatings13050901
Chicago/Turabian StyleWang, Yuyang, Jing Wang, Jing Dong, and Ye Tian. 2023. "Capacitive Ferrosoferric Oxide as an Anode to Enhance the Electrical Energy Output and Storage of Microbial Fuel Cells" Coatings 13, no. 5: 901. https://doi.org/10.3390/coatings13050901







