Heterogeneous Fenton-like Catalyzation of Nanoscale Schwertmannite for Sulfamethoxazole Degradation
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Preparation and Characterization of Nano-SWT
2.3. Catalytic Degradation of SMX
2.4. Analytical Methods
3. Results
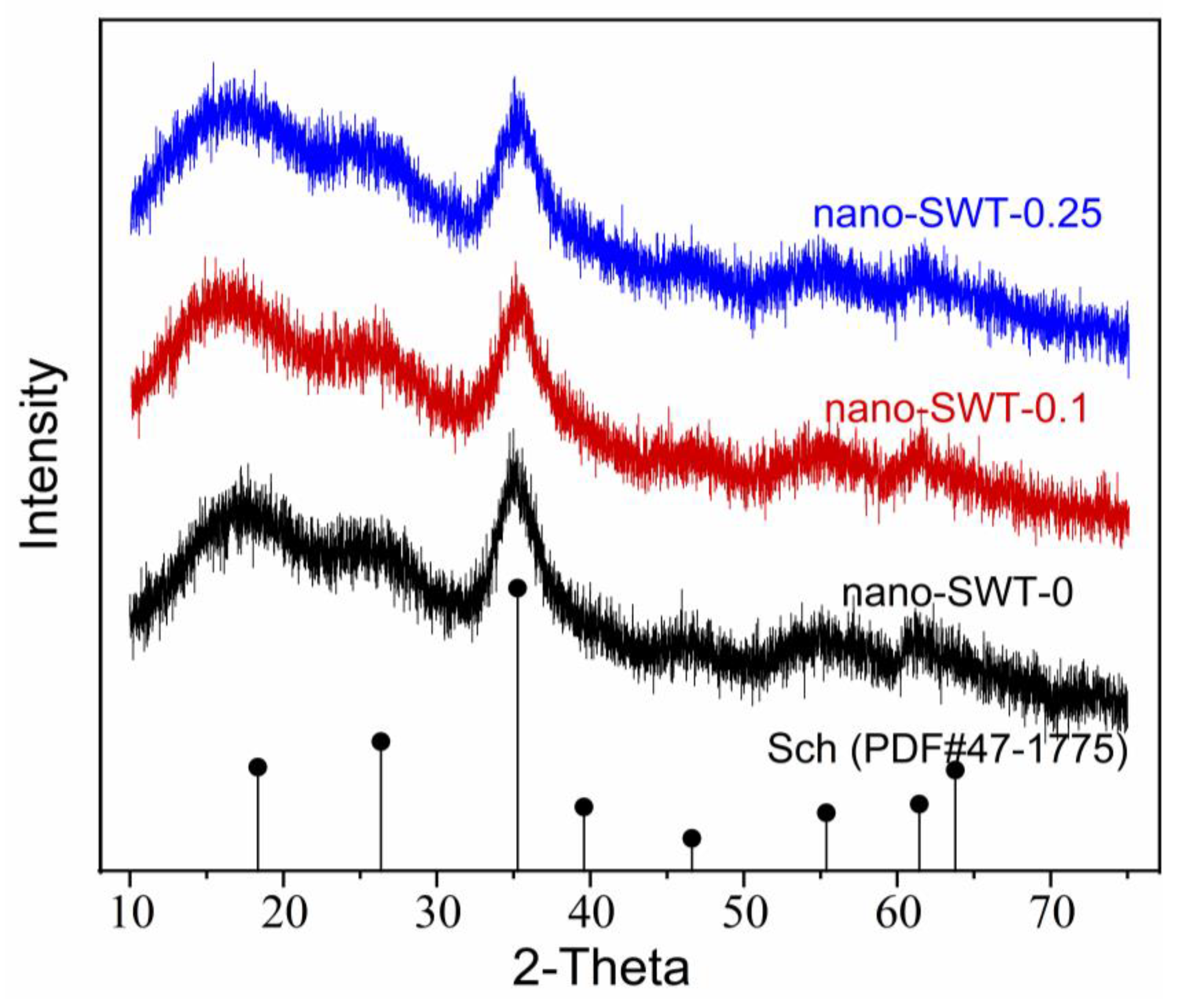
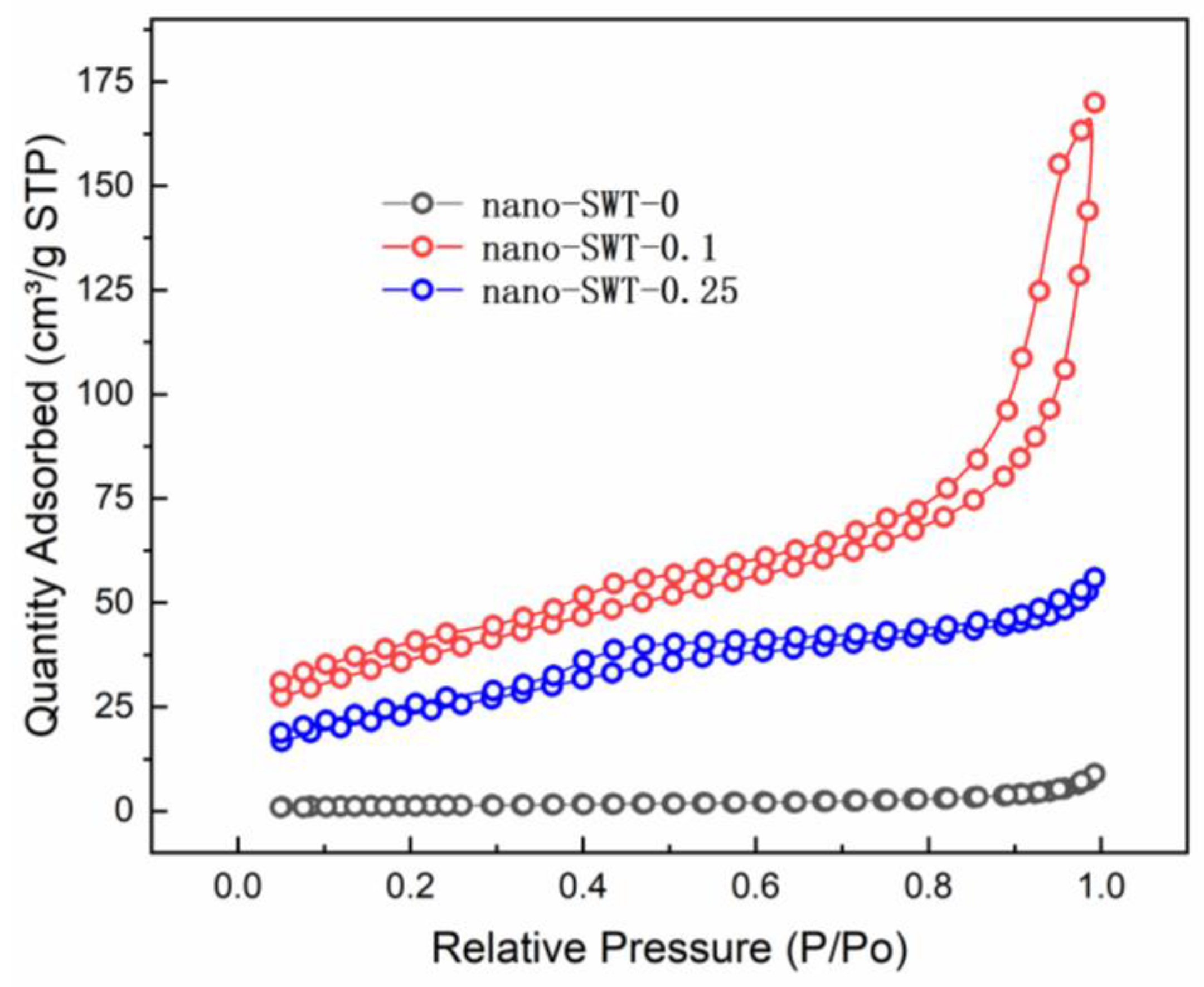
3.1. Characterizations of Prepared Schwertmannite
3.2. Catalytic Performances of Catalyst for SMX Degradation
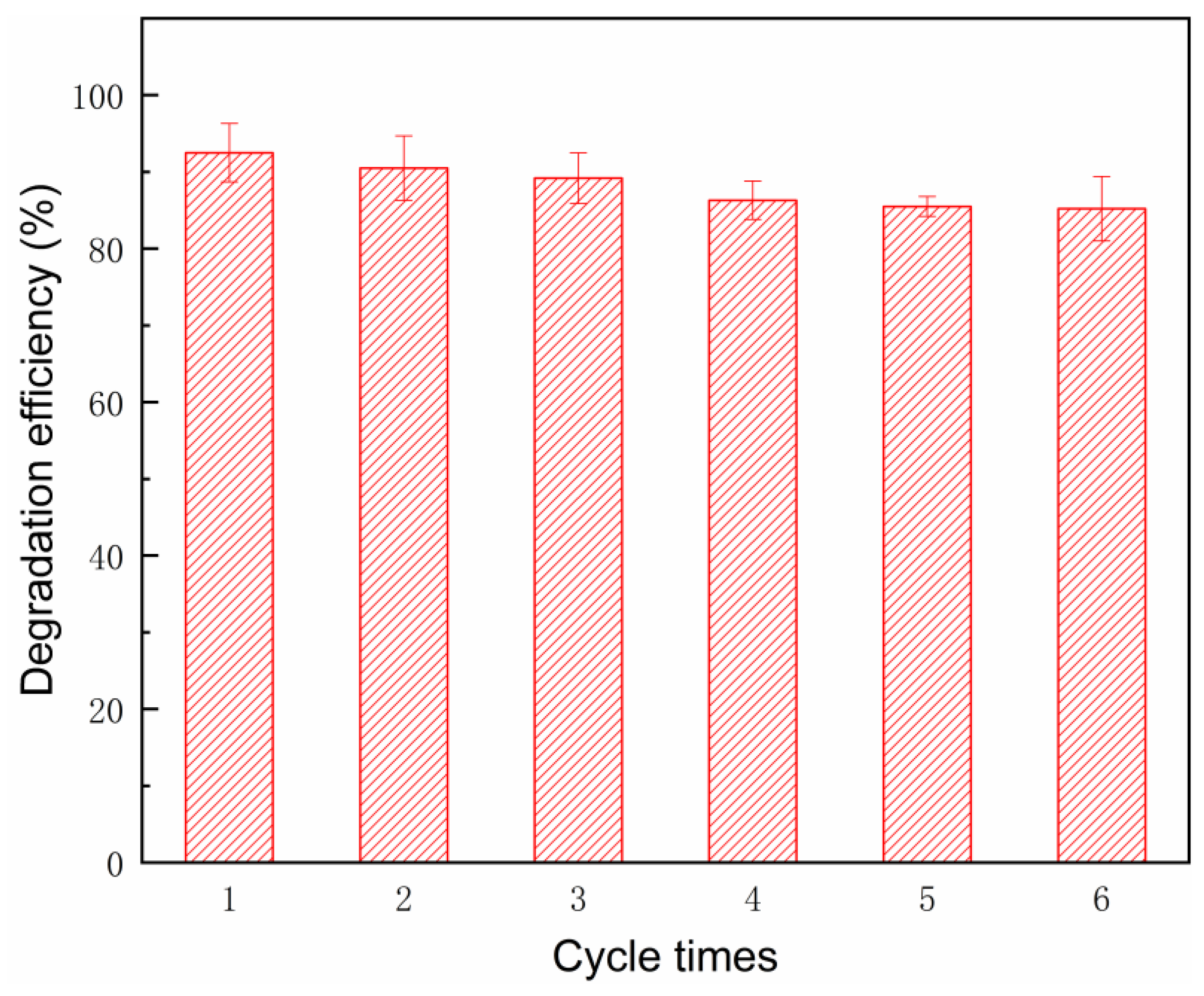
3.3. Stability and Reusability of the Nano-SWT in the SMX Degradation Process
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Lee, K.; Kim, T.H.; Kim, T.H.; Lee, J.; Yu, S. Enhancement of TOC removal efficiency of sulfamethoxazole using catalysts in the radiation treatment: Effects of band structure and electrical properties of radiocatalysts. Sep. Purif. Technol. 2023, 312, 123390. [Google Scholar] [CrossRef]
- Yan, S.; Zhan, L.; Meng, X.; Wang, D.; Wang, X.; Zheng, G.; Lu, J.; Zhou, L. Role of schwertmannite or jarosite in photocatalytic degradation of sulfamethoxazole in ultraviolet/peroxydisulfate system. Sep. Purif. Technol. 2021, 274, 118991. [Google Scholar] [CrossRef]
- Gong, H.; Chu, W. Determination and toxicity evaluation of the generated products in sulfamethoxazole degradation by UV/CoFe2O4/TiO2. J. Hazard. Mater. 2016, 314, 197–203. [Google Scholar] [CrossRef] [PubMed]
- Oh, D.; Lee, C.S.; Kang, Y.G.; Chang, Y.S. Hydroxylamine-assisted peroxymonosulfate activation using cobalt ferrite for sulfamethoxazole degradation. Chem. Eng. J. 2020, 386, 123751. [Google Scholar] [CrossRef]
- Meffe, R.; de Bustamante, I. Emerging organic contaminants in surface water and groundwater: A first overview of the situation in Italy. Sci. Total Environ. 2014, 481, 280–295. [Google Scholar] [CrossRef] [PubMed]
- Burke, V.; Richter, D.; Greskowiak, J.; Mehrtens, A.; Schulz, L.; Massmann, G. Occurrence of antibiotics in surface and groundwater of a drinking water catchment area in Germany. Water Environ. Res. 2016, 88, 652–659. [Google Scholar] [CrossRef]
- Pisharody, L.; Gopinath, A.; Malhotra, M.; Nidheesh, P.V.; Kumar, M.S. Occurrence of organic micropollutants in municipal landfill leachate and its effective treatment by advanced oxidation processes. Chemosphere 2022, 287, 132216. [Google Scholar] [CrossRef]
- Lama, G.; Meijide, J.; Sanromán, A.; Pazos, M. Heterogeneous Advanced Oxidation Processes: Current Approaches for Wastewater Treatment. Catalysts 2022, 12, 344. [Google Scholar] [CrossRef]
- Munoz, M.; De Pedro, Z.M.; Casas, J.A.; Rodriguez, J.J. Preparation of magnetite-based catalysts and their application in heterogeneous Fenton oxidation—A review. Appl. Catal. B Environ. 2015, 176, 249–265. [Google Scholar] [CrossRef] [Green Version]
- Xu, X.R.; Zhao, Z.Y.; Li, X.Y.; Gu, J.D. Chemical oxidative degradation of methyl tert-butyl ether in aqueous solution by Fenton’s reagent. Chemosphere 2004, 55, 73–79. [Google Scholar] [CrossRef]
- Neyens, E.; Baeyens, J. A review of classic Fenton’s peroxidation as an advanced oxidation technique. J. Hazard. Mater. 2003, 98, 33–50. [Google Scholar] [CrossRef]
- Zhu, Y.; Zhu, R.; Xi, Y.; Zhu, J.; Zhu, G.; He, H. Strategies for enhancing the heterogeneous Fenton catalytic reactivity: A review. Appl. Catal. B Environ. 2019, 255, 117739. [Google Scholar] [CrossRef]
- Hartmann, M.; Kullmann, S.; Keller, H. Wastewater treatment with heterogeneous Fenton-type catalysts based on porous materials. J. Mater. Chem. 2010, 20, 9002–9017. [Google Scholar] [CrossRef]
- Babuponnusami, A.; Muthukumar, K. Removal of phenol by heterogenous photo electro Fenton-like process using nano-zero valent iron. Sep. Purif. Technol. 2012, 98, 130–135. [Google Scholar] [CrossRef]
- Ganiyu, S.O.; Zhou, M.; Martínez-Huitle, C.A. Heterogeneous electro-Fenton and photoelectro-Fenton processes: A critical review of fundamental principles and application for water/wastewater treatment. Appl. Catal. B Environ. 2018, 235, 103–129. [Google Scholar] [CrossRef]
- Jin, D.; Wang, X.; Liu, L.; Liang, J.; Zhou, L. A novel approach for treating acid mine drainage through forming schwertmannite driven by a mixed culture of Acidiphilium multivorum and Acidithiobacillus ferrooxidans prior to lime neutralization. J. Hazard. Mater. 2020, 400, 123108. [Google Scholar] [CrossRef]
- Meng, X.; Yan, S.; Wu, W.; Zheng, G.; Zhou, L. Heterogeneous Fenton-like degradation of phenanthrene catalyzed by schwertmannite biosynthesized using Acidithiobacillus ferrooxidans. Rsc. Adv. 2017, 7, 21638–21648. [Google Scholar] [CrossRef] [Green Version]
- Wang, W.M.; Song, J.; Han, X. Schwertmannite as a new Fenton-like catalyst in the oxidation of phenol by H2O2. J. Hazard. Mater. 2013, 262, 412–419. [Google Scholar] [CrossRef]
- Cui, Q.; Li, Y.; Chai, S.; Zhang, W.; Zuo, Q.; He, C. Enhanced catalytic activation of H2O2 by CNTs/SCH through rapid Fe (III)/Fe (II) redox couple circulation: Insights into the role of functionalized multiwalled CNTs. Sep. Purif. Technol. 2022, 282, 120000. [Google Scholar] [CrossRef]
- Yang, Z.; Zhu, P.; Yan, C.; Wang, D.; Fang, D.; Zhou, L. Biosynthesized Schwertmannite@ Biochar composite as a heterogeneous Fenton-like catalyst for the degradation of sulfanilamide antibiotics. Chemosphere 2021, 266, 129175. [Google Scholar] [CrossRef]
- Guo, J.; Dong, C.; Zhang, J.; Lan, Y. Biogenic synthetic schwertmannite photocatalytic degradation of acid orange 7 (AO7) assisted by citric acid. Sep. Purif. Technol. 2015, 143, 27–31. [Google Scholar] [CrossRef]
- Duan, H.; Liu, Y.; Yin, X.; Bai, J.; Qi, J. Degradation of nitrobenzene by Fenton-like reaction in a H2O2/schwertmannite system. Chem. Eng. J. 2016, 283, 873–879. [Google Scholar] [CrossRef]
- Qiao, X.X.; Yu, K.; Xu, J.Y.; Cai, Y.L.; Li, Y.F.; Cao, H.L.; Lü, J. Engineered nanoscale schwertmannites as Fenton–like catalysts for highly efficient degradation of nitrophenols. Appl. Surf. Sci. 2021, 548, 149248. [Google Scholar] [CrossRef]
- Bigham, J.M.; Schwertmann, U.; Traina, S.J.; Winland, R.L.; Wolf, M. Schwertmannite and the chemical modeling of iron in acid sulfate waters. Geochim. Et Cosmochim. Acta 1996, 60, 2111–2121. [Google Scholar] [CrossRef]
- Liao, Y.; Zhou, L.; Liang, J.; Xiong, H. Biosynthesis of schwertmannite by Acidithiobacillus ferrooxidans cell suspensions under different pH condition. Mater. Sci. Eng. C 2009, 29, 211–215. [Google Scholar] [CrossRef]
- Fan, C.; Guo, C.; Zeng, Y.; Tu, Z.; Ji, Y.; Reinfelder, J.R.; Chen, M.; Huang, W.; Lu, G.; Yi, X.; et al. The behavior of chromium and arsenic associated with redox transformation of schwertmannite in AMD environment. Chemosphere 2019, 222, 945–953. [Google Scholar] [CrossRef]
- Jönsson, J.; Persson, P.; Sjöberg, S.; Lövgren, L. Schwertmannite precipitated from acid mine drainage: Phase transformation, sulphate release and surface properties. Appl. Geochem. 2005, 20, 179–191. [Google Scholar] [CrossRef]
- Wu, M.; Gong, Y.; Nie, T.; Zhang, J.; Wang, R.; Wang, H.; He, B. Template-free synthesis of nanocage-like gC3N4 with high surface area and nitrogen defects for enhanced photocatalytic activity. J. Mater. Chem. A 2019, 7, 5324–5332. [Google Scholar] [CrossRef]
- Mian, M.M.; Liu, G. Activation of peroxymonosulfate by chemically modified sludge biochar for the removal of organic pollutants: Understanding the role of active sites and mechanism. Chem. Eng. J. 2020, 392, 123681. [Google Scholar] [CrossRef]
- Leonard, N.; Ju, W.; Sinev, I.; Steinberg, J.; Luo, F.; Varela, A.S.; Cuenya, B.R.; Strasser, P. The chemical identity, state and structure of catalytically active centers during the electrochemical CO2 reduction on porous Fe–nitrogen–carbon (Fe–N–C) materials. Chem. Sci. 2018, 9, 5064–5073. [Google Scholar] [CrossRef] [Green Version]
- Huo, C.; Ouyang, J.; Yang, H. CuO nanoparticles encapsulated inside Al-MCM-41 mesoporous materials via direct synthetic route. Sci. Rep. 2014, 4, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Vradman, L.; Landau, M.V.; Kantorovich, D.; Koltypin, Y.; Gedanken, A. Evaluation of metal oxide phase assembling mode inside the nanotubular pores of mesostructured silica. Microporous Mesoporous Mater. 2005, 79, 307–318. [Google Scholar] [CrossRef]
- Yan, S.; Zheng, G.; Meng, X.; Zhou, L. Assessment of catalytic activities of selected iron hydroxysulphates biosynthesized using Acidithiobacillus ferrooxidans for the degradation of phenol in heterogeneous Fenton-like reactions. Sep. Purif. Technol. 2017, 185, 83–93. [Google Scholar] [CrossRef]
- Chen, H.; Gao, B.; Li, H. Removal of sulfamethoxazole and ciprofloxacin from aqueous solutions by graphene oxide. J. Hazard. Mater. 2015, 282, 201–207. [Google Scholar] [CrossRef]
- Tab, A.; Bellal, B.; Belabed, C.; Dahmane, M.; Trari, M. Visible light assisted photocatalytic degradation and mineralization of Rhodamine B in aqueous solution by Ag3PO4. Optik 2020, 214, 164858. [Google Scholar] [CrossRef]
- Daneshvar, N.; Salari, D.; Khataee, A.R. Photocatalytic degradation of azo dye acid red 14 in water on ZnO as an alternative catalyst to TiO2. J. Photochem. Photobiol. A Chem. 2004, 162, 317–322. [Google Scholar] [CrossRef]
- Mao, S.; Sun, X.; Qi, H.; Sun, Z. Cu2O nanoparticles anchored on 3D bifunctional CNTs/copper foam cathode for electrocatalytic degradation of sulfamethoxazole over a broad pH range. Sci. Total Environ. 2021, 793, 148492. [Google Scholar] [CrossRef]
- Oyekunle, D.T.; Gendy, E.A.; Ifthikar, J.; Chen, Z. Heterogeneous activation of persulfate by metal and non-metal catalyst for the degradation of sulfamethoxazole: A review. Chem. Eng. J. 2022, 437, 135277. [Google Scholar] [CrossRef]
- Nie, M.; Yan, C.; Xiong, X.; Wen, X.; Yang, X.; Dong, W. Degradation of chloramphenicol using a combination system of simulated solar light, Fe2+ and persulfate. Chem. Eng. J. 2018, 348, 455–463. [Google Scholar] [CrossRef]
- Shang, K.; Wang, X.; Li, J.; Wang, H.; Lu, N.; Jiang, N.; Wu, Y. Synergetic degradation of Acid Orange 7 (AO7) dye by DBD plasma and persulfate. Chem. Eng. J. 2017, 311, 378–384. [Google Scholar] [CrossRef]
- Zhou, Q.; Song, C.; Wang, P.; Zhao, Z.; Li, Y.; Zhan, S. Generating dual-active species by triple-atom sites through peroxymonosulfate activation for treating micropollutants in complex water. Proc. Natl. Acad. Sci. USA 2023, 120, e2300085120. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Xu, L.; Bai, X.; Jin, P. Enhanced visible-light activation of persulfate by Ti3+ self-doped TiO2/graphene nanocomposite for the rapid and efficient degradation of micropollutants in water. J. Hazard. Mater. 2019, 365, 107–117. [Google Scholar] [CrossRef] [PubMed]
- Mo, F.; Song, C.; Zhou, Q.; Xue, W.; Ouyang, S.; Wang, Q.; Wang, J. The optimized Fenton-like activity of Fe single-atom sites by Fe atomic clusters–mediated electronic configuration modulation. Proc. Natl. Acad. Sci. USA 2023, 120, e2300281120. [Google Scholar] [CrossRef] [PubMed]
- Burbano, A.A.; Dionysiou, D.D.; Suidan, M.T.; Richardson, T.L. Oxidation kinetics and effect of pH on the degradation of MTBE with Fenton reagent. Water Res. 2005, 39, 107–118. [Google Scholar] [CrossRef]
- Meng, X.; Wang, X.; Zhang, C.; Yan, S.; Zheng, G.; Zhou, L. Co-adsorption of As (III) and phenanthrene by schwertmannite and Fenton-like regeneration of spent schwertmannite to realize phenanthrene degradation and As (III) oxidation. Environ. Res. 2021, 195, 110855. [Google Scholar] [CrossRef]
- Meng, X.; Zhang, C.; Zhuang, J.; Zheng, G.; Zhou, L. Assessment of schwertmannite, jarosite and goethite as adsorbents for efficient adsorption of phenanthrene in water and the regeneration of spent adsorbents by heterogeneous fenton-like reaction. Chemosphere 2020, 244, 125523. [Google Scholar] [CrossRef]
- Regenspurg, S.; Brand, A.; Peiffer, S. Formation and stability of schwertmannite in acidic mining lakes. Geochim. Cosmochim. Acta 2004, 68, 1185–1197. [Google Scholar] [CrossRef]
- Xu, Z.; Liang, J.; Zhou, L. Photo-Fenton-like degradation of azo dye methyl orange using synthetic ammonium and hydronium jarosite. J. Alloys Compd. 2013, 546, 112–118. [Google Scholar] [CrossRef]



| Sample | Fe (wt.%) | S (wt.%) | Molar Ratio (Fe/S) | n | Formulae | Bet (m2/g) |
|---|---|---|---|---|---|---|
| Nano-SWT-0 | 46.88 | 6.67 | 7.03 | 1.14 | Fe8O8(OH)5.92(SO4)1.14 | 4.58 |
| Nano-SWT-0.1 | 39.73 | 5.34 | 7.44 | 1.08 | Fe8O8(OH)5.84(SO4)1.08 | 130.12 |
| Nano-SWT-0.25 | 30.08 | 4.17 | 7.21 | 1.11 | Fe8O8(OH)5.78(SO4)1.11 | 85.40 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Meng, X.; Wang, L.; Yang, Y.; Song, Y.; Yuan, C. Heterogeneous Fenton-like Catalyzation of Nanoscale Schwertmannite for Sulfamethoxazole Degradation. Coatings 2023, 13, 1097. https://doi.org/10.3390/coatings13061097
Meng X, Wang L, Yang Y, Song Y, Yuan C. Heterogeneous Fenton-like Catalyzation of Nanoscale Schwertmannite for Sulfamethoxazole Degradation. Coatings. 2023; 13(6):1097. https://doi.org/10.3390/coatings13061097
Chicago/Turabian StyleMeng, Xiaoqing, Lin Wang, Ying Yang, Yuqi Song, and Cansheng Yuan. 2023. "Heterogeneous Fenton-like Catalyzation of Nanoscale Schwertmannite for Sulfamethoxazole Degradation" Coatings 13, no. 6: 1097. https://doi.org/10.3390/coatings13061097
APA StyleMeng, X., Wang, L., Yang, Y., Song, Y., & Yuan, C. (2023). Heterogeneous Fenton-like Catalyzation of Nanoscale Schwertmannite for Sulfamethoxazole Degradation. Coatings, 13(6), 1097. https://doi.org/10.3390/coatings13061097





