Bimetallic 3D Nickel-Manganese/Titanium Bifunctional Electrocatalysts for Efficient Hydrogen and Oxygen Evolution Reaction in Alkaline and Acidic Media
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Fabrication of Catalysts
2.3. Characterization of Catalysts
2.4. Electrochemical Measurements
3. Results and Discussions
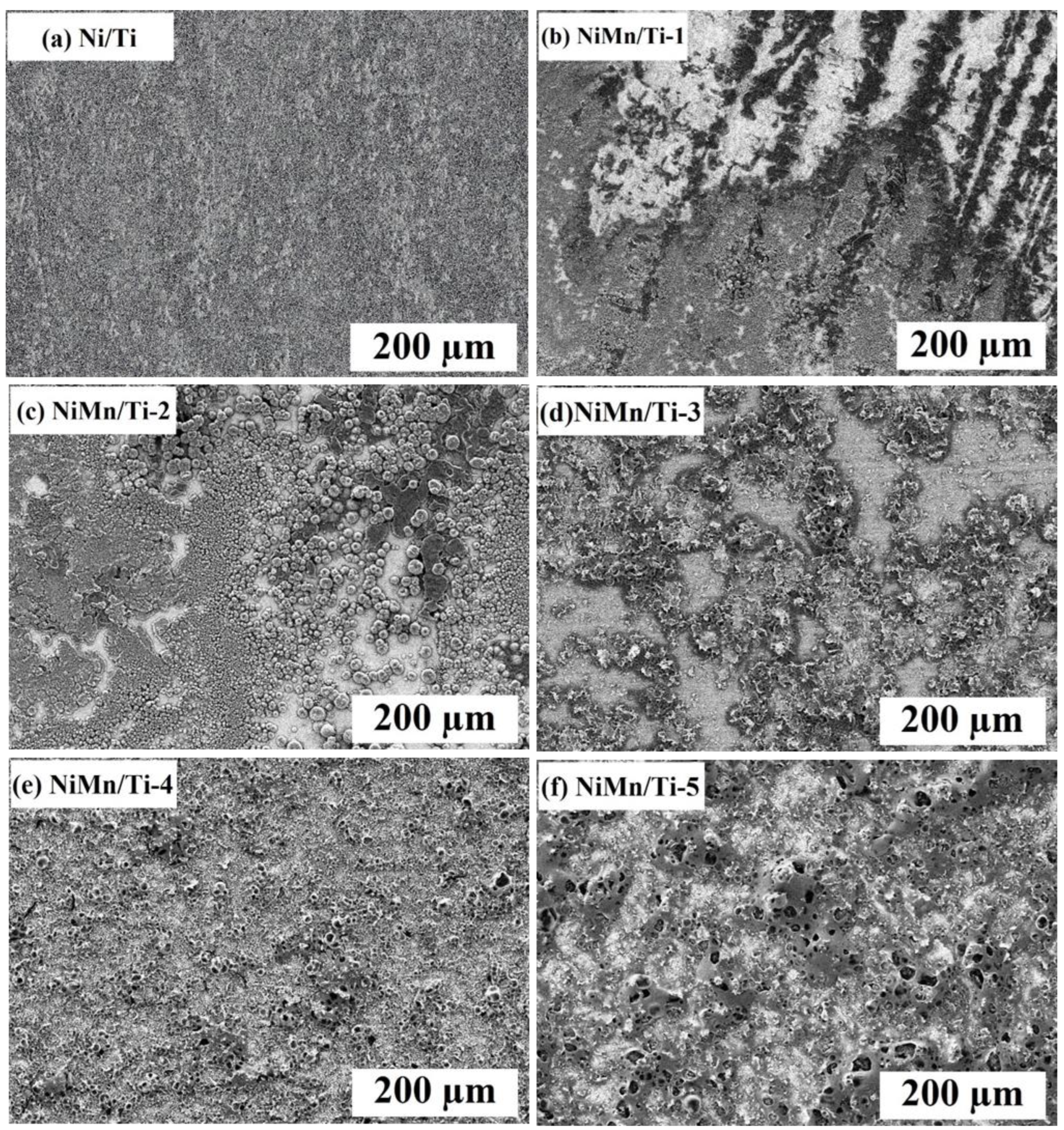
3.1. Microstructure and Morphology Studies
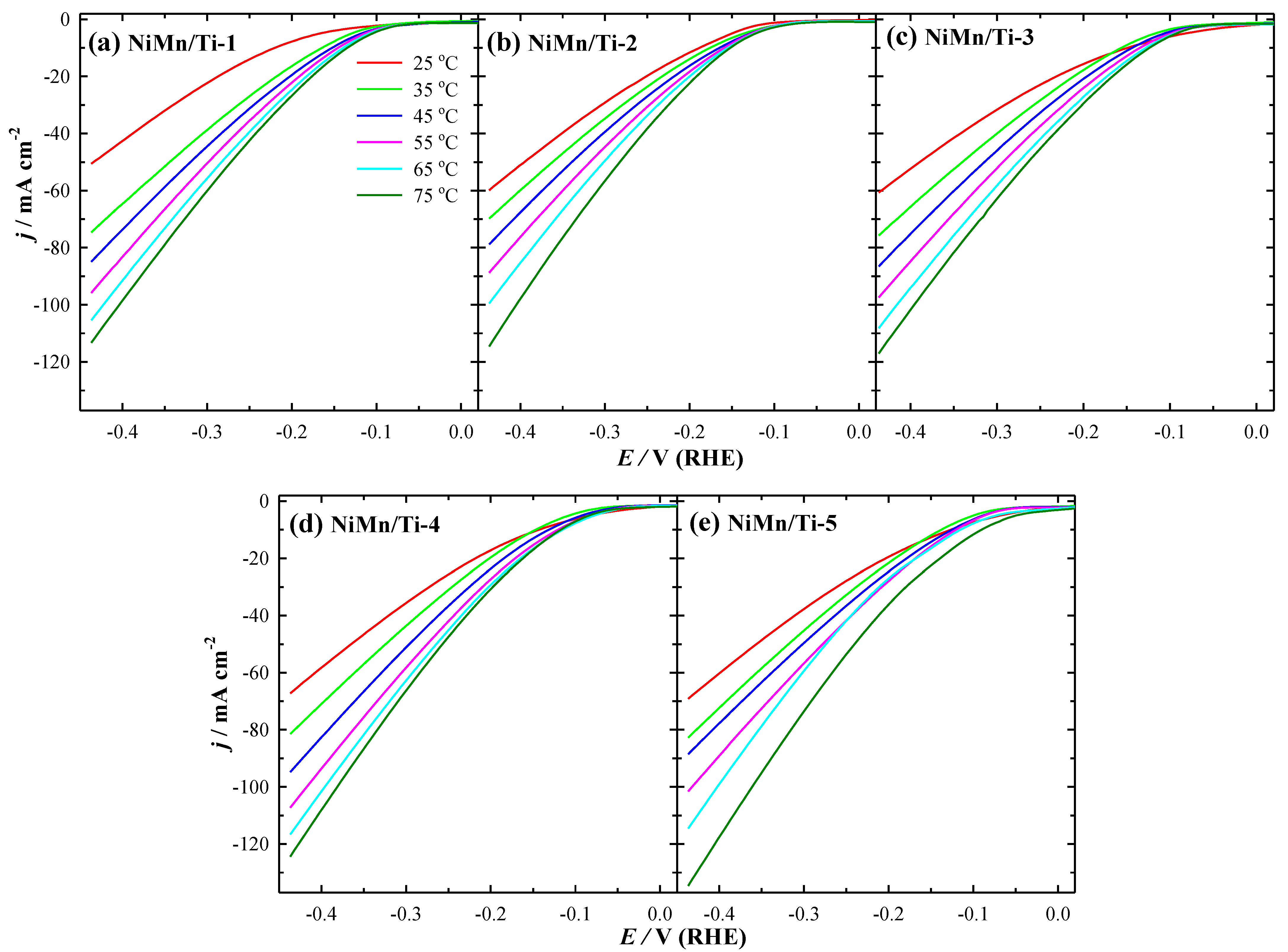
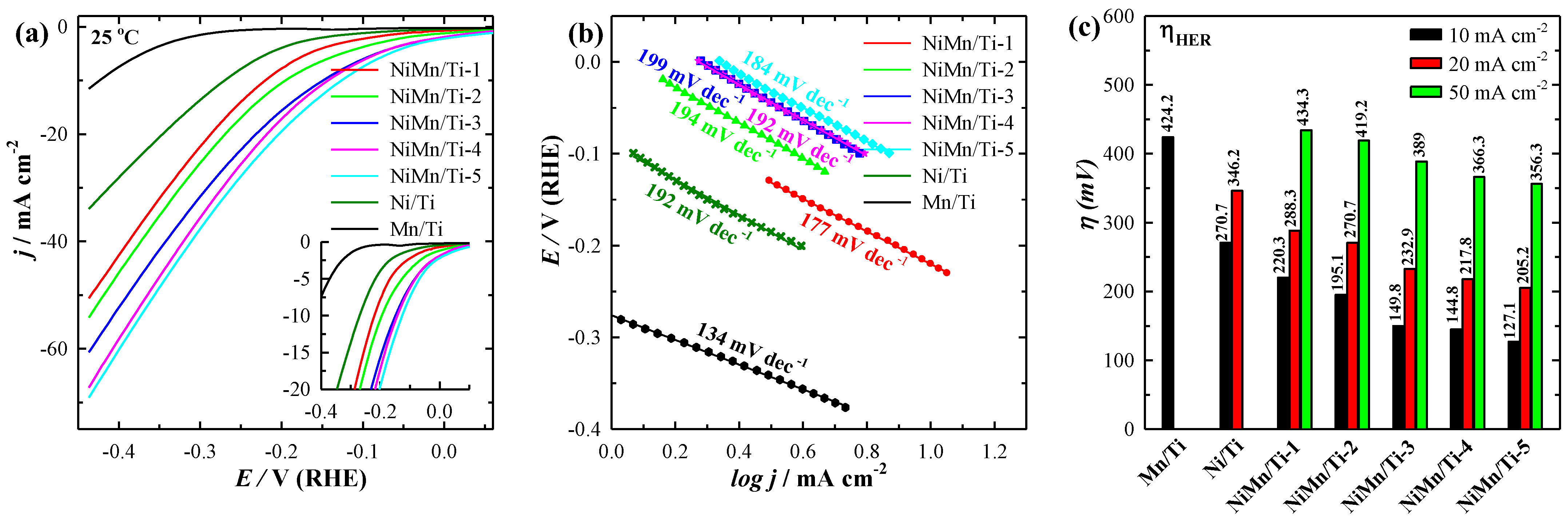
3.2. Electrocatalytic Activity towards HER
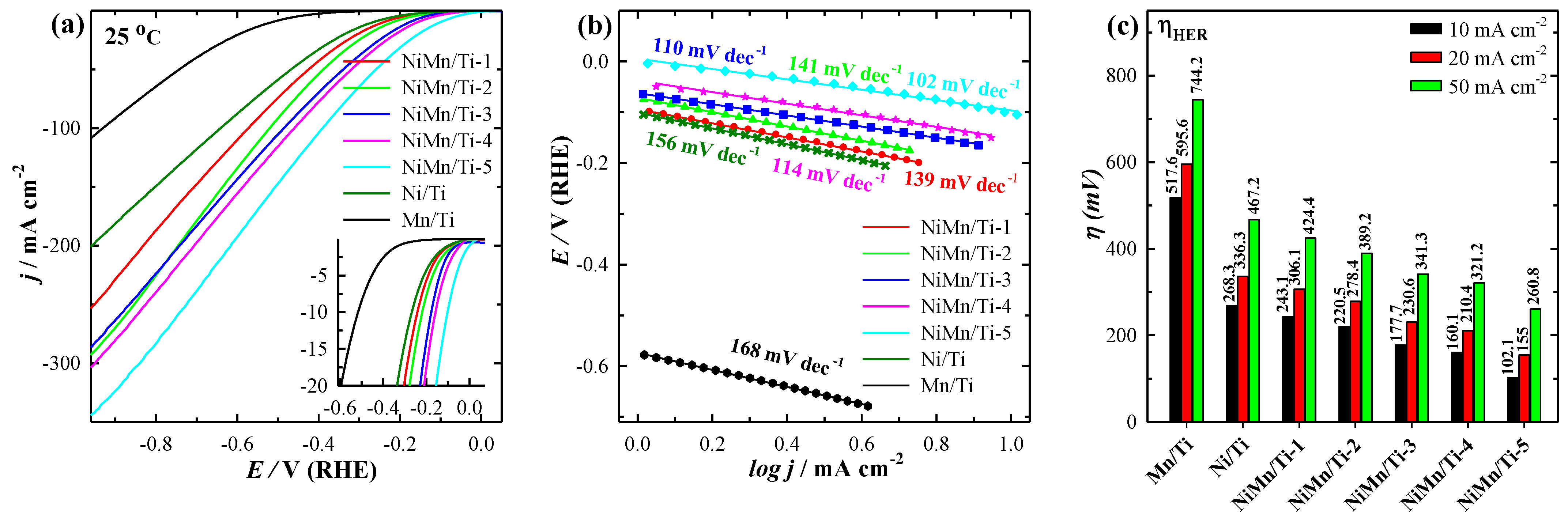
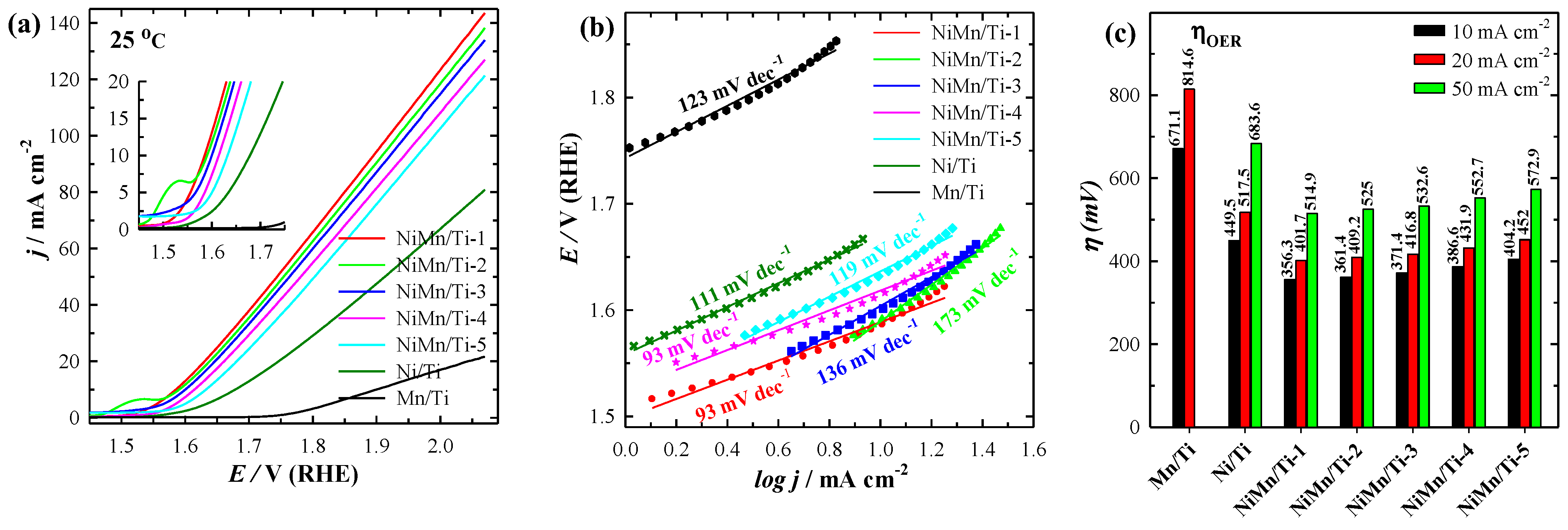
3.3. Electrocatalytic Activity towards OER
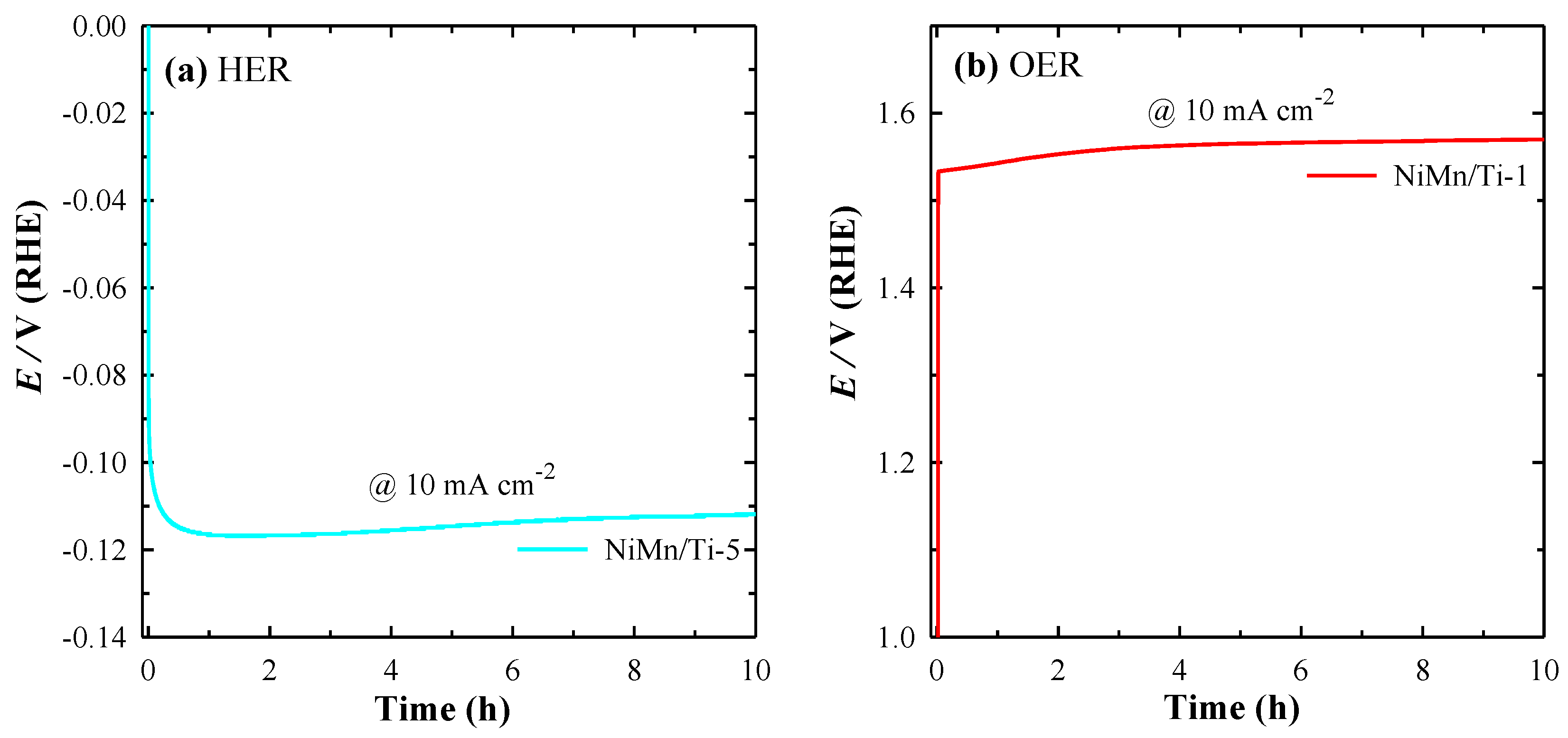
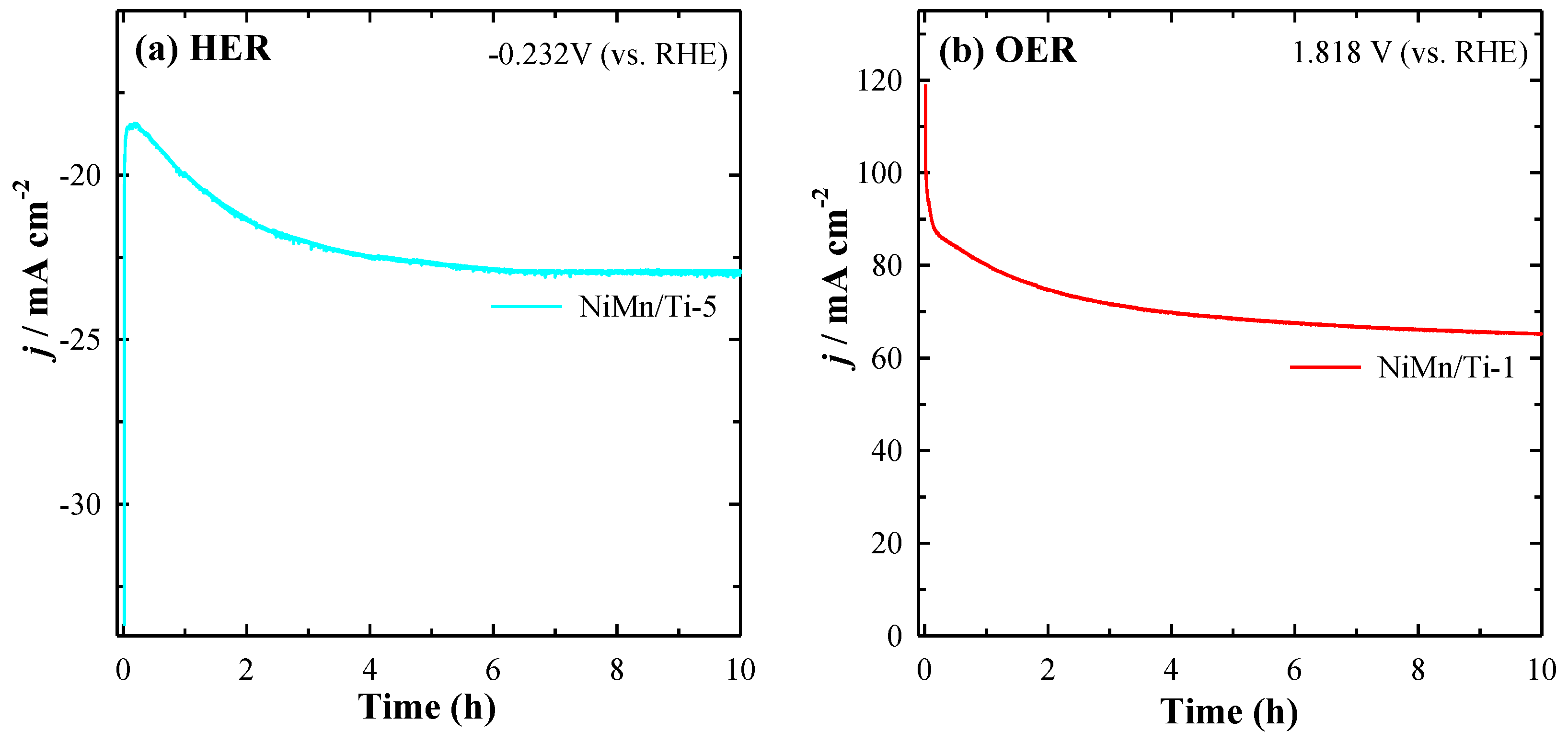
3.4. Electrocatalytic Stability Studies for HER and OER
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zhou, Z.; Pei, Z.; Wei, L.; Zhao, S.; Jian, X.; Chen, Y. Electrocatalytic hydrogen evolution under neutral pH conditions: Current understandings, recent advances, and future prospects. Energy Environ. Sci. 2020, 13, 3185–3206. [Google Scholar] [CrossRef]
- Zhu, L.; Li, C.; Li, H.; Li, H.; Wu, Z.; Huang, Y.; Zhu, X.; Sun, Y. Adjustable antiperovskite cobalt-based nitrides as efficient electrocatalysts for overall water splitting. J. Mater. Chem. A 2022, 10, 15520–15527. [Google Scholar] [CrossRef]
- Ali, A.; Long, F.; Shen, P.K. Innovative strategies for overall water splitting using nanostructured transition metal electrocatalysts. Electrochem. Energy Rev. 2022, 5, 1. [Google Scholar] [CrossRef]
- Yan, Y.; Wang, P.; Lin, J.; Cao, J.; Qi, J. Modification strategies on transition metal-based electrocatalysts for efficient water splitting. J. Energy Chem. 2021, 58, 446–462. [Google Scholar] [CrossRef]
- Cao, X.; Wang, T.; Jiao, L. Transition-metal (Fe, Co, and Ni)-based nanofiber electrocatalysts for water splitting. Adv. Fiber Mater. 2021, 3, 210–228. [Google Scholar] [CrossRef]
- Guo, Y.; Park, T.; Yi, J.W.; Henzie, J.; Kim, J.; Wang, Z.; Jiang, B.; Bando, Y.; Sugahara, Y.; Tang, J.; et al. Nanoarchitectonics for transition-metal-sulfide-based electrocatalysts for water splitting. Adv. Mater. 2019, 31, 1807134. [Google Scholar] [CrossRef]
- Yu, Y.; Zhou, J.; Sun, Z. Novel 2D Transition-Metal Carbides: Ultrahigh Performance Electrocatalysts for Overall Water Splitting and Oxygen Reduction. Adv. Funct. Mater. 2020, 30, 2000570. [Google Scholar] [CrossRef]
- Su, H.; Jiang, J.; Song, S.; An, B.; Li, N.; Gao, Y.; Ge, L. Recent progress on design and applications of transition metal chalcogenide-associated electrocatalysts for the overall water splitting. Chin. J. Catal. 2023, 44, 7–49. [Google Scholar] [CrossRef]
- Peng, X.; Yan, Y.; Jin, X.; Huang, C.; Jin, W.; Gao, B.; Chu, P.K. Recent advance and prospectives of electrocatalysts based on transition metal selenides for efficient water splitting. Nano Energy 2020, 78, 105234. [Google Scholar] [CrossRef]
- Ali, A.; Shen, P.K. Nonprecious metal’s graphene-supported electrocatalysts for hydrogen evolution reaction: Fundamentals to applications. Carbon Energy 2020, 2, 99–121. [Google Scholar] [CrossRef] [Green Version]
- Sun, H.; Xu, X.; Kim, H.; Jung, W.; Zhou, W.; Shao, Z. Electrochemical water splitting: Bridging the gaps between fundamental research and industrial applications. Energy Environ. Mater. 2022, 2022, e12441. [Google Scholar] [CrossRef]
- Fu, H.C.; Varadhan, P.; Tsai, M.L.; Li, W.; Ding, Q.; Lin, C.H.; Bonifazi, M.; Fratalocchi, A.; Jin, S.; He, J.H. Improved performance and stability of photoelectrochemical water-splitting Si system using a bifacial design to decouple light harvesting and electrocatalysis. Nano Energy 2020, 70, 104478. [Google Scholar] [CrossRef]
- Kumar, M.; Meena, B.; Subramanyam, P.; Suryakala, D.; Subrahmanyam, C. Recent trends in photoelectrochemical water splitting: The role of cocatalysts. NPG Asia Mater. 2022, 14, 88. [Google Scholar] [CrossRef]
- Wang, S.; Yang, P.; Sun, X.; Xing, H.; Hu, J.; Chen, P.; Cui, Z.; Zhu, W.; Ma, Z. Synthesis of 3D heterostructure Co-doped Fe2P electrocatalyst for overall seawater electrolysis. Appl. Catal. B Environ. 2021, 297, 120386. [Google Scholar] [CrossRef]
- Zhao, Y.; You, J.; Wang, L.; Bao, W.; Yao, R. Recent advances in Ni3S2-based electrocatalysts for oxygen evolution reaction. Int. J. Hydrogen Energy 2021, 46, 39146–39182. [Google Scholar] [CrossRef]
- Li, Y.; Sun, Y.; Qin, Y.; Zhang, W.; Wang, L.; Luo, M.; Yang, H.; Guo, S. Recent advances on water-splitting electrocatalysis mediated by noble-metal-based nanostructured materials. Adv. Energy Mater. 2020, 10, 1903120. [Google Scholar] [CrossRef]
- Xu, H.; Zhao, Y.; He, G.; Chen, H. Race on engineering noble metal single-atom electrocatalysts for water splitting. Int. J. Hydrogen Energy 2022, 47, 14257–14279. [Google Scholar] [CrossRef]
- Zhang, J.; Lian, J.; Jiang, Q.; Wang, G. Boosting the OER/ORR/HER activity of Ru-doped Ni/Co oxides heterostructure. Chem. Eng. J. 2022, 439, 135634. [Google Scholar] [CrossRef]
- Lin, S.; Yu, Y.; Sun, D.; Meng, F.; Chu, W.; Huang, L.; Ren, J.; Su, Q.; Ma, S.; Xu, B. FeNi2P three-dimensional oriented nanosheet array bifunctional catalysts with better full water splitting performance than the full noble metal catalysts. J. Colloid Interface Sci. 2022, 608, 2192–2202. [Google Scholar] [CrossRef]
- Sun, H.; Xu, X.; Song, Y.; Zhou, W.; Shao, Z. Designing high-valence metal sites for electrochemical water splitting. Adv. Funct. Mater. 2021, 31, 2009779. [Google Scholar] [CrossRef]
- Chatenet, M.; Pollet, B.G.; Dekel, D.R.; Dionigi, F.; Deseure, J.; Millet, P.; Braatz, R.D.; Bazant, M.Z.; Eikerling, M.; Staffell, I.; et al. Water electrolysis: From textbook knowledge to the latest scientific strategies and industrial developments. Chem. Soc. Rev. 2022, 51, 4583–4762. [Google Scholar] [CrossRef] [PubMed]
- Luo, W.; Wang, Y.; Cheng, C. Ru-based electrocatalysts for hydrogen evolution reaction: Recent research advances and perspectives. Mater. Today Phys. 2020, 15, 100274. [Google Scholar] [CrossRef]
- Gong, Y.; Yao, J.; Wang, P.; Li, Z.; Zhou, H.; Xu, C. Perspective of hydrogen energy and recent progress in electrocatalytic water splitting. Chin. J. Chem. Eng. 2022, 43, 282–296. [Google Scholar] [CrossRef]
- Darband, G.B.; Aliofkhazraei, M.; Rouhaghdam, A.S.; Kiani, M.A. Three-dimensional Ni-Co alloy hierarchical nanostructure as efficient non-noble-metal electrocatalyst for hydrogen evolution reaction. Appl. Surf. Sci. 2019, 465, 846–862. [Google Scholar] [CrossRef]
- Darband, G.B.; Aliofkhazraei, M.; Rouhaghdam, A.S. Facile electrodeposition of ternary Ni-Fe-Co alloy nanostructure as a binder free, cost-effective and durable electrocatalyst for high-performance overall water splitting. J. Colloid Interface Sci. 2019, 547, 407–420. [Google Scholar] [CrossRef]
- Nie, M.; Sun, H.; Gao, Z.D.; Li, Q.; Xue, Z.H.; Luo, J.; Liao, J.M. Co–Ni nanowires supported on porous alumina as an electrocatalyst for the hydrogen evolution reaction. Electrochem. Commun. 2020, 115, 106719. [Google Scholar] [CrossRef]
- Xu, Y.; Dong, X.; Miao, J.; Wang, S.; Liu, Z.; Zhai, Z.; Zhang, L.; Liu, Z. Facile preparation of Ni, Co-alloys supported on porous carbon spheres for supercapacitors and hydrogen evolution reaction application. Int. J. Hydrogen Energy 2020, 45, 1466–1476. [Google Scholar] [CrossRef]
- Ashraf, M.A.; Li, C.; Pham, B.T.; Zhang, D. Electrodeposition of Ni–Fe–Mn ternary nanosheets as affordable and efficient electrocatalyst for both hydrogen and oxygen evolution reactions. Int. J. Hydrogen Energy 2020, 45, 24670–24683. [Google Scholar] [CrossRef]
- Zhou, J.; Xiao, H.; Weng, W.; Gu, D.; Xiao, W. Interfacial confinement of Ni-V2O3 in molten salts for enhanced electrocatalytic hydrogen evolution. J. Energy Chem. 2020, 50, 280–285. [Google Scholar] [CrossRef]
- Browne, M.P.; Sofer, Z.; Pumera, M. Layered and two dimensional metal oxides for electrochemical energy conversion. Energy Environ. Sci. 2019, 12, 41–58. [Google Scholar] [CrossRef] [Green Version]
- Li, C.; Zhang, B.; Li, Y.; Hao, S.; Cao, X.; Yang, G.; Wu, J.; Huang, Y. Self-assembled Cu-Ni bimetal oxide 3D in-plane epitaxial structures for highly efficient oxygen evolution reaction. Appl. Catal. B Environ. 2019, 244, 56–62. [Google Scholar] [CrossRef]
- Yu, M.; Budiyanto, E.; Tüysüz, H. Principles of water electrolysis and recent progress in cobalt-, nickel-, and iron-based oxides for the oxygen evolution reaction. Angew. Chem. Int. Ed. 2022, 61, e202103824. [Google Scholar]
- Ma, X.; Wei, P.; Yang, Y.; Kang, H.; Guo, D.; Liu, L. One-pot synthesis of Ni-Co layered double hydroxide nanosheets as efficient catalysts for oxygen evolution reaction. Mater. Today Commun. 2019, 20, 100596. [Google Scholar] [CrossRef]
- Gao, G.; Wang, K.; Wang, X. Peony flower-like CuxS@NiMn LDH heterostructure as an efficient electrocatalyst for the oxygen evolution reaction. Int. J. Hydrogen Energy 2023, 48, 1347–1359. [Google Scholar] [CrossRef]
- Li, H.; Wang, X.; Wang, T.; Xiao, F. A facile, green and time-saving method to prepare partially crystalline NiFe layered double hydroxide nanosheets on nickel foam for superior OER catalysis. J. Alloys Compd. 2020, 844, 156224. [Google Scholar] [CrossRef]
- Zhu, W.; Chen, W.; Yu, H.; Zeng, Y.; Ming, F.; Liang, H.; Wang, Z. NiCo/NiCo–OH and NiFe/NiFe–OH core shell nanostructures for water splitting electrocatalysis at large currents. Appl. Catal. B Environ. 2020, 278, 119326. [Google Scholar] [CrossRef]
- Li, L.F.; Li, Y.F.; Liu, Z.P. Oxygen evolution activity on NiOOH catalysts: Four-coordinated Ni cation as the active site and the hydroperoxide mechanism. ACS Catal. 2020, 10, 2581–2590. [Google Scholar] [CrossRef]
- Ullah, H.; Loh, A.; Trudgeon, D.P.; Li, X. Density functional theory study of NiFeCo trinary oxy-hydroxides for an efficient and stable oxygen evolution reaction catalyst. ACS Omega 2020, 5, 20517–20524. [Google Scholar] [CrossRef]
- Vandichel, M.; Laasonen, K.; Kondov, I. Oxygen evolution and reduction on Fe-doped NiOOH: Influence of solvent, dopant position and reaction mechanism. Top. Catal. 2020, 63, 833–845. [Google Scholar] [CrossRef]
- Pi, C.; Huang, C.; Yang, Y.; Song, H.; Zhang, X.; Zheng, Y.; Gao, B.; Fu, J.; Chu, P.K.; Huo, K. In situ formation of N-doped carbon-coated porous MoP nanowires: A highly efficient electrocatalyst for hydrogen evolution reaction in a wide pH range. Appl. Catal. B Environ. 2020, 263, 118358. [Google Scholar] [CrossRef]
- Yan, Q.; Chen, X.; Wei, T.; Wang, G.; Zhu, M.; Zhuo, Y.; Cheng, K.; Ye, K.; Zhu, K.; Yan, J.; et al. Hierarchical edge-rich nickel phosphide nanosheet arrays as efficient electrocatalysts toward hydrogen evolution in both alkaline and acidic conditions. ACS Sustain. Chem. Eng. 2019, 7, 7804–7811. [Google Scholar] [CrossRef]
- Ma, B.; Yang, Z.; Chen, Y.; Yuan, Z. Nickel cobalt phosphide with three-dimensional nanostructure as a highly efficient electrocatalyst for hydrogen evolution reaction in both acidic and alkaline electrolytes. Nano Res. 2019, 12, 375–380. [Google Scholar] [CrossRef]
- Ge, R.; Huo, J.; Liao, T.; Liu, Y.; Zhu, M.; Li, Y.; Zhang, J.; Li, W. Hierarchical molybdenum phosphide coupled with carbon as a whole pH-range electrocatalyst for hydrogen evolution reaction. Appl. Catal. B Environ. 2020, 260, 118196. [Google Scholar] [CrossRef]
- Zhou, M.; Sun, Q.; Shen, Y.; Ma, Y.; Wang, Z.; Zhao, C. Fabrication of 3D microporous amorphous metallic phosphides for high-efficiency hydrogen evolution reaction. Electrochim. Acta 2019, 306, 651–659. [Google Scholar] [CrossRef]
- Zhu, L.; Liu, L.; Huang, G.; Zhao, Q. Hydrogen evolution over N-doped CoS2 nanosheets enhanced by superaerophobicity and electronic modulation. Appl. Surf. Sci. 2020, 504, 144490. [Google Scholar] [CrossRef]
- Guo, X.; Liu, Z.; Liu, F.; Zhang, J.; Zheng, L.; Hu, Y.; Mao, J.; Liu, H.; Xue, Y.; Tang, C. Sulfur vacancy-tailored NiCo2S4 nanosheet arrays for the hydrogen evolution reaction at all pH values. Catal. Sci. Technol. 2020, 10, 1056–1065. [Google Scholar] [CrossRef]
- Chen, S.; Liang, W.; Wang, X.; Zhao, Y.; Wang, S.; Li, Z.; Wang, S.; Hou, L.; Jiang, Y.; Gao, F. P-doped MOF-derived CoNi bimetallic sulfide electrocatalyst for highly-efficiency overall water splitting. J. Alloys Compd. 2023, 931, 167575. [Google Scholar] [CrossRef]
- Zahra, R.; Pervaiz, E.; Baig, M.M.; Rabi, O. Three-dimensional hierarchical flowers-like cobalt-nickel sulfide constructed on graphitic carbon nitride: Bifunctional non-noble electrocatalyst for overall water splitting. Electrochim. Acta 2022, 418, 140346. [Google Scholar] [CrossRef]
- Wang, J.; Shao, H.; Ren, S.; Hu, A.; Li, M. Fabrication of porous Ni-Co catalytic electrode with high performance in hydrogen evolution reaction. Appl. Surf. Sci. 2021, 539, 148045. [Google Scholar] [CrossRef]
- Pan, Q.Q.; Xu, C.Y.; Li, X.; Zhang, J.F.; Hu, X.L.; Geng, Y.; Su, Z.M. Porous Ni-Mo bimetallic hybrid electrocatalyst by intermolecular forces in precursors for enhanced hydrogen generation. Chem. Eng. J. 2021, 405, 126962. [Google Scholar] [CrossRef]
- Ros, C.; Murcia-López, S.; Garcia, X.; Rosado, M.; Arbiol, J.; Llorca, J.; Morante, J.R. Facing seawater splitting challenges by regeneration with Ni−Mo−Fe bifunctional electrocatalyst for hydrogen and oxygen evolution. ChemSusChem 2021, 14, 2872–2881. [Google Scholar] [CrossRef]
- Zhang, Z.; Wu, Y.; Zhang, D. Potentiostatic electrodeposition of cost-effective and efficient Ni–Fe electrocatalysts on Ni foam for the alkaline hydrogen evolution reaction. Int. J. Hydrogen Energy 2022, 47, 1425–1434. [Google Scholar] [CrossRef]
- Sun, J.; Yu, B.; Tan, F.; Yang, W.; Cheng, G.; Zhang, Z. High throughput preparation of Ni–Mo alloy thin films as efficient bifunctional electrocatalysts for water splitting. Int. J. Hydrogen Energy 2022, 47, 15764–15774. [Google Scholar] [CrossRef]
- Badrnezhad, R.; Nasri, F.; Pourfarzad, H.; Jafari, S.K. Effect of iron on Ni–Mo–Fe composite as a low-cost bifunctional electrocatalyst for overall water splitting. Int. J. Hydrogen Energy 2021, 46, 3821–3832. [Google Scholar] [CrossRef]
- Niu, J.; Yue, Y.; Yang, C.; Wang, Y.; Qin, J.; Zhang, X.; Wu, Z.S. Ultrarapid synthesis Ni-Cu bifunctional electrocatalyst by self-etching electrodeposition for high-performance water splitting reaction. Appl. Surf. Sci. 2021, 561, 150030. [Google Scholar] [CrossRef]
- Gao, W.; Zou, Y.; Zang, Y.; Zhao, X.; Zhou, W.; Dai, Y.; Liu, H.; Wang, J.J.; Ma, Y.; Sang, Y. Magnetic-field-regulated Ni-Fe-Mo ternary alloy electrocatalysts with enduring spin polarization enhanced oxygen evolution reaction. Chem. Eng. J. 2023, 455, 140821. [Google Scholar] [CrossRef]
- Yuan, H.; Zheng, S.; Sang, S.; Yang, J.; Sun, J.; Ma, Z.; Wang, X. Three-dimensional hierarchical nanoporous (Mn, Ni)-Doped Cu2S architecture towards high-efficiency overall water splitting. Int. J. Hydrogen Energy 2022, 47, 11827–11840. [Google Scholar] [CrossRef]
- Luo, J.; Guo, W.H.; Zhang, Q.; Wang, X.H.; Shen, L.; Fu, H.C.; Wu, L.L.; Chen, X.H.; Luo, H.Q.; Li, N.B. One-pot synthesis of Mn–Fe bimetallic oxide heterostructures as bifunctional electrodes for efficient overall water splitting. Nanoscale 2020, 12, 19992–20001. [Google Scholar] [CrossRef]
- Xu, P.; Qiu, L.; Wei, L.; Liu, Y.; Yuan, D.; Wang, Y.; Tsiakaras, P. Efficient overall water splitting over Mn doped Ni2P microflowers grown on nickel foam. Catal. Today 2020, 355, 815–821. [Google Scholar] [CrossRef]
- Li, Y.; Dong, Z.; Jiao, L. Multifunctional transition metal-based phosphides in energy-related electrocatalysis. Adv. Energy Mater. 2020, 10, 1902104. [Google Scholar] [CrossRef]
- Gbadamasi, S.; Mohiuddin, M.; Krishnamurthi, V.; Verma, R.; Khan, M.W.; Pathak, S.; Kalantar-Zadeh, K.; Mahmood, N. Interface chemistry of two-dimensional heterostructures–fundamentals to applications. Chem. Soc. Rev. 2021, 50, 4684–4729. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Tian, X.; Zhong, Z.; Kang, L.; Yao, J. In-situ growth of iron/nickel phosphides hybrid on nickel foam as bifunctional electrocatalyst for overall water splitting. J. Power Sources 2019, 424, 42–51. [Google Scholar] [CrossRef]
- Sahoo, D.P.; Das, K.K.; Mansingh, S.; Sultana, S.; Parida, K. Recent progress in first row transition metal Layered double hydroxide (LDH) based electrocatalysts towards water splitting: A review with insights on synthesis. Coord. Chem. Rev. 2022, 469, 214666. [Google Scholar] [CrossRef]
- Sumboja, A.; Chen, J.; Zong, Y.; Lee, P.S.; Liu, Z. NiMn layered double hydroxides as efficient electrocatalysts for the oxygen evolution reaction and their application in rechargeable Zn–air batteries. Nanoscale 2017, 9, 774–780. [Google Scholar] [CrossRef]
- Wang, P.; Qi, J.; Li, C.; Li, W.; Wang, T.; Liang, C. Hierarchical CoNi2S4@NiMn-layered double hydroxide heterostructurenanoarrays on superhydrophilic carbon cloth for enhanced overall water splitting. Electrochim. Acta 2020, 345, 136247. [Google Scholar] [CrossRef]
- Wang, P.; Qi, J.; Chen, X.; Li, C.; Li, W.; Wang, T.; Liang, C. Three-dimensional heterostructured NiCoP@NiMn-layered double hydroxide arrays supported on Ni foam as a bifunctional electrocatalyst for overall water splitting. ACS Appl. Mater. Interfaces 2019, 12, 4385–4395. [Google Scholar] [CrossRef]
- Yang, L.; Chen, L.; Yang, D.; Yu, X.; Xue, H.; Feng, L. NiMn layered double hydroxide nanosheets/NiCo2O4 nanowires with surface rich high valence state metal oxide as an efficient electrocatalyst for oxygen evolution reaction. J. Power Sources 2018, 392, 23–32. [Google Scholar] [CrossRef]
- Wang, Y.; Liu, X.; Zhang, N.; Qiu, G.; Ma, R. Cobalt-doped Ni–Mn layered double hydroxide nanoplates as high-performance electrocatalyst for oxygen evolution reaction. Appl. Clay Sci. 2018, 165, 277–283. [Google Scholar] [CrossRef]
- Khan, R.; Mehran, M.T.; Baig, M.M.; Sarfraz, B.; Naqvi, S.R.; Niazi, M.B.K.; Khan, M.Z.; Khoja, A.H. 3D hierarchical Heterostructured LSTN@NiMn-layered double hydroxide as a bifunctional water splitting electrocatalyst for hydrogen production. Fuel 2021, 285, 119174. [Google Scholar] [CrossRef]
- Jiang, D.; Ma, W.; Yang, R.; Quan, B.; Li, D.; Meng, S.; Chen, M. Nickel–manganese bimetallic phosphides porous nanosheet arrays as highly active bifunctional hydrogen and oxygen evolution electrocatalysts for overall water splitting. Electrochim. Acta 2020, 329, 135121. [Google Scholar] [CrossRef]
- Zhang, G.; Ge, H.; Zhao, L.; Liu, J.; Wang, F.; Fan, S.; Li, G. NiMn1.5PO4 thin layer supported on Ni foam as a highly efficient bifunctional electrocatalyst for overall water splitting. Electrochim. Acta 2021, 367, 137567. [Google Scholar] [CrossRef]
- Maleki, M.; Darband, G.B.; Rouhaghdam, A.S.; Andaveh, R.; Kazemi, Z.M. Mn-incorporated nickel selenide: An ultra-active bifunctional electrocatalyst for hydrogen evolution and urea oxidation reactions. Chem. Commun. 2022, 58, 3545–3548. [Google Scholar] [CrossRef] [PubMed]
- Taherinia, D.; Hatami, H.; Valadi, F.M. Trimetallic Co-Ni-Mn metal-organic framework as an efficient electrocatalyst for alkaline oxygen evolution reaction. J. Electroanal. Chem. 2022, 922, 116720. [Google Scholar] [CrossRef]
- Luo, L.; Huang, H.; Yang, Y.; Gong, S.; Li, Y.; Wang, Y.; Luo, W.; Li, Z. Nickel and manganese oxide heterostructure nanoparticles supported by carbon nanotube for highly efficient oxygen evolution reaction catalysis. Appl. Surf. Sci. 2022, 575, 151699. [Google Scholar] [CrossRef]
- Luo, Y.; Wang, P.; Zhang, G.; Wu, S.; Chen, Z.; Ranganathan, H.; Sun, S.; Shi, Z. Mn-doped nickel–iron phosphide heterointerface nanoflowers for efficient alkaline freshwater/seawater splitting at high current densities. Chem. Eng. J. 2023, 454, 140061. [Google Scholar] [CrossRef]
- Huang, H.; Hu, X.; Hou, Z.; Yang, D.; Xiang, D.; Hu, L. Interfacial construction and lattice distortion-triggered bifunctionality of Mn-NiS/Mn-Ni3S4 for H2 production. Fuel 2022, 328, 125337. [Google Scholar] [CrossRef]
- Hatami, E.; Toghraei, A.; Darband, G.B. Electrodeposition of Ni–Fe micro/nano urchin-like structure as an efficient electrocatalyst for overall water splitting. Int. J. Hydrogen Energy 2021, 46, 9394–9405. [Google Scholar] [CrossRef]
- Kim, C.; Lee, S.; Kim, S.H.; Park, J.; Kim, S.; Kwon, S.H.; Bae, J.S.; Park, Y.S.; Kim, Y. Cobalt–iron–phosphate hydrogen evolution reaction electrocatalyst for solar-driven alkaline seawater electrolyzer. Nanomataterials 2021, 11, 2989. [Google Scholar] [CrossRef]
- Sun, Y.Y.; Jiang, M.Y.; Wu, L.K.; Hou, G.Y.; Tang, Y.P.; Liu, M. Ultra-thin NiFeSe nanosheets as a highly efficient bifunctional electrocatalyst for overall water splitting. Sustain. Energy Fuels 2020, 4, 582–588. [Google Scholar] [CrossRef]
- Zhu, M.; Bai, X.; Yan, Q.; Yan, Y.; Zhu, K.; Ye, K.; Yan, J.; Cao, D.; Huang, X.; Wang, G. Iron molybdenum selenide supported on reduced graphene oxide as an efficient hydrogen electrocatalyst in acidic and alkaline media. J. Colloid Interface Sci. 2021, 602, 384–393. [Google Scholar] [CrossRef]
- Laszczyńska, A.; Tylus, W.; Szczygieł, I. Electrocatalytic properties for the hydrogen evolution of the electrodeposited Ni–Mo/WC composites. Int. J. Hydrogen Energy 2021, 46, 22813–22831. [Google Scholar] [CrossRef]
- Dai, Z.; Du, X.; Zhang, X. The synthesis of Ni-Co-Fe-Se@NiCo-LDH nanoarrays on Ni foam as efficient overall water splitting electrocatalyst. J. Alloys Compd. 2023, 946, 169451. [Google Scholar] [CrossRef]
- Van Phuc, T.; Jana, J.; Ravi, N.; Kang, S.G.; Chung, J.S.; Choi, W.M.; Hur, S.H. Highly active Ni/Co-metal organic framework bifunctional electrocatalyst for water splitting reaction. Int. J. Hydrogen Energy 2022, 47, 22787–22795. [Google Scholar] [CrossRef]
- Bao, F.; Kemppainen, E.; Dorbandt, I.; Bors, R.; Xi, F.; Schlatmann, R.; van de Krol, R.; Calnan, S. Understanding the Hydrogen Evolution Reaction Kinetics of Electrodeposited Nickel-Molybdenum in Acidic, Near-Neutral, and Alkaline Conditions. ChemElectroChem 2021, 8, 195–208. [Google Scholar] [CrossRef]
- Hussain, S.; Rabani, I.; Vikraman, D.; Feroze, A.; Karuppasamy, K.; Haq, Z.U.; Seo, Y.S.; Chun, S.H.; Kim, H.S.; Jung, J. Hybrid design using carbon nanotubes decorated with Mo2C and W2C nanoparticles for supercapacitors and hydrogen evolution reactions. ACS Sustain. Chem. Eng. 2020, 8, 12248–12259. [Google Scholar] [CrossRef]
- Yang, C.; Zhao, R.; Xiang, H.; Wu, J.; Zhong, W.; Li, W.; Zhang, Q.; Yang, N.; Li, X. Ni-activated transition metal carbides for efficient hydrogen evolution in acidic and alkaline solutions. Adv. Energy Mater. 2020, 10, 2002260. [Google Scholar] [CrossRef]
- Caliskan, S.; Wang, A.; Qin, F.; House, S.D.; Lee, J.K. Molybdenum Carbide-Reduced Graphene Oxide Composites as Electrocatalysts for Hydrogen Evolution. ACS Appl. Nano Mater. 2022, 5, 3790–3798. [Google Scholar] [CrossRef]
- Sun, Y.; Peng, F.; Zhang, L.; Jiang, B.; Dou, H.; Zhang, N.; Xu, M.; Yang, N. Hierarchical Nitrogen-doped Mo2C Nanoparticle-in-microflower Electrocatalyst: In Situ Synthesis and Efficient Hydrogen-evolving Performance in Alkaline and Acidic Media. ChemCatChem 2020, 12, 6040–6049. [Google Scholar] [CrossRef]
- Eiler, K.; Suriñach, S.; Sort, J.; Pellicer, E. Mesoporous Ni-rich Ni–Pt thin films: Electrodeposition, characterization and performance toward hydrogen evolution reaction in acidic media. Appl. Catal. B Environ. 2020, 265, 118597. [Google Scholar] [CrossRef]
- El-Refaei, S.M.; Russo, P.A.; Schultz, T.; Koch, N.; Pinna, N. Dual doping of MoP with M (Mn, Fe) and S to achieve high hydrogen evolution reaction activity in both acidic and alkaline media. ChemCatChem 2021, 13, 4392–4402. [Google Scholar] [CrossRef]
- Song, H.; Guo, S.; Zhang, X.; Yang, Y.; Gao, B.; Pi, Y.; Pi, C.; Chu, P.K.; Huo, K. In-Situ and controllable construction of Mo2N embedded Mo2C nanobelts as robust electrocatalyst for superior pH-universal hydrogen evolution reaction. J. Alloys Compd. 2022, 918, 165611. [Google Scholar] [CrossRef]
- Chen, T.; Qian, M.; Tong, X.; Liao, W.; Fu, Y.; Dai, H.; Yang, Q. Nanosheet self-assembled NiCoP microflowers as efficient bifunctional catalysts (HER and OER) in alkaline medium. Int. J. Hydrogen Energy 2021, 46, 29889–29895. [Google Scholar] [CrossRef]
- Bhat, K.S.; Nagaraja, H.S. In situ synthesis of copper sulfide-nickel sulfide arrays on three-dimensional nickel foam for overall water splitting. ChemistrySelect 2020, 5, 2455–2464. [Google Scholar] [CrossRef]
- Li, Z.; Wang, X.; Wang, X.; Lin, Y.; Meng, A.; Yang, L.; Li, Q. Mn-Cd-S@amorphous-Ni3S2 hybrid catalyst with enhanced photocatalytic property for hydrogen production and electrocatalytic OER. Appl. Surf. Sci. 2019, 491, 799–806. [Google Scholar] [CrossRef]
- Li, B.; Li, Z.; He, F.; Pang, Q.; Shen, P. One-pot preparation of Ni3S2@3-D graphene free-standing electrode by simple Q-CVD method for efficient oxygen evolution reaction. Int. J. Hydrogen Energy 2019, 44, 30806–30819. [Google Scholar] [CrossRef]

| Catalysts | Concentration (M) | Plating Conditions | ||||
|---|---|---|---|---|---|---|
| NiSO4 6H2O | MnCl2 4H2O | (NH4)2SO4 | H3BO3 | Parameters | Values | |
| Ni/Ti | 0.2 | - | 0.5 | 0.3 | Current densities | 50 mA cm−2 |
| Mn/Ti | - | 1.0 | 0.5 | 0.3 | 500 mA cm−2 | |
| NiMn/Ti-1 | 0.2 | 0.2 | 0.5 | 0.3 | Time | 3 min |
| NiMn/Ti-2 | 0.2 | 0.4 | 0.5 | 0.3 | ||
| NiMn/Ti-3 | 0.2 | 0.6 | 0.5 | 0.3 | Temperature | 25 °C |
| NiMn/Ti-4 | 0.2 | 0.8 | 0.5 | 0.3 | ||
| NiMn/Ti-5 | 0.2 | 1.0 | 0.5 | 0.3 | pH | ~1 |
| Catalyst | Ni Loading (µgNicm−2) | Mn Loading (µgMncm−2) | Total Metal Loading (µgmetalcm−2) | Wt.% | |
|---|---|---|---|---|---|
| Ni | Mn | ||||
| Mn/Ti | - | 21.5 | 21.5 | - | 100 |
| Ni/Ti | 300.25 | - | 300.25 | 100 | - |
| NiMn/Ti-1 | 86.55 | 13.43 | 99.98 | 86.56 | 13.44 |
| NiMn/Ti-2 | 126.4 | 40.55 | 166.95 | 75.71 | 24.29 |
| NiMn/Ti-3 | 269.7 | 105.25 | 374.95 | 71.93 | 28.07 |
| NiMn/Ti-4 | 448.45 | 374.4 | 822.85 | 54.49 | 45.51 |
| NiMn/Ti-5 | 538 | 685.5 | 1223.5 | 43.97 | 56.03 |
| Catalysts | j (mA cm−2) in Different Temperatures (°C) at −0.432 V(vs. RHE) | j (mA µg−1) at 25 °C | η10 (mV) at 25 °C | Tafel Slope (mV dec−1) | |||||
|---|---|---|---|---|---|---|---|---|---|
| 25 | 35 | 45 | 55 | 65 | 75 | ||||
| Mn/Ti | 11.53 | - | - | - | - | - | 0.54 | 424.2 | 134 |
| Ni/Ti | 33.95 | - | - | - | - | - | 0.11 | 270.7 | 192 |
| NiMn/Ti-1 | 50.58 | 74.57 | 84.92 | 95.81 | 105.39 | 113.28 | 0.51 | 220.3 | 177 |
| NiMn/Ti-2 | 59.85 | 69.79 | 78.84 | 88.75 | 99.49 | 114.56 | 0.36 | 195.1 | 194 |
| NiMn/Ti-3 | 60.65 | 75.68 | 86.56 | 97.46 | 108.15 | 117.03 | 0.16 | 149.8 | 199 |
| NiMn/Ti-4 | 67.25 | 81.47 | 94.82 | 107.25 | 116.65 | 124.41 | 0.08 | 144.8 | 192 |
| NiMn/Ti-5 | 69.12 | 82.75 | 88.56 | 101.6 | 114.59 | 134.67 | 0.06 | 127.1 | 184 |
| Catalysts | j (mA cm−2) at 25 °C | j (mA µg−1) at 25 °C | η10 (mV) at 25 °C | Tafel Slope (mV dec−1) |
|---|---|---|---|---|
| Mn/Ti | 108.67 | 5.05 | 517.6 | 168 |
| Ni/Ti | 201.13 | 0.67 | 268.3 | 156 |
| NiMn/Ti-1 | 253.59 | 2.54 | 243.1 | 139 |
| NiMn/Ti-2 | 293.17 | 1.76 | 220.5 | 141 |
| NiMn/Ti-3 | 286.79 | 0.77 | 177.7 | 110 |
| NiMn/Ti-4 | 303.79 | 0.37 | 160.1 | 114 |
| NiMn/Ti-5 | 344.59 | 0.28 | 102.1 | 102 |
| Catalysts | j (mA cm−2) in Different Temperatures (°C) at 2.068 V | j (mA µg−1) at 25 °C | η10 (mV) at 25 °C | Tafel Slope (mV·dec−1) | |||||
|---|---|---|---|---|---|---|---|---|---|
| 25 | 35 | 45 | 55 | 65 | 75 | ||||
| Mn/Ti | 21.64 | - | - | - | - | - | 1.0 | 671.1 | 123 |
| Ni/Ti | 80.89 | - | - | - | - | - | 0.27 | 449.5 | 111 |
| NiMn/Ti-1 | 143.54 | 177.03 | 188.96 | 216.81 | 253.04 | 316.7 | 1.44 | 356.3 | 93 |
| NiMn/Ti-2 | 138.28 | 170.03 | 195.53 | 232.16 | 262.74 | 289.38 | 0.83 | 361.4 | 173 |
| NiMn/Ti-3 | 133.94 | 148.77 | 172.7 | 198.04 | 223.88 | 249.68 | 0.36 | 371.4 | 136 |
| NiMn/Ti-4 | 126.97 | 140.93 | 160.16 | 181.08 | 202.56 | 224.84 | 0.15 | 386.6 | 93 |
| NiMn/Ti-5 | 121.35 | 134.16 | 153.26 | 172.24 | 194.09 | 219.53 | 0.1 | 404.2 | 119 |
| Catalysts | Overpotential@Current Density (mV@mA cm−2) | Tafel Slope (mV dec−1) | Temperature (°C) | Electrolyte | Ref. |
|---|---|---|---|---|---|
| Ni-Mn/Cu | 101@10 | 118 | - | 1 M KOH | [28] |
| Ni-Fe-Mn/Cu | 64@10 | 68 | |||
| Ni-Fe/NF | 142@10 | 133.3 | - | 1 M KOH | [52] |
| Ni1Mn1P | 160@10 | 109 | - | 1 M KOH | [70] |
| Ni2Mn1P | 120@10 | 82 | |||
| Ni3Mn1P | 140@10 | 93 | |||
| Mn-doped Ni2P | 160@10 | 124.27 | - | 1 M KOH | [75] |
| Mn-doped Fe2P | 136@10 | 142.34 | |||
| Mn-doped Ni2P/Fe2P | 90@10 | 115.41 | |||
| Mn-Ni(OH)2 | 197@10 | 134.5 | - | 1 M KOH | [76] |
| NiSx | 172@10 | 111.9 | |||
| Mn-NiSx | 94.2@10 | 71.5 | |||
| Ni-Fe/Cu | 124@10 | 114 | - | 1 M KOH | [77] |
| (Co,Fe)PO4 | 122@10 | 71 | - | 1 M KOH | [78] |
| NiFe10Se10@NF | 154@10 | 129.3 | - | 1 M KOH | [79] |
| FeSe2-MoSe2(1-1)/rGO | 178@10 | 80 | 1 M KOH | [80] | |
| Ni–Mo/WC 3 | 134@10 | 163 | 25 | 1 M KOH | [81] |
| Ni-Co-Se@NiCo-LDH/NF | 189@10 | 124.09 | - | 1 M KOH | [82] |
| Ni-Co-Fe-Se@NiCo-LDH/NF | 113@10 | 44.87 | |||
| NC-1@CoO/NF | 241@10 | 155 | - | 1 M KOH | [83] |
| NC-2@CoO/NF | 139@10 | 96 | |||
| NC-3@CoO/NF | 192@10 | 189 | |||
| NC-4@CoO/NF | 196@10 | 141 | |||
| NiMo/FTO | 154@10 | 152 | 25 | 1 M KOH | [84] |
| W2C@CNT | 125@10 | 104 | - | 1 M KOH | [85] |
| Mo2C@CNT | 118@10 | 92 | |||
| Ni-GF/VC | 128@10 | 80 | - | 1 M KOH | [86] |
| Ni-GF/Fe3C | 93@10 | 63 | |||
| NiMn/Ti-1 | 220.3@10 | 177 | 25 | 1 M KOH | This work |
| NiMn/Ti-2 | 195.1@10 | 194 | |||
| NiMn/Ti-3 | 149.8@10 | 199 | |||
| NiMn/Ti-4 | 144.8@10 | 192 | |||
| NiMn/Ti-5 | 127.1@10 | 184 | |||
| NiMn/Ti-1 | 243.1@10 | 139 | 25 | 0.5 M H2SO4 | This Work |
| NiMn/Ti-2 | 220.5@10 | 141 | |||
| NiMn/Ti-3 | 177.7@10 | 110 | |||
| NiMn/Ti-4 | 160.1@10 | 114 | |||
| NiMn/Ti-5 | 102.1@10 | 102 | |||
| NiMo/FTO | 140@20 | 118 | 25 | 0.5 M H2SO4 | [84] |
| W2C@CNT | 155@10 | 85 | - | 0.5 M H2SO4 | [85] |
| Mo2C@CNT | 121@10 | 77 | |||
| Ni-GF/VC | 111@10 | 86 | - | 0.5 M H2SO4 | [86] |
| Ni-GF/Fe3C | 112@10 | 97 | |||
| Mo2C-RGO (3.0 wt %) | 125@10 | 89 | 25 | 0.5 M H2SO4 | [87] |
| Mo2C P/Mo2C F | 118@10 | 48.6 | - | 0.5 M H2SO4 | [88] |
| Ni-Pt nanofilm | 90@10 | 49 | 25 | 0.5 M H2SO4 | [89] |
| MoP | 115@10 | 87 | - | 0.5 M H2SO4 | [90] |
| FeS-MoP | 89@10 | 70 | |||
| MnS-MoP | 88@10 | 68 | |||
| Mo2N-Mo2C/NC | 114@10 | 62 | - | 0.5 M H2SO4 | [91] |
| Catalysts | Overpotential@Current Density (mV@mA cm−2) | Tafel Slope (mV dec−1) | Electrolyte | Ref. |
|---|---|---|---|---|
| CoNi2S4 (GCN)30/NF | 340@30 | 93.21 | 1.0 M KOH | [48] |
| CoNi2S4 (GCN)50/NF | 310@30 | 49.86 | ||
| CoNi2S4 (GCN)100/NF | 350@30 | 109.01 | ||
| NMF-6 (Ni52.3Mo37.4Fe10.2) | 344@10 | - | 1.0 M KOH | [54] |
| Ni1Mn1 LDH | 420@10 | 41 | 1.0 M KOH | [64] |
| Ni3Mn1 LDH | 350@10 | 40 | ||
| Ni5Mn1 LDH | 390@10 | 40 | ||
| NiMn LDH/NiCo2O4 | 310@10 | 99 | 1.0 M KOH | [67] |
| Ni-Mn LDH | 385@10 | 80 | 1.0 M KOH | [68] |
| 21.1% Co-doped Ni-Mn LDH | 310@10 | 59 | ||
| Ni1Mn1P | 250@20 | 63 | 1.0 M KOH | [70] |
| Ni2Mn1P | 340@20 | 93 | ||
| Ni3Mn1P | 330@20 | 89 | ||
| Ni0.95|Mn0.05O/CNT | 293@10 | 55.6 | 1.0 M KOH | [74] |
| Ni0.83|Mn0.17O/CNT | 316@10 | 63.5 | ||
| NC-1@CoO/NF | 340@10 | 93 | 1.0 M KOH | [83] |
| NC-2@CoO/NF | 290@10 | 82 | ||
| NC-3@CoO/NF | 335@10 | 127 | ||
| NC-4@CoO/NF | 370@10 | 91 | ||
| Ni1.5Co1.5P/MF | 314@10 | 71 | 1.0 M KOH | [92] |
| Ni2Co1P/MF | 342@10 | 83 | ||
| Ni1Co2P/MF | 387@10 | 114 | ||
| Ni3S2/NF | 362@10 | 56.5 | 1.0 M KOH | [93] |
| Cu2S-Ni3S2/NF | 329@10 | 44.11 | ||
| MCS@a-Ni3S2 | 333@10 | 150.1 | 1.0 M KOH | [94] |
| Ni3S2@3-D GNs | 305@10 | 50 | 1.0 M KOH | [95] |
| NiMn/Ti-1 | 356.3@10 | 93 | 1.0 M KOH at 25 °C | This work |
| NiMn/Ti-2 | 361.4@10 | 173 | ||
| NiMn/Ti-3 | 371.4@10 | 136 | ||
| NiMn/Ti-4 | 386.6@10 | 93 | ||
| NiMn/Ti-5 | 404.2@10 | 119 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Barua, S.; Balčiūnaitė, A.; Vaičiūnienė, J.; Tamašauskaitė-Tamašiūnaitė, L.; Norkus, E. Bimetallic 3D Nickel-Manganese/Titanium Bifunctional Electrocatalysts for Efficient Hydrogen and Oxygen Evolution Reaction in Alkaline and Acidic Media. Coatings 2023, 13, 1102. https://doi.org/10.3390/coatings13061102
Barua S, Balčiūnaitė A, Vaičiūnienė J, Tamašauskaitė-Tamašiūnaitė L, Norkus E. Bimetallic 3D Nickel-Manganese/Titanium Bifunctional Electrocatalysts for Efficient Hydrogen and Oxygen Evolution Reaction in Alkaline and Acidic Media. Coatings. 2023; 13(6):1102. https://doi.org/10.3390/coatings13061102
Chicago/Turabian StyleBarua, Sukomol, Aldona Balčiūnaitė, Jūrate Vaičiūnienė, Loreta Tamašauskaitė-Tamašiūnaitė, and Eugenijus Norkus. 2023. "Bimetallic 3D Nickel-Manganese/Titanium Bifunctional Electrocatalysts for Efficient Hydrogen and Oxygen Evolution Reaction in Alkaline and Acidic Media" Coatings 13, no. 6: 1102. https://doi.org/10.3390/coatings13061102
APA StyleBarua, S., Balčiūnaitė, A., Vaičiūnienė, J., Tamašauskaitė-Tamašiūnaitė, L., & Norkus, E. (2023). Bimetallic 3D Nickel-Manganese/Titanium Bifunctional Electrocatalysts for Efficient Hydrogen and Oxygen Evolution Reaction in Alkaline and Acidic Media. Coatings, 13(6), 1102. https://doi.org/10.3390/coatings13061102









