In-Situ Production of Metal Matrix Composites Layers by TIG Surface Alloying to Improve Wear Resistance of Ductile Cast Iron Using a Buffer-Layer and Post Weld Heat Treatment
Abstract
:1. Introduction
- Preheating to prevent cold cracks due to the solidification process (3.2);
- Application of a buffer layer produced in situ to prevent shrinkage and porosity defects due to mismatches between the melt pool of the substrate and the powders used (3.2);
- Overcoming balling defects due to the first-time surface modification (3.3);
- The effects of PWHT on the layers, PMZ, and HAZ of the samples (3.5–3.7);
- Types of in-situ compounds formed in the MMC layers of ductile cast iron (3.8);
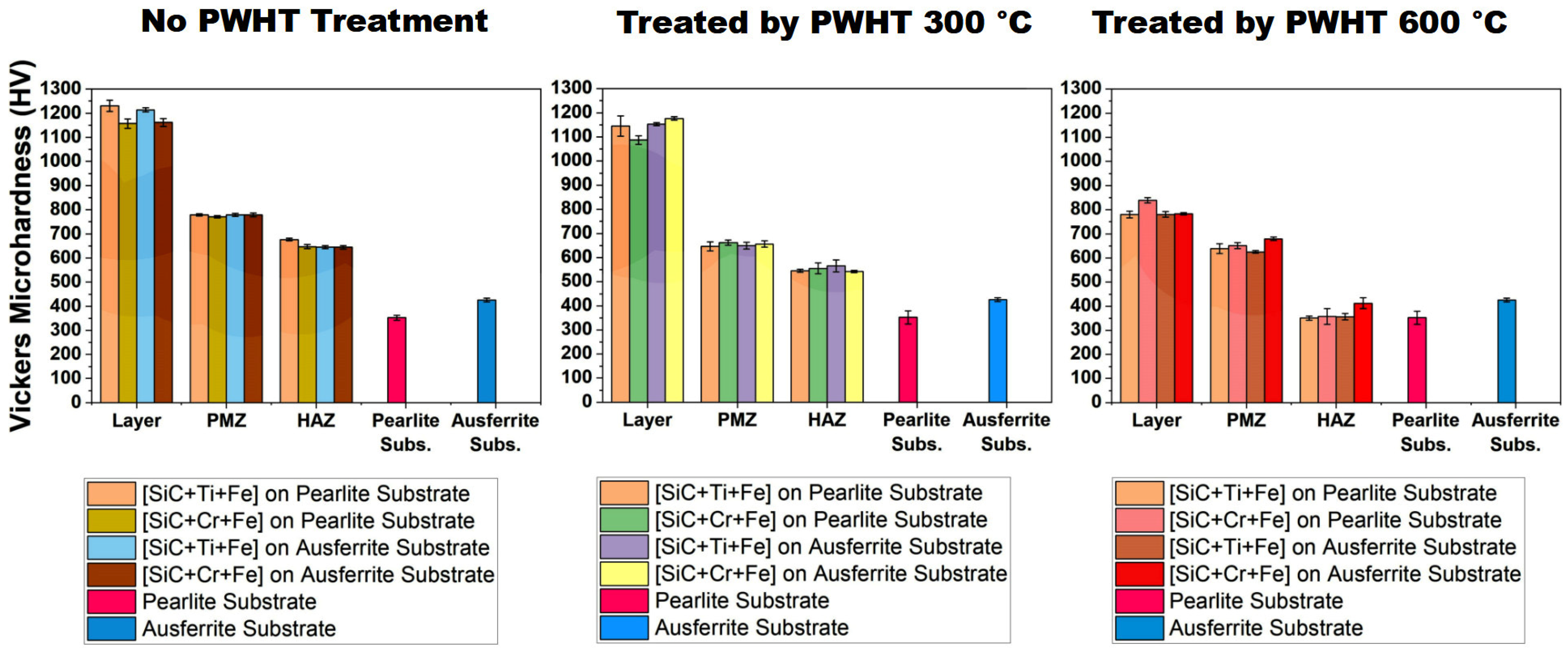
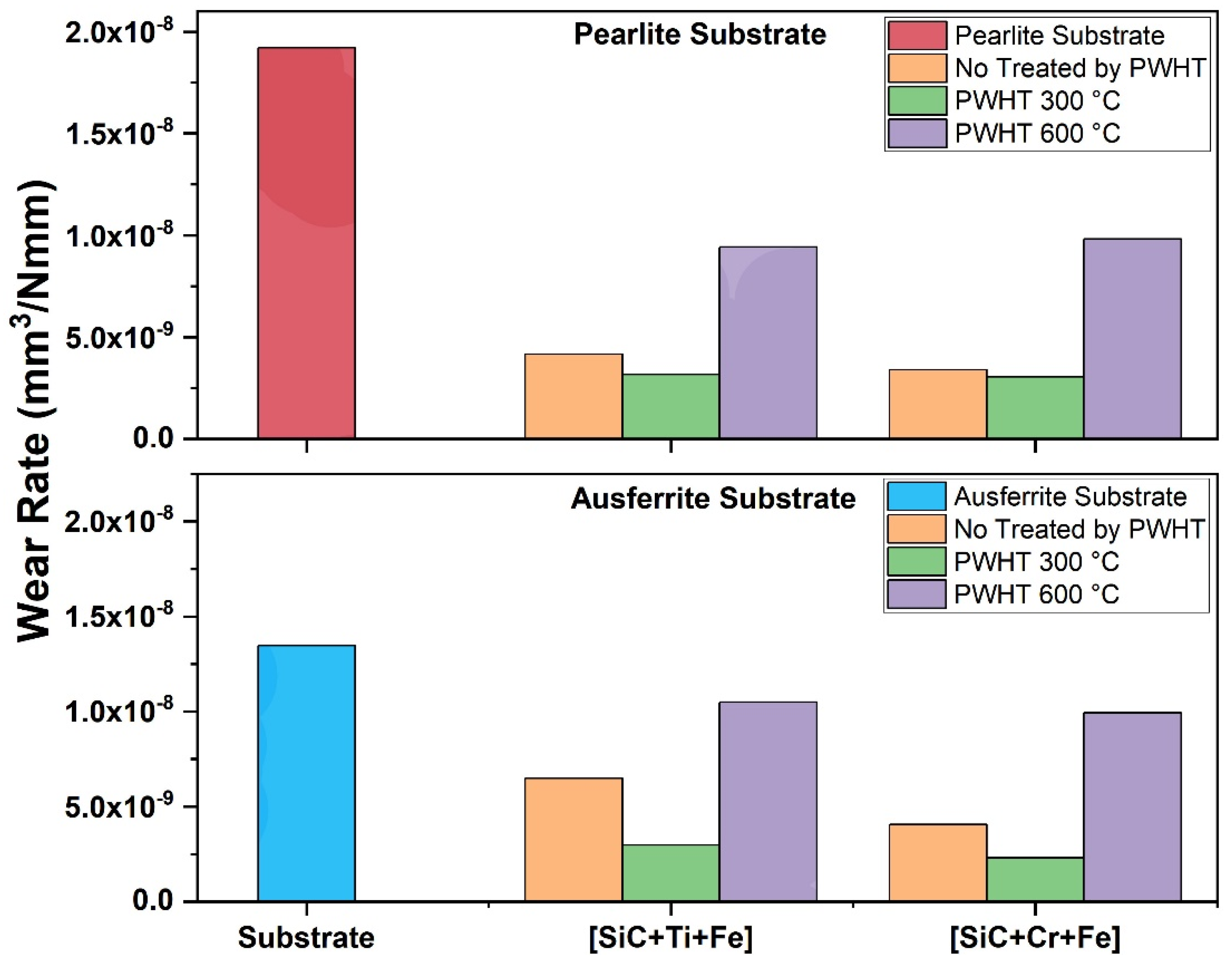
- The influence of PWHT on properties such as microhardness and wear resistance (3.9);
- Verifying whether the methodology adopted was efficient to produce in-situ MMC layers with high hardness and superior wear resistance compared to ductile cast iron substrate without any modification (4).
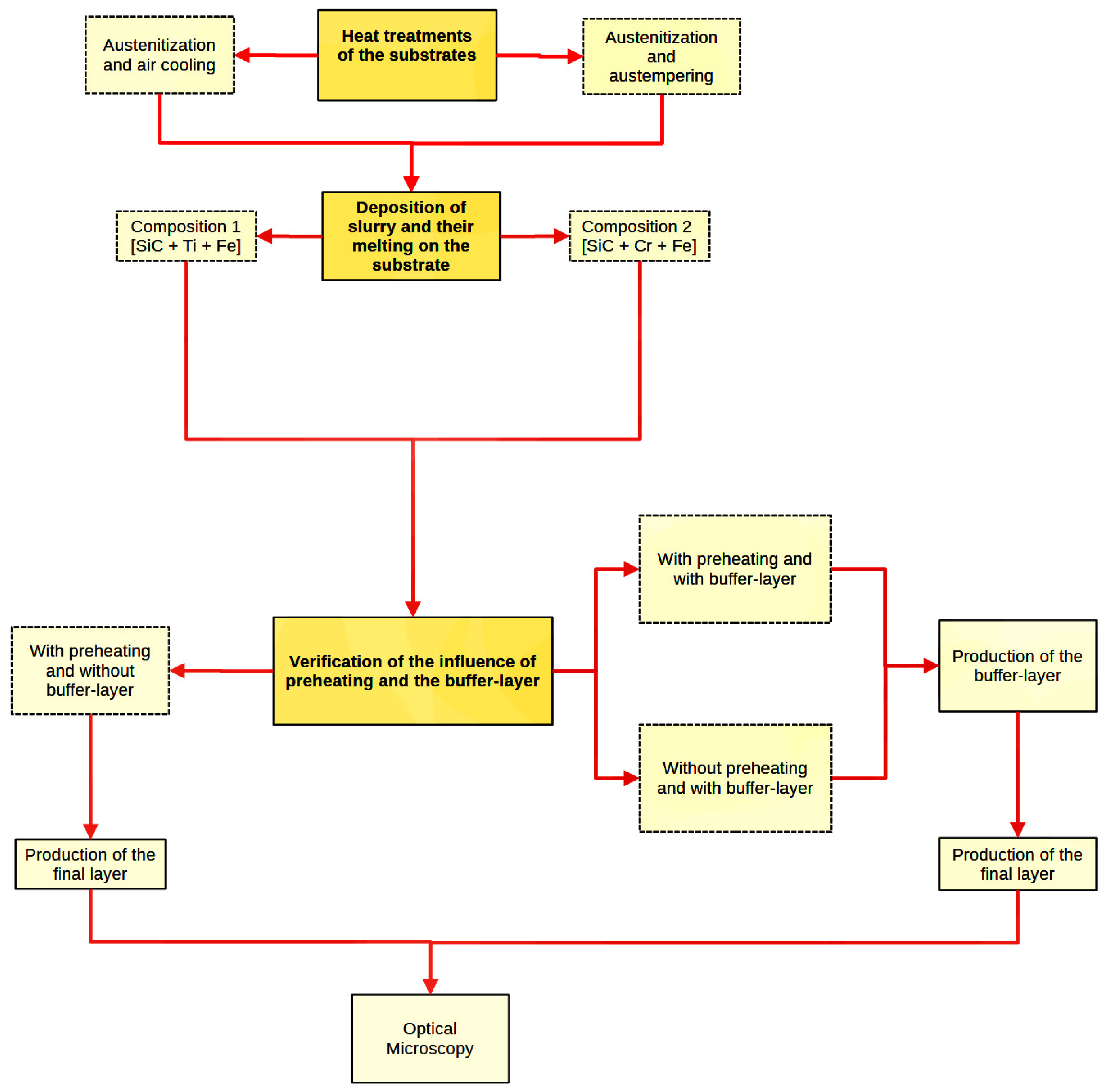
2. Materials and Methods
2.1. Initial Heat Treatments
2.2. Determination of the Specimens Preheating Temperature
2.3. Configuration of the Production Equipment
2.4. Direction and Sequence of Tracks in Layer Formation
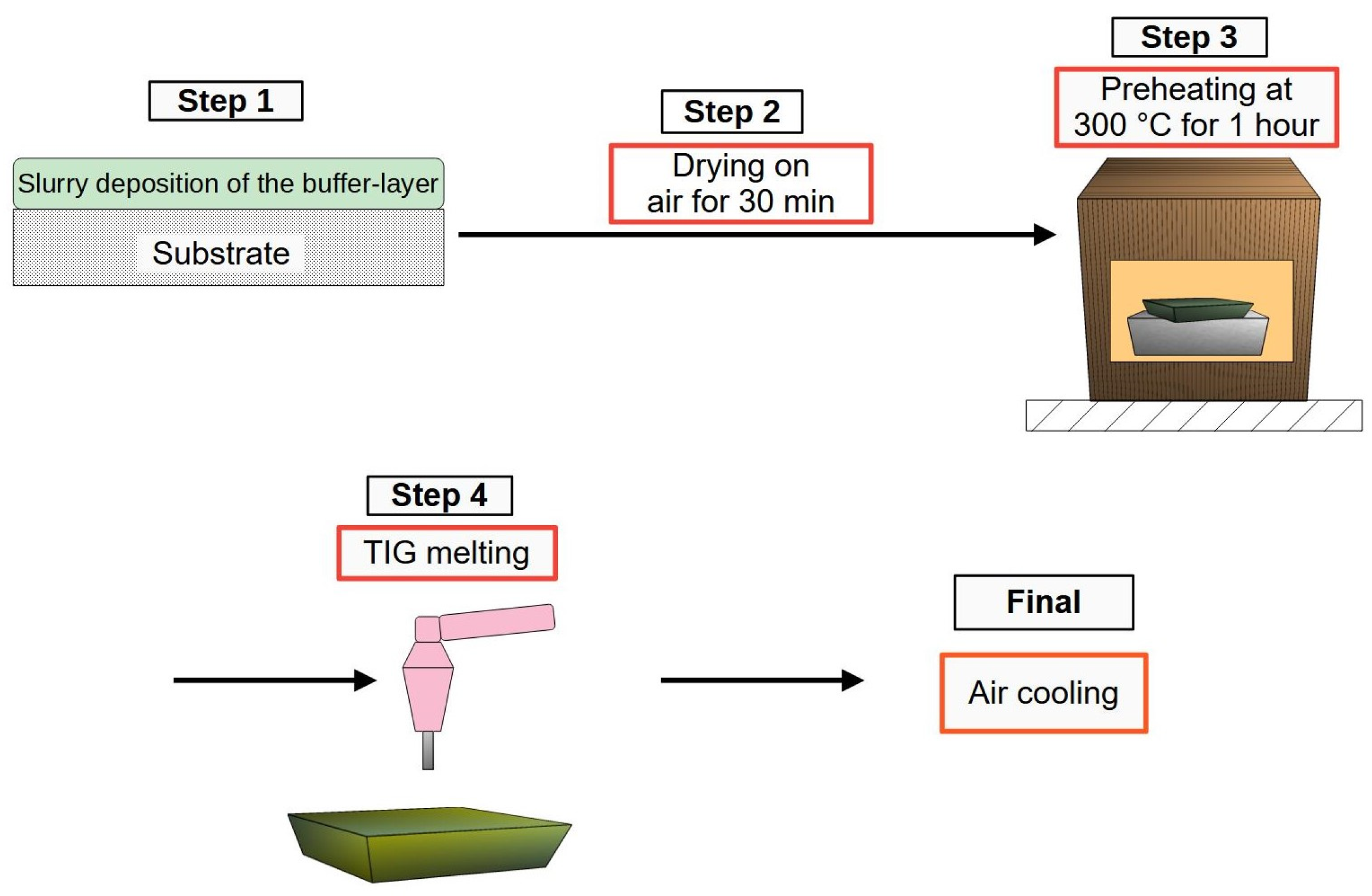
2.5. Slurry Production and Deposition
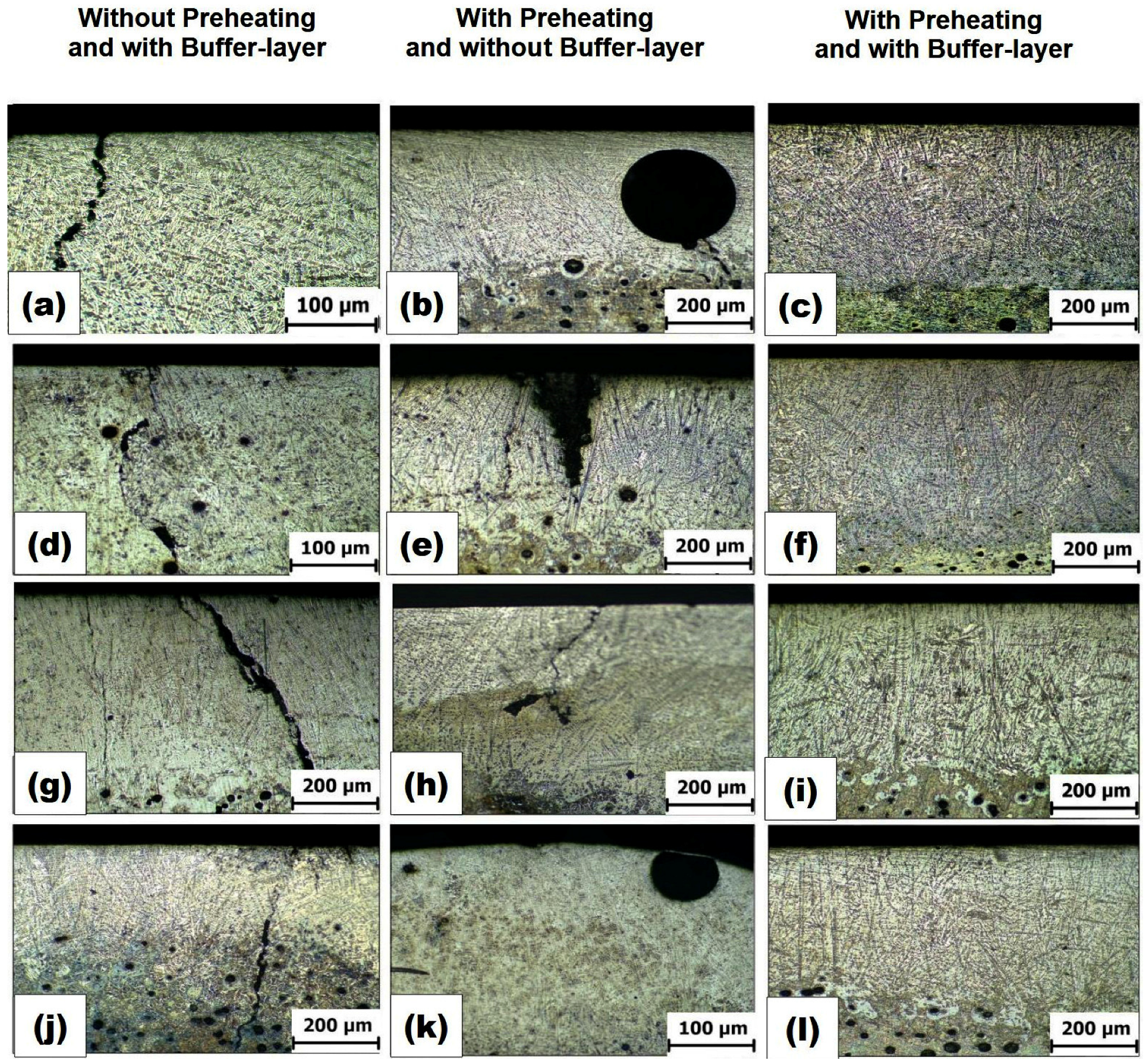
2.6. Verification of the Influence of Preheating (on Crack Prevention) and a Buffer Layer (on Final Layer Bond)
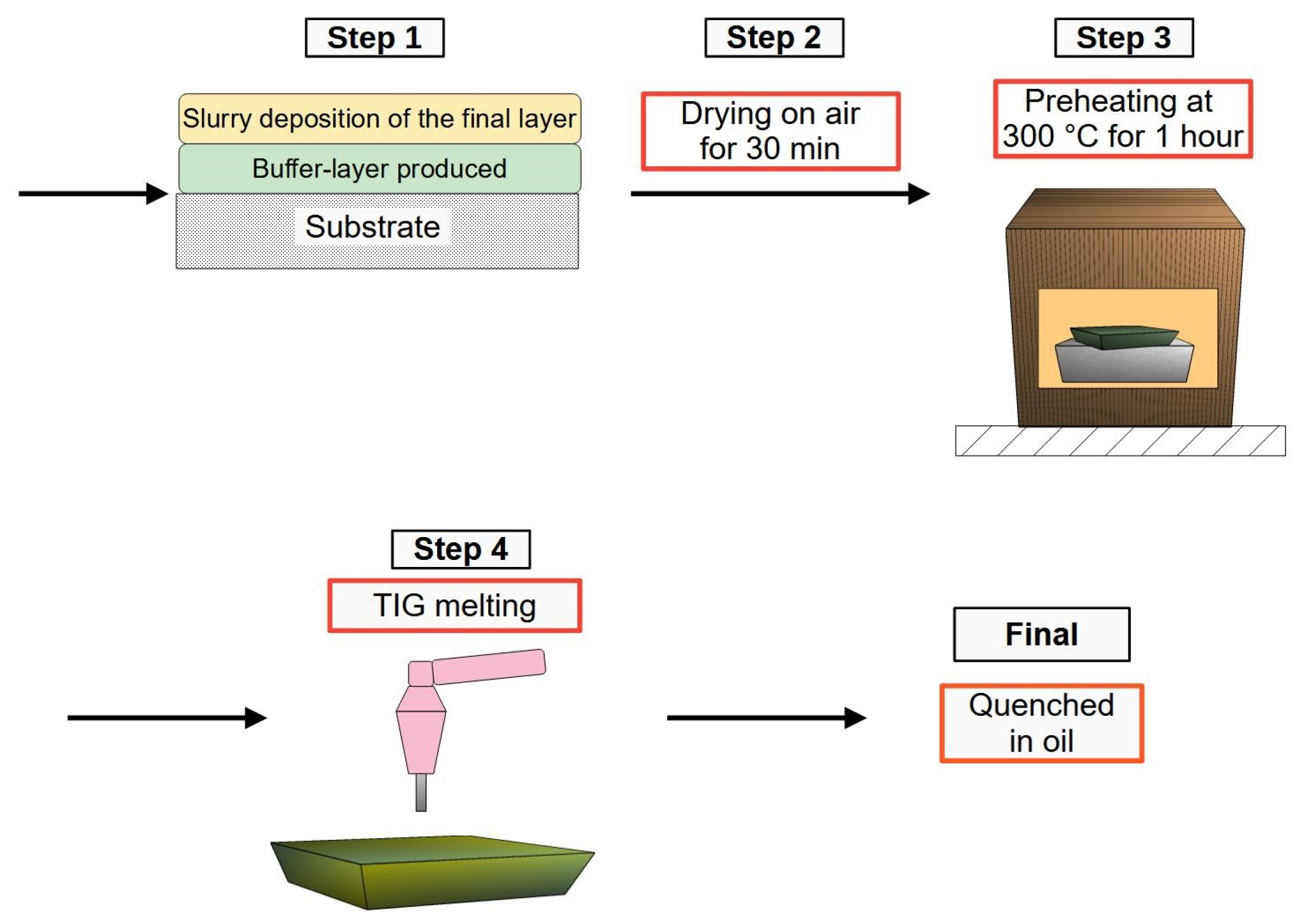
2.7. Production of the Final Layer by Applying Preheating and Using a Buffer Layer
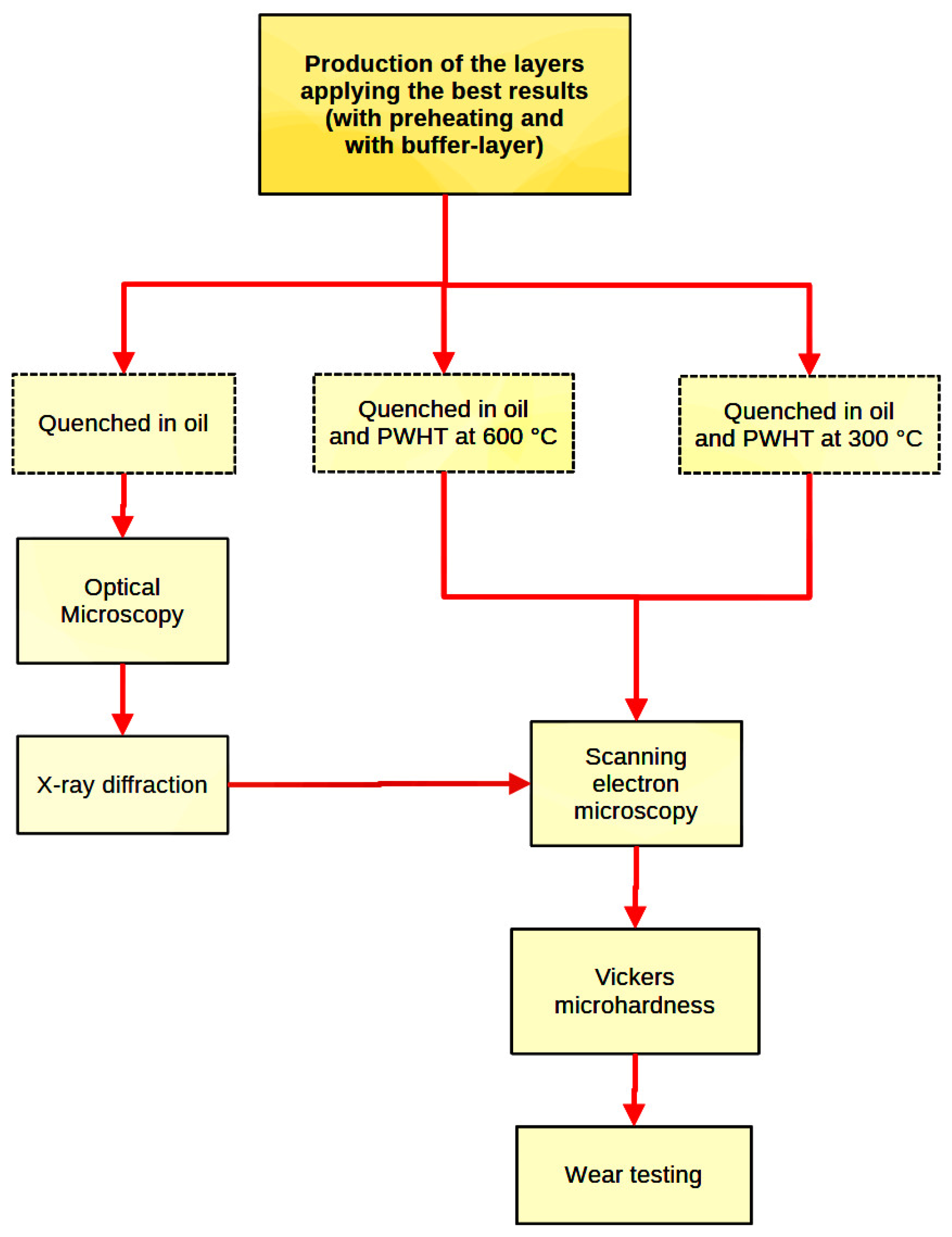
2.8. X-ray Diffraction (XRD) and Energy Dispersion X-ray Spectroscopy (EDS)
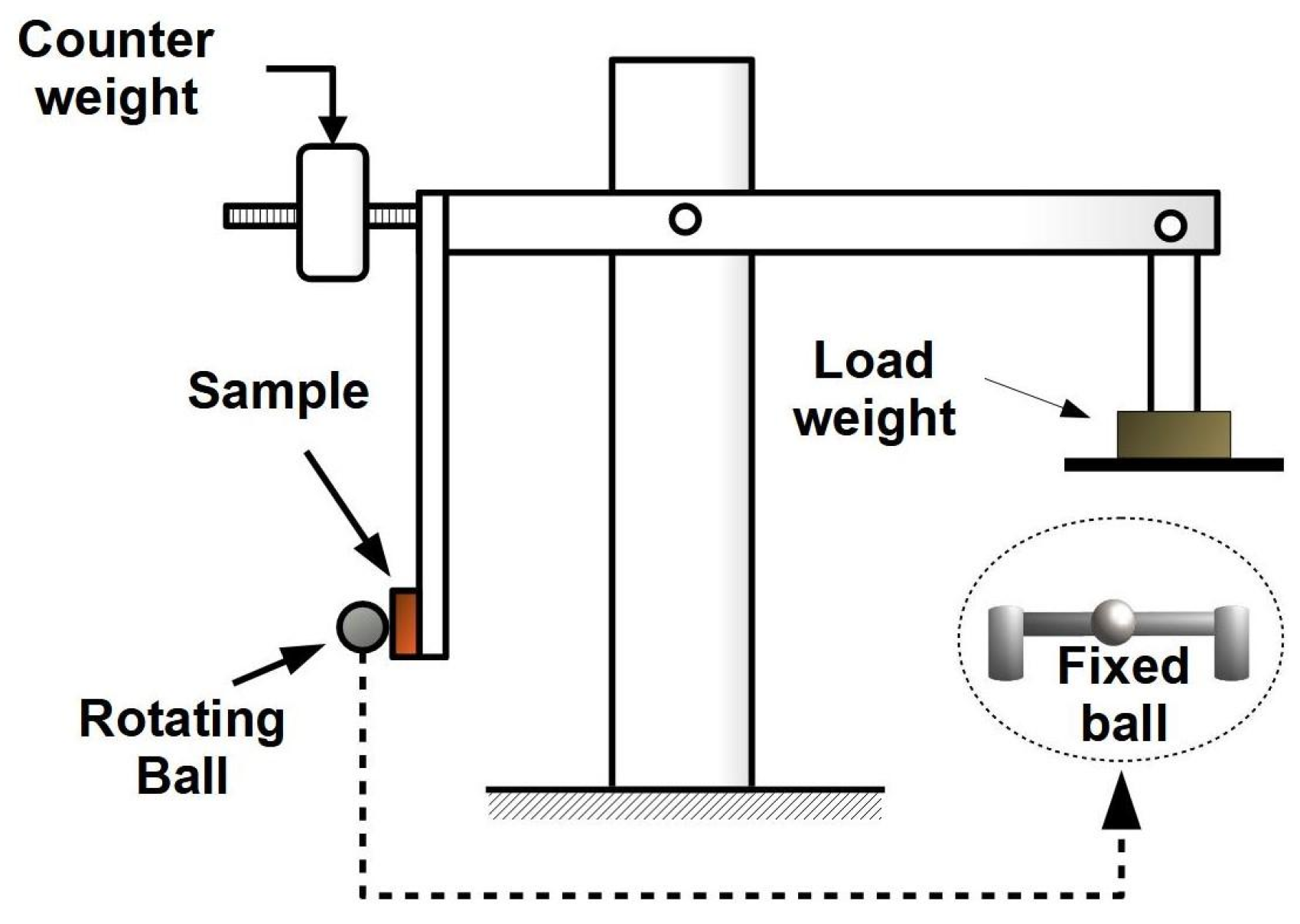
2.9. Vickers Microhardness and Wear Resistance Tests
3. Results and Discussion
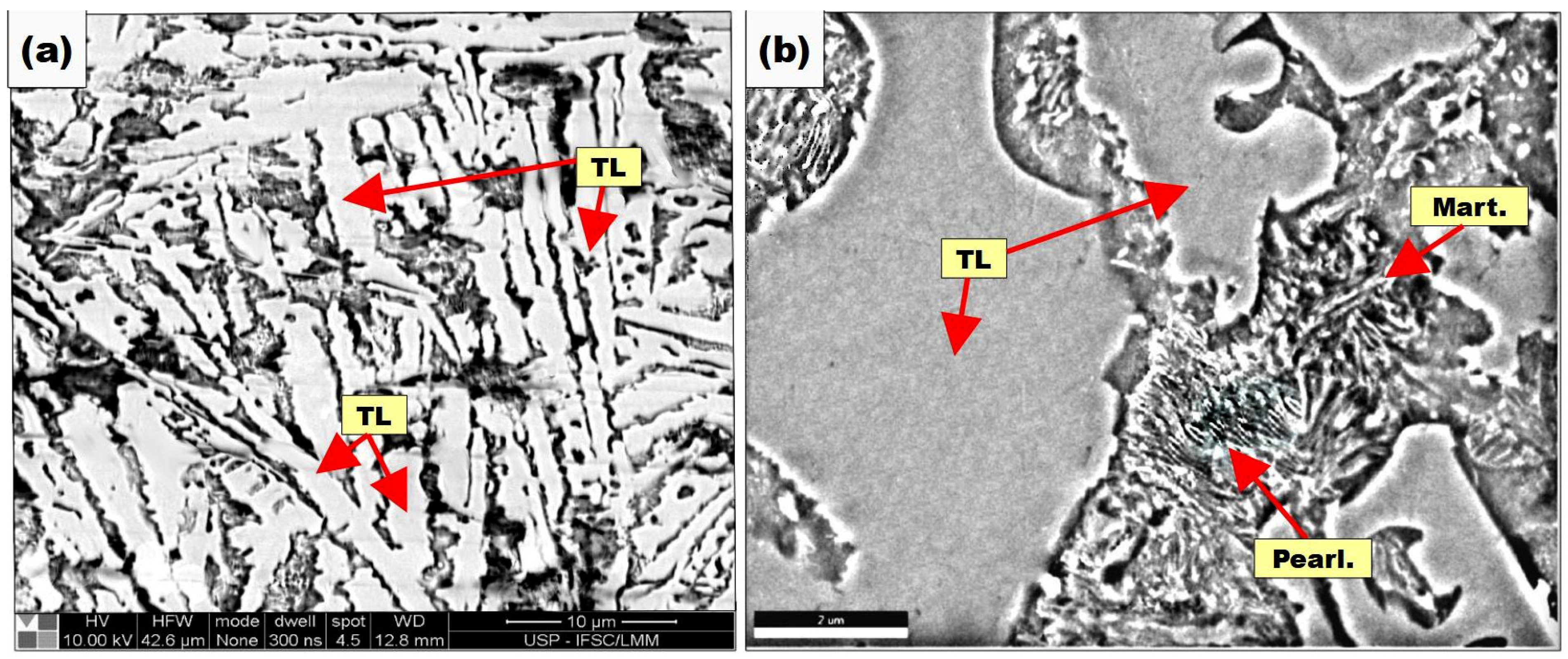
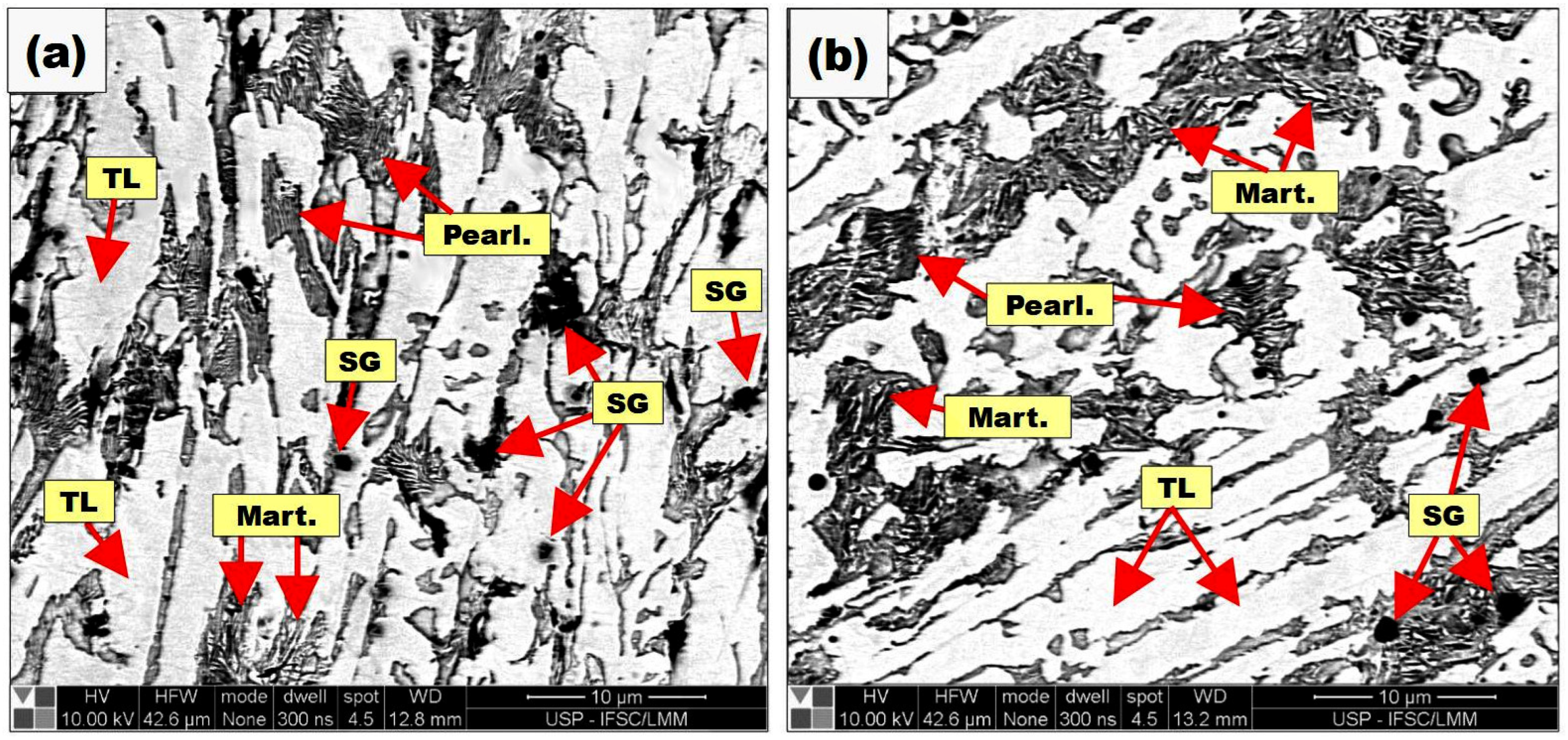
3.1. Chemical and Micrographic Analysis of Samples
3.2. Evaluation of the Influence of the Preheating and Application of Buffer Layer
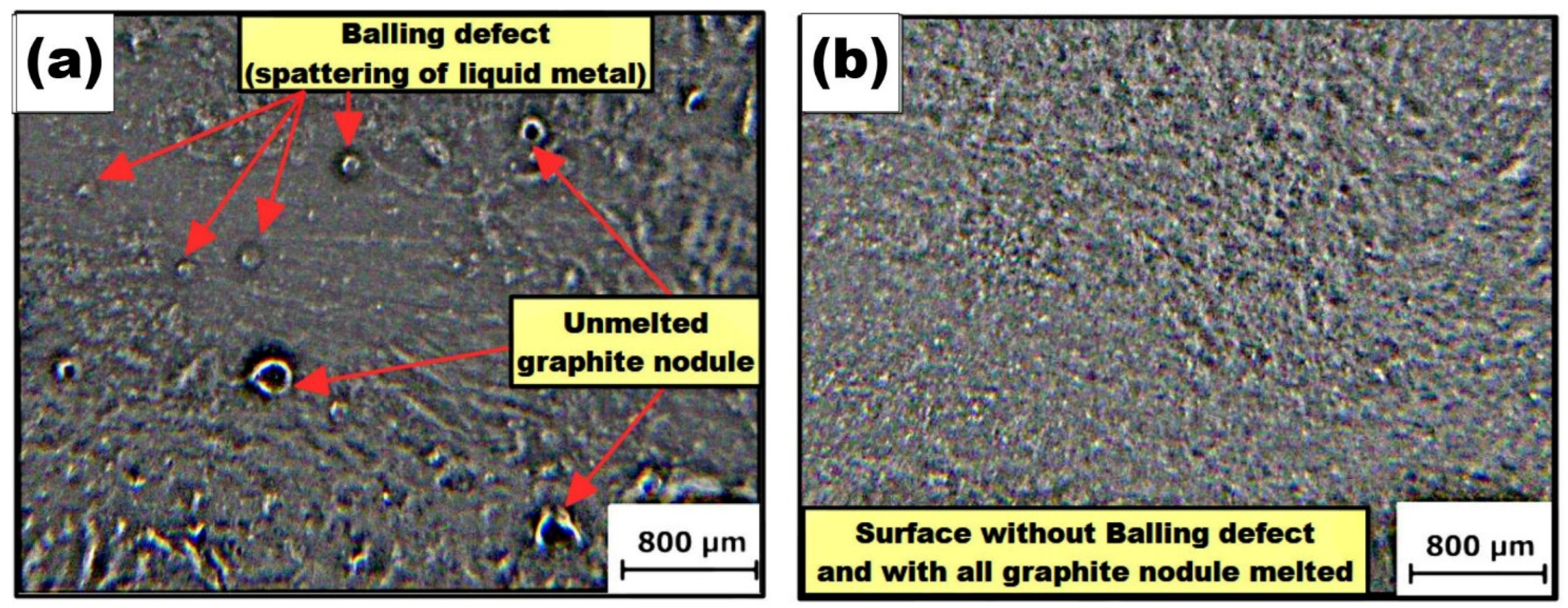
3.3. Elimination of Superficial Balling Defects
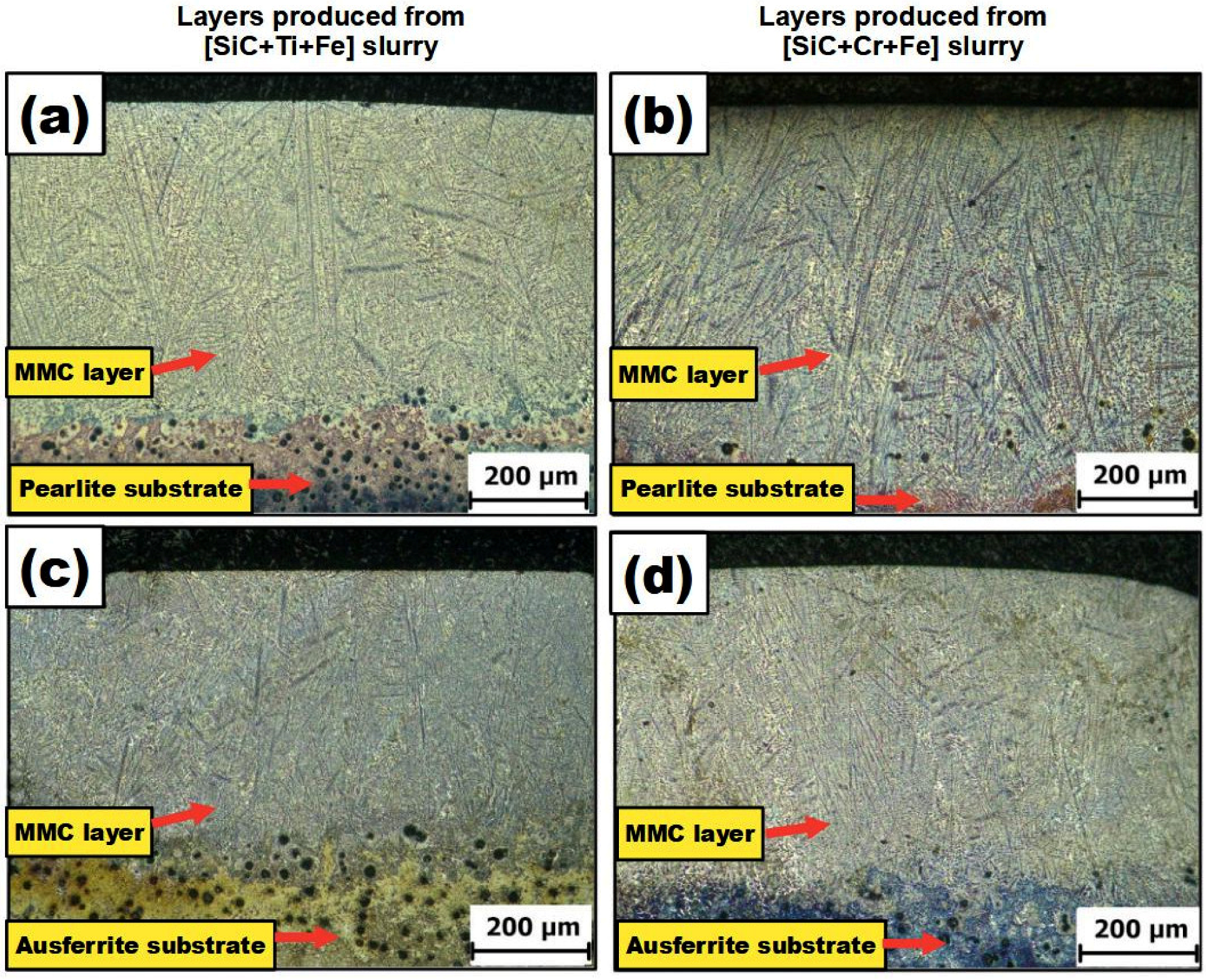
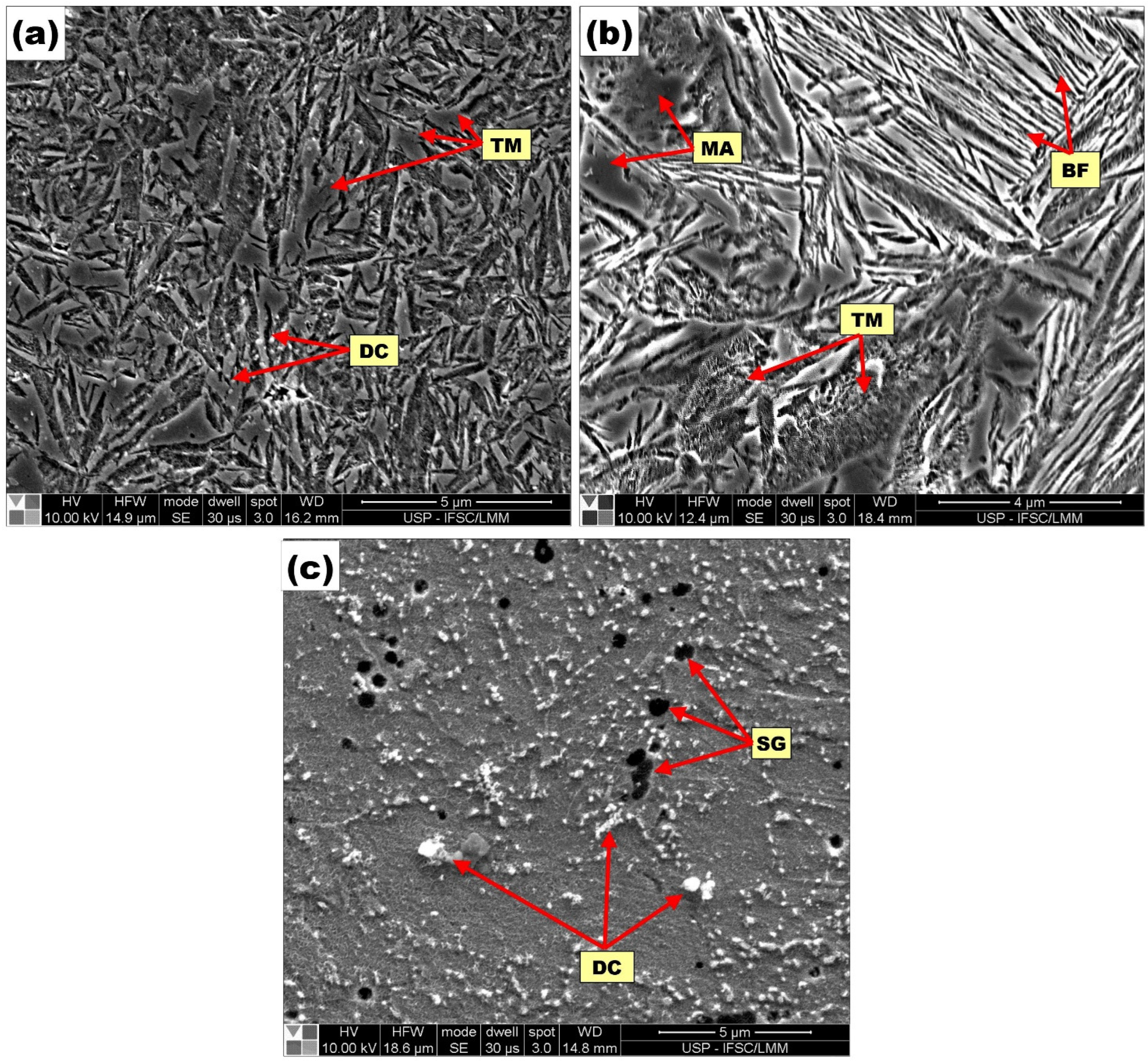
3.4. Metallographic and SEM Characterization of Final Layers Produced on the Buffer Layer
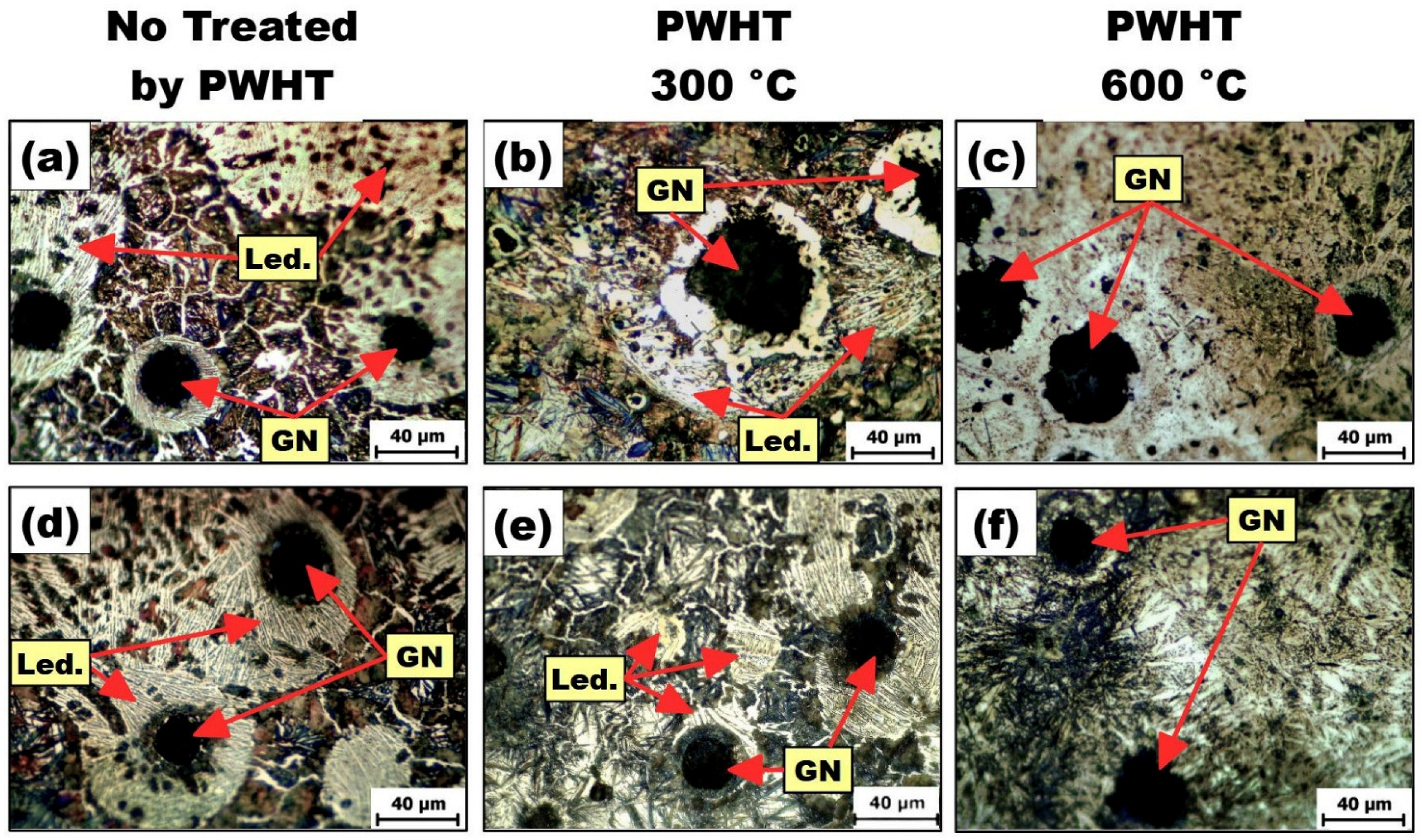
3.5. Effects of PWHT Treatments on Layers
3.6. Effects of PWHT Treatments on PMZ
3.7. Effects of PWHT Treatments on HAZ
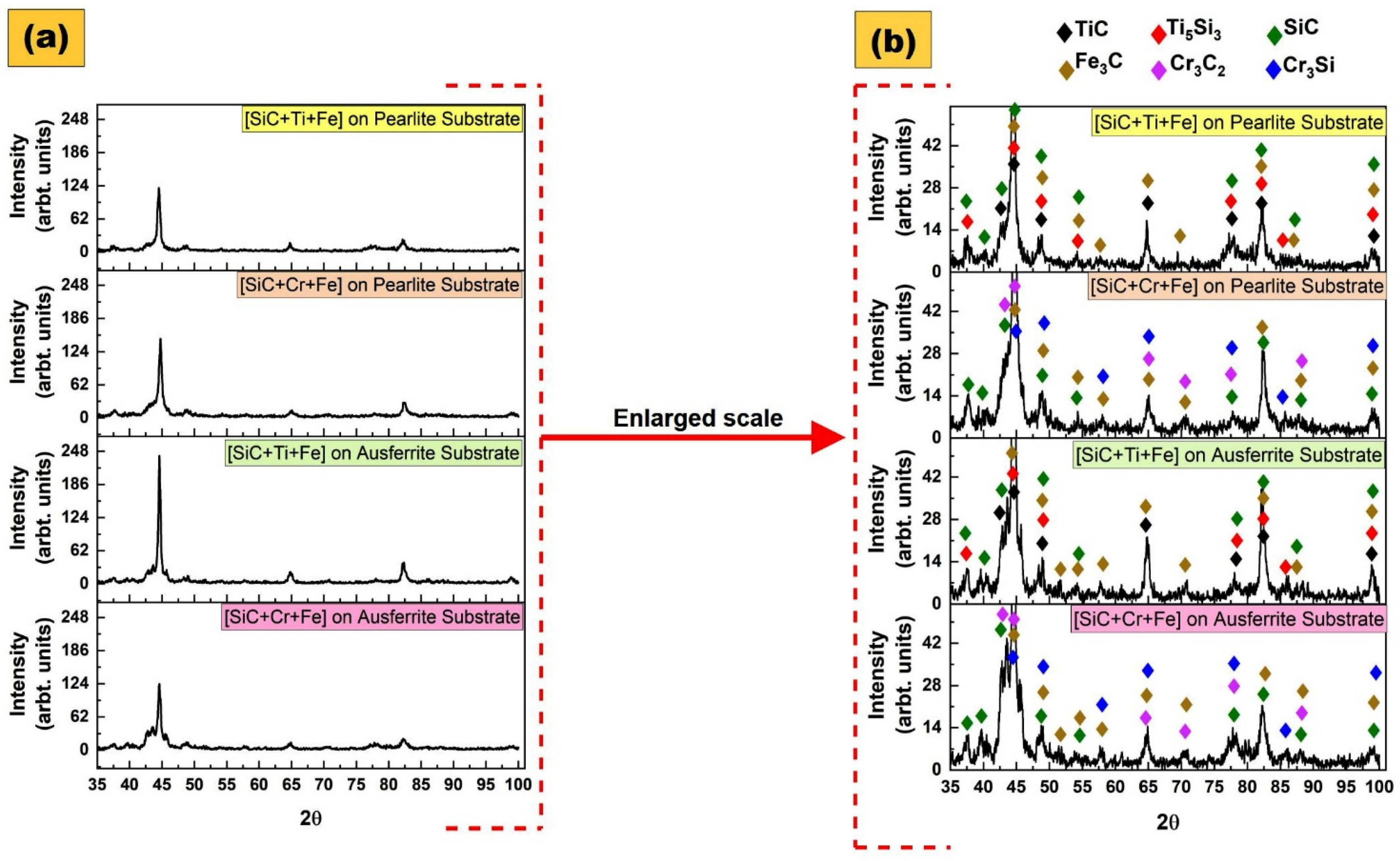
3.8. X-ray Diffraction for Determination of In-Situ Formed Compounds
3.9. Vickers Microhardness and Determination of the Wear Resistance of the Layers
4. Conclusions
- The combination of preheating and a buffer layer resulted in layers without cracks and porosity. Specifically, preheating at 300 °C of samples before fusion of the surface was effective in avoiding crack formation in ductile cast iron samples. Additionally, the use of a buffer layer constituted by ferroalloy was successful in preventing defects, such shrinkage and porosity.
- Balling defects were overcome since they typically originate when the first modification is made by applying fusion to the surface. They were overcome by applying a final layer that completed the spreading of the liquid metal over the surface of the substrate.
- The use of PWHT influenced the microconstituents formed on the layers, PMZ, and HAZ of the samples. The whole presentation of all microconstituents formed appears in the Results section. However, it is worth mentioning that any possibility of adverse effects of HAZ were eliminated with the application of PWHT at 300 °C. This statement can be made since all the microconstituents produced have great beneficial properties. The types of in-situ compounds formed, such as TiC, Ti5Si3, SiC, Fe3C, Cr3C2, and Cr3Si, promote the improvement of microhardness of the MMC layers, as well as their resistance to wear compared to the substrates without layers modified.
- The post-weld heat treatment at 300 °C significantly improved the hardness and wear resistance of the layers, showing better results compared to all other conditions and the substrate samples without layers.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
| Abbreviation | Long Name |
|---|---|
| CTE | Coefficients of Thermal Expansion |
| HAZ | Heat-Affected Zone |
| MMC | Metal Matrix Composites |
| PMZ | Partial Melted Zone |
| PWHT | Post-Weld Heat Treatment |
| TBW | Temper Bead Welding |
References
- Li, X.; Liu, J.; Xiong, J.; Yang, L.; Gou, Q.; Song, X.; Guo, Z.; Hua, T.; Liang, M. Wear and corrosion resistant Mn-doped austenitic cast iron prepared by powder metallurgy method. J. Mater. Res. Technol. 2020, 9, 6376–6385. [Google Scholar] [CrossRef]
- Rebasa, N.; Dommarco, R.; Sikora, J. Wear resistance of high nodule count ductile iron. Wear 2002, 253, 855–861. [Google Scholar] [CrossRef]
- Liu, J.H.; Hao, X.Y.; Li, G.L.; Liu, G.S. Microvoid evaluation of ferrite ductile iron under strain. Mater. Lett. 2002, 56, 748–755. [Google Scholar] [CrossRef]
- Dommarco, R.C.; Sousa, M.E.; Sikora, J.A. Abrasion resistance of high nodule count ductile iron with different matrix microstructures. Wear 2004, 257, 1185–1192. [Google Scholar] [CrossRef]
- Abedi, H.R.; Fareghi, A.; Saghafian, H.; Kheirandish, S.H. Sliding wear behavior of a ferritic–pearlitic ductile cast iron with different nodule count. Wear 2010, 268, 622–628. [Google Scholar] [CrossRef]
- Al-Asadi, M.M.; Al-Tameemi, H.A. A review of tribological properties and deposition methods for selected hard protective coatings. Tribol. Int. 2022, 176, 107919. [Google Scholar] [CrossRef]
- Zhang, D.; Li, Y.; Du, X.; Fan, H.; Gao, F. Microstructure and tribological performance of boride layers on ductile cast iron under dry sliding conditions. Eng. Fail. Anal. 2022, 134, 106080. [Google Scholar] [CrossRef]
- Giacomelli, R.O.; Salvaro, D.B.; Binder, C.; Klein, A.N.; De Mello, J.D.B. DLC deposited onto nitrided grey and nodular cast iron substrates: An unexpected tribological behaviour. Tribol. Int. 2018, 121, 460–467. [Google Scholar] [CrossRef]
- Vackel, A.; Sampath, S. Fatigue behavior of thermal sprayed WCCoCr-steel systems: Role of process and deposition parameters. Surf. Coat. Technol. 2017, 315, 408–416. [Google Scholar] [CrossRef] [Green Version]
- Zhang, X.; Sun, C.; Pan, T.; Flood, A.; Zhang, Y.; Li, L.; Liou, F. Additive manufacturing of copper—H13 tool steel bimetallic structures via Ni-based multi-interlayer. Addit. Manuf. 2020, 36, 101474. [Google Scholar]
- Uday, M.B.; Ahmad-Fauzi, M.N.; Alias, M.N.; Srithar, R. Current Issues and Problems in the Joining of Ceramic to Metal. In Joining Technologies; Ishak, M., Ed.; IntechOpen: London, UK, 2016; pp. 159–193. [Google Scholar]
- Molobi, E.; Sacks, M.; Theron, M. Crack mitigation in laser engineered net shaping of WC-10wt%FeCr cemented carbides. Addit. Manuf. Lett. 2021, 2, 100028. [Google Scholar] [CrossRef]
- Rathod, D.W.; Pandey, S.; Aravindan, S.; Singh, P.K. Metallurgical Behaviour and Carbon Diffusion in Buttering Deposits Prepared With and Without Buffer Layers. Acta Metall. Sin. 2017, 30, 120–132. [Google Scholar] [CrossRef] [Green Version]
- Yi, R.; Chen, C.; Shi, C.; Li, Y.; Li, H.; Ma, Y. Research advances in residual thermal stress of ceramic/metal brazes. Ceram. Int. 2021, 47, 20807–20820. [Google Scholar] [CrossRef]
- Kim, D.K.; Woo, W.; Kim, E.Y.; Choi, S.H. Microstructure and mechanical characteristics of multi-layered materials composed of 316L stainless steel and ferritic steel produced by direct energy deposition. J. Alloy. Compd. 2019, 774, 896–907. [Google Scholar] [CrossRef]
- Ranjan, R.; Kumar Das, A. Protection from corrosion and wear by different weld cladding techniques: A review. Mater. Today Proc. 2022, 57, 1687–1693. [Google Scholar] [CrossRef]
- Balaguru, S.; Gupta, M. Hardfacing studies of Ni alloys: A critical review. J. Mater. Res. Technol. 2021, 10, 1210–1242. [Google Scholar] [CrossRef]
- Chatterjee, S.; Pal, T.K. Weld procedural effect on the performance of iron based hardfacing deposits on cast iron substrate. J. Mater. Process. Technol. 2006, 173, 61–69. [Google Scholar] [CrossRef]
- Wu, Y.; Schmitt, T.; Bousser, E.; Vernhes, L.; Khelfaoui, F.; Perez, G.; Klemberg-Sapieha, J.E.; Brochu, M. Microstructural and mechanical characterization of Stellite-hardfaced coatings with two types of buffer layers. Surf. Coat. Technol. 2020, 390, 125611. [Google Scholar] [CrossRef]
- Ahn, D.G. Hardfacing technologies for improvement of wear characteristics of hot working tools: A review. Int. J. Precis. Eng. Manuf. 2013, 14, 1271–1283. [Google Scholar] [CrossRef]
- Tjong, S.C.; Ma, Z.Y. Microstructural and mechanical characteristics of in situ metal matrix composites. Mater. Sci. Eng. R Rep. 2000, 29, 49–113. [Google Scholar] [CrossRef]
- Oukach, S.; Pateyron, B.; Pawłowski, L. Physical and chemical phenomena occurring between solid ceramics and liquid metals and alloys at laser and plasma composite coatings formation: A review. Surf. Sci. Rep. 2019, 74, 213–241. [Google Scholar] [CrossRef]
- Storozhenko, M.; Umanskyi, O.; Krasovskyy, V.; Antonov, M.; Terentjev, O. Wetting and interfacial behaviour in the TiB2-NiCrBSiC system. J. Alloy. Compd. 2019, 778, 15–22. [Google Scholar] [CrossRef]
- Wang, Y.; Sun, C.; Sun, J.; Zhao, W.; Dong, L.; Li, L.; Meng, F. Erosion behavior of arc sprayed FeTi/CrB MMC coating at elevated temperature. Surf. Coat. Technol. 2015, 262, 141–147. [Google Scholar] [CrossRef]
- Chen, X.; Qin, X.; Zhu, Z.; Gao, K. Microstructural evolution and wear properties of the continual local induction cladding NiCrBSi coatings. J. Mater. Process. Technol. 2018, 262, 257–268. [Google Scholar] [CrossRef]
- Tang, W.M.; Zheng, Z.X.; Ding, H.F.; Jin, Z.H. A study of the solid state reaction between silicon carbide and iron. Mater. Chem. Phys. 2002, 74, 258–264. [Google Scholar] [CrossRef]
- Li, J.; Ru, H.; Yang, H.; Liu, Y. Liquid-solid reactions and microstructure of SiC-5120 steel composite brake material. Metall. Mater. Trans. A: Phys. Metall. Mater. Sci. 2012, 43, 658–664. [Google Scholar] [CrossRef]
- Davis, J.R. Preface. In Alloying Understanding the Basics, 1st ed.; Davis, J.R., Ed.; ASM International: Cleveland, OH, USA, 2001; p. xiii. [Google Scholar]
- Hajbagheri, F.A.; Bozorg, S.F.K.; Amadeh, A.A. Microstructure and wear assessment of TIG surface alloying of CP-titanium with silicon. J. Mater. Sci. 2008, 43, 5720–5727. [Google Scholar] [CrossRef]
- Ogbonna, O.S.; Akinlabi, S.A.; Madushele, N.; Mashinini, P.M.; Abioye, A.A. Application of MIG and TIG Welding in Automobile Industry. J. Phys. Conf. Ser. 2019, 1378, 042065. [Google Scholar] [CrossRef]
- Amirsadeghi, A.; Sohi, M.H. Comparison of the influence of molybdenum and chromium TIG surface alloying on the microstructure, hardness and wear resistance of ADI. J. Mater. Process. Technol. 2008, 201, 673–677. [Google Scholar] [CrossRef]
- Kılınç, B.; Kocaman, E.; Şen, Ş.; Şen, U. Effect of vanadium content on the microstructure and wear behavior of Fe(13-x)VxB7 (x = 0–5) based hard surface alloy layers. Mater. Charact. 2021, 179, 111324. [Google Scholar] [CrossRef]
- Voigt, R.C.; Loper, C.R. A Study of Heat-Affected Zone Structures in Ductile Cast Iron; WRC: Shaker Heights, OH, USA, 1983. [Google Scholar]
- Chen, T.; Li, W.; Liu, D.; Xiong, Y.; Zhu, X. Effects of heat treatment on microstructure and mechanical properties of TiC/TiB composite bioinert ceramic coatings in-situ synthesized by laser cladding on Ti6Al4V. Ceram. Int. 2021, 47, 755–768. [Google Scholar] [CrossRef]
- Lu, F.; Liu, P.; Ji, H.; Ding, Y.; Xu, X.; Gao, Y. Dramatically enhanced impact toughness in welded 10%Cr rotor steel by high temperature post-weld heat treatment. Mater. Charact. 2014, 92, 149–158. [Google Scholar] [CrossRef]
- Marques, E.S.V.; Silva, F.J.G.; Paiva, O.C.; Pereira, A.B. Improving the mechanical strength of ductile cast iron welded joints using different heat treatments. Materials 2019, 12, 2263. [Google Scholar] [CrossRef] [Green Version]
- Wang, B.; Barber, G.C.; Tao, C.; Sun, X.; Ran, X. Characteristics of tempering response of austempered ductile iron. J. Mater. Res. Technol. 2018, 7, 198–202. [Google Scholar] [CrossRef]
- ANSI/AWS D11.2-89. In Guide for Welding Iron Casting, 8th ed.; American Welding Society: Miami, FL, USA, 1991; pp. 227–280.
- Gouveia, R.M.; Silva, F.J.G.; Paiva, O.C.; De Fátima Andrade, M.; Pereira, L.A.; Moselli, P.C.; Papis, K.J.M. Comparing the structure and mechanical properties of welds on ductile cast iron (700 MPa) under different heat treatment conditions. Metals 2018, 8, 72. [Google Scholar] [CrossRef] [Green Version]
- Altomare, A.; Corriero, N.; Cuocci, C.; Falcicchio, A.; Moliterni, A.; Rizzi, R. QUALX2.0: A qualitative phase analysis software using the freely available database POW_COD. J. Appl. Cryst. 2015, 48, 598–603. [Google Scholar] [CrossRef]
- Trezona, R.; Hutchings, I. Three-body abrasive wear testing of soft materials. Wear 1999, 233, 209–221. [Google Scholar] [CrossRef]
- Triani, R.M.; Mariani, F.E.; Gomes, L.F.D.A.; De Oliveira, P.G.B.; Totten, G.E.; Casteletti, L.C. Improvement of the Tribological Characteristics of AISI 8620, 8640 and 52100 Steels through Thermo-Reactive Treatments. Lubricants 2019, 7, 63. [Google Scholar] [CrossRef] [Green Version]
- Petersen, D.; Link, R.; Rutherford, K.; Hutchings, I. Theory and Application of a Micro-Scale Abrasive Wear Test. J. Test. Eval. 1997, 25, 250. [Google Scholar] [CrossRef]
- Ramos, H.E.L.; Franco, A.R.; Vieira, E.A. Influence of plasma nitriding pressure on microabrasive wear resistance of a microalloyed steel. J. Mater. Res. Technol. 2019, 8, 1694–1700. [Google Scholar] [CrossRef]
- Zhou, C.; Zhao, S.; Wang, Y.; Liu, F.; Gao, W.; Lin, X. Mitigation of pores generation at overlapping zone during laser cladding. J. Mater. Process. Technol. 2015, 216, 369–374. [Google Scholar] [CrossRef]
- Soysal, T.; Kou, S. Effect of filler metals on solidification cracking susceptibility of Al alloys 2024 and 6061. J. Mater. Process. Technol. 2019, 266, 421–428. [Google Scholar] [CrossRef]
- Li, Y.; Dong, S.; Yan, S.; Liu, X.; Li, E.; He, P.; Xu, B. Elimination of voids by laser remelting during laser cladding Ni based alloy on gray cast iron. Opt. Laser Technol. 2019, 112, 30–38. [Google Scholar] [CrossRef]
- Rahman Rashid, R.A.; Abaspour, S.; Palanisamy, S.; Matthews, N.; Dargusch, M.S. Metallurgical and geometrical characterisation of the 316L stainless steel clad deposited on a mild steel substrate. Surf. Coat. Technol. 2017, 327, 174–184. [Google Scholar] [CrossRef]
- Liu, S.; Guo, H. Balling behavior of selective laser melting (SLM) magnesium alloy. Materials 2020, 13, 3632. [Google Scholar] [CrossRef] [PubMed]
- Li, R.; Liu, J.; Shi, Y.; Wang, L.; Jiang, W. Balling behavior of stainless steel and nickel powder during selective laser melting process. Int. J. Adv. Manuf. Technol. 2012, 59, 1025–1035. [Google Scholar] [CrossRef]
- Lashgari, H.R.; Xue, Y.; Onggowarsito, C.; Kong, C.; Li, S. Microstructure, Tribological Properties and Corrosion Behaviour of Additively Manufactured 17-4PH Stainless Steel: Effects of Scanning Pattern, Build Orientation, and Single vs. Double scan. Mater. Today Commun. 2020, 25, 101535. [Google Scholar] [CrossRef]
- Weng, Z.; Wang, A.; Wang, Y.; Xiong, D.; Tang, H. Diode laser cladding of Fe-based alloy on ductile cast iron and related interfacial behavior. Surf. Coat. Technol. 2016, 286, 64–71. [Google Scholar] [CrossRef]
- Xu, J.; Lin, X.; Guo, P.; Dong, H.; Wen, X.; Li, Q.; Xue, L.; Huang, W. The initiation and propagation mechanism of the overlapping zone cracking during laser solid forming of IN-738LC superalloy. J. Alloy. Compd. 2018, 749, 859–870. [Google Scholar] [CrossRef]
- Orłowicz, A.W.; Trytek, A. Effect of rapid solidification on sliding wear of iron castings. Wear 2003, 254, 154–163. [Google Scholar] [CrossRef]
- Mardaras, E.; González-Martínez, R.; Bayon, R.; Nastac, L.; Méndez, S. Surface modification of ductile iron produced by an innovative in-situ casting technique. Int. J. Cast Met. Res. 2020, 33, 103–111. [Google Scholar] [CrossRef]
- Stefanescu, D.M. ASM Handbook; White, C., Ed.; ASM International: Detroit, MI, USA, 2017; pp. 1–23. [Google Scholar]
- Askeland, D.R.; Birer, N. Secondary Graphite Formation in Tempered Nodular Cast Iron Weldments. Weld. J. 1979, 58, 337–342. [Google Scholar]
- Pereira, H.B.; Tschiptschin, A.P.; Goldenstein, H.; Azevedo, C.R.F. Effect of the Austenitization Route on the Bainitic Reaction Kinetics and Tensile Properties of an Alloyed Austempered Ductile Iron. Int. J. Met. 2021, 15, 1442–1455. [Google Scholar] [CrossRef]
- Wang, H.; Yu, S. Influence of heat treatment on microstructure and sliding wear resistance of high chromium cast iron electroslag hardfacing layer. Surf. Coat. Technol. 2017, 319, 182–190. [Google Scholar] [CrossRef]
- Li, Y.; Dong, S.; Yan, S.; Liu, X.; He, P.; Xu, B. Microstructure evolution during laser cladding Fe-Cr alloy coatings on ductile cast iron. Opt. Laser Technol. 2018, 108, 255–264. [Google Scholar] [CrossRef]
- El-Banna, E.; Nageda, M.; El-Saadat, M. Study of restoration by welding of pearlitic ductile cast iron. Mater. Lett. 2000, 42, 311–320. [Google Scholar] [CrossRef]
- Tamizi, M.; Pouranvari, M.; Movahedi, M. Welding metallurgy of martensitic advanced high strength steels during resistance spot welding. Sci. Technol. Weld. Join. 2017, 22, 327–335. [Google Scholar] [CrossRef]
- Krauss, G. Steels: Processing, Structure, and Performance; ASM International: Novelty, OH, USA, 2015. [Google Scholar]
- Gelfi, M.; Pola, A.; Girelli, L.; Zacco, A.; Masotti, M.; La Vecchia, G.M. Effect of heat treatment on microstructure and erosion resistance of white cast irons for slurry pumping applications. Wear 2019, 428–429, 438–448. [Google Scholar] [CrossRef]
- Avishan, B.; Garcia-Mateo, C.; Yazdani, S.; Caballero, F.G. Retained austenite thermal stability in a nanostructured bainitic steel. Mater. Charact. 2013, 81, 105–110. [Google Scholar] [CrossRef] [Green Version]
- Avishan, B.; Mohammadzadeh Khoshkebari, S.; Yazdani, S. Effect of pre-existing martensite within the microstructure of nano bainitic steel on its mechanical properties. Mater. Chem. Phys. 2021, 260, 124160. [Google Scholar] [CrossRef]
- Karam-Abian, M.; Zarei-Hanzaki, A.; Abedi, H.; Ghodrat, S.; Hajy-Akbary, F.; Kestens, L. The Effect of Martensite-Austenite Constituent Characteristics on the Mechanical Behavior of Quenched-Partitioned Steel at Room Temperature. Steel Res. Int. 2019, 90, 1800399. [Google Scholar] [CrossRef]
- Nishikawa, A.S.; Miyamoto, G.; Furuhara, T.; Tschiptschin, A.P.; Goldenstein, H. Phase transformation mechanisms during Quenching and Partitioning of a ductile cast iron. Acta Mater. 2019, 179, 1–16. [Google Scholar] [CrossRef]
- De Oliveira, P.G.B.; Triani, R.M.; Filho, A.I.; Neto, A.L.; Totten, G.E.; Casteletti, L.C. Production of Niobium Carbide Layers on High-Strength Bainitic Steels by Thermochemical Treatment of Thermoreactive Diffusion Followed by Austempering. Steel Res. Int. 2022, 93, 2100352. [Google Scholar] [CrossRef]
- Melado, A.C.; Nishikawa, A.S.; Goldenstein, H.; Giles, M.A.; Reed, P.A.S. Effect of microstructure on fatigue behaviour of advanced high strength ductile cast iron produced by quenching and partitioning process. Int. J. Fatigue 2017, 104, 397–407. [Google Scholar] [CrossRef] [Green Version]




| Type 1 | Type 2 | ||||
|---|---|---|---|---|---|
| Powder | Buffer Layer | Final Layer | Powder | Buffer Layer | Final Layer |
| SiC + Ti-Fe | 20% wt + 80% wt | 80% wt + 20% wt | SiC + Cr-Fe | 20% wt + 80% wt | 80% wt + 20% wt |
| Element | wt % | Element | wt % |
|---|---|---|---|
| Fe | Balance | Mn | 0.41 |
| C | 3.49 | Cu | 0.31 |
| Si | 2.70 | Mg | 0.03 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Triani, R.M.; Neto, J.B.T.D.R.; De Oliveira, P.G.B.; Rêgo, G.C.; Neto, A.L.; Casteletti, L.C. In-Situ Production of Metal Matrix Composites Layers by TIG Surface Alloying to Improve Wear Resistance of Ductile Cast Iron Using a Buffer-Layer and Post Weld Heat Treatment. Coatings 2023, 13, 1137. https://doi.org/10.3390/coatings13071137
Triani RM, Neto JBTDR, De Oliveira PGB, Rêgo GC, Neto AL, Casteletti LC. In-Situ Production of Metal Matrix Composites Layers by TIG Surface Alloying to Improve Wear Resistance of Ductile Cast Iron Using a Buffer-Layer and Post Weld Heat Treatment. Coatings. 2023; 13(7):1137. https://doi.org/10.3390/coatings13071137
Chicago/Turabian StyleTriani, Rafael Magalhães, José Benedito Tosoni Decarlis Rodrigues Neto, Pedro Gabriel Bonella De Oliveira, Galtiere Corrêa Rêgo, Amadeu Lombardi Neto, and Luiz Carlos Casteletti. 2023. "In-Situ Production of Metal Matrix Composites Layers by TIG Surface Alloying to Improve Wear Resistance of Ductile Cast Iron Using a Buffer-Layer and Post Weld Heat Treatment" Coatings 13, no. 7: 1137. https://doi.org/10.3390/coatings13071137
APA StyleTriani, R. M., Neto, J. B. T. D. R., De Oliveira, P. G. B., Rêgo, G. C., Neto, A. L., & Casteletti, L. C. (2023). In-Situ Production of Metal Matrix Composites Layers by TIG Surface Alloying to Improve Wear Resistance of Ductile Cast Iron Using a Buffer-Layer and Post Weld Heat Treatment. Coatings, 13(7), 1137. https://doi.org/10.3390/coatings13071137







