1. Introduction
Production of high-quality hot asphalt concrete mix is impossible without the application of a carefully selected, finely ground filler fraction of less than 0.063 mm. This asphalt concrete component should perform the following functions due to the presence of a large specific surface area (filler aggregate should be 90%–95% of the total surface area of grains that are part of asphalt concrete): increase the number of contacts between asphalt concrete structural components; fill small pores between asphalt concrete larger particles; and transfer bitumen from bulk state to film, forming from bitumen asphalt binder–mastic binding (asphalt binder–mastic). The most common variant of filler aggregate in the world that performs the described functions is mineral powder (MP) from the main carbonate sedimentary rocks (limestone, dolomites, dolomitized limestone, and their varieties) containing calcium carbonate (CaCO
3) depending on the following categories: more than 70 (CC
70), more than 80 (CC
80), or more than 90 (CC
90) wt.%. The category is chosen depending on the used asphalt concrete mix and such external parameters as traffic intensity, pavement traffic load, climatic factors, and surface texture and traffic noise emissions [
1,
2,
3,
4,
5,
6,
7,
8,
9].
MP absorbs a significant part of petroleum bitumen due to the high adsorbing surface, thus giving asphalt concrete the necessary characteristics: mechanical strength and resistance to deformation, which significantly improves the road surface quality and increases its service life. This significantly improves the quality of the resulting asphalt concrete, and the entire future road surface.
Many researchers [
9,
10,
11,
12,
13,
14,
15,
16,
17,
18,
19,
20] suggest the application other filler aggregate options, such as cement or waste cement dust, various types of ash and slag, gabbro, trachybasalt, quartz sandstone, rocks from postglacial deposits, activated carbon powder, sugar industry waste materials, and many others.
Industrial by-products containing combustible residues are used as alternative fuel. Wastes from paper sludge (PS) also belong to them. Currently, this waste in the form of wet sludge is mostly stored in dumps, which initiates the problem of disposal and environmental pollution. Partly after drying, this waste is used in road construction to consolidate the soil, in the ceramic industry, and in agriculture. It is also incinerated, forming the so-called wastepaper sludge ash (WSA).
Previous studies [
21,
22,
23] have investigated the application of WSA as a substitute for traditional limestone mineral powder (LMP) in hot mix asphalt (HMA) preparation. It has been proven [
21] that this material can completely or partially replace traditional LMP for HMA. As a result of replacing 50 wt.% of LMP with WSA, we obtain asphalt concrete material, which is not significantly inferior to the characteristics of asphalt concrete using LMP without foreign inclusions. Therefore, the task was to bring WSA to the conditional composition for the complete replacement of LMP in hot asphalt concrete without deterioration, and possibly with improvement in the properties of the latter. To carry this out, the authors proposed to activate WSA. As is known, depending on the formation mechanism of new active centers on the surface of the filler aggregate, activation is divided into the following categories: physical (mechanical activation); chemical; and physico-chemical. Mechanoactivation is based on the change in reactivity at the contact surface of mineral materials under the action of mechanical forces [
24]. Mechanoactivation is carried out by a ball, drum, hammer, planetary, centrifugal shock, and jet mills [
25].
Chemical activation methods [
8,
26,
27,
28] involve obtaining activated mineral powder (AMP) by adding activators to the raw material or to the crushed material, followed by grinding and mixing in mills. For activation by this method, cationic (higher aliphatic (fatty)) amines and aminoamides or their salts, as well as quaternary ammonium salts compounds) and anionic surfactants, such as higher carboxylic acids (synthetic fatty acids C
17-C
20, cubic residues of synthetic fatty acids), organic binders (bitumen and modified bitumen), bitumen plasticizers (tar and fuel oil, industrial oils, oils of vegetable industrial crops, some fuels), oligomers solutions (urea-formaldehyde resin, epoxy resins and polymer-containing wastes from epoxy resin production, rubber mainly with low molecular weight), inorganic substances (cement, slaked lime, various rocks), chemical reagents (calcium chloride solutions, salt solutions of iron, copper, and lead) are used.
The essence of physico-chemical activation is that the process of grinding raw materials is accompanied by the treatment of mineral powder with a mixture of bitumen and an activator. Moreover, the activator is injected at a time when the chemical activity of the newly formed surfaces is maximum.
In our case, the chemical activation of WSA by acid tar in pure (AT) and neutralized form (NAT) in a ball mill was chosen. AT was chosen as the activator because this by-product of oil refining technically belongs to the group of bitumen plasticizers, and ecologically, the problem of its utilization is not completely solved. In the refining industry, sulfuric acid was used extensively as a reagent to purify unwanted components of distillate and residual oil fractions, paraffins, kerosene, and gasoline. Purification was performed to remove unsaturated hydrocarbons, sulfur- and nitrogen-containing compounds, and resinous compounds from oil fractions, the presence of which degrades the performance of petroleum products. As a result of this purification, sufficient amounts of AT were formed, which were not utilized but merged into the pits that are still used nowadays [
29,
30,
31,
32,
33].
The authors hypothesized that the combined application of WSA and AT causes the formation of a qualitative AMP in which the concentration of active centers can increase by an average of 2.5–3 times. To test the effectiveness of the proposed new AMP, it was decided to design the composition of stone mastic asphalt (SMA) as the composition of this material requires high content of a mineral powder.
3. Results and Discussion
To obtain the so-called activated mineral powders, which are used in the production of SMA, industrial waste was used, namely paper (WSA and oil refining) AT. This solves two problems: the first is ecological since these wastes (especially AT) pollute the environment during their open storage; and the second problem is the shortage of quality materials for road construction. In other words, this work is dedicated to the creation of a comprehensive approach for the gradual and effective disposal of harmful acid tar dumps and paper production waste in order to obtain new ecological road-construction materials. The study of these components was carried out at the first stage.
3.1. Properties of WSA and LMP, as a Filler Aggregate for SMA
First, elemental WSA and LMP were determined using XRF analysis (
Figure 2 and
Figure 3,
Table 7). LMP was studied in parallel with WSA because it is a traditional mineral powder.
The obtained results (
Table 7) show that WSA has a lower content of Ca and higher contents of Si and Al compared to traditional LMP, indicating the presence of sand (SiO
2) and alumina (Al
2O
3) in WSA. Therefore, it is necessary to study the mineralogical composition of WSA.
It is known that the mineralogical composition of the obtained WSA significantly depends on the technology and conditions of PS firing. Then, the mineralogical composition of PS (
Figure 4,
Table 8) and WSA (
Figure 5,
Table 9) was determined using XRD analysis.
Table 8 shows that PS mainly consists of calcite (70.1 wt.%). Calcite undergoes thermal and thermochemical dissociation during firing. Calcite transformation as a result of firing is confirmed by its decrease from 70.1 wt.% (
Table 8) to 25.5 wt.% (
Table 9).
The theoretical temperature of thermal dissociation of CaCO
3 is 910 °C:
Thermochemical dissociation of CaCO
3 begins in the presence of reactive active phases in PS, particularly kaolinite (15.1 wt.%;
Table 8), already at a temperature of 600–700 °C, according to the following scheme:
The passage of this reaction indicates the presence of Larnite (13.8 wt.%) and Dmitryivanovite (6.3 wt.%) phases in WSA (
Table 9). This process is the beginning of solid phase interactions, due to which the phase composition of WSA is formed.
Figure 6 shows the PS derivative diagram, which characterizes the processes occurring in the solid-fuel heat generator during the burning of PS briquettes.
PS briquettes that burn in the combustion chamber have an even higher temperature in their core, which promotes sintering and its subsequent slag under the influence of combustion products and carbon dioxide from CaCO3 decarburization.
The derivative diagram illustrates the dynamics and phase transformations of PS combustion. At the intersection points of the DTA curve with its “zero” line, five characteristic zones are distinguished (
Figure 6):
- (1)
evaporation of moisture and physically bound H2O (0–200 °C);
- (2)
combustion of volatiles (200–480 °C);
- (3)
coke burning and calcite interaction with kaolinite (480–700 °C);
- (4)
calcite decarburization (700–800 °C);
- (5)
formation of Ca silicates and Ca aluminates (>800 °C), which proves their high content in WSA (
Table 9).
The humidity of PS was W = 4% (according to the projections of the TG curve points in the first zone) and the yield of volatile substances was 96 − 64 = 32% (according to the TG projection in the second zone).
Comparing
Table 8 and
Table 9, the mineralogical composition of WSA is much more complex compared to PS and depends on many factors.
Also, for comparison, the mineralogical composition of traditional LMP (
Figure 7;
Table 10), which is used to obtain different types of SMA, was determined using XRD analysis.
Commercial LMP belongs to the highest category in terms of calcium carbonate content according to [
1], namely CC
90, as it contains more than 90 wt. % calcium carbonate (99 wt.%;
Table 10). PS is more similar in mineralogical composition to traditional LMP than WSA, but has significant disadvantages:
- (1)
high humidity;
- (2)
high flammability due to the presence of volatile substances (already ignites at 80–100 °C);
- (3)
impossibility of achieving the required granulometric composition (≤0.063 mm).
Therefore, fired PS, i.e., WSA, which is devoid of all these disadvantages, was used as a traditional substitute for MR in the production of SMA.
3.2. Activator for LMP and WSA
As LMP and WSA activators, other wastes were used, already from oil refineries, such as AT. The expediency of neutralizing BP and obtaining NAT was also studied. To study the chemical structure of these activators, FTIR spectra were recorded for AT and NAT (
Figure 8).
AT and NAT consist of three main components:
- (1)
hydrocarbons (R–H);
- (2)
interaction products of Hydrocarbons with sulfuric acid (R–O–SO2–R’; R–O–SO2–O–R’);
- (3)
acids (R–O–SO2–OH; H2SO4) in the case of AT and salts (R–O–SO2–ONa; Na2SO4) in the case of NAT.
The interpretation of the main absorption bands of the FTIR spectra of AT and NAT is given in
Table 11.
3.3. Activated Filler Aggregate
LMP and WSA were activated by AT and NAT in a ball mill. After that, the analysis of the obtained AMP was carried out—the wettability index was determined as shown in
Figure 9 and
Figure 10.
Figure 9 and
Figure 10 show that deactivated LMP and WSA are hydrophilic, that is, they were well wetted by water. All obtained AMP samples were hydrophobic, that is, they were poorly wetted by water, which is the main requirement for AMP.
AMP application in road construction makes it possible to significantly increase the resistance of such roads to the effects of moisture and frost, and as a result, reduce the occurrence of cracks and increase the service life of such a coating.
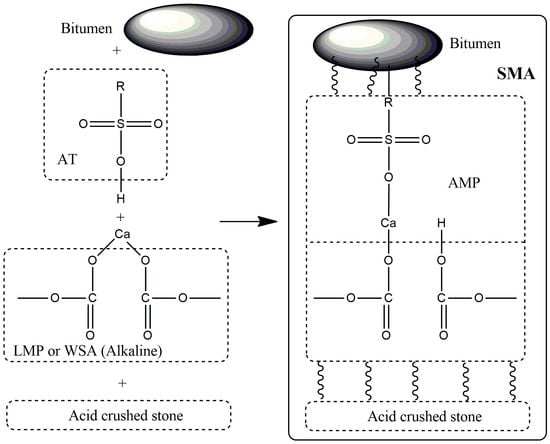
Figure 11 shows possible chemical transformations in SMA using MP activated by AT.
AT can be neutralized directly in MP (LMP or WSA), while a strong bond between AT and MP is formed, that is, chemical activation takes place. The presence of acids (R–O–SO
2–OH; H
2SO
4) in AT was confirmed by FTIR spectroscopy (
Table 11), and the presence of alkaline components in LMP and WSA was confirmed by XRD analysis (
Table 10 and
Table 9, respectively). For example, the following chemical reactions are possible between acids and calcite during AT activation:
This interaction does not occur with NAT as an activator.
In our opinion, the formed AMPs do not pose a significant threat to the environment since the content of the AT activator in AMPs is insignificant (5 wt. % over MP mass) and chemical neutralization of sulfonic acids contained in AT is also possible. However, it is advisable to investigate the aspects related to environmental impact, which will be studied in further research.
3.4. Stone Mastic Asphalt
To determine the influence of WSA and LMP on the physical and mechanical properties of SMA, six compositions of SMA 8 were selected (
Table 12).
The analysis of
Table 12 indicates that WSA can completely replace traditional LMP since the main physical and mechanical properties of SMA 8 using WSA are practically the same as using traditional LMP. However, the content of the pure phase of calcite in WSA (25.5 wt.%) (
Table 9) and LMP (99.1 wt.%) (
Table 11) differs significantly. In other words, the low content of calcium carbonate in WSA does not lead to a negative impact on the main physical and mechanical parameters of the obtained SMA 8.
During the activation of LMP and WSA (
Table 12), AT decreases water saturation, and in the case of WSA, this decrease is much greater (from 2.3 to 1.1 vol %), which indicates the high hydrophobicity of AMP (
Figure 10) and the undergoing chemical activation (
Table 12). Compression strength at 20 °C also increases slightly with the activation of AT.
The use of NAT for the activation of LMP and WSA is impractical, as all the investigated physical and mechanical properties of SMA 8 deteriorate, due to the impossibility of chemical activation (NAT + LMP or WSA).
4. Conclusions
The current study was conducted by means of the application of oil refining (acid tar) and paper (wastepaper sludge ash) waste to obtain new road materials. Therefore, two problems are solved: the first is ecological, and the second is a shortage of building materials.
First, the study of these materials was carried out using X-ray diffraction analysis, and the mineralogical composition of wastepaper sludge ash was established; compared to the composition of traditional limestone mineral powder, it is much more complex. In addition to the calcite phase, it also contains other phases in significant quantities. Using FTIR spectroscopy, the presence of free sulfuric acid and organic sulfonic acids was established in acid tar.
The chemical activation process of wastepaper sludge ash and limestone mineral powder with original and neutralized acid tar was carried out in a ball mill. According to the Ukrainian method, it was established that the inactivated wastepaper sludge ash and limestone mineral powder are hydrophilic, that is, well wetted with water, and the obtained activated samples are hydrophobic, i.e., poorly wetted with water, which is the main requirement for the production of activated mineral powder.
The obtained results show that wastepaper sludge ash can completely replace traditional limestone mineral powder since the main physical and mechanical parameters of stone mastic asphalt (SMA 8) with their use are practically the same.
The activation efficiency of the initial acid tar of wastepaper sludge ash and limestone mineral powder has been proven, allowing for SMA 8 to increase water resistance, and in the case of wastepaper sludge ash, this reduction is much greater (from 2.3 to 1.1 vol %), which indicates it is highly hydrophobic. The use of neutralized acid tar for activation is not advisable, because there is a deterioration of all the investigated physical and mechanical properties of SMA 8.
The strength characteristics of stone mastic asphalt SMA 8 with activated filler aggregate (using AT) increase slightly by 0.1–0.3 MPa, but water saturation decreases by 17–52%.
The obtained research results are valid for other types of asphalt concrete with high probability.