Lignin and Starch Derivatives with Selenium Nanoparticles for the Efficient Reduction of Dyes and as Polymer Fillers
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
2.2.1. Preparation of Sodium Starch Octenyl Succinate
2.2.2. Synthesis of SeNPs
2.2.3. Preparation of SeNPs–Matrix/Polymer
2.2.4. SEM—Surface Composition and Imaging
2.2.5. Catalytic Properties
2.2.6. Thermogravimetric Analysis
2.2.7. Mechanical Properties
2.2.8. Optical Microscopy
2.2.9. Atomic Force Microscopy (AFM)
3. Results and Discussion
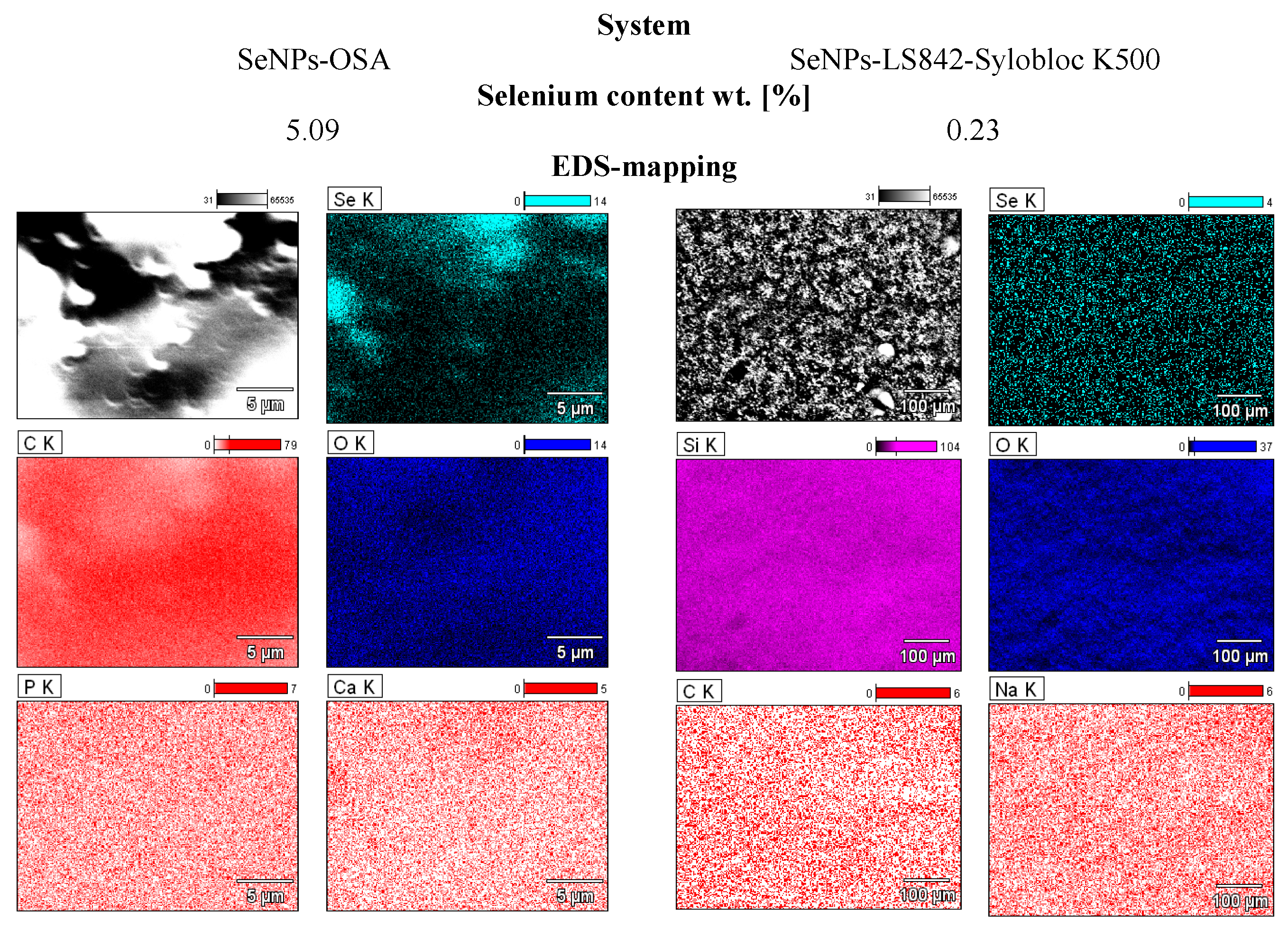
3.1. Energy Dispersion Microanalysis (EDS) and SEM Imaging (Filler)
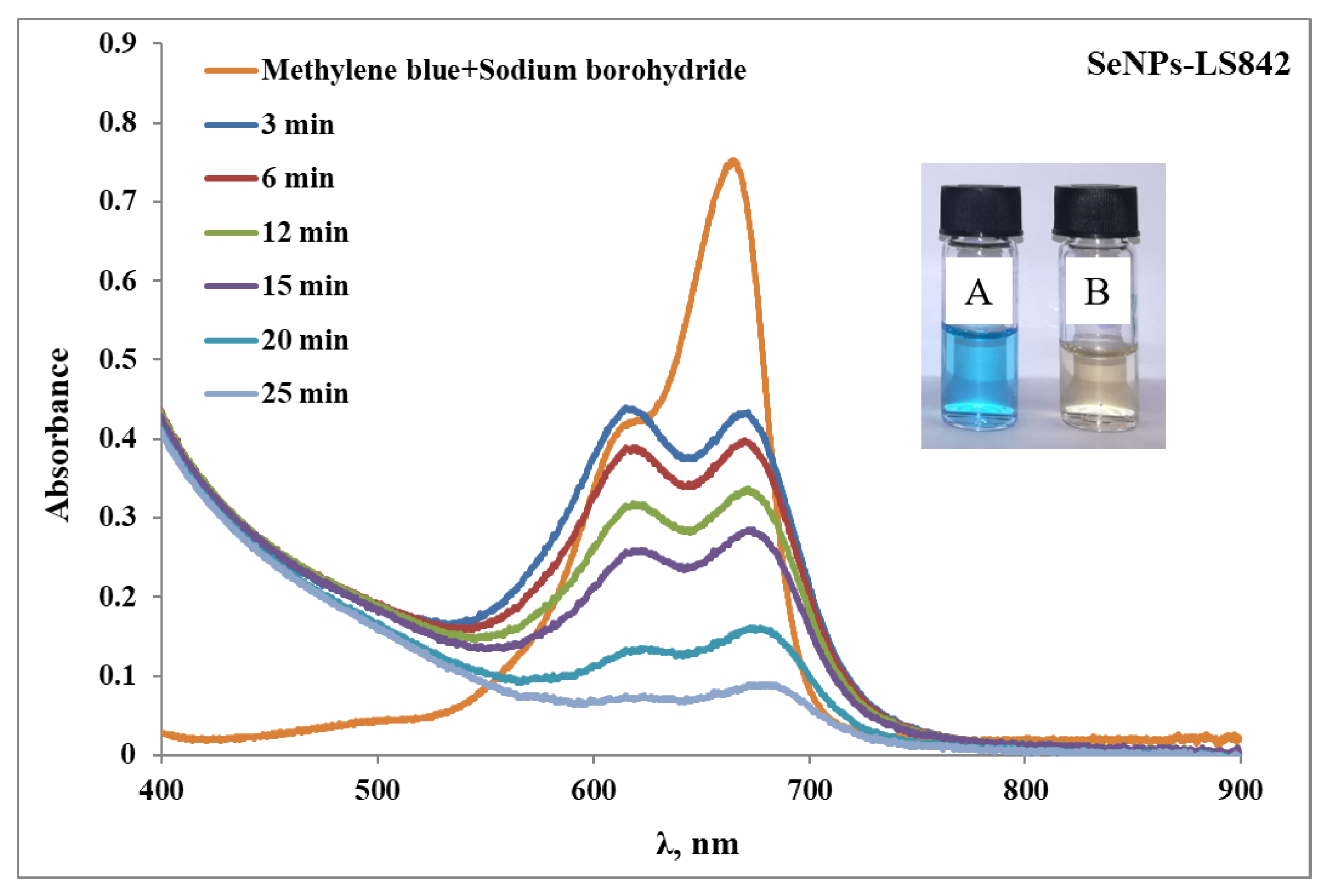
3.2. Selenium as a Catalyst for Reduction Reactions
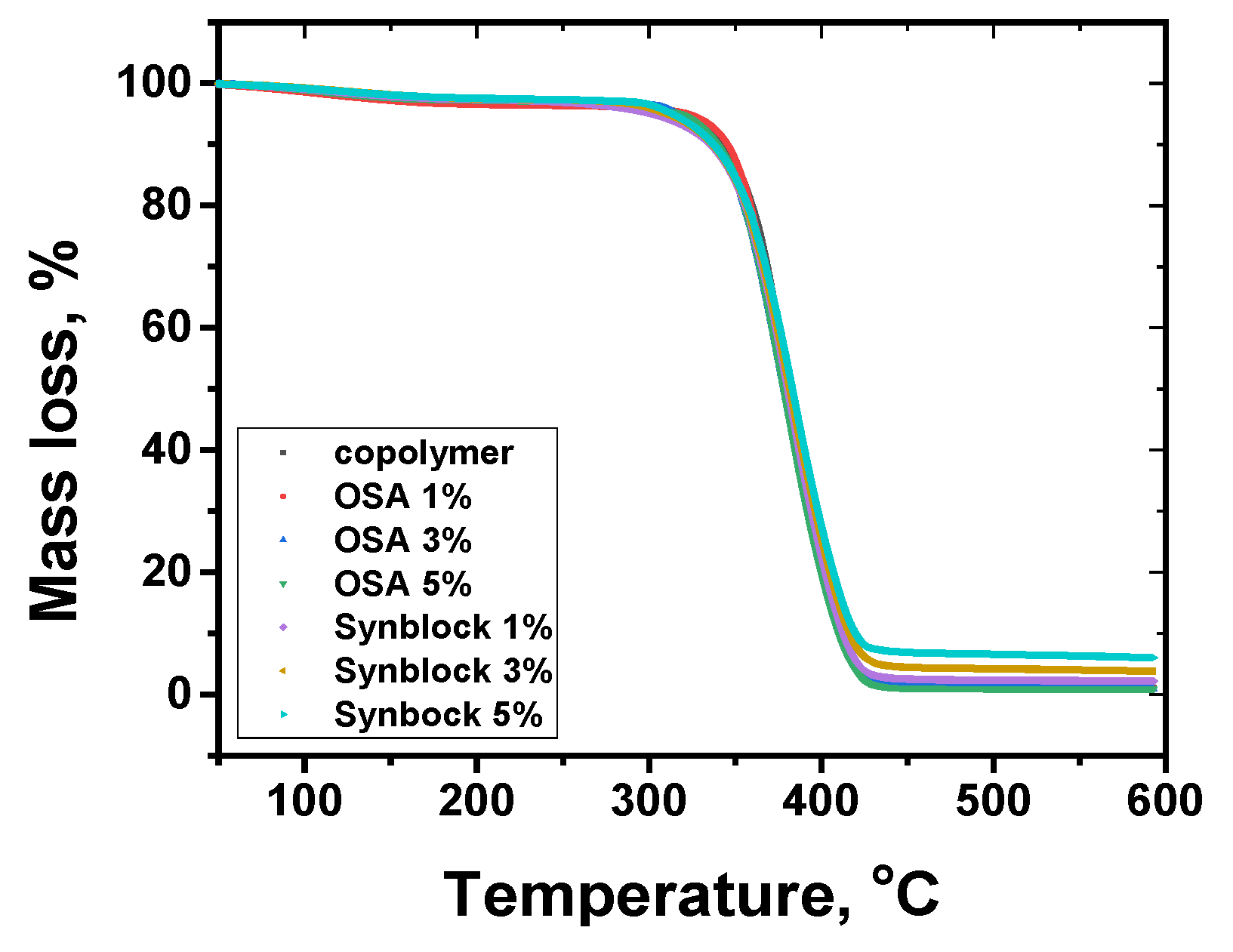
3.3. Thermogravimetric Analysis
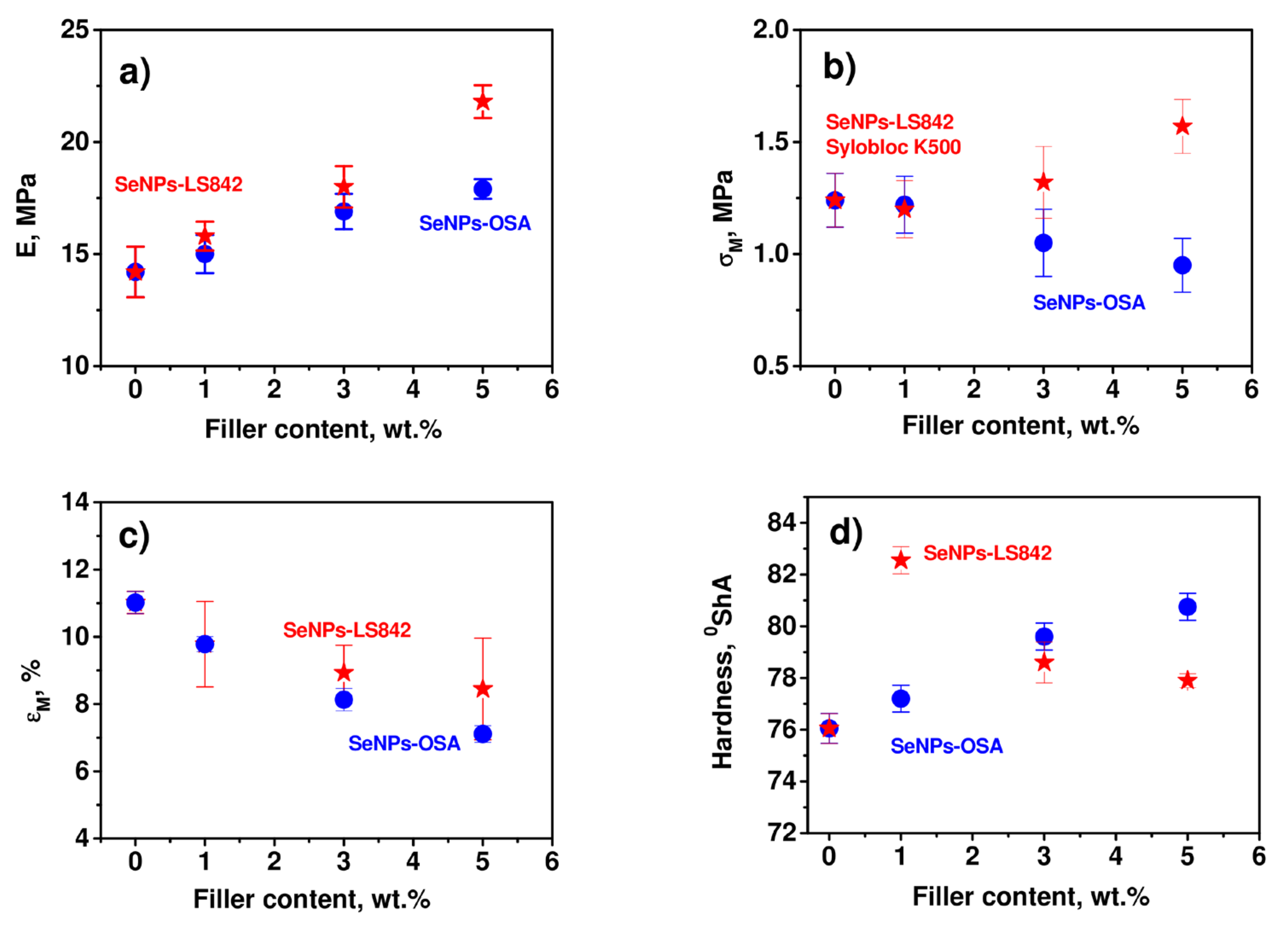
3.4. Influence of the SeNPs–Carrier System on the Mechanical Parameters of the Composite Materials
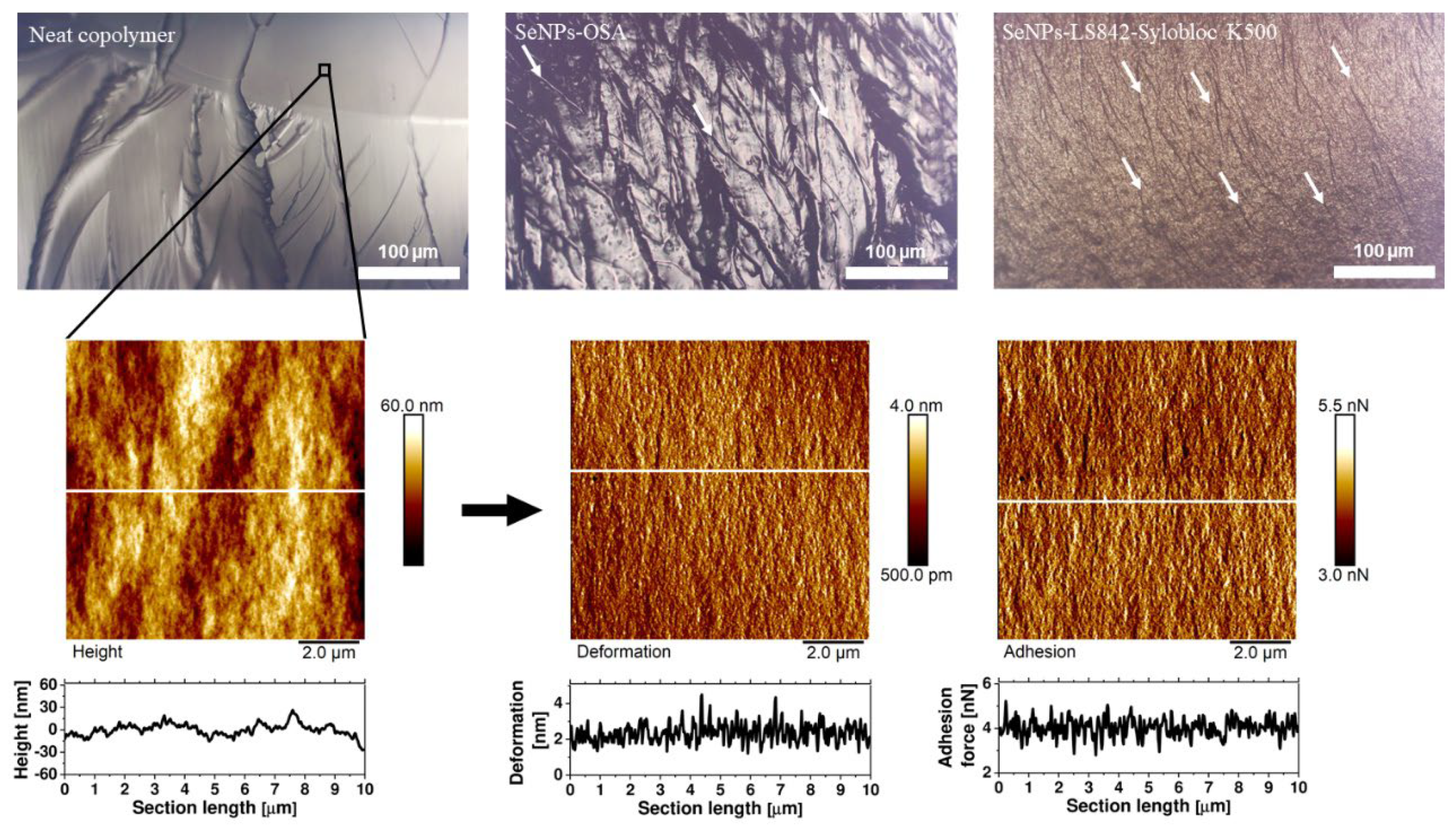
3.5. AFM and Optical Microscopy Analysis of the Composites
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Presentato, A.; Piacenza, E.; Anikovskiy, M.; Cappelletti, M.; Zannoni, D.; Turner, R.J. Biosynthesis of selenium-nanoparticles and -nanorods as a product of selenite bioconversion by the aerobic bacterium Rhodococcus aetherivorans BCP1. New Biotechnol. 2018, 41, 1–8. [Google Scholar] [CrossRef]
- Diko, C.S.; Zhang, H.; Lian, S.; Fan, S.; Li, Z.; Qu, Y. Optimal synthesis conditions and characterization of selenium nanoparticles in Trichoderma sp. WL-Go culture broth. Mater. Chem. Phys. 2020, 246, 122583. [Google Scholar] [CrossRef]
- Vahdati, M.; Moghadam, T.T. Synthesis and Characterization of Selenium Nanoparticles-Lysozyme Nanohybrid System with Synergistic Antibacterial Properties. Sci. Rep. 2020, 10, 510. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tripathi, R.M.; Hameed, P.; Rao, R.P.; Shrivastava, N.; Mittal, J.; Mohapatra, S. Biosynthesis of Highly Stable Fluorescent Selenium Nanoparticles and the Evaluation of Their Photocatalytic Degradation of Dye. Bionanoscience 2020, 10, 389–396. [Google Scholar] [CrossRef]
- Jamróz, E.; Kulawik, P.; Kopel, P.; Balková, R.; Hynek, D.; Bytesnikova, Z.; Gagic, M.; Milosavljevic, V.; Adam, V. Intelligent and active composite films based on furcellaran: Structural characterization, antioxidant and antimicrobial activities. Food Packag. Shelf Life 2019, 22, 100405. [Google Scholar] [CrossRef]
- Menon, S.; Ks, S.D.; Santhiya, R.; Rajeshkumar, S.; Kumar, V. Selenium nanoparticles: A potent chemotherapeutic agent and an elucidation of its mechanism. Colloids Surf. B Biointerfaces 2018, 170, 280–292. [Google Scholar] [CrossRef]
- Umar, A.; Khan, M.S.; Alam, S.; Zekker, I.; Burlakovs, J.; Rubin, S.S.; Bhowmick, G.D.; Kallistova, A.; Pimenov, N.; Zahoor, M. Synthesis and Characterization of Pd-Ni Bimetallic Nanoparticles as Efficient Adsorbent for the Removal of Acid Orange 8 Present in Wastewater. Water 2021, 13, 1095. [Google Scholar] [CrossRef]
- Lai, S.K.M.; Tang, H.-W.; Lau, K.C.; Ng, K.M. Nanosecond UV Laser Ablation of Gold Nanoparticles: Enhancement of Ion Desorption by Thermal-Driven Desorption, Vaporization, or Phase Explosion. J. Phys. Chem. C 2016, 120, 20368–20377. [Google Scholar] [CrossRef]
- Panahi-Kalamuei, M.; Salavati-Niasari, M.; Zarghami, Z.; Mousavi-Kamazani, M.; Taqriri, H.; Mohsenikia, A. Synthesis and characterization of Se nanostructures via co-precipitation, hydrothermal, microwave and sonochemical routes using novel starting reagents for solar cells. J. Mater. Sci. Mater. Electron. 2015, 26, 2851–2860. [Google Scholar] [CrossRef]
- Qiao, L.; Dou, X.; Yan, S.; Zhang, B.; Xu, C. Biogenic selenium nanoparticles synthesized by Lactobacillus casei ATCC 393 alleviate diquat-induced intestinal barrier dysfunction in C57BL/6 mice through their antioxidant activity. Food Funct. 2020, 11, 3020–3031. [Google Scholar] [CrossRef]
- Mates, I.; Antoniac, I.; Laslo, V.; Vicas, S.; Brocks, M.; Luminita, F.; Milea, C.; Mohan, A.; Cavalu, S. Selenium nanoparticles: Production, characterization and possible applications in biomedicine and food science. Sci. Bull. B Chem. Mater. Sci. UPB 2019, 81, 205–216. [Google Scholar]
- Alam, H.; Khatoon, N.; Raza, M.; Ghosh, P.C.; Sardar, M. Synthesis and Characterization of Nano Selenium Using Plant Biomolecules and Their Potential Applications. BioNanoScience 2019, 9, 96–104. [Google Scholar] [CrossRef]
- Sohouli, E.; Irannejad, N.; Ziarati, A.; Ehrlich, H.; Rahimi-Nasrabadi, M.; Ahmadi, F.; Luque, R. Application of polysaccharide-based biopolymers as supports in photocatalytic treatment of water and wastewater: A review. Environ. Chem. Lett. 2022, 20, 3789–3809. [Google Scholar] [CrossRef]
- Mukherjee, S.; Mukhopadhyay, S. Recent progress in sulfur-containing technical lignin-based polymer composites. Express Polym. Lett. 2023, 17, 120–151. [Google Scholar] [CrossRef]
- Ndwandwe, B.K.; Malinga, S.P.; Kayitesi, E.; Dlamini, B.C. Selenium nanoparticles–Enhanced potato starch film for active food packaging application. Int. J. Food Sci. Technol. 2022, 57, 6512–6521. [Google Scholar] [CrossRef]
- Modrzejewska-Sikorska, A.; Konował, E.; Klapiszewski, Ł.; Nowaczyk, G.; Jurga, S.; Jesionowski, T.; Milczarek, G. Lignosulfonate-stabilized selenium nanoparticles and their deposition on spherical silica. Int. J. Biol. Macromol. 2017, 103, 403–408. [Google Scholar] [CrossRef]
- Hu, T.Q. Chemical Modification, Properties, and Usage of Lignin; Springer: New York, NY, USA, 2002. [Google Scholar]
- Belgacem, A.G.M.N. Monomers, Polymers and Composites from Renewable Resources, 1st ed.; Elsevier: Amsterdam, The Netherlands, 2008. [Google Scholar]
- Qiu, X.; Kong, Q.; Zhou, M.; Yang, D. Aggregation behavior of sodium lignosulfonate in water solution. J. Phys. Chem. B 2010, 114, 15857–15861. [Google Scholar] [CrossRef] [PubMed]
- Vishtal, A.; Kraslawski, A. Challenges in industrial applications of technical lignins. BioResources 2011, 6, 3547–3568. [Google Scholar] [CrossRef]
- Vanholme, R.; Morreel, K.; Ralph, J.; Boerjan, W. Lignin engineering. Curr. Opin. Plant Biol. 2008, 11, 278–285. [Google Scholar] [CrossRef] [PubMed]
- Pang, Y.X.; Qiu, X.Q.; Yang, D.J.; Lou, H.M. Influence of oxidation, hydroxymethylation and sulfomethylation on the physicochemical properties of calcium lignosulfonate. Colloids Surf. A Physicochem. Eng. Asp. 2008, 312, 154–159. [Google Scholar] [CrossRef]
- Yan, M.; Yang, D.; Deng, Y.; Chen, P.; Zhou, H.; Qiu, X. Influence of pH on the behavior of lignosulfonate macromolecules in aqueous solution. Colloids Surf. A Physicochem. Eng. Asp. 2010, 371, 50–58. [Google Scholar] [CrossRef]
- Deng, Y.; Wu, Y.; Qian, Y.; Ouyang, X.; Yang, D.; Qiu, X. Adsorption and desorption behaviors of lignosulfonate during the self-assembly of multilayers. BioResources 2010, 2010, 1178–1196. [Google Scholar]
- Konował, E.; Modrzejewska-Sikorska, A.; Milczarek, G. Synthesis and multifunctional properties of lignosulfonate-stabilized gold nanoparticles. Mater. Lett. 2015, 159, 451–454. [Google Scholar] [CrossRef]
- Modrzejewska-Sikorska, A.; Konował, E.; Cichy, A.; Nowicki, M.; Jesionowski, T.; Milczarek, G. The effect of silver salts and lignosulfonates in the synthesis of lignosulfonate-stabilized silver nanoparticles. J. Mol. Liq. 2017, 240, 80–86. [Google Scholar] [CrossRef]
- Modrzejewska-Sikorska, A.; Konował, E. Silver and gold nanoparticles as chemical probes of the presence of heavy metal ions. J. Mol. Liq. 2020, 302, 112559. [Google Scholar] [CrossRef]
- Sweedman, M.C.; Tizzotti, M.J.; Schäfer, C.; Gilbert, R.G. Structure and physicochemical properties of octenyl succinic anhydride modified starches: A review. Carbohydr. Polym. 2013, 92, 905–920. [Google Scholar] [CrossRef]
- Caldwell, C.G.; Wurzburg, O.B. Polysaccharide Derivatives of Substituted Dicarboxylic Acids. U.S. Patent 2,661,349, 1 December 1953. [Google Scholar]
- Khatami, M.; Iravani, S. Green and Eco-Friendly Synthesis of Nanophotocatalysts: An Overview. Comments Inorg. Chem. 2021, 41, 133–187. [Google Scholar] [CrossRef]
- Nguyen, N.T.T.; Nguyen, L.M.; Nguyen, T.T.T.; Liew, R.K.; Nguyen, D.T.C.; van Tran, T. Recent advances on botanical biosynthesis of nanoparticles for catalytic, water treatment and agricultural applications: A review. Sci. Total Environ. 2022, 827, 154160. [Google Scholar] [CrossRef]
- Asl, F.D.; Mousazadeh, M.; Azimzadeh, M.; Ghaani, M. Mesoporous selenium nanoparticles for therapeutic goals: A review. J. Nanoparticle Res. 2022, 24, 210. [Google Scholar] [CrossRef]
- Shah, C.P.; Singh, K.K.; Kumar, M.; Bajaj, P.N. Vinyl monomers-induced synthesis of polyvinyl alcohol-stabilized selenium nanoparticles. Mater. Res. Bull. 2010, 45, 56–62. [Google Scholar] [CrossRef]
- Tsivileva, O.; Pozdnyakov, A.; Ivanova, A. Polymer Nanocomposites of Selenium Biofabricated Using Fungi. Molecules 2021, 26, 3657. [Google Scholar] [CrossRef] [PubMed]
- Prochaska, K.; Kędziora, P.; Le Thanh, J.; Lewandowicz, G. Surface properties of enzymatic hydrolysis products of octenylsuccinate starch derivatives. Food Hydrocoll. 2007, 21, 654–659. [Google Scholar] [CrossRef]
- González-Henríquez, C.M.; Del Pizarro, G.C.; Sarabia-Vallejos, M.A.; Terraza, C.A.; López-Cabaña, Z.E. In situ-preparation and characterization of silver-HEMA/PEGDA hydrogel matrix nanocomposites: Silver inclusion studies into hydrogel matrix. Arab. J. Chem. 2019, 12, 1413–1423. [Google Scholar] [CrossRef] [Green Version]
- Hutter, J.L.; Bechhoefer, J. Calibration of atomic-force microscope tips. Rev. Sci. Instrum. 1993, 64, 1868–1873. [Google Scholar] [CrossRef] [Green Version]
- Aso, O.; Eguiazábal, J.I.; Nazábal, J. The influence of surface modification on the structure and properties of a nanosilica filled thermoplastic elastomer. Compos. Sci. Technol. 2007, 67, 2854–2863. [Google Scholar] [CrossRef]


| Sample Name | Synthesis and Composition | |
|---|---|---|
| SeNPs-LS842 (liquid sample) | 40 mL (5.5 g/L) SeO2 20 mL (42.2 g/L) C6H8O6—reducer 13.33 mL (18.75 g/L) sodium salt of lignosulfonic acid (LS)—liquid stabilizer | The components were stirred for 24 h at room temperature and then allowed to stand quiescent for a few days to complete the reaction. |
| SeNPs-OSA (solid sample) | 40 mL (5.5 g/L) SeO2 20 mL (42.2 g/L) C6H8O6—reducer 3 g sodium starch octenyl succinate (OSA)—solid stabilizer | |
| SeNPs-LS842-Sylobloc K500 (solid sample) | 40 mL (5.5 g/L) SeO2 20 mL (42.2 g/L) C6H8O6—reducer 13.33 mL (18.75 g/L) sodium salt of lignosulfonic acid (LS)—liquid stabilizer Commercial silica Sylobloc K500 used as a solid matrix | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Modrzejewska-Sikorska, A.; Robakowska, M.; Konował, E.; Gojzewski, H.; Gierz, Ł.; Wieczorek, B.; Warguła, Ł.; Łykowski, W. Lignin and Starch Derivatives with Selenium Nanoparticles for the Efficient Reduction of Dyes and as Polymer Fillers. Coatings 2023, 13, 1185. https://doi.org/10.3390/coatings13071185
Modrzejewska-Sikorska A, Robakowska M, Konował E, Gojzewski H, Gierz Ł, Wieczorek B, Warguła Ł, Łykowski W. Lignin and Starch Derivatives with Selenium Nanoparticles for the Efficient Reduction of Dyes and as Polymer Fillers. Coatings. 2023; 13(7):1185. https://doi.org/10.3390/coatings13071185
Chicago/Turabian StyleModrzejewska-Sikorska, Anna, Mariola Robakowska, Emilia Konował, Hubert Gojzewski, Łukasz Gierz, Bartosz Wieczorek, Łukasz Warguła, and Wiktor Łykowski. 2023. "Lignin and Starch Derivatives with Selenium Nanoparticles for the Efficient Reduction of Dyes and as Polymer Fillers" Coatings 13, no. 7: 1185. https://doi.org/10.3390/coatings13071185
APA StyleModrzejewska-Sikorska, A., Robakowska, M., Konował, E., Gojzewski, H., Gierz, Ł., Wieczorek, B., Warguła, Ł., & Łykowski, W. (2023). Lignin and Starch Derivatives with Selenium Nanoparticles for the Efficient Reduction of Dyes and as Polymer Fillers. Coatings, 13(7), 1185. https://doi.org/10.3390/coatings13071185












