Structure and Properties of NbMoCrTiAl High-Entropy Alloy Coatings Formed by Plasma-Assisted Vacuum Arc Deposition
Abstract
1. Introduction
2. Materials and Methods
3. Results
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Cantor, B.; Chang, I.T.H.; Knight, P.; Vincent, A.J.B. Microstructural development in equiatomic multicomponent alloys. Mater. Sci. Eng. A 2004, 375–377, 213–218. [Google Scholar] [CrossRef]
- Yeh, J.W.; Chen, S.K.; Lin, S.-J.; Gan, J.Y.; Chin, T.S.; Shun, T.-T.; Tsau, C.H.; Chang, S.Y. Nanostructured High-Entropy Alloys with Multiple Principal Elements: Novel Alloy Design Concepts and Outcomes. Adv. Eng. Mater. 2004, 6, 299–303. [Google Scholar] [CrossRef]
- Chen, T.K.; Shun, T.T.; Yeh, J.W.; Wong, M.S. Nanostructured nitride films of multi-element high-entropy alloys by reactive DC sputtering. Surf. Coat. Technol. 2004, 188–189, 193–200. [Google Scholar] [CrossRef]
- Chen, T.-K.; Wong, M.-S.; Shun, T.-T.; Yeh, J.-W. Nanostructured nitride films of multi-element high-entropy alloys by reactive DC sputtering. Surf. Coat. Technol. 2005, 200, 1361–1365. [Google Scholar] [CrossRef]
- Kao, Y.-F.; Lee, T.-D.; Chen, S.-K.; Chang, Y.-S. Electrochemical passive properties of AlxCoCrFeNi (x = 0, 0.25, 0.50, 1.00) alloys in sulfuric acids. Corros. Sci. 2010, 52, 1026–1034. [Google Scholar] [CrossRef]
- Ye, Q.; Feng, K.; Li, Z.; Lu, F.; Li, R.; Huang, J.; Wu, Y. Microstructure and corrosion properties of CrMnFeCoNi high entropy alloy coating. Appl. Surf. Sci. 2017, 396, 1420–1426. [Google Scholar] [CrossRef]
- Senkov, O.N.; Wilks, G.B.; Scott, J.M.; Miracle, D.B. Mechanical properties of Nb25Mo25Ta25W25 and V20Nb20Mo20Ta20W20 refractory high entropy alloys. Intermetallics 2011, 19, 698–706. [Google Scholar] [CrossRef]
- Wang, W.-R.; Wang, W.-L.; Wang, S.-C.; Tsai, Y.-C.; Lai, C.-H.; Yeh, J.-W. Effects of Al addition on the microstructure and mechanical property of AlxCoCrFeNi high-entropy alloys. Intermetallics 2012, 26, 44–51. [Google Scholar] [CrossRef]
- Qiao, J.W.; Ma, S.G.; Huang, E.W.; Chuang, C.P.; Liaw, P.K.; Zhang, Y. Microstructural Characteristics and Mechanical Behaviors of AlCoCrFeNi High-Entropy Alloys at Ambient and Cryogenic Temperatures. Mater. Sci. Forum 2011, 688, 419–425. [Google Scholar] [CrossRef]
- Laktionova, M.A.; Tabchnikova, E.D.; Tang, Z.; Liaw, P.K. Mechanical properties of the high-entropy alloy Ag0.5CoCrCuFeNi at temperatures of 4.2–300 K. Low Temp. Phys. 2013, 39, 630–632. [Google Scholar] [CrossRef]
- Wu, J.-M.; Lin, S.-J.; Yeh, J.-W.; Chen, S.-K.; Huang, Y.-S.; Chen, H.-C. Adhesive wear behavior of AlxCoCrCuFeNi high-entropy alloys as a function of aluminum content. Wear 2006, 261, 513–519. [Google Scholar] [CrossRef]
- Chuang, M.-H.; Tsai, M.-H.; Wang, W.-R.; Lin, S.-J.; Yeh, J.-W. Microstructure and wear behavior of AlxCo1.5CrFeNi1.5Tiy high-entropy alloys. Acta Mater. 2011, 59, 6308–6317. [Google Scholar] [CrossRef]
- Zhou, Y.J.; Zhang, Y.; Wang, Y.L.; Chen, G.L. Solid solution alloys of AlCoCrFeNiTix with excellent room-temperature mechanical properties. Appl. Phys. Lett. 2007, 90, 181904. [Google Scholar] [CrossRef]
- Zhang, Y.; Zuo, T.T.; Tang, Z.; Gao, M.C.; Dahmen, K.A.; Liaw, P.K.; Lu, Z.P. Microstructures and properties of high-entropy alloys. Prog. Mater. Sci. 2014, 61, 1–93. [Google Scholar] [CrossRef]
- Lai, C.-H.; Lin, S.-J.; Yeh, J.-W.; Chang, S.-Y. Preparation and characterization of AlCrTaTiZr multi-element nitride coatings. Surf. Coat. Technol. 2006, 201, 3275–3280. [Google Scholar] [CrossRef]
- Lin, C.; Duh, J.; Yeh, J.-W. Multi-component nitride coatings derived from Ti–Al–Cr–Si–V target in RF magnetron sputter. Surf. Coat. Technol. 2007, 201, 6304–6308. [Google Scholar] [CrossRef]
- Chang, H.-W.; Huang, P.-K.; Davison, A.; Yeh, J.-W.; Tsau, C.-H.; Yang, C.-C. Nitride films deposited from an equimolar Al–Cr–Mo–Si–Ti alloy target by reactive direct current magnetron sputtering. Thin Solid Film. 2008, 516, 6402–6408. [Google Scholar] [CrossRef]
- Tsai, M.-H.; Lai, C.-H.; Yeh, J.-W.; Gan, J.-Y. Effects of nitrogen flow ratio on the structure and properties of reactively sputtered (AlMoNbSiTaTiVZr)Nx coatings. J. Phys. D Appl. Phys. 2008, 41, 235402. [Google Scholar] [CrossRef]
- Huang, P.-K.; Yeh, J.-W. Effects of nitrogen content on structure and mechanical properties of multi-element (AlCrNbSiTiV)N coating. Surf. Coat. Technol. 2009, 203, 1891–1896. [Google Scholar] [CrossRef]
- Pogrebnjak, A.D.; Bagdasaryan, A.A.; Yakushchenko, I.V.; Beresnev, V.M. The structure and properties of high-entropy alloys and nitride coatings based on them. Russ. Chem. Rev. 2014, 83, 1027. [Google Scholar] [CrossRef]
- Praveen, S.; Kim, H.-S. High-Entropy Alloys: Potential Candidates for High-Temperature Applications—An Overview. Adv. Eng. Mater. 2018, 20, 1700645. [Google Scholar] [CrossRef]
- Pickering, E.J.; Jones, N.G. High-entropy alloys: A critical assessment of their founding principles and future prospects. Int. Mater. Rev. 2016, 61, 183–202. [Google Scholar] [CrossRef]
- Nene, S.S.; Liu, K.; Frank, M.; Mishra, R.S.; Brennan, R.E.; Cho, K.C.; Li, Z.; Raabe, D. Enhanced strength and ductility in a friction stir processing engineered dual phase high entropy alloy. Sci. Rep. 2017, 7, 16167. [Google Scholar] [CrossRef]
- Li, Z.; Körmann, F.; Grabowski, B.; Neugebauer, J.; Raabe, D. Ab initio assisted design of quinary dual-phase high-entropy alloys with transformation-induced plasticity. Acta Mater. 2017, 136, 262–270. [Google Scholar] [CrossRef]
- Basu, S.; Li, Z.; Pradeep, K.G.; Raabe, D. Strain Rate Sensitivity of a TRIP-Assisted Dual-Phase High-Entropy Alloy. Front. Mater. 2018, 5, 30. [Google Scholar] [CrossRef]
- Li, Z.; Tasan, C.C.; Springer, H.; Gault, B.; Raabe, D. Interstitial atoms enable joint twinning and transformation induced plasticity in strong and ductile high-entropy alloys. Sci. Rep. 2017, 7, 40704. [Google Scholar] [CrossRef] [PubMed]
- Youssef, K.; Roberto, S.R. Applications of salt solutions before and after harvest affect the quality and incidence of postharvest gray mold of ‘Italia’ table grapes. Postharvest Biol. Technol. 2014, 87, 95–102. [Google Scholar] [CrossRef]
- Miracle, D.B.; Miller, J.D.; Senkov, O.N.; Woodward, C.; Uchic, M.D.; Tiley, J. Exploration and Development of High Entropy Alloys for Structural Applications. Entropy 2014, 16, 494–525. [Google Scholar] [CrossRef]
- Ashby, M.; Cebon, D.; Shercliff, H. Materials: Engineering, Science, Processing and Design; Butterworth-Heinemann: Oxford, UK, 2007. [Google Scholar]
- Takeuchi, A.; Amiya, K.; Wada, T.; Yubuta, K.; Zhang, W.; Makino, A. Entropies in Alloy Design for High-Entropy and Bulk Glassy Alloys. Entropy 2013, 15, 3810–3821. [Google Scholar] [CrossRef]
- Inoue, A.; Zhang, T.; Masumoto, T. Glass-forming ability of alloys. J. Non-Cryst. Solids 1993, 156–158, 473–480. [Google Scholar] [CrossRef]
- Glicksman, M.E. Principles of Solidification: An Introduction to Modern Casting and Crystal Growth Concepts; Springer: New York, NY, USA, 2010. [Google Scholar]
- Kumar, A.; Gupta, M. An Insight into Evolution of Light Weight High Entropy Alloys: A Review. Metals 2016, 6, 199. [Google Scholar] [CrossRef]
- Komarov, F.F.; Pogrebnyak, A.D.; Konstantinov, S.V. Radiation Resistance of high-entropy nanostructured (Ti, Hf, Zr, V, Nb)N coatings. Tech. Phys. 2015, 60, 1519–1524. [Google Scholar] [CrossRef]
- Tsai, D.-C.; Lin Huang, Y.; Lin, S.-R.; Liang, S.-C.; Shieu, F.-S. Effect of nitrogen flow ratios on the structure and mechanical properties of (TiVCrZrY)N coatings prepared by reactive magnetron sputtering. Appl. Surf. Sci. 2010, 257, 1361–1367. [Google Scholar] [CrossRef]
- Liang, S.-C.; Chang, Z.-C.; Tsai, D.-C.; Lin, Y.-C.; Sung, H.-H.; Deng, M.-J.; Shieu, F.-S. Effects of substrate bias on structure and mechanical properties of (TiVCrZrHf)N coatings. Appl. Surf. Sci. 2011, 257, 7709–7713. [Google Scholar] [CrossRef]
- Pogrebnjak, A.; Yakuschenko, I.; Bagdasaryan, A.; Bondar, O.; Krause-Rehberg, R.; Abadias, G.; Chartier, P.; Oyoshi, K.; Takeda, Y.; Beresnev, V.; et al. Microstructure, physical and chemical properties of nanostructured (Ti-Hf-Zr-V-Nb)N coatings under different deposition conditions. Mater. Chem. Phys. 2014, 147, 1079–1091. [Google Scholar] [CrossRef]
- Beresnev, V.M.; Nemchenko, U.S.; Litovchenko, S.V.; Sobol, O.V.; Mejlekhov, A.A.; Postel’nik, A.A.; Gorban, G.F.; Stolbovoj, V.A.; Kolesnikov, D.A.; Novikov, V.Y. The influence of nitrogen pressure on the structure of condensates, obtained at vacuum-arc deposition from high entropy alloy AlCrTiZrNbY. Vopr. At. Nauk. I Tekhniki 2016, 2-102, 86–91. [Google Scholar]
- Lai, C.-H.; Tsai, M.-H.; Lin, S.-J.; Yeh, J.-W. Influence of substrate temperature on structure and mechanical, properties of multi-element (AlCrTaTiZr)N coatings. Surf. Coat. Technol. 2007, 201, 6993–6998. [Google Scholar] [CrossRef]
- Serdiuk, I.; Andreev, A.A.; Gorban’, V.; Stolbovoi, V.; Sobol’, O.; Krapivka, N.; Fil’chikov, V. Reproducibility of the Single-Phase Structural State of the Multielement High-Entropy Ti–V–Zr–Nb–Hf System and Related Superhard Nitrides Formed by the Vacuum-Arc Method. Tech. Phys. Lett. 2012, 38, 616–619. [Google Scholar] [CrossRef]
- Gromov, V.E.; Ivanov, Y.F.; Osintsev, K.A.; Shlarova, Y.A.; Panchenko, I.A. High-entropy alloys: Structure and properties. Rusajns: Moscow, Russia, 2022; 204p. [Google Scholar]
- Gromov, V.E.; Konovalov, S.V.; Ivanov, Y.F.; Osintsev, K.A. Structure and Properties of High-Entropy Alloys; Springer: Cham, Switzerland, 2021; 110p. [Google Scholar] [CrossRef]
- Cherenda, N.N.; Rogovaya, I.S.; Shymanski, V.I.; Uglov, V.V.; Saladukhin, I.A.; Astashynski, V.M.; Kuzmitski, A.M.; Ivanov, Y.F.; Petrikova, E.A. Elemental and phase compositions and mechanical properties of titanium surface layer alloyed by zr, nb, and al under the action of compression plasma flows. High Temp. Mater. Process. Int. Q. High-Technol. Plasma Process. 2022, 26, 1–9. [Google Scholar] [CrossRef]
- Tarbokov, V.A.; Slobodyan, M.; Pavlov, S.; Smolyanskiy, E.; Uglov, V.; Remnev, G.E. Changes in adhesion of crn coatings on zr-1%nb alloy substrates preliminary irradiated with high-intense pulsed ion beams. High Temp. Mater. Process. Int. Q. High-Technol. Plasma Process. 2022, 26, 7–19. [Google Scholar] [CrossRef]
- Nikolaev, A.G.; Gushenets, V.I.; Bugaev, A.S.; Oks, E.M.; Vizir, A.V.; Yushkov, G.Y. Dc planar magnetron sputter deposition of boron thin films and their properties. High Temp. Mater. Process. Int. Q. High-Technol. Plasma Process. 2023, 27, 51–56. [Google Scholar] [CrossRef]
- Ivanov, Y.F.; Shugurov, V.V.; Petrikova, E.A.; Krysina, O.V.; Prokopenko, N.A.; Tolkachev, O.S.; Petyukevich, M.S. Electron-ion-plasma synthesis of multilayer cr-b films. High Temp. Mater. Process. Int. Q. High-Technol. Plasma Process. 2023, 27, 11–18. [Google Scholar] [CrossRef]
- Krysina, O.V.; Ivanov, Y.F.; Koval, N.N.; Prokopenko, N.A. The influence of plasma assistance modes on characteristics and composition of pvd zirconium nitride coatings. High Temp. Mater. Process. Int. Q. High-Technol. Plasma Process. 2023, 27, 19–31. [Google Scholar] [CrossRef]
- Tyunkov, A.V.; Andronov, A.A.; Oks, E.M.; Yushkov, Y.G.; Zolotukhin, D.B. Analysis of formation and composition of boron-based protective coatings obtained by electron-beam deposition at fore-vacuum pressures. High Temp. Mater. Process. Int. Q. High-Technol. Plasma Process. 2023, 27, 65–73. [Google Scholar] [CrossRef]
- Ivanov, Y.F.; Koval, N.N.; Krysina, O.V.; Baumbach, T.; Doyle, S.; Slobodsky, T.; Timchenko, N.A.; Galimov, R.M.; Shmakov, A.N. Superhard nanocrystalline Ti–Cu–N coatings deposited by vacuum arc evaporation of a sintered cathode. Surf. Coat. Technol. 2012, 207, 430–434. [Google Scholar] [CrossRef]
- Krysina, O.V.; Koval, N.N.; Shmakov, A.N.; Vinokurov, Z.S. In situ X-ray diffraction investigation of nitride coatings at high-temperature oxidation. J. Phys. Conf. Ser. 2016, 669, 012034. [Google Scholar] [CrossRef]
- Timchenko, N.A.; Zubavichus, Y.V.; Krysina, O.V.; Kuznetsov, S.I.; Syrtanov, M.S.; Bondarenko, S.V. Local structure of titanium nitride-based coatings. Journal of Surface Investigation. X-Ray Synchrotron Neutron Tech. 2016, 10, 425–428. [Google Scholar] [CrossRef]
- Schroeder, J.L.; Thomson, W.; Howard, B.; Schell, N.; Näslund, L.-Å.; Rogström, L.; Johansson-Jõesaar, M.P.; Ghafoor, N.; Odén, M.; Nothnagel, E.; et al. Industry-relevant magnetron sputtering and cathodic arc ultra-high vacuum deposition system for in situ x-ray diffraction studies of thin film growth using high energy synchrotron radiation. Rev. Sci. Instrum. 2015, 86, 095113. [Google Scholar] [CrossRef]
- Shugurov, V.V.; Koval, N.N.; Krysina, O.V.; Prokopenko, N.A. QUINTA equipment for ion-plasma modification of materials and products surface and vacuum arc plasma-assisted deposition of coatings. J. Phys. Conf. Ser. 2019, 1393, 012131. [Google Scholar] [CrossRef]
- Ivanov, Y.F.; Koval, N.N.; Akhmadeev, Y.H.; Uglov, V.V.; Shugurov, V.V.; Petrikova, E.A.; Krysina, O.V.; Prokopenko, N.A.; Azhazha, I.I. Structure and Properties of Multi-Layer Films of High-Entropy Metals Deposited by the Ion-Plasma Method. Russ. Phys. J. 2022, 64, 2207–2213. [Google Scholar] [CrossRef]
- Chang, Y.-Y.; Chung, C.-H. Tribological and Mechanical Properties of Multicomponent CrVTiNbZr(N) Coatings. Coatings 2021, 11, 41. [Google Scholar] [CrossRef]
- Wang, Y.; Li, M.; Wang, H.; Shao, G.; Zhu, J.; Liu, W.; Wang, H.; Fan, B.; Xu, H.; Lu, H.; et al. The Fabrication and Mechanical Properties of Laminated ZrB2-Mo5SiB2 Ceramics with an Mo-Mo5SiB2 Interlayer. Metals 2021, 11, 2018. [Google Scholar] [CrossRef]
- Padamata, S.K.; Yasinskiy, A.; Yanov, V.; Saevarsdottir, G. Magnetron Sputtering High-Entropy Alloy Coatings: A Mini-Review. Metals 2022, 12, 319. [Google Scholar] [CrossRef]
- Devarajan, D.K.; Rangasamy, B.; Amirtharaj Mosas, K.K. State-of-the-Art Developments in Advanced Hard Ceramic Coatings Using PVD Techniques for High-Temperature Tribological Applications. Ceramics 2023, 6, 301–329. [Google Scholar] [CrossRef]
- Grigoriev, S.N.; Sobol, O.V.; Beresnev, V.M.; Serdyuk, I.V.; Pogrebnyak, A.D.; Kolesnikov, D.A.; Nemchenko, U.S. Tribological characteristics of (TiZrHfVNbTa)N coatings applied using the vacuum arc deposition method. J. Frict. Wear 2014, 35, 359–364. [Google Scholar] [CrossRef]

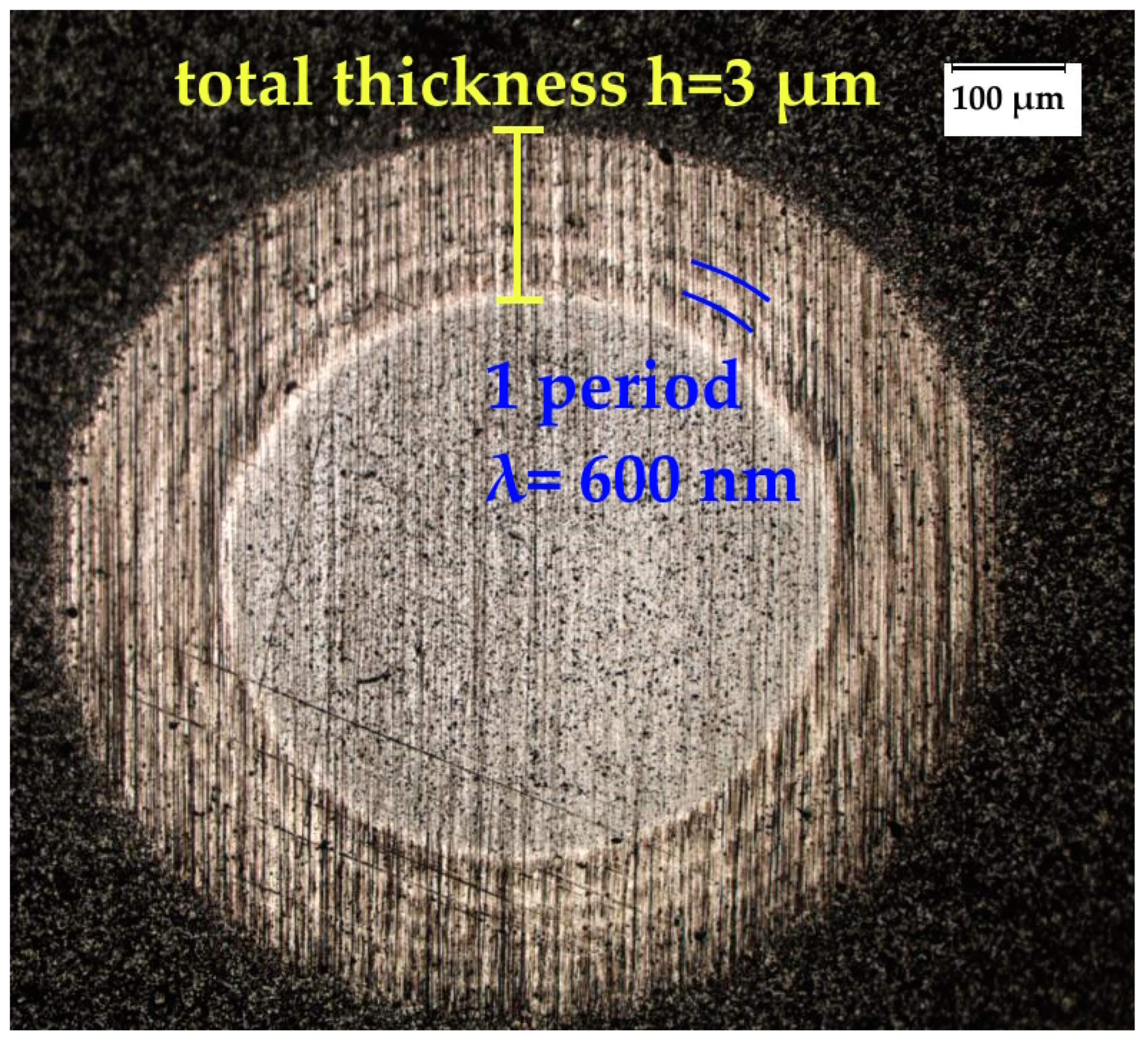
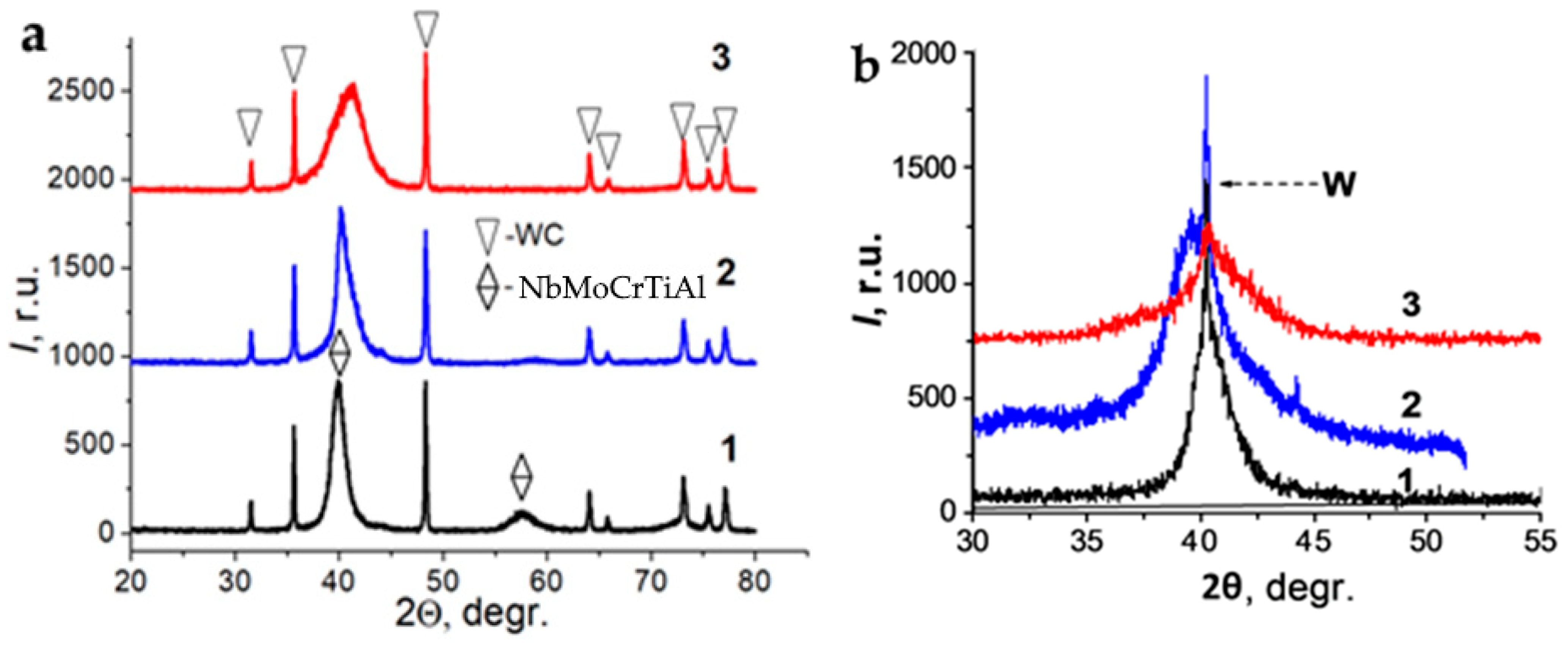

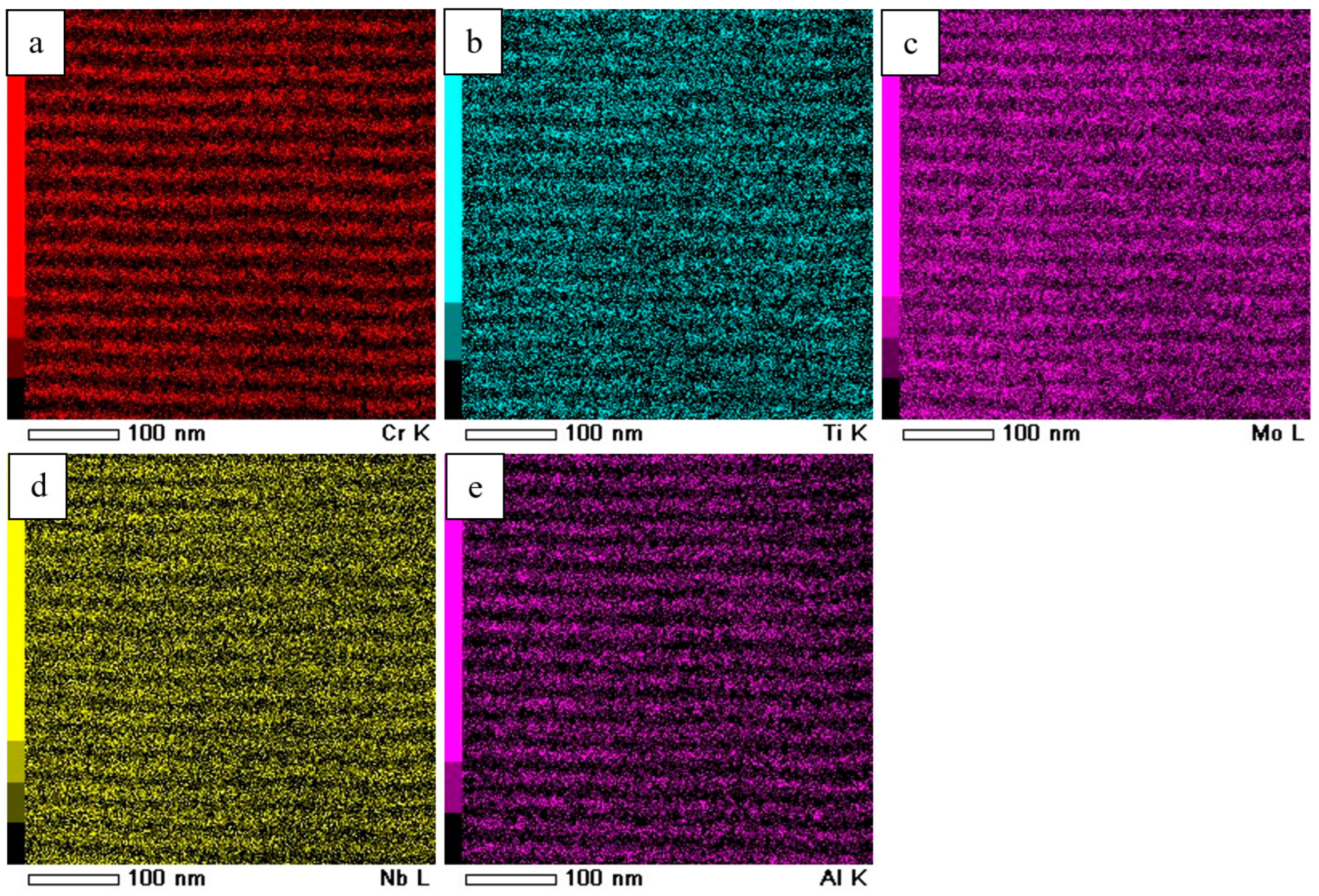
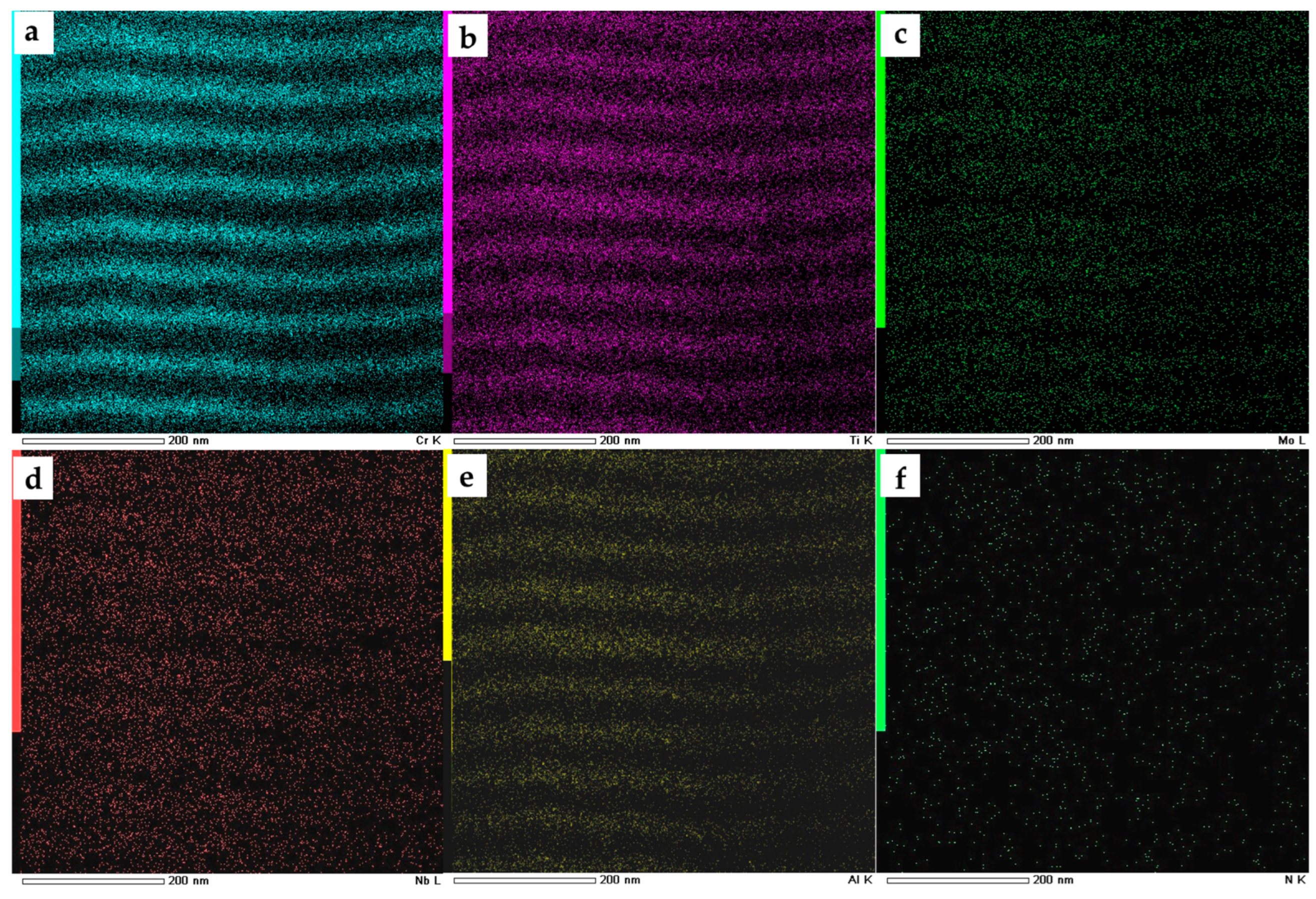
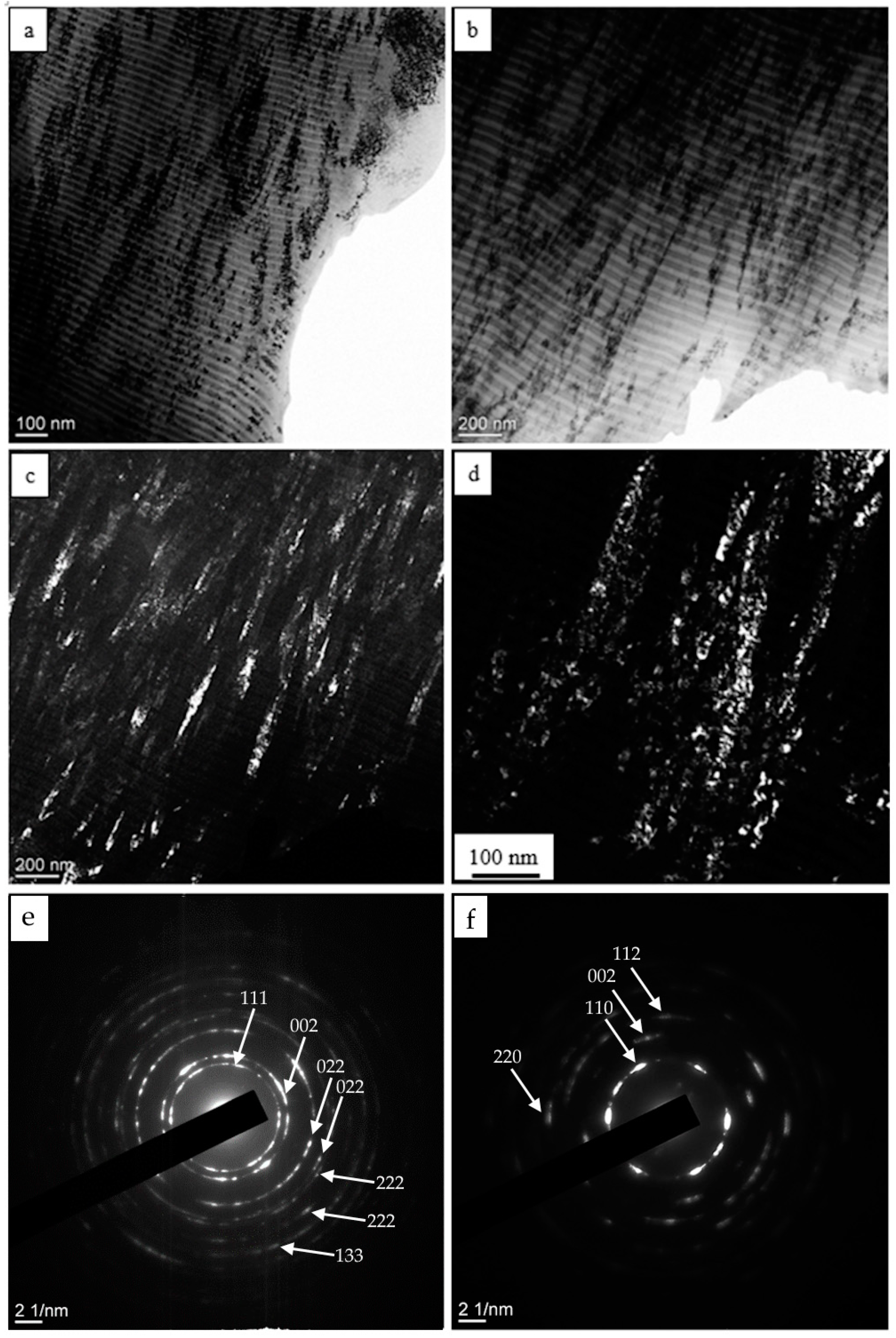
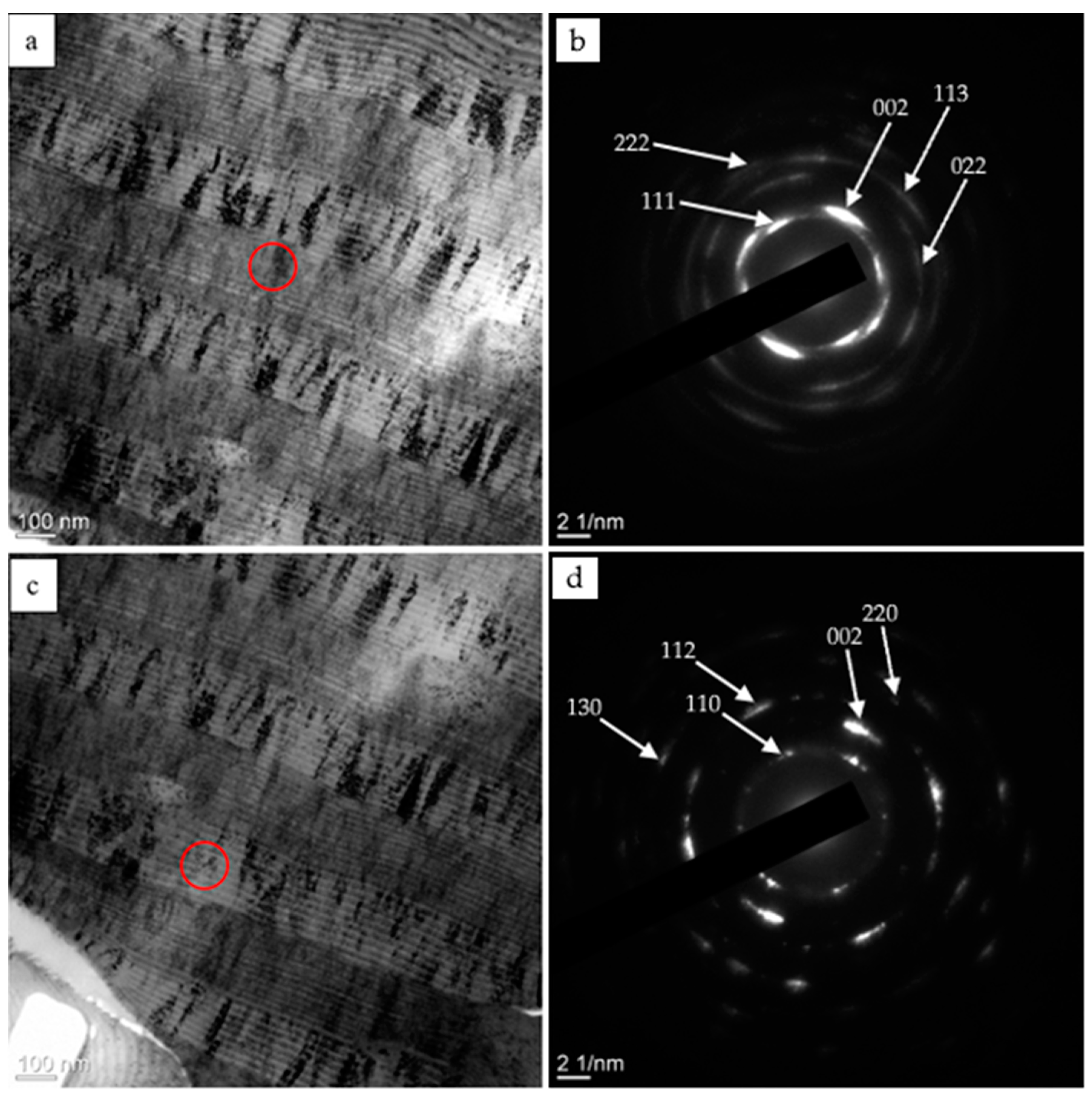
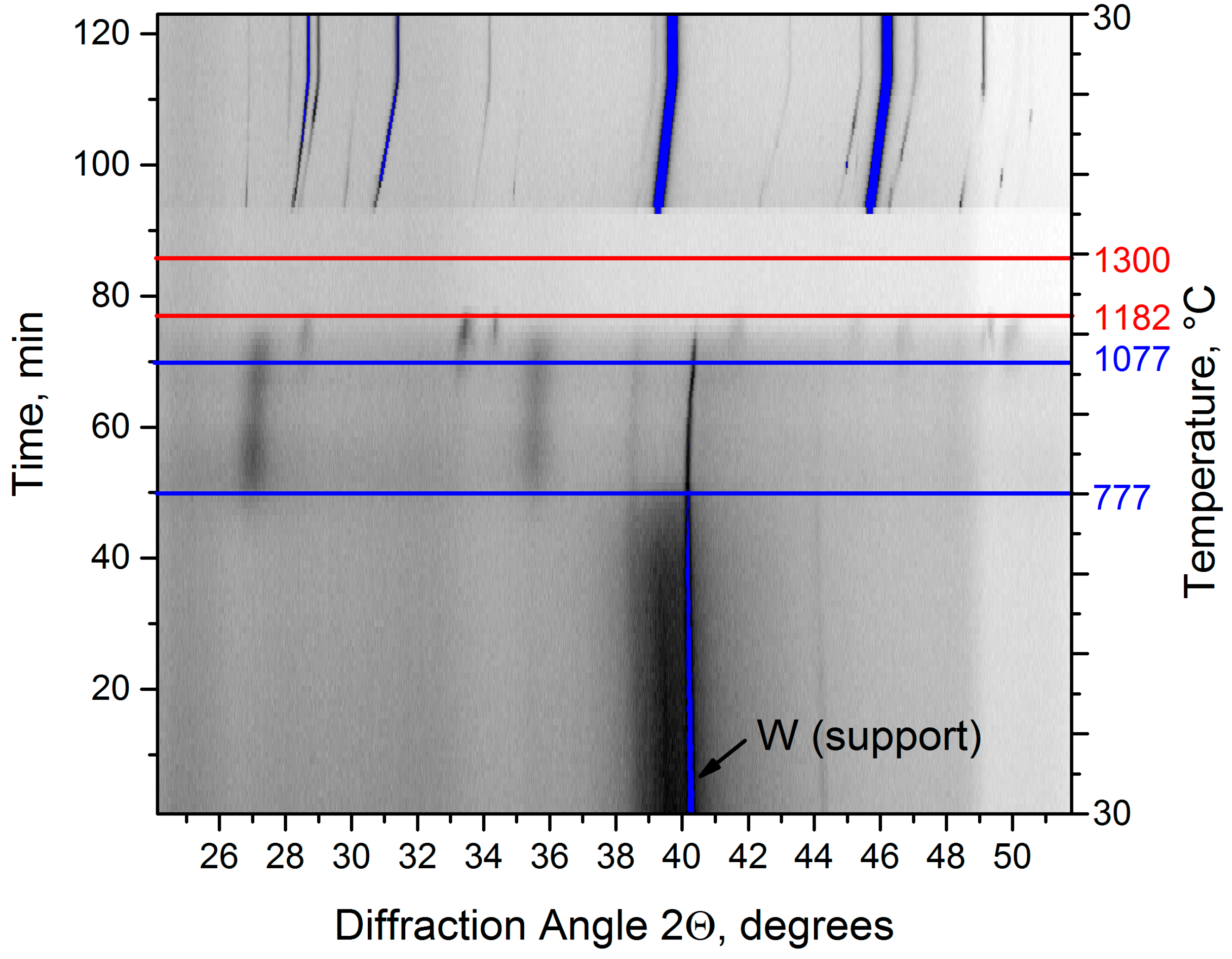
| Evaporated Cathode | Discharge Current, A | Coating Composition, Type | Coating Growth Rate, µm/h ± 10%. |
|---|---|---|---|
| Cr | 150 | Cr, metal | 2.2 |
| CrN, ceramic | 2.4 | ||
| TiAl | 180 | TiAl, metal | 2.4 |
| (Ti,Al)N, ceramic | 5.2 | ||
| Mo | 90 | Mo, metal | 1.1 |
| MoN, ceramic | 1.4 | ||
| Nb | 120 | Nb, metal | 0.9 |
| NbN, ceramic | 2 | ||
| Cr, TiAl, Mo, and Nb together | Discharge currents are indicated above | NbMoCrTiAl, metal | 6.3 |
| (Nb,Mo,Cr,Ti,Al)N, ceramic | 6 | ||
| NbMoCrTiAl/(Nb,Mo,Cr,Ti,Al)N, cermet | 6.2 |
| HEA Coating Type | Elements, at % | |||||
|---|---|---|---|---|---|---|
| Al | Ti | Cr | Nb | Mo | N | |
| Metal | 16.0 | 17.4 | 19.3 | 19.3 | 28.0 | 0.0 |
| Cermet | 9.7 | 17.2 | 11.2 | 17.7 | 21.1 | 23.1 |
| Ceramic | 13.9 | 6.8 | 9.3 | 6.8 | 7.8 | 55.4 |
| Coating Type | HV, GPa | E, GPa | k, 10−5, mm3N−1m−1 | μ |
|---|---|---|---|---|
| Metal | 15 | 113 | 15 | 0.64 |
| Cermet | 27 | 176 | 0.64 | 0.20 |
| Ceramic | 43 | 326 | 0.65 | 0.47 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ivanov, Y.F.; Akhmadeev, Y.K.; Prokopenko, N.A.; Krysina, O.V.; Koval, N.N.; Petrikova, E.A.; Tolkachev, O.S.; Shugurov, V.V.; Uglov, V.V.; Shmakov, A.N. Structure and Properties of NbMoCrTiAl High-Entropy Alloy Coatings Formed by Plasma-Assisted Vacuum Arc Deposition. Coatings 2023, 13, 1191. https://doi.org/10.3390/coatings13071191
Ivanov YF, Akhmadeev YK, Prokopenko NA, Krysina OV, Koval NN, Petrikova EA, Tolkachev OS, Shugurov VV, Uglov VV, Shmakov AN. Structure and Properties of NbMoCrTiAl High-Entropy Alloy Coatings Formed by Plasma-Assisted Vacuum Arc Deposition. Coatings. 2023; 13(7):1191. https://doi.org/10.3390/coatings13071191
Chicago/Turabian StyleIvanov, Yurii F., Yuriy Kh. Akhmadeev, Nikita A. Prokopenko, Olga V. Krysina, Nikolai N. Koval, Elizaveta A. Petrikova, Oleg S. Tolkachev, Vladimir V. Shugurov, Vladimir V. Uglov, and Alexander N. Shmakov. 2023. "Structure and Properties of NbMoCrTiAl High-Entropy Alloy Coatings Formed by Plasma-Assisted Vacuum Arc Deposition" Coatings 13, no. 7: 1191. https://doi.org/10.3390/coatings13071191
APA StyleIvanov, Y. F., Akhmadeev, Y. K., Prokopenko, N. A., Krysina, O. V., Koval, N. N., Petrikova, E. A., Tolkachev, O. S., Shugurov, V. V., Uglov, V. V., & Shmakov, A. N. (2023). Structure and Properties of NbMoCrTiAl High-Entropy Alloy Coatings Formed by Plasma-Assisted Vacuum Arc Deposition. Coatings, 13(7), 1191. https://doi.org/10.3390/coatings13071191









