Thin 1,2,4-Triazole Films for the Inhibition of Carbon Steel Corrosion in Sulfuric Acid Solution
Abstract
1. Introduction
2. Materials and Methods
2.1. Methods
2.1.1. Gravimetric Method
2.1.2. Atomic Force Microscopy (AFM) Method
2.1.3. Determination of the Amount of Hydrogen Absorbed by the Metal by Means of Vacuum Extraction
2.1.4. Determination of Mechanical Properties (Ductility) of Steel
2.1.5. Voltammetric Studies
2.1.6. Permeation Test
2.1.7. Electrochemical Impedance Spectroscopy (EIS)
2.1.8. XPS Studies of Steel Surface
2.1.9. Studies on the Protective Aftereffect of Thin Films of 3ST on Steel
3. Results and Discussion
3.1. Effect of the Inhibitor on the Corrosion and Mechanical Properties of Steel
3.2. Effect of the CI on the State of Steel Surface
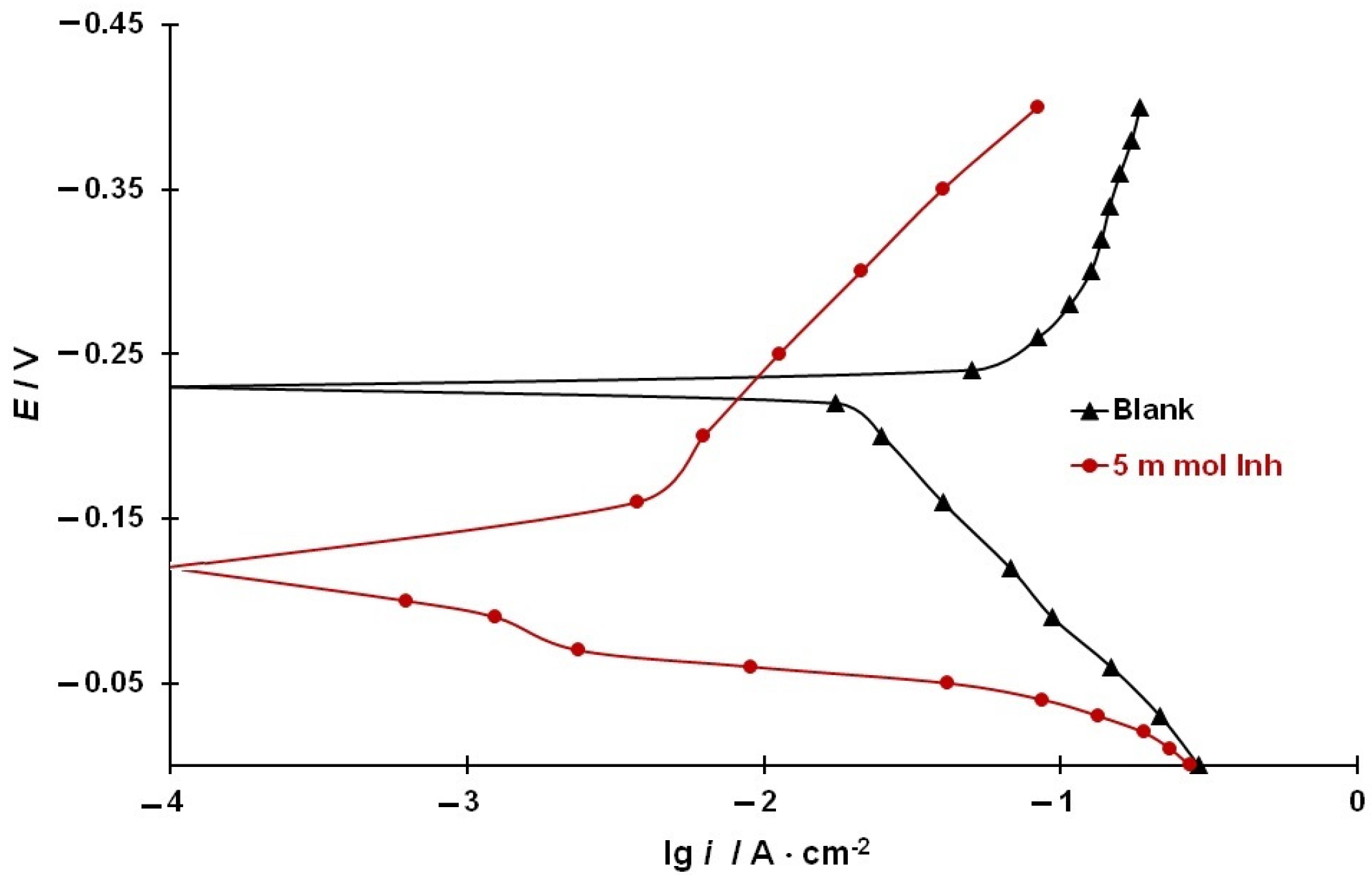
3.3. Effect of the CI on the Rates of Electrode Reactions on Steel
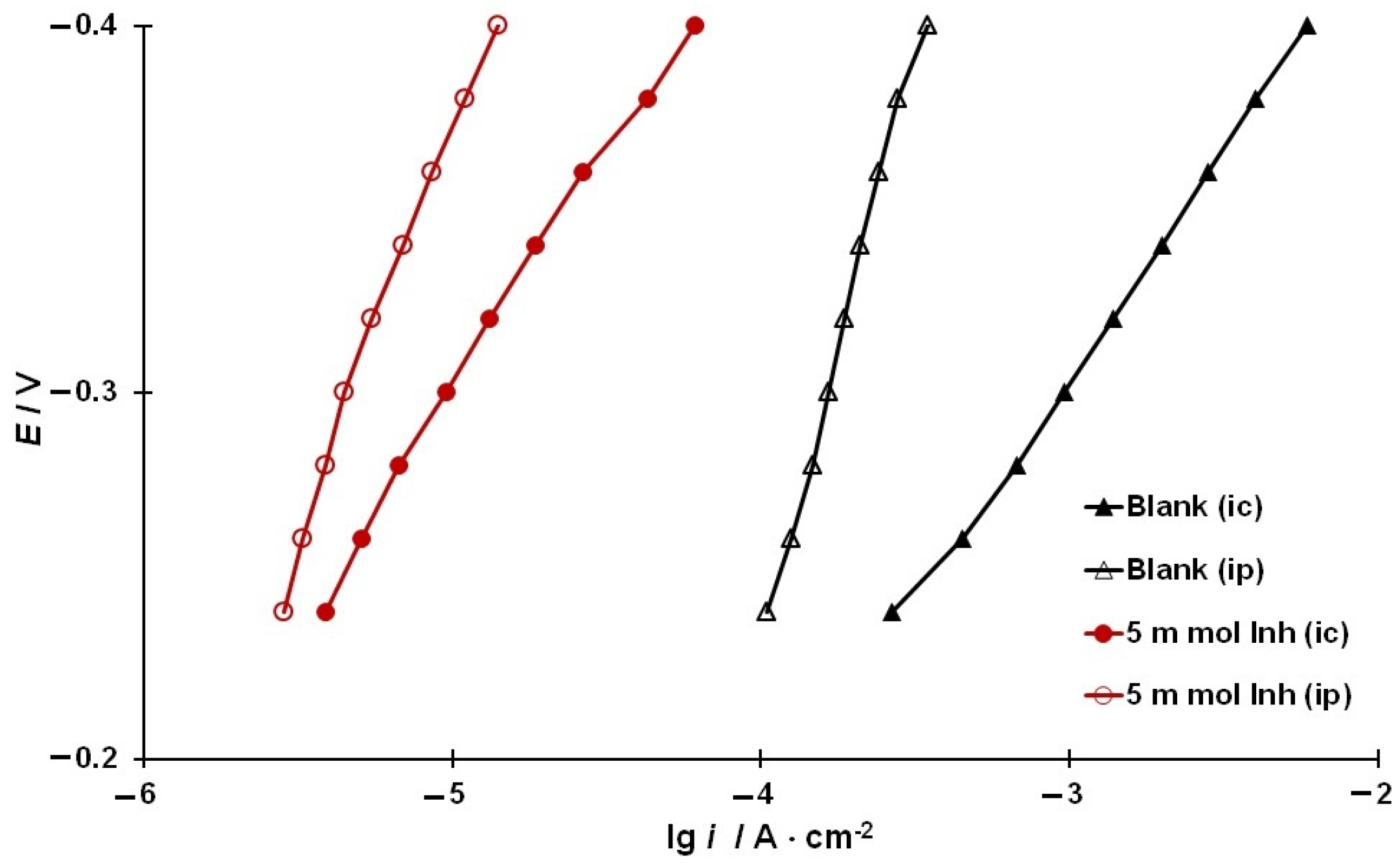
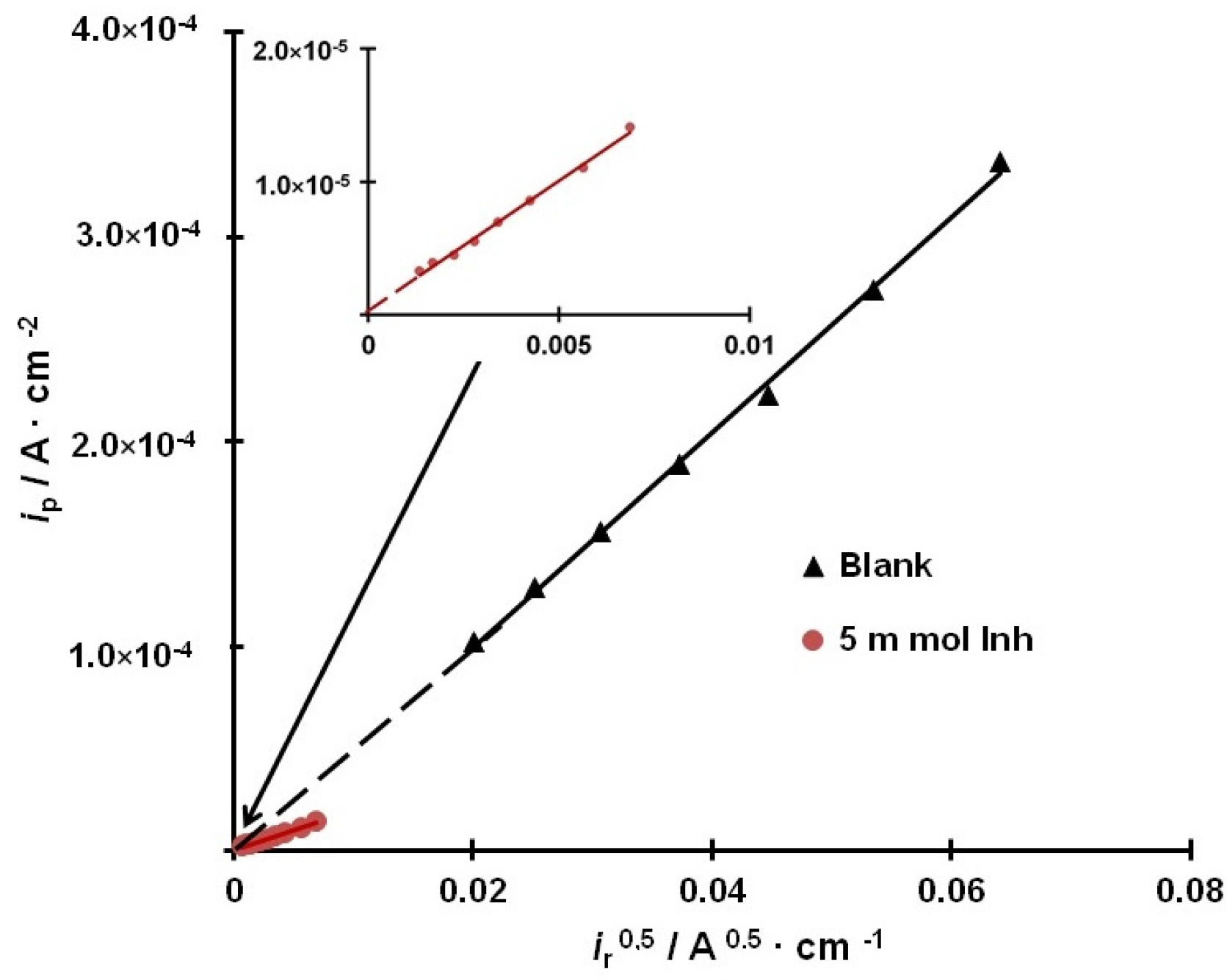
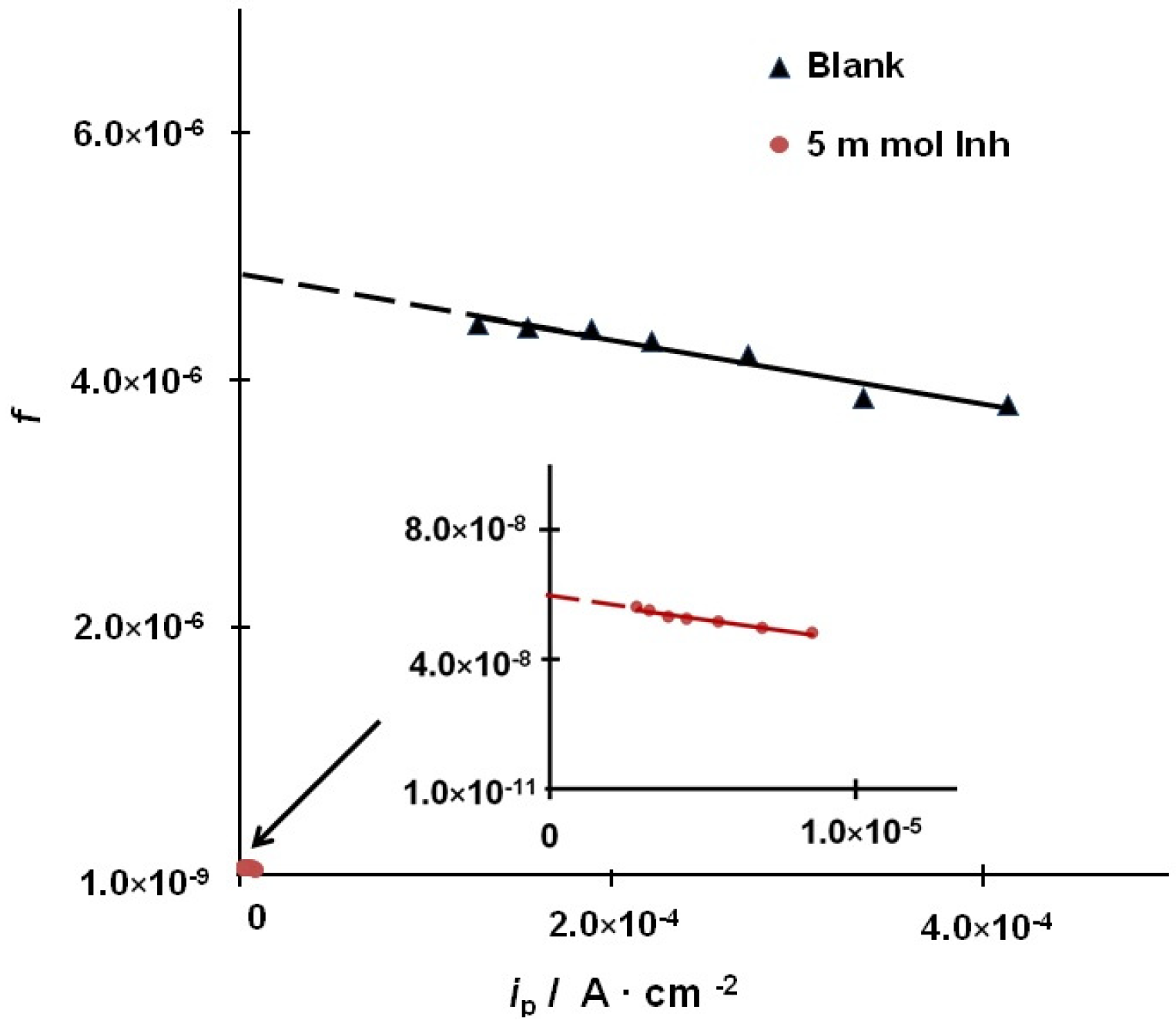
3.4. Kinetics of Hydrogen Evolution and Permeation into the Metal
3.5. Rate Constants of the Main Stages of Hydrogen Evolution and Permeation into Steel
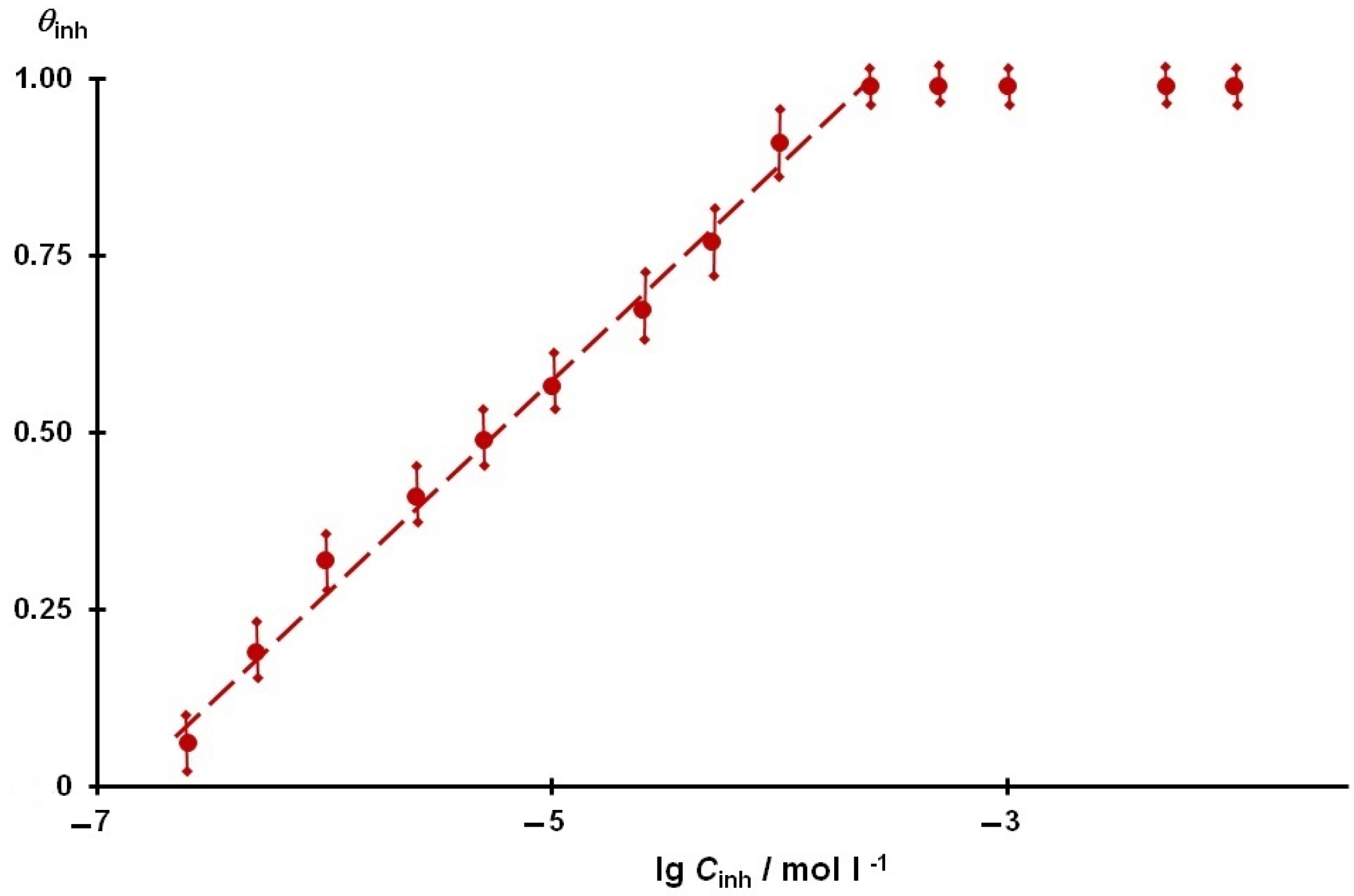
3.6. The Adsorption Interaction of the Steel Surface with the Inhibitor
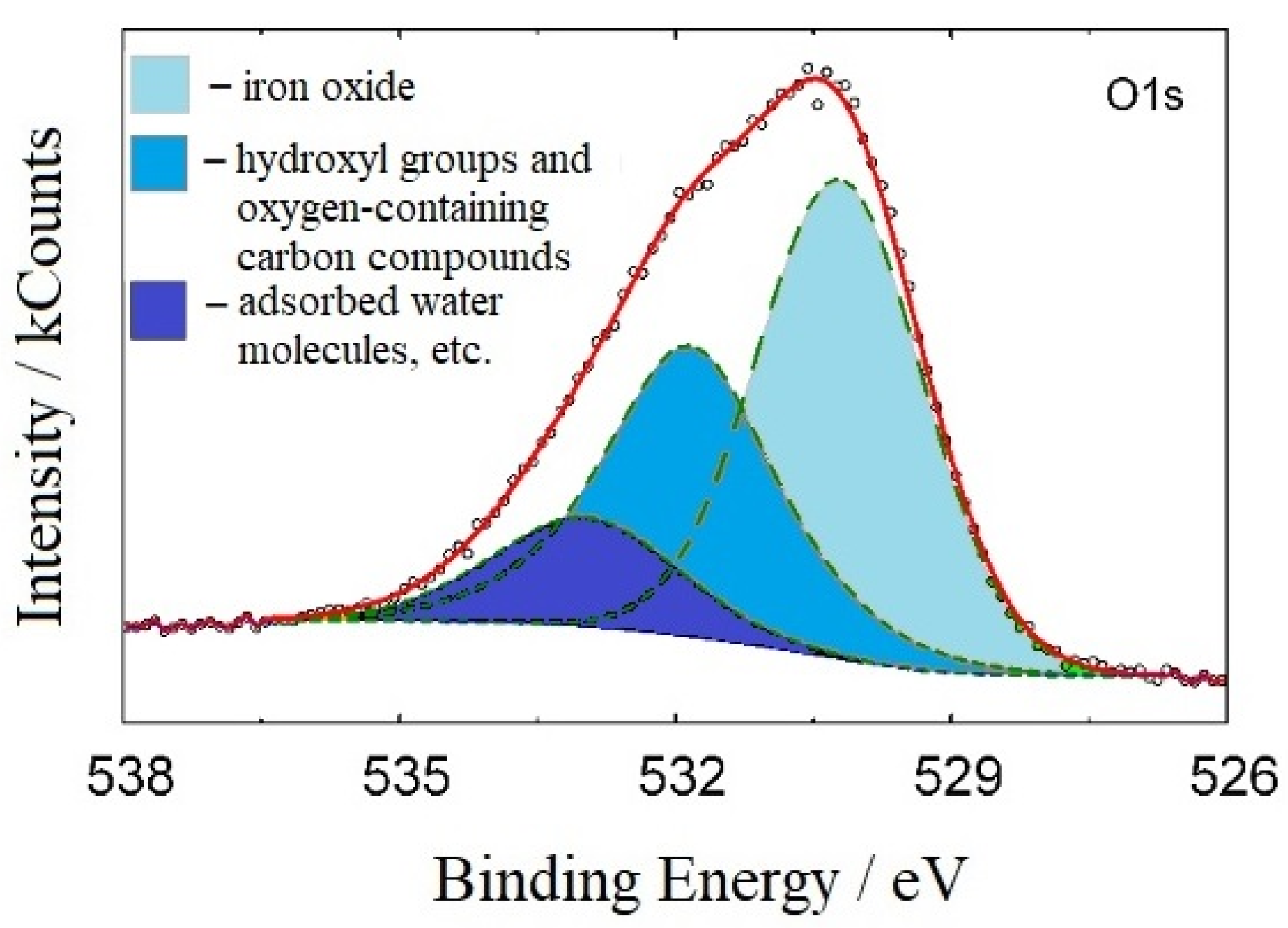
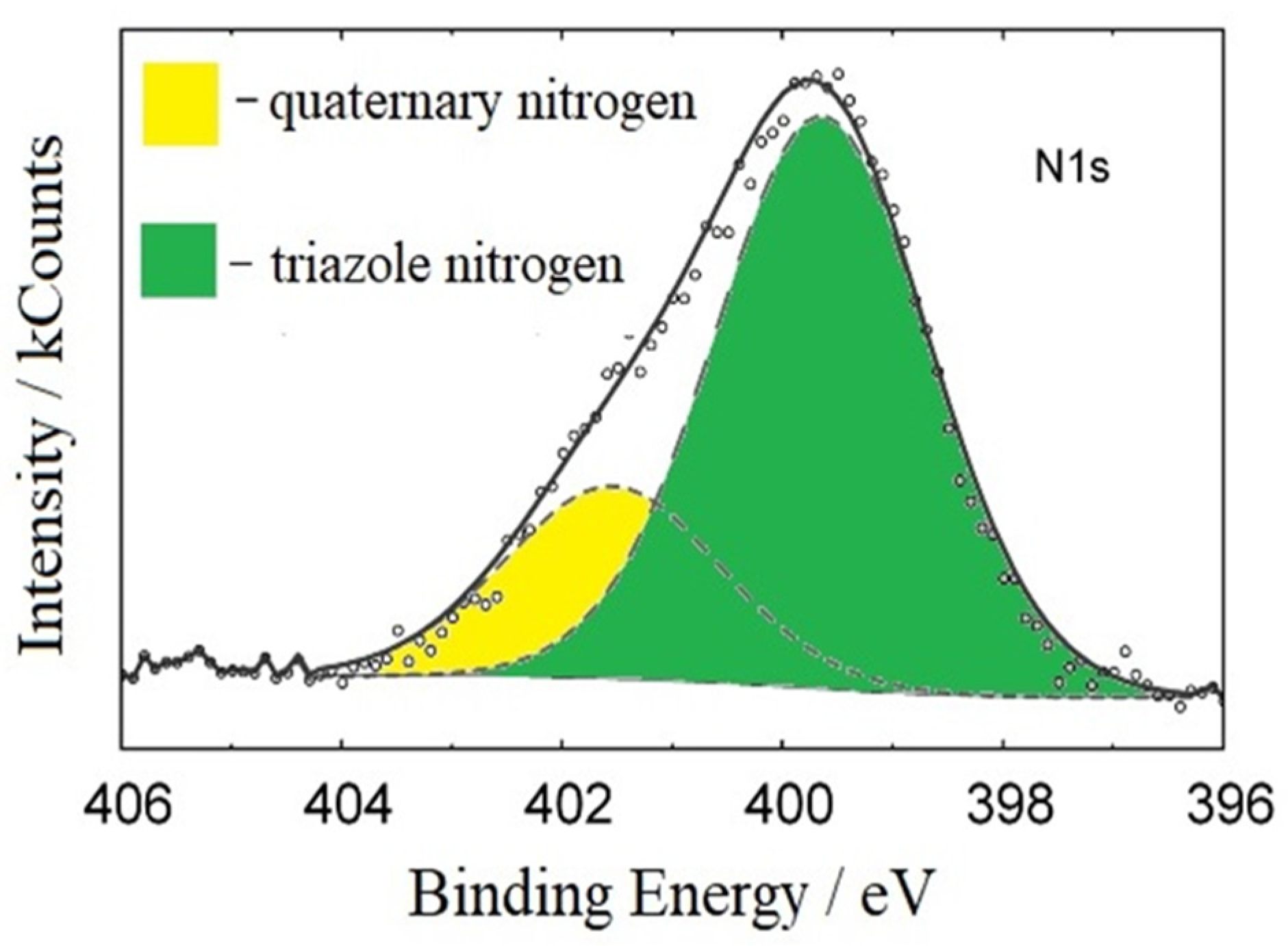
3.7. The Protective Layers Formed by 3ST on the Steel
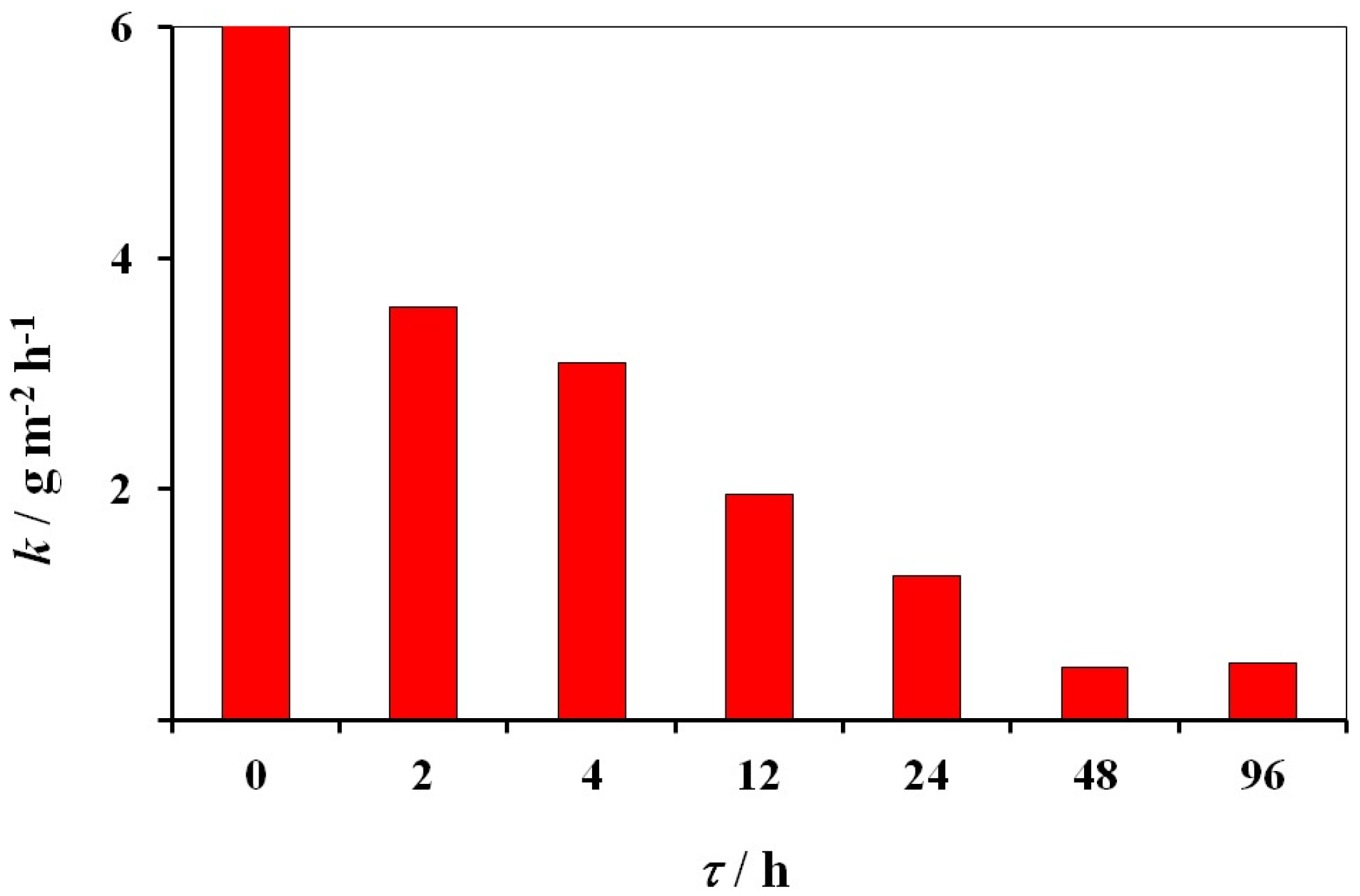
3.8. Protective Aftereffect of Thin 3ST Films
4. Conclusions
- Steel protection with the 3-substituted 1,2,4-triazole (3ST) in an H2SO4 solution is accompanied by the formation of a polymolecular protective layer of CI molecules up to 4 nm thick on the metal. The lower 3ST monolayer that is adjacent to the metal is chemically bound to it. The overlying layers are bound to that layer and to each other by physical interaction. The efficiency of 3ST as an inhibitor of steel corrosion and hydrogen absorption by steel is determined by the specific features of its inhibitory action mechanism involving the formation of an organic protective layer on the metal surface. The formation of protective layers is confirmed by XPS and AFM data and by the results of studies on their protective aftereffects.
- The rate constants of the main stages of hydrogen evolution and permeation into steel, both in the background H2SO4 solution and in a solution containing 3ST, were calculated. It was shown that CI decreased the rate of the H+ ion discharge reaction and the amount of hydrogen absorbed by steel.
- Due to the reduction in the rate of hydrogen permeation into the metal by 3ST, the plastic properties of steel are partially preserved when it corrodes in H2SO4 solutions, whereas complete loss of metal ductility occurs in the background environment.
- Lastly, 3ST significantly slows down the anodic reaction of steel in H2SO4 solution. This effect, along with the inhibition of the cathodic process, determines its efficiency as an inhibitor of steel corrosion in H2SO4 solutions.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kuznetsov, Y.I.; Redkina, G.V. Thin Protective Coatings on Metals Formed by Organic Corrosion Inhibitors in Neutral Media. Coatings 2022, 12, 149. [Google Scholar] [CrossRef]
- Shapagina, N.A.; Dushik, V.V. Application of Electrophoretic Deposition as an Advanced Technique of Inhibited Polymer Films Formation on Metals from Environmentally Safe Aqueous Solutions of Inhibited Formulations. Materials 2022, 16, 19. [Google Scholar] [CrossRef] [PubMed]
- Petrunin, M.; Maksaeva, L.; Gladkikh, N.; Makarychev, Y.; Maleeva, M.; Yurasova, T.; Nazarov, A. Thin Benzotriazole Films for Inhibition of Carbon Steel Corrosion in Neutral Electrolytes. Coatings 2020, 10, 362. [Google Scholar] [CrossRef]
- Gladkikh, N.; Petrunin, M.; Maksaeva, L.; Yurasova, T. Adsorption of Organosilanes on the Surface of Aluminium and the Formation of Organosilane Films to Protect It from Corrosion. Materials 2021, 14, 5757. [Google Scholar] [CrossRef] [PubMed]
- Semiletov, A.M.; Chirkunov, A.A.; Grafov, O.Y.; Kuznetsov, Y.I. Stability of Supehydrophobic Layers Formed by Organic Acids on the Surface of Aluminum Alloy 6063. Coatings 2022, 12, 1468. [Google Scholar] [CrossRef]
- Poling, G.W. Infrared Studies of Protective Films Formed by Acetylenic Corrosion Inhibitors. J. Electrochem. Soc. 1967, 114, 1209–1214. [Google Scholar] [CrossRef]
- Barmatov, E.; La Terra, F.; Hughes, T. Mechanism of degradation of propargyl alcohol by acid-catalysed hydrolysis and corrosion inhibition efficiency of propargyl alcohol intermediates for carbon steel in hydrochloric acid. Mater. Chem. Phys. 2021, 272, 125048. [Google Scholar] [CrossRef]
- Growcock, F.B.; Lopp, V.R. The inhibition of steel corrosion in hydrochloric acid with 3-phenyl-2-propyn-1-ol. Corros. Sci. 1988, 28, 397–410. [Google Scholar] [CrossRef]
- Growcock, F.B.; Lopp, V.R.; Jasinski, R.J. Corrosion Protection of Oilfield Steel with 1-Phenyl-2-Propyn-1-ol. J. Electrochem. Soc. 1988, 135, 823–827. [Google Scholar] [CrossRef]
- Bartos, M.; Kapusta, S.D.; Hackerman, N. A Study of Polymerization of Propargyl Alcohol on Steel. J. Electrochem. Soc. 1993, 140, 2604–2605. [Google Scholar] [CrossRef]
- Aramaki, K.; Fujioka, E. Surface-Enhanced Raman Scattering Spectroscopy Studies on the Inhibition Mechanism of Propargyl Alcohol for Iron Corrosion in Hydrochloric Acid. Corrosion 1996, 52, 83–91. [Google Scholar] [CrossRef]
- Frignani, A.; Monticelli, C.; Zucchi, F.; Trabanelli, G. Acetylenic alcohols as inhibitors of iron acid corrosion. Improvement of the inhibition efficiency of a class of substances based on their action mechanism. Int. J. Corros. Scale Inhib. 2014, 3, 105–119. [Google Scholar] [CrossRef]
- Aramaki, K.; Fujioka, E. Spectroscopic investigations on the inhibition mechanism of propargyl alcohol for iron corrosion in hydrochloric acid at elevated temperatures. Corrosion 1997, 53, 319–326. [Google Scholar] [CrossRef]
- Growcock, F.B.; Lopp, V.R. Film Formation on Steel in Cinnamaldehyde-Inhibited Hydrochloric Acid. Corrosion 1988, 44, 248–254. [Google Scholar] [CrossRef]
- Gao, J.; Weng, Y.; Salitanate; Li, F.; Hong, Y. Corrosion inhibition of α,β-unsaturated carbonyl compounds on steel in acid medium. Pet. Sci. 2009, 6, 201–207. [Google Scholar] [CrossRef]
- Kumar, D.; Muralidhar, V.K.; Jain, V.; Rai, B. Integrating experiments, DFT and characterization for comprehensive corrosion inhibition studies—A case for cinnamaldehyde as an excellent green inhibitor for steels in acidic media. Corros. Sci. 2022, 208, 110623. [Google Scholar] [CrossRef]
- Avdeev, Y.G.; Kuznetsov, Y.I.; Buryak, A.K. Inhibition of steel corrosion by unsaturated aldehydes in solutions of mineral acids. Corros. Sci. 2013, 69, 50–60. [Google Scholar] [CrossRef]
- Avdeev, Y.G.; Kuznetsov, Y.I. Inhibitor protection of steel corrosion in acid solutions at high temperatures. A review. Part 2. Int. J. Corros. Scale Inhib. 2020, 9, 867–902. [Google Scholar] [CrossRef]
- Obot, I.B.; Meroufel, A.; Onyeachu, I.B.; Alenazi, A.; Sorour, A.A. Corrosion inhibitors for acid cleaning of desalination heat exchangers: Progress, challenges and future perspectives. J. Mol. Liq. 2019, 296, 111760. [Google Scholar] [CrossRef]
- Verma, C.; Quraishi, M.A.; Ebenso, E.E. Corrosive electrolytes. Int. J. Corros. Scale Inhib. 2020, 9, 1261–1276. [Google Scholar] [CrossRef]
- Avdeev, Y.G.; Gorichev, I.G.; Luchkin, A.Y. Effect of IFKhAN-92 inhibitor on scale removal during sulfuric acid pickling of steel. Int. J. Corros. Scale Inhib. 2012, 1, 26–37. [Google Scholar] [CrossRef]
- Schmitt, G. Application of Inhibitors for Acid Media: Report prepared for the European Federation of Corrosion Working Party on Inhibitors. Br. Corros. J. 1984, 19, 165–176. [Google Scholar] [CrossRef]
- Pradhan, A.; Vishwakarma, M.; Dwivedi, S.K. A review: The impact of hydrogen embrittlement on the fatigue strength of high strength steel. Mater. Today: Proceed. 2020, 26, 3015–3019. [Google Scholar] [CrossRef]
- Ohaeri, E.; Eduok, U.; Szpunar, J. Hydrogen related degradation in pipeline steel: A review. Int. J. Hydrog. Energy 2018, 43, 14584–14617. [Google Scholar] [CrossRef]
- Liu, Q.; Zhou, Q.; Venezuela, J.; Zhang, M.; Wang, J.; Atrens, A. A review of the influence of hydrogen on the mechanical properties of DP, TRIP, and TWIP advanced high-strength steels for auto construction. Corros. Rev. 2016, 34, 127–152. [Google Scholar] [CrossRef]
- Lunarska, E.; Nikiforov, K. Hydrogen Degradation of the Refinery and Electric Power Installations. Corros. Rev. 2008, 26, 173–213. [Google Scholar] [CrossRef]
- Ramamurthy, S.; Atrens, A. Stress corrosion cracking of high-strength steels. Corros. Rev. 2013, 31, 1–31. [Google Scholar] [CrossRef]
- Avdeev, Y.G.; Nenasheva, T.A.; Luchkin, A.Y.; Marshakov, A.I.; Kuznetsov, Y.I. Effect of Quaternary Ammonium Salts and 1,2,4-Triazole Derivatives on Hydrogen Absorption by Mild Steel in Hydrochloric Acid Solution. Materials 2022, 15, 6989. [Google Scholar] [CrossRef]
- Muralidharan, S.; Quraishi, M.A.; Iyer, S.V.K. The effect of molecular structure on hydrogen permeation and the corrosion inhibition of mild steel in acidic solutions. Corros. Sci. 1995, 37, 1739–1750. [Google Scholar] [CrossRef]
- Hari Kumar, S.; Vivekanand, P.A.; Kamaraj, P. The inhibitive effect of cloxacillin on mild steel corrosion in 2 N Sulphuric acid medium. Mater. Today Proc. 2021, 36, 898–902. [Google Scholar] [CrossRef]
- Ansari, K.R.; Chauhan, D.S.; Singh, A.; Saji, V.S.; Quraishi, M.A. Corrosion inhibitors for acidizing process in oil and gas sectors. In Corrosion Inhibitors in the Oil and Gas Industry; Saji, V.S., Umoren, S.A., Eds.; Wiley-VCH Verlag GmbH & Co. KGaA: Weinheim, Germany, 2020; pp. 153–176. [Google Scholar] [CrossRef]
- Quraishi, M.A.; Chauhan, D.S.; Saji, V.S. Heterocyclic corrosion inhibitors for acid environments. In Heterocyclic Organic Corrosion Inhibitors; Quraishi, M.A., Chauhan, D.S., Saji, V.S., Eds.; Elsevier Inc.: Amsterdam, The Netherlands, 2020; pp. 87–131. [Google Scholar] [CrossRef]
- Phadke Swathi, N.; Alva, V.D.P.; Samshuddin, S. A Review on 1,2,4-Triazole Derivatives as Corrosion Inhibitors. J. Bio- Tribo-Corros. 2017, 3, 42. [Google Scholar] [CrossRef]
- Avdeev, Y.G.; Kuznetsov, Y.I. Nitrogen-containing five-membered heterocyclic compounds as corrosion inhibitors for metals in solutions of mineral acids—An overview. Int. J. Corros. Scale Inhib. 2021, 10, 480–540. [Google Scholar] [CrossRef]
- Devanathan, M.A.V.; Stachurski, Z. The adsorption and diffusion of electrolytic hydrogen in palladium. Proceed. R. Society. Ser. A Math. Phys. Sci. 1962, 270A, 90–540. [Google Scholar] [CrossRef]
- Devanathan, M.A.V.; Stachurski, Z. The Mechanism of Hydrogen Evolution on Iron in Acid Solutions by Determination of Permeation Rates. J. Electrochem. Soc. 1964, 111, 619–623. [Google Scholar] [CrossRef]
- Wagner, C.D.; Davis, L.E.; Zeller, M.V.; Taylor, J.A.; Raymond, R.H.; Gale, L.H. Empirical atomic sensitivity factors for quantitative analysis by electron spectroscopy for chemical analysis. Surf. Interf. Analys. 1981, 3, 211–225. [Google Scholar] [CrossRef]
- Shirley, D.A. High-Resolution X-Ray Photoemission Spectrum of the Valence Bands of Gold. Phys. Rev. B 1972, 5, 4709–4714. [Google Scholar] [CrossRef]
- Avdeev, Y.G.; Kuznetsov, D.S.; Makarychev, Y.B.; Kazansky, L.P. Protective Aftereffect of IFKhAN-92 Inhibitor for Corrosion of Nickel–Chromium Steel in Hydrochloric Acid. Prot. Met. Phys. Chem. Surf. 2020, 56, 1264–1269. [Google Scholar] [CrossRef]
- Iyer, R.N.; Pickering, H.W.; Zamanzadeh, M. Analysis of hydrogen evolution and entry into metals for the discharge-recombination process. J. Electrochem. Soc. 1989, 136, 2463–2470. [Google Scholar] [CrossRef]
- Popov, B.N.; Lee, J.-W.; Djukic, M.B. Chapter 7. Hydrogen permeation and hydrogen-induced cracking. In Handbook of Environmental Degradation of Materials, 3rd ed.; Kutz, M., Ed.; Elsevier Inc.: Amsterdam, The Netherlands, 2018; pp. 133–162. [Google Scholar] [CrossRef]
- Damaskin, B.B.; Afanas’ev, B.N. Current state of the theory of the effect of the adsorption of organic substances on the kinetics of electrochemical reactions. Sov. Elektrokhimiya 1977, 13, 1099–1117. (In Russian) [Google Scholar]
- Marshakov, A.I.; Nenasheva, T.A. Effect of sorbed hydrogen on iron dissolution in the presence of tetraethylammonium cations. Prot. Met. Phys. Chem. Surf. 2002, 38, 556–562. [Google Scholar] [CrossRef]
- Afanas’ev, B.N.; Skobochkina, Y.P.; Serdyukova, G.G. Physicochemical Bases of the Action of Corrosion Inhibitors; Publishing House of UdGu: Moscow, Russia, 1990; p. 20. (In Russian) [Google Scholar]
- Kiuchi, K.; McLellan, R.B. The solubility and diffusivity of hydrogen in well-annealed and deformed iron. Acta Metall. 1983, 31, 961–984. [Google Scholar] [CrossRef]
- Mohai, M. XPS Multi Quant: Multimodel XPS quantification software. Surf. Interface Anal. 2004, 36, 828–832. [Google Scholar] [CrossRef]
- Cumpson, P.J.; Seah, M.P. Elastic Scattering Corrections in AES and XPS. II. Estimating Attenuation Lengths and Conditions Required for their Valid Use in Overlayer/Substrate Experiments. Surf. Interface Anal. 1997, 25, 430–446. [Google Scholar] [CrossRef]



| Solution | ρ, g m−2·h−1 | Zcor, % | , mol cm−3 | *, % | π, % |
|---|---|---|---|---|---|
| Background | 35.3 | - | 1.92 × 10−5 | - | - * |
| CI | 0.71 | 98.0 | 4.20 × 10−7 | 97.8 | 32 |
| No. | Treatment | Microphotographs (400 × 600 μm) | AFM Images (25 × 25 μm) |
|---|---|---|---|
| 1 | None | 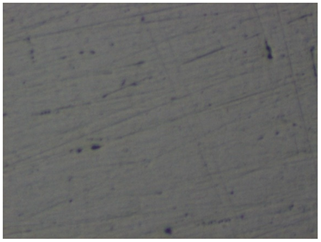 | 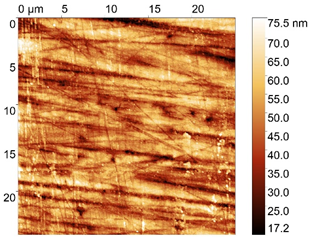 |
| 2 | 2 M H2SO4 | 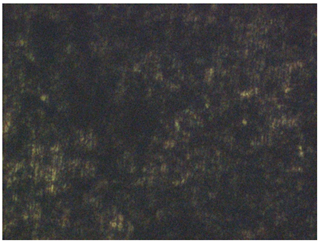 | 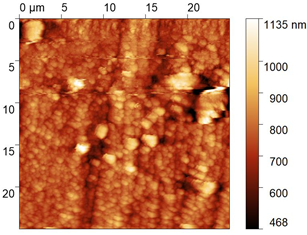 |
| 3 | 2 M H2SO4 + 5mM CI | 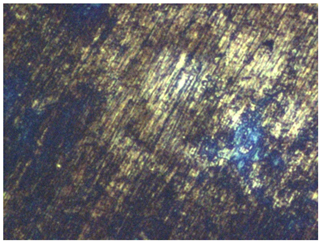 |  |
| Additive | k1,i, 9 mol cm−2 s−1 | k, cm3 mol−1 | kr, mol cm−2 s−1 | θH × 100 | mol cm−3 |
|---|---|---|---|---|---|
| None (background) | 1.14 × 10−8 | 1.50 × 105 | 7.05 × 10−6 | 3.65 | 2.34 × 10−7 |
| CI | 9.94 × 10−11 | 3.69 × 106 | 9.46 × 10−8 | 1.61 | 6.39 × 10−9 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Avdeev, Y.G.; Nenasheva, T.A.; Luchkin, A.Y.; Marshakov, A.I.; Kuznetsov, Y.I. Thin 1,2,4-Triazole Films for the Inhibition of Carbon Steel Corrosion in Sulfuric Acid Solution. Coatings 2023, 13, 1221. https://doi.org/10.3390/coatings13071221
Avdeev YG, Nenasheva TA, Luchkin AY, Marshakov AI, Kuznetsov YI. Thin 1,2,4-Triazole Films for the Inhibition of Carbon Steel Corrosion in Sulfuric Acid Solution. Coatings. 2023; 13(7):1221. https://doi.org/10.3390/coatings13071221
Chicago/Turabian StyleAvdeev, Yaroslav G., Tatyana A. Nenasheva, Andrey Yu. Luchkin, Andrey I. Marshakov, and Yurii I. Kuznetsov. 2023. "Thin 1,2,4-Triazole Films for the Inhibition of Carbon Steel Corrosion in Sulfuric Acid Solution" Coatings 13, no. 7: 1221. https://doi.org/10.3390/coatings13071221
APA StyleAvdeev, Y. G., Nenasheva, T. A., Luchkin, A. Y., Marshakov, A. I., & Kuznetsov, Y. I. (2023). Thin 1,2,4-Triazole Films for the Inhibition of Carbon Steel Corrosion in Sulfuric Acid Solution. Coatings, 13(7), 1221. https://doi.org/10.3390/coatings13071221






