Study on the Self-Repairing Effect of Nanoclay in Powder Coatings for Corrosion Protection
Abstract
:1. Introduction
2. Materials and Methods
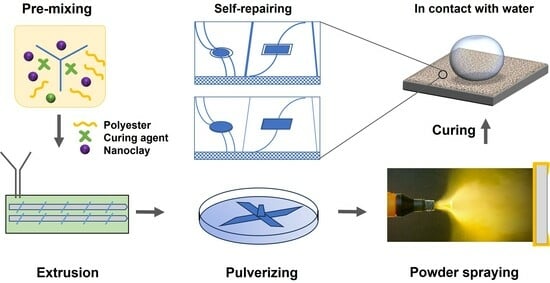
2.1. Materials and Coating Formulations
2.2. Preparation of Powder-Coated Panels
2.3. Coating Performance Evaluations
2.4. Anti-Corrosive Performance Evaluations
2.4.1. Neutral Salt Spray Tests
2.4.2. Electrochemical Tests and Degree of Rusting
2.5. Characterization Techniques
3. Results and Discussion
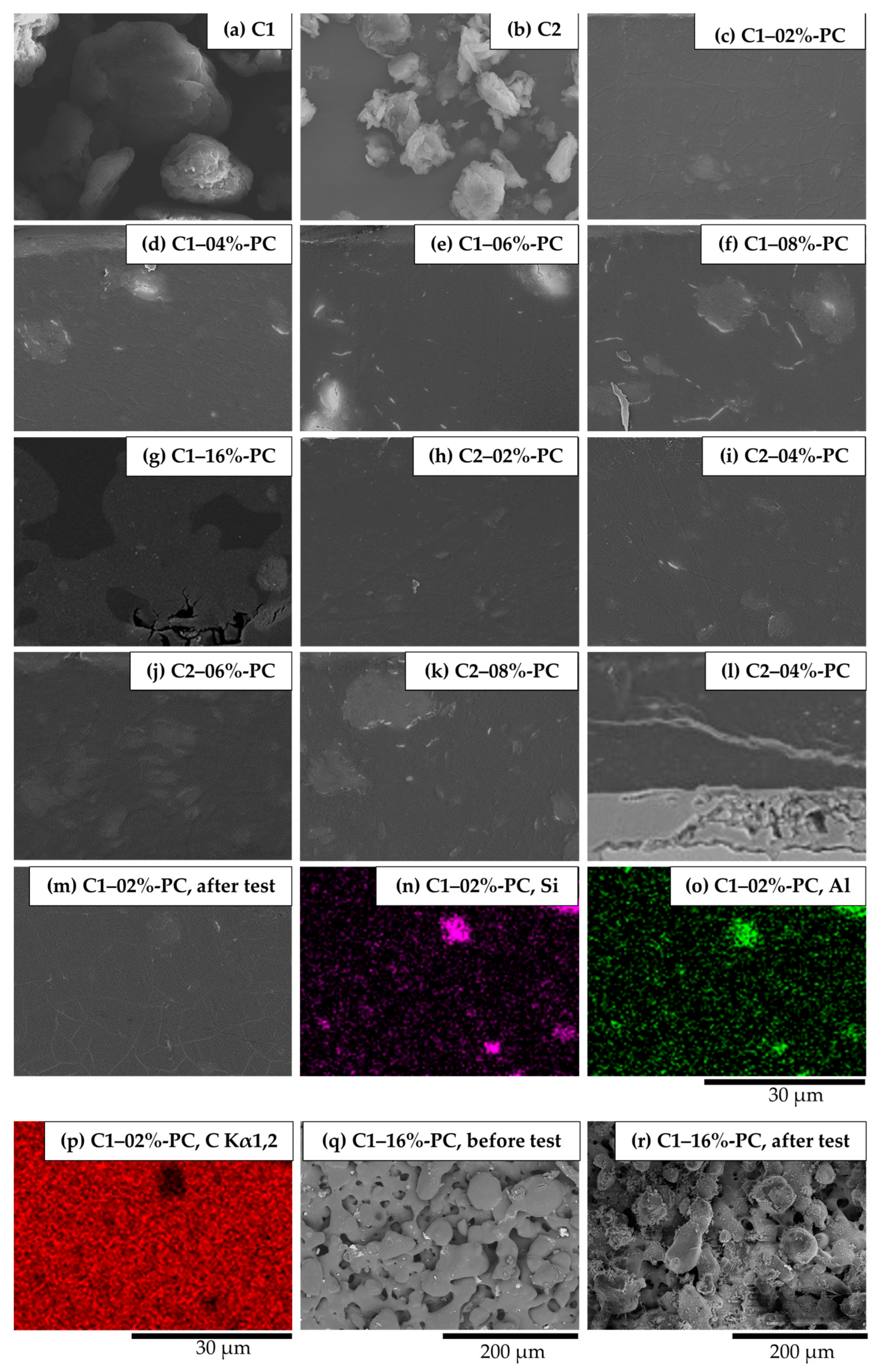
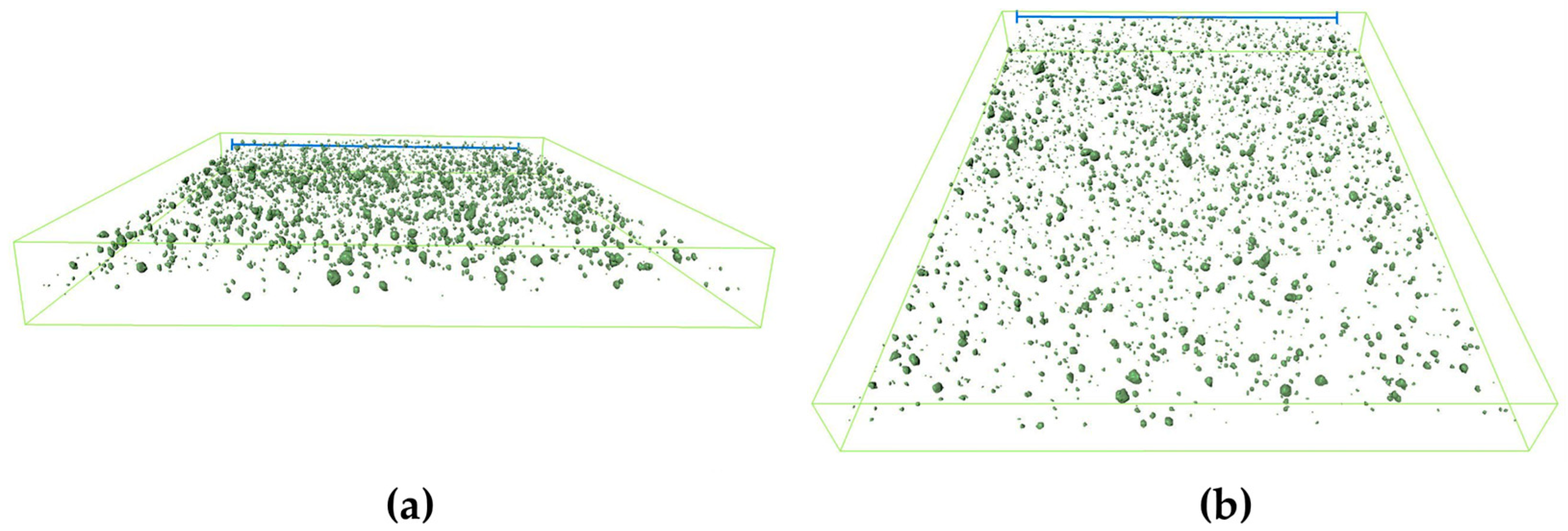
3.1. Coating Morphologies
3.2. Coating Properties
3.3. Electrochemical Measurement Results
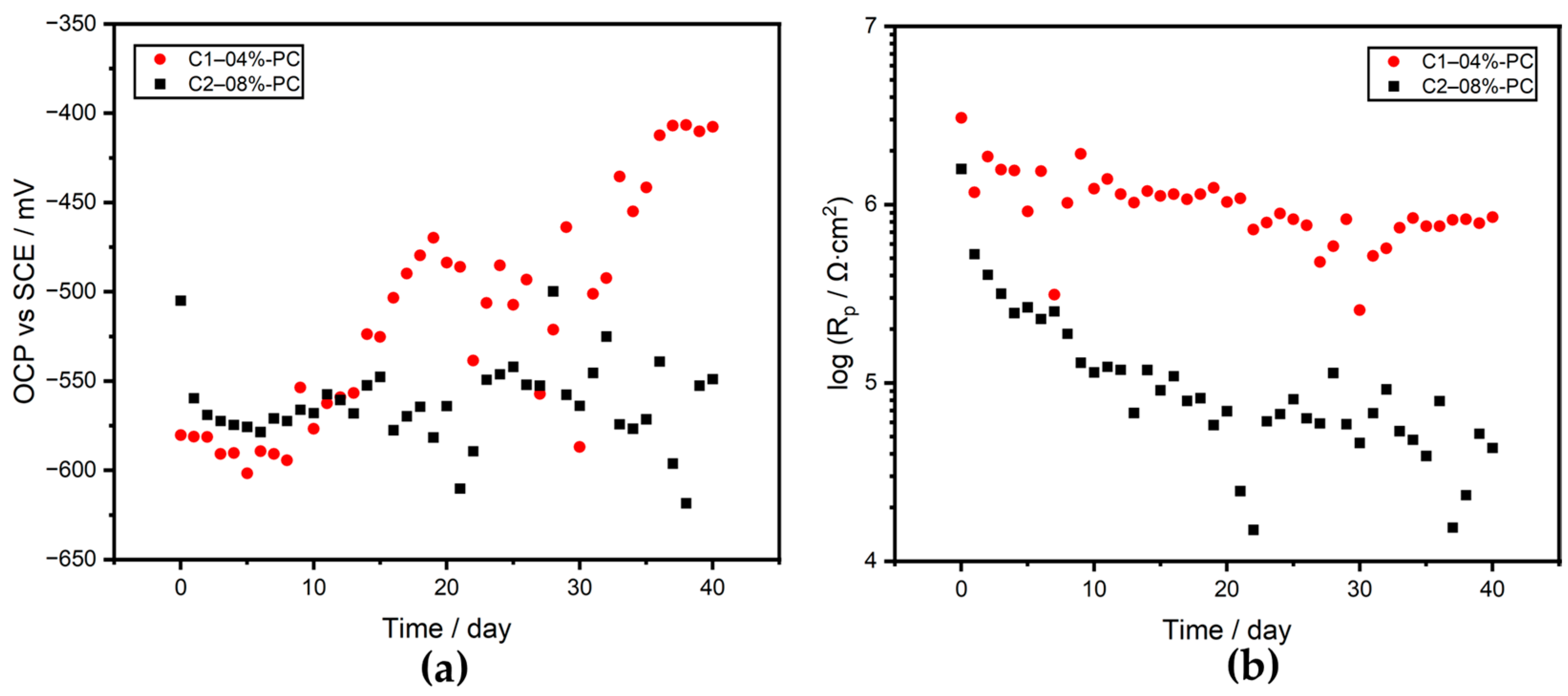
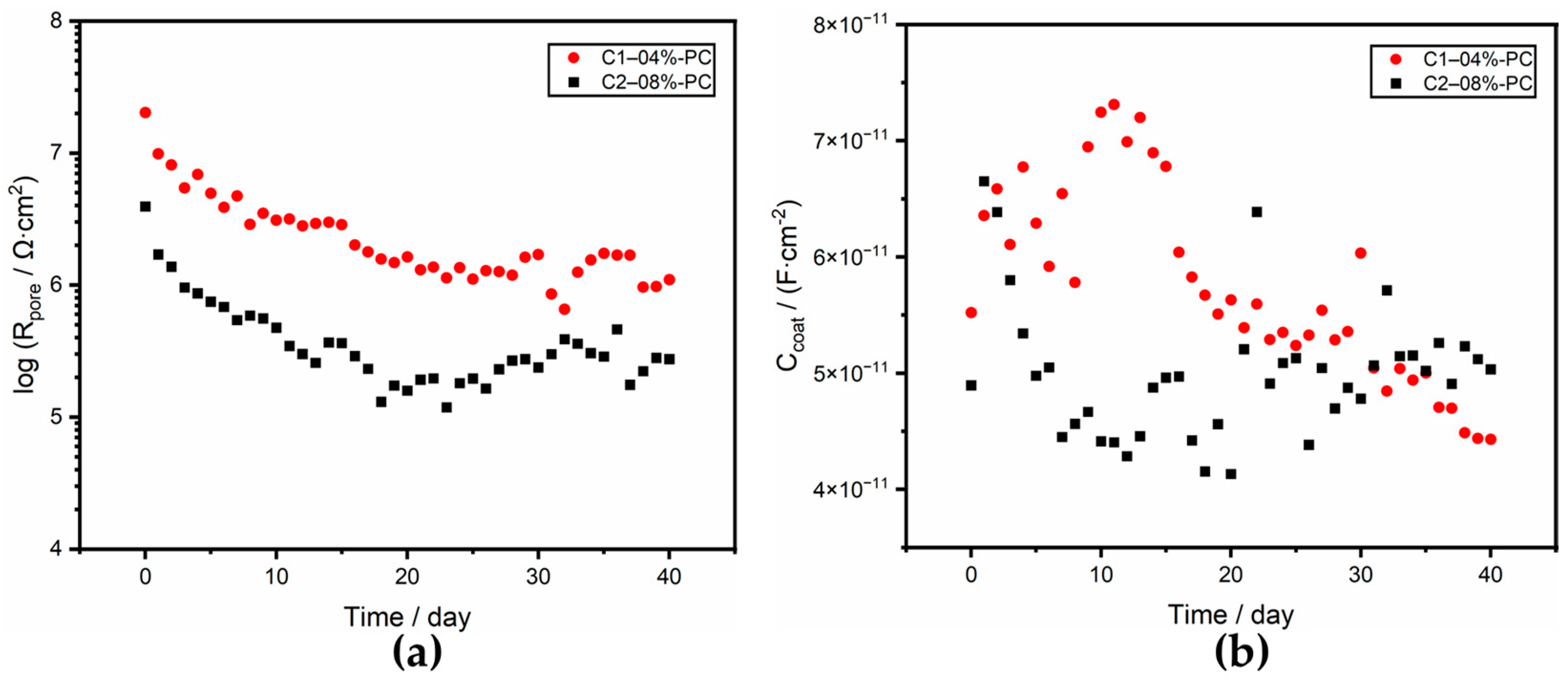
3.3.1. OCP and Rp
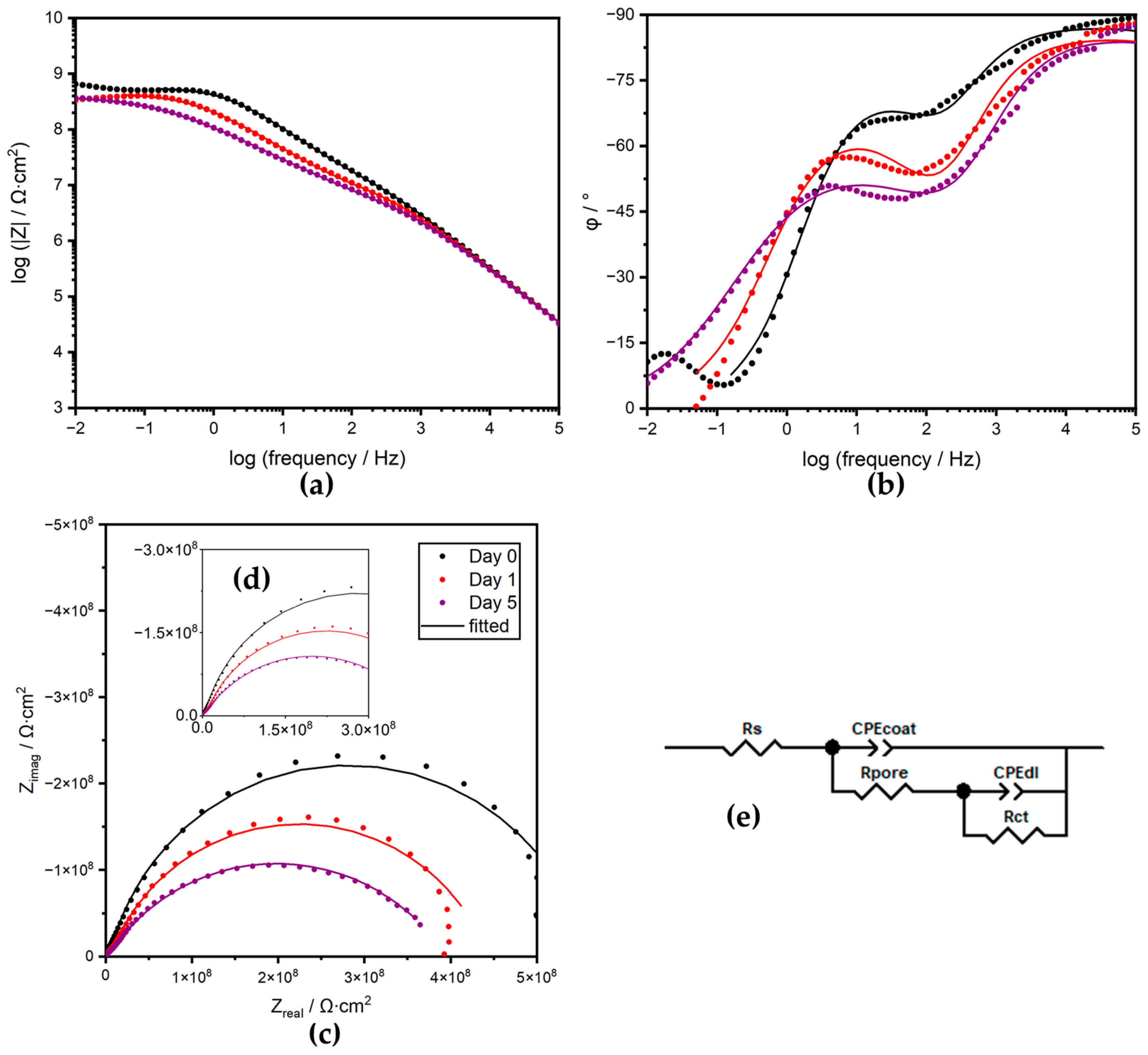
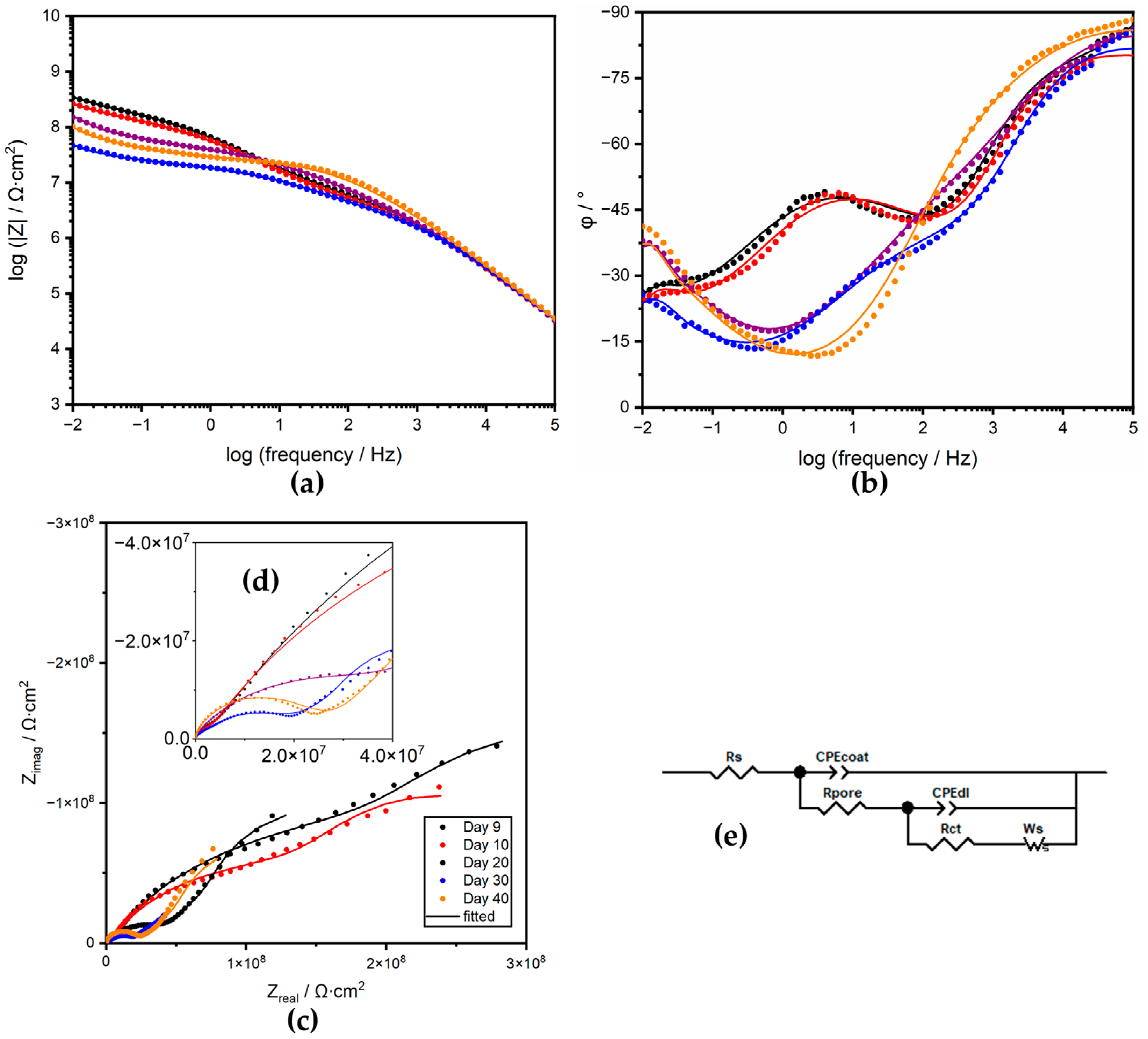
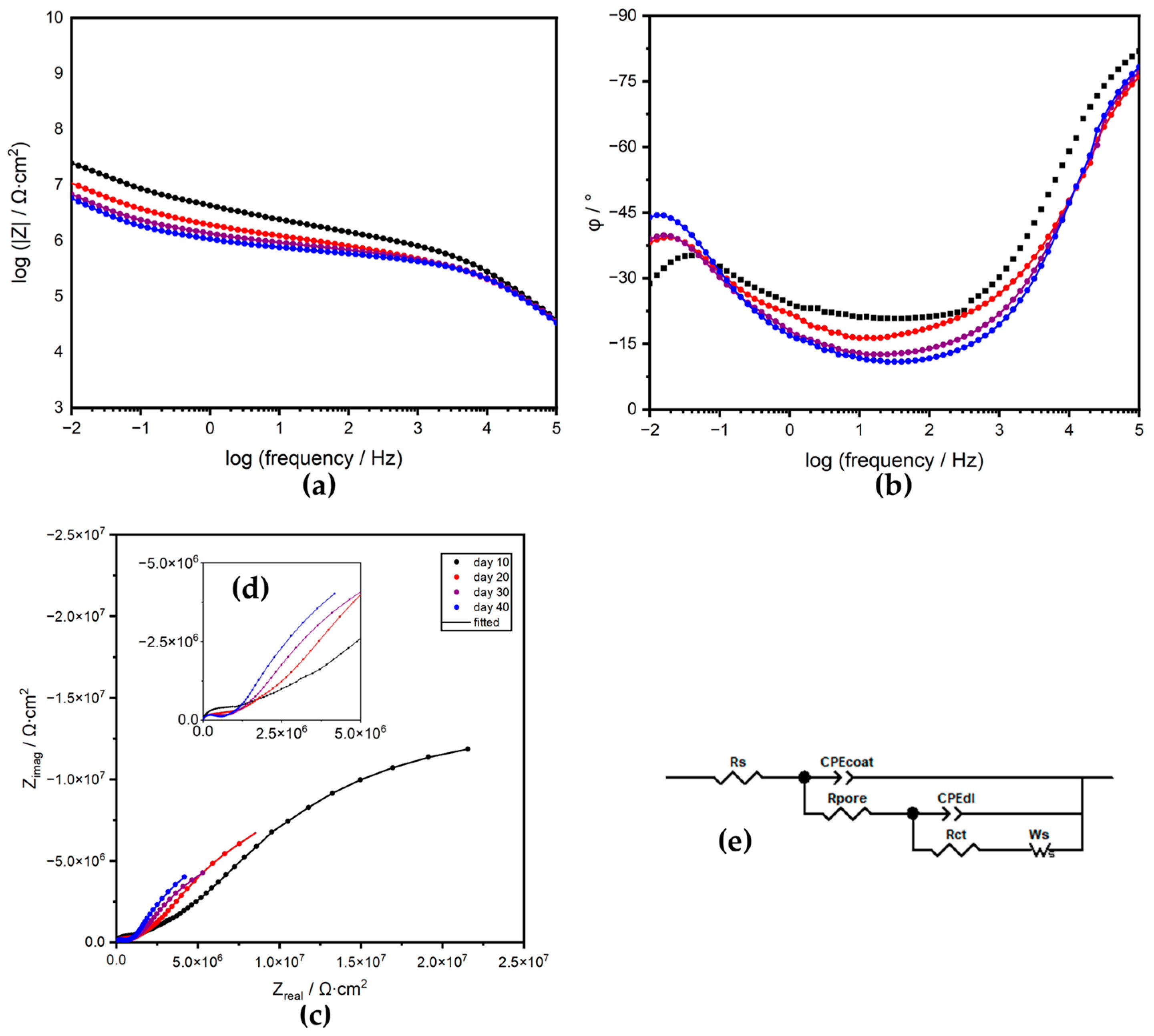
3.3.2. Electrical Equivalent Circuit Analysis
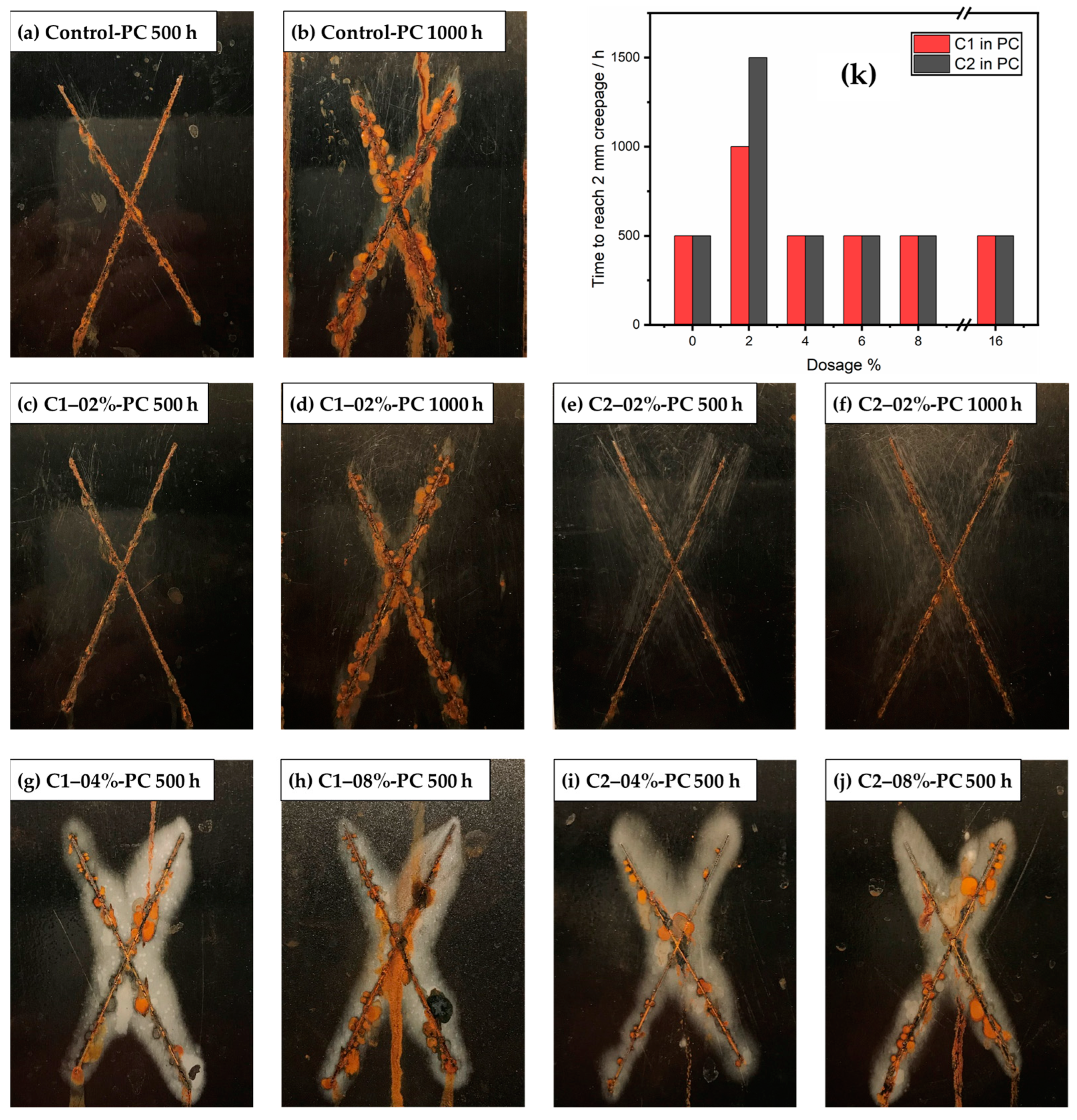
3.4. Neutral Salt Spray Test Results
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sørensen, P.A.; Kiil, S.; Dam-Johansen, K.; Weinell, C.E. Anti-corrosive Coatings: A Review. J. Coat. Technol. Res. 2009, 6, 135–176. [Google Scholar] [CrossRef]
- Fotovvati, B.; Namdari, N.; Dehghanghadikolaei, A. On Coating Techniques for Surface Protection: A Review. J. Manuf. Mater. Process. 2019, 3, 28. [Google Scholar] [CrossRef]
- Zhang, H.; Yang, M.S.; Bhuiyan, M.T.I.; Zhu, J. CHAPTER 15: Green Chemistry for Automotive Coatings: Sustainable Applications; The Royal Society of Chemistry: London, UK, 2019; Volume 2019, ISBN 9781782629948. [Google Scholar]
- Crapper, G. Powder Coatings. Polymer Science: A Comprehensive Reference; Elsevier: Amsterdam, The Netherlands, 2012; Volume 10, pp. 541–566. ISBN 9780080878621. [Google Scholar]
- Turner, S.; Baskir, J.; Nunez, C. Powder Coatings: A Technology Review. Pollut. Prev. Rev. 1999, Spring, 7–21. [Google Scholar]
- Schoff, C.K. Organic Coatings: The Paradoxical Materials. Prog. Org. Coat. 2005, 52, 21–27. [Google Scholar] [CrossRef]
- Misev, T.A.; Van Der Linde, R. Powder Coatings Technology: New Developments at the Turn of the Century. Prog. Org. Coat. 1997, 34, 160–168. [Google Scholar] [CrossRef]
- Liberto, N. Powder Coatings: Not Just Another Pretty Finish. Powder Coat. 2011, 1–7. [Google Scholar]
- Spyrou, E. Powder Coatings: Chemistry and Technology, 3rd Revise ed.; Vincentz Network GmbH & Co KG: Hannover, Germany, 2012; ISBN 978-3-86630-824-4. [Google Scholar]
- Yang, S.; Huang, J.; Chen, J.; Noël, J.J.; Barker, I.; Henderson, J.D.; He, P.; Zhang, H.; Zhang, H.; Zhu, J. A Comparative Study on the Anti-Corrosive Performance of Zinc Phosphate in Powder Coatings. Coatings 2022, 12, 217. [Google Scholar] [CrossRef]
- Huttunen-Saarivirta, E.; Vaganov, G.V.; Yudin, V.E.; Vuorinen, J. Characterization and Corrosion Protection Properties of Epoxy Powder Coatings Containing Nanoclays. Prog. Org. Coat. 2013, 76, 757–767. [Google Scholar] [CrossRef]
- Farshchi, N.; Gedan-Smolka, M. Polyurethane Powder Coatings: A Review of Composition and Characterization. Ind. Eng. Chem. Res. 2020, 59, 15121–15132. [Google Scholar] [CrossRef]
- Tan, B.; Thomas, N.L. Tortuosity Model to Predict the Combined Effects of Crystallinity and Nano-Sized Clay Mineral on the Water Vapour Barrier Properties of Polylactic Acid. Appl. Clay Sci. 2017, 141, 46–54. [Google Scholar] [CrossRef]
- Uddin, F. Clays, Nanoclays, and Montmorillonite Minerals. Metall. Mater. Trans. A Phys. Metall. Mater. Sci. 2008, 39, 2804–2814. [Google Scholar] [CrossRef]
- Wang, N.; Cheng, K.; Wu, H.; Wang, C.; Wang, Q.; Wang, F. Effect of Nano-Sized Mesoporous Silica MCM-41 and MMT on Corrosion Properties of Epoxy Coating. Prog. Org. Coat. 2012, 75, 386–391. [Google Scholar] [CrossRef]
- Al-Shahrani, A.; Taie, I.; Fihri, A.; Alabedi, G. Polymer-Clay Nanocomposites for Corrosion Protection. In Current Topics in the Utilization of Clay in Industrial and Medical Applications; InTech: London, UK, 2018. [Google Scholar] [CrossRef]
- Kotal, M.; Bhowmick, A.K. Polymer Nanocomposites from Modified Clays: Recent Advances and Challenges. Prog. Polym. Sci. 2015, 51, 127–187. [Google Scholar] [CrossRef]
- Ganjaee Sari, M.; Shamshiri, M.; Ramezanzadeh, B. Fabricating an Epoxy Composite Coating with Enhanced Corrosion Resistance through Impregnation of Functionalized Graphene Oxide-Co-Montmorillonite Nanoplatelet. Corros. Sci. 2017, 129, 38–53. [Google Scholar] [CrossRef]
- Szociński, M.; Darowicki, K. Performance of Zinc-Rich Coatings Evaluated Using AFM-Based Electrical Properties Imaging. Prog. Org. Coat. 2016, 96, 58–64. [Google Scholar] [CrossRef]
- Ding, R.; Li, W.; Wang, X.; Gui, T.; Li, B.; Han, P.; Tian, H.; Liu, A.; Wang, X.; Liu, X.; et al. A Brief Review of Corrosion Protective Films and Coatings Based on Graphene and Graphene Oxide. J. Alloys Compd. 2018, 764, 1039–1055. [Google Scholar] [CrossRef]
- Zheng, H.; Shao, Y.; Wang, Y.; Meng, G.; Liu, B. Reinforcing the Corrosion Protection Property of Epoxy Coating by Using Graphene Oxide–Poly(Urea–Formaldehyde) Composites. Corros. Sci. 2017, 123, 267–277. [Google Scholar] [CrossRef]
- Giménez, R.; Serrano, B.; San-Miguel, V.; Cabanelas, J.C. Recent Advances in MXene/Epoxy Composites: Trends and Prospects. Polymers 2022, 14, 1170. [Google Scholar] [CrossRef]
- Navarchian, A.H.; Joulazadeh, M.; Karimi, F. Investigation of Corrosion Protection Performance of Epoxy Coatings Modified by Polyaniline/Clay Nanocomposites on Steel Surfaces. Prog. Org. Coat. 2014, 77, 347–353. [Google Scholar] [CrossRef]
- Arianpouya, N.; Shishesaz, M.; Arianpouya, M.; Nematollahi, M. Evaluation of Synergistic Effect of Nanozinc/Nanoclay Additives on the Corrosion Performance of Zinc-Rich Polyurethane Nanocomposite Coatings Using Electrochemical Properties and Salt Spray Testing. Surf. Coat. Technol. 2013, 216, 199–206. [Google Scholar] [CrossRef]
- Faheem, U. Montmorillonite: An Introduction to Properties and Utilization; InTech: London, UK, 2016; Volume 1, p. 13. [Google Scholar] [CrossRef]
- Williams, G.; McMurray, H.N. Inhibition of Corrosion Driven Delamination on Iron by Smart-Release Bentonite Cation-Exchange Pigments Studied Using a Scanning Kelvin Probe Technique. Prog. Org. Coat. 2017, 102, 18–28. [Google Scholar] [CrossRef]
- Mills, D.J.; Jamali, S.S. The Best Tests for Anti-Corrosive Paints. And Why: A Personal Viewpoint. Prog. Org. Coat. 2017, 102, 8–17. [Google Scholar] [CrossRef]
- Grundmeier, G.; Schmidt, W.; Stratmann, M. Corrosion Protection by Organic Coatings: Electrochemical Mechanism and Novel Methods of Investigation. Electrochim. Acta 2000, 45, 2515–2533. [Google Scholar] [CrossRef]
- Guo, F.; Aryana, S.; Han, Y.; Jiao, Y. A Review of the Synthesis and Applications of Polymer-Nanoclay Composites. Appl. Sci. 2018, 8, 1696. [Google Scholar] [CrossRef]
- Pandey, J.K.; Reddy, K.R.; Mohanty, A.K.; Misra, M. Handbook of Polymernanocomposites. Processing, Performance and Application Volume A: Layered Silicates; Springer Science & Business Media: Berlin/Heidelberg, Germany, 2014; ISBN 9783642386497. [Google Scholar]
- Lee, K.-S.; Kobayashi, S. (Eds.) Polymer Materials Block-Copolymers, Nanocomposites, Organic/Inorganic Hybrids, Polymethylenes; Springer: Berlin/Heidelberg, Germany, 2010; ISBN 978-3-642-13626-6. [Google Scholar]
- Lee, K.M.; Han, C.D. Effect of Hydrogen Bonding on the Rheology of Polycarbonate/Organoclay Nanocomposites. Polymer 2003, 44, 4573–4588. [Google Scholar] [CrossRef]
- Tan, B.; Thomas, N.L. A Review of the Water Barrier Properties of Polymer/Clay and Polymer/Graphene Nanocomposites. J. Membr. Sci. 2016, 514, 595–612. [Google Scholar] [CrossRef]
- Fischer, H. Polymer Nanocomposites: From Fundamental Research to Specific Applications. Mater. Sci. Eng. C 2003, 23, 763–772. [Google Scholar] [CrossRef]
- Huang, C.; Qian, X.; Yang, R. Thermal Conductivity of Polymers and Polymer Nanocomposites. Mater. Sci. Eng. R Rep. 2018, 132, 1–22. [Google Scholar] [CrossRef]
- Raju, A.; Lakshmi, V.; Vishnu Prataap, R.K.; Resmi, V.G.; Rajan, T.P.D.; Pavithran, C.; Prasad, V.S.; Mohan, S. Adduct Modified Nano-Clay Mineral Dispersed Polystyrene Nanocomposites as Advanced Corrosion Resistance Coatings for Aluminum Alloys. Appl. Clay Sci. 2016, 126, 81–88. [Google Scholar] [CrossRef]
- Miccichè, F.; Fischer, H.; Varley, R.; van der Zwaag, S. Moisture Induced Crack Filling in Barrier Coatings Containing Montmorillonite as an Expandable Phase. Surf. Coat. Technol. 2008, 202, 3346–3353. [Google Scholar] [CrossRef]
- Seyedmehdi, S.A.; Zhang, H.; Zhu, J. Effect of Nanoclay on Electrical and Mechanical Properties of Polyurethane Conductive Coatings Filled with Nickel-Coated Carbon Fibers. Polym. Eng. Sci. 2014, 54, 1120–1125. [Google Scholar] [CrossRef]
- Mirabedini, S.M.; Kiamanesh, A. The Effect of Micro and Nano-Sized Particles on Mechanical and Adhesion Properties of a Clear Polyester Powder Coating. Prog. Org. Coat. 2013, 76, 1625–1632. [Google Scholar] [CrossRef]
- Feller, J.F.; Bruzaud, S.; Grohens, Y. Influence of Clay Nanofiller on Electrical and Rheological Properties of Conductive Polymer Composite. Mater. Lett. 2004, 58, 739–745. [Google Scholar] [CrossRef]
- Tyagi, S.; Ray, A.K.; Sood, Y.V. Surface Characteristics of Coating Layers Formed by Coating Pigments with Different Particle Sizes and Size Distribution. J. Coat. Technol. Res. 2010, 7, 747–756. [Google Scholar] [CrossRef]
- Kalendová, A. Effects of Particle Sizes and Shapes of Zinc Metal on the Properties of Anti-corrosive Coatings. Prog. Org. Coat. 2003, 46, 324–332. [Google Scholar] [CrossRef]
- Bierwagen, G.P.; Sanders, T.E. Studies of the Effects of Particle Size Distribution on the Packing Efficiency of Particles. Powder Technol. 1974, 10, 111–119. [Google Scholar] [CrossRef]
- Scully, J.R. Electrochemical Impedance of Organic-Coated Steel: Correlation of Impedance Parameters with Long-Term Coating Deterioration. J. Electrochem. Soc. 1989, 136, 979–990. [Google Scholar] [CrossRef]
- Ma, J.; Xu, J.; Gu, L.; Lai, G.; Chen, B.; Liu, S.; Zhao, H. Corrosion resistance of UV-cured urethane acrylate coatings containing dibutyl-substituted poly (3, 4-propylenedioxythiophene) as a corrosion inhibitor. Int. J. Electrochem. Sci. 2015, 10, 10104–10118. [Google Scholar] [CrossRef]
- Cubides, Y.; Castaneda, H. Corrosion Protection Mechanisms of Carbon Nanotube and Zinc-Rich Epoxy Primers on Carbon Steel in Simulated Concrete Pore Solutions in the Presence of Chloride Ions. Corros. Sci. 2016, 109, 145–161. [Google Scholar] [CrossRef]
- Sekine, I. Recent Evaluation of Corrosion Protective Paint Films by Electrochemical Methods. Prog. Org. Coat. 1997, 31, 73–80. [Google Scholar] [CrossRef]
- Sykes, J.M. A Variant of the Brasher-Kingsbury Equation. Corros. Sci. 2004, 46, 515–517. [Google Scholar] [CrossRef]
- Sharma, M.; Gao, S.; Mäder, E.; Sharma, H.; Wei, L.Y.; Bijwe, J. Carbon Fiber Surfaces and Composite Interphases. Compos. Sci. Technol. 2014, 102, 35–50. [Google Scholar] [CrossRef]
- ASTM D609-00; Standard Practice for Preparation of Cold-Rolled Steel Panels for Testing Paint, Varnish, Conversion Coatings, and Related Coating Products. ASTM International: West Conshohocken, PA, USA, 2017; pp. 1–3.
- ASTM D7091-13; Standard Practice for Nondestructive Measurement of Dry Film Thickness of Nonmagnetic Coatings Applied to Ferrous Metals and Nonmagnetic, Nonconductive Coatings Applied to Non-Ferrous Metals. ASTM International: West Conshohocken, PA, USA, 2013; pp. 1–7.
- ASTM D523; Standard Test Method for Specular Gloss. ASTM International: West Conshohocken, PA, USA, 2014; pp. 1–5.
- ASTM D5767-18; Standard Test Method for Instrumental Measurement of Distinctness-of-Image (DOI) Gloss of Coated Surfaces. ASTM International: West Conshohocken, PA, USA, 2018; pp. 1–7.
- ASTM D3359; Standard Test Methods for Measuring Adhesion by Tape Test. ASTM International: West Conshohocken, PA, USA, 2009; pp. 1–8.
- ASTM D3363-05; Standard Test Method for Film Hardness by Pencil Test. ASTM International: West Conshohocken, PA, USA, 2005.
- ASTM D 2794; Standard Test Method for Resistance of Organic Coatings to the Effects of Rapid Deformation (Impact). ASTM International: West Conshohocken, PA, USA, 1993.
- ASTM B 117; Standard Practice for Operating Salt Spray (Fog) Apparatus. ASTM International: West Conshohocken, PA, USA, 2016.
- ASTM D1654; Standard Test Method for Evaluation of Painted or Coated Specimens Subjected to Corrosive Environments. ASTM International: West Conshohocken, PA, USA, 2008; pp. 1–4.
- Hack, H.P.; Scully, J.R. Defect Area Determination of Organic Coated Steels in Seawater Using the Breakpoint Frequency Method. J. Electrochem. Soc. 1991, 138, 233–238. [Google Scholar] [CrossRef]
- McIntyre, J.M.; Pham, H.Q. Electrochemical Impedance Spectroscopy; a Tool for Organic Coatings Optimizations. Prog. Org. Coat. 1996, 27, 201–207. [Google Scholar] [CrossRef]
- Musiani, M.; Orazem, M.E.; Pébère, N.; Tribollet, B.; Vivier, V. Determination of Resistivity Profiles in Anti-Corrosion Coatings from Constant-Phase-Element Parameters. Prog. Org. Coat. 2014, 77, 2076–2083. [Google Scholar] [CrossRef]
- Croll, S.G. Electrolyte Transport in Polymer Barrier Coatings: Perspectives from Other Disciplines. Prog. Org. Coat. 2018, 124, 41–48. [Google Scholar] [CrossRef]
- Scully, J.R.; Hensley, S.T. Lifetime Prediction for Organic Coatings on Steel and a Magnesium Alloy Using Electrochemical Impedance Methods. Corrosion 1994, 50, 705–716. [Google Scholar] [CrossRef]
- Tsai, C.H.; Mansfeld, F. Determination of Coating Deterioration with EIS: Part II. Development of a Method for Field Testing of Protective Coatings. Corrosion 1993, 49, 726–737. [Google Scholar] [CrossRef]
- Kelly, R.G.; Scully, J.R.; Shoesmith, D.; Buchheit, R.G. Electrochemical Techniques in Corrosion Science and Engineering; CRC Press: Boca Raton, FL, USA, 2002; ISBN 9780203909133. [Google Scholar]
- Yuan, X.; Yue, Z.F.; Chen, X.; Wen, S.F.; Li, L.; Feng, T. EIS Study of Effective Capacitance and Water Uptake Behaviors of Silicone-Epoxy Hybrid Coatings on Mild Steel. Prog. Org. Coat. 2015, 86, 41–48. [Google Scholar] [CrossRef]
- Mansfeld, F.; Buchheit, R.G.; Cunningham, M.; Jensen, H.; Kendig, M.; Martinez, M.A. Discussion: A Correlation between Salt Spray and Electrochemical Impedance Spectroscopy Test Results for Conversion-Coated Aluminum Alloys. Corrosion 1998, 54, 595–597. [Google Scholar] [CrossRef]
- Moreno, C.; Hernández, S.; Santana, J.J.; González-Guzmán, J.; Souto, R.M.; González, S. Characterization of Water Uptake by Organic Coatings Used for the Corrosion Protection of Steel as Determined from Capacitance Measurements. Int. J. Electrochem. Sci. 2012, 7, 8444–8457. [Google Scholar] [CrossRef]
- Brasher, D.M.; Kingsbury, A.H. Electrical Measurements in the Study of Immersed Paint Coatings on Metal. I. Comparison between Capacitance and Gravimetric Methods of Estimating Water-Uptake. J. Appl. Chem. 1954, 4, 62. [Google Scholar] [CrossRef]
- Miszczyk, A.; Darowicki, K. Water Uptake in Protective Organic Coatings and Its Reflection in Measured Coating Impedance. Prog. Org. Coat. 2018, 124, 296–302. [Google Scholar] [CrossRef]
- Deflorian, F.; Fedrizzi, L.; Bonora, P.L. Determination of the Reactive Area of Organic Coated Metals Using the Breakpoint Method. Corrosion 1994, 50, 741–743. [Google Scholar] [CrossRef]
- Yin, K.M.; Lu, L.I. Parametric Study on the Electrochemical Impedance Spectroscopy of Organic-Coated Steels in Hydrochloric Acid Solutions. J. Coat. Technol. 2003, 75, 65–72. [Google Scholar] [CrossRef]
- Sahu, S.C.; Samantara, A.K.; Seth, M.; Parwaiz, S.; Singh, B.P.; Rath, P.C.; Jena, B.K. A Facile Electrochemical Approach for Development of Highly Corrosion Protective Coatings Using Graphene Nanosheets. Electrochem. Commun. 2013, 32, 22–26. [Google Scholar] [CrossRef]
- Liu, J.; Liu, T.; Guo, Z.; Guo, N.; Lei, Y.; Chang, X.; Yin, Y. Promoting Barrier Performance and Cathodic Protection of Zinc-Rich Epoxy Primer via Single-Layer Graphene. Polymers 2018, 10, 591. [Google Scholar] [CrossRef]
- Song, Z.; Qian, B. Effect of Graphene on the Electrochemical Protection of Zinc-Rich Coatings. Mater. Corros. 2018, 69, 1854–1860. [Google Scholar] [CrossRef]
- Liu, S.; Gu, L.; Zhao, H.; Chen, J.; Yu, H. Corrosion Resistance of Graphene-Reinforced Waterborne Epoxy Coatings. J. Mater. Sci. Technol. 2016, 32, 425–431. [Google Scholar] [CrossRef]
- Walter, G.W. A Critical Review of d.c. Electrochemical Tests for Painted Metals. Corros. Sci. 1986, 26, 39–47. [Google Scholar] [CrossRef]
- Ding, J.; Zhao, H.; Xu, B.; Zhao, X.; Su, S.; Yu, H. Superanticorrosive Graphene Nanosheets through π Deposition of Boron Nitride Nanodots. ACS Sustain. Chem. Eng. 2019, 7, 10900–10911. [Google Scholar] [CrossRef]
- Ye, Y.; Zhang, D.; Li, J.; Liu, T.; Pu, J.; Zhao, H.; Wang, L. One-Step Synthesis of Superhydrophobic Polyhedral Oligomeric Silsesquioxane-Graphene Oxide and Its Application in Anti-Corrosion and Anti-Wear Fields. Corros. Sci. 2019, 147, 9–21. [Google Scholar] [CrossRef]
- Askari, F.; Ghasemi, E.; Ramezanzadeh, B.; Mahdavian, M. Mechanistic Approach for Evaluation of the Corrosion Inhibition of Potassium Zinc Phosphate Pigment on the Steel Surface: Application of Surface Analysis and Electrochemical Techniques. Dye. Pigment. 2014, 109, 189–199. [Google Scholar] [CrossRef]
- ASTM-D610; Standard Practice for Evaluating Degree of Rusting on Painted Steel Surfaces. ASTM International: West Conshohocken, PA, USA, 2008; pp. 1–6.
- Tronto, J.; Cludia, A.; Naal, Z.; Barros Valim, J. Conducting Polymers/Layered Double Hydroxides Intercalated Nanocomposites. In Materials Science—Advanced Topics; InTech: London, UK, 2013. [Google Scholar]
- ISO 25178-1; Geometrical Product Specifications (GPS)—Surface Texture: Areal—Part 1: Indication of Surface Texture. International Organization for Standardization: Geneva, Switzerland, 2016; pp. 1–25.
- Amand, S.; Musiani, M.; Orazem, M.E.; Pébère, N.; Tribollet, B.; Vivier, V. Constant-Phase-Element Behavior Caused by Inhomogeneous Water Uptake in Anti-Corrosion Coatings. Electrochim. Acta 2013, 87, 693–700. [Google Scholar] [CrossRef]
- Touzain, S.; Le Thu, Q.; Bonnet, G. Evaluation of Thick Organic Coatings Degradation in Seawater Using Cathodic Protection and Thermally Accelerated Tests. Prog. Org. Coat. 2005, 52, 311–319. [Google Scholar] [CrossRef]
- Hsu, C.H.; Mansfeld, F. Technical Note: Concerning the Conversion of the Constant Phase Element Parameter Y0 into a Capacitance. Corrosion 2001, 57, 747–748. [Google Scholar] [CrossRef]
- Taylor, S.R. Incentives for Using Local Electrochemical Impedance Methods in the Investigation of Organic Coatings. Prog. Org. Coat. 2001, 43, 141–148. [Google Scholar] [CrossRef]
- Upadhyay, V.; Harkal, U.D.; Webster, D.C.; Bierwagen, G.P. Preliminary Investigation of the Impact of Polymer Composition on Electrochemical Properties of Coatings as Determined by Electrochemical Impedance Spectroscopy. J. Coat. Technol. Res. 2013, 10, 865–878. [Google Scholar] [CrossRef]
- Misawa, T.; Hashimoto, K.; Shimodaira, S. The Mechanism of Formation of Iron Oxide and Oxyhydroxides in Aqueous Solutions at Room Temperature. Corros. Sci. 1974, 14, 131–149. [Google Scholar] [CrossRef]






| Code | Additive | Particle Size/µm | Density/(g/cm3) | ||
|---|---|---|---|---|---|
| D10, V | D50, V | D90, V | |||
| C1 | Claytone® HT | 4.49 | 18.38 | 46.10 | 1.70 |
| C2 | CLOISITE® 30B | 1.91 | 8.64 | 23.08 | 1.98 |
| Component | Composition | Content/wt.% |
|---|---|---|
| Resin | Carboxylated polyester | 90.8 |
| Curing agent | TGIC | 6.8 |
| Flow and leveling agent | polyacrylate | 1.6 |
| Degassing agent | Benzoin | 0.8 |
| Formula Code | Nanoclay/wt.% | Binder/wt.% |
|---|---|---|
| Control-PC | 0.0 | 100.0 |
| C1–02%-PC | 2.0 | 98.0 |
| C1–04%-PC | 4.0 | 96.0 |
| C1–06%-PC | 6.0 | 94.0 |
| C1–08%-PC | 8.0 | 92.0 |
| C1–16%-PC | 16.0 | 84.0 |
| C2–02%-PC | 2.0 | 98.0 |
| C2–04%-PC | 4.0 | 96.0 |
| C2–06%-PC | 6.0 | 94.0 |
| C2–08%-PC | 8.0 | 92.0 |
| C2–16%-PC | 16.0 | 84.0 |
| Time | CPEcoat | Rpore | Calculated Ccoat | CPEdl | Rct | Ws, RD | Ws, TD | Ws, P | χ2 | ||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Days | Qc/ Ω−1∙cm−2∙sα | αcoat | Ω∙cm−2 | F∙cm−2 | Qd/ Ω−1∙cm−2∙sα | αdl | Ω∙cm−2 | Ω∙cm2∙sP | s | ||
| 0 | 6.71 × 10−11 | 0.971 | 2.02 × 107 | 5.52 × 10−11 | 2.16 × 10−10 | 0.804 | 5.41 × 108 | 7.04 × 10−4 | |||
| 1 | 9.58 × 10−11 | 0.945 | 9.87 × 106 | 6.35 × 10−11 | 8.30 × 10−10 | 0.751 | 4.35 × 108 | 1.53 × 10−3 | |||
| 5 | 9.87 × 10−11 | 0.944 | 4.95 × 106 | 6.29 × 10−11 | 2.42 × 10−9 | 0.625 | 3.91 × 108 | 5.22 × 10−4 | |||
| 9 | 1.34 × 10−10 | 0.921 | 3.49 × 106 | 6.95 × 10−11 | 4.34 × 10−9 | 0.602 | 2.88 × 108 | 2.88 × 108 | 4.49 × 103 | 0.500 | 7.56 × 10−4 |
| 10 | 8.42 × 10−11 | 0.909 | 3.09 × 106 | 7.24 × 10−11 | 4.45 × 10−9 | 0.620 | 1.55 × 108 | 2.13 × 108 | 4.01 × 103 | 0.500 | 7.56 × 10−4 |
| 20 | 5.09 × 10−9 | 0.957 | 1.63 × 106 | 5.63 × 10−11 | 3.46 × 10−9 | 0.534 | 4.46 × 107 | 2.02 × 108 | 3.51 × 103 | 0.558 | 2.06 × 10−4 |
| 30 | 1.12 × 10−10 | 0.932 | 1.70 × 106 | 6.03 × 10−11 | 7.11 × 10−9 | 0.538 | 2.04 × 107 | 4.52 × 107 | 5.54 × 103 | 0.500 | 4.52 × 10−4 |
| 40 | 4.43 × 10−11 | 1.000 | 1.10 × 106 | 4.43 × 10−11 | 1.12 × 10−9 | 0.559 | 2.53 × 107 | 1.65 × 108 | 7.42 × 103 | 0.554 | 1.68 × 10−4 |
| Time | CPEcoat | Rpore | Calculated Ccoat | CPEdl | Rct | Ws, RD | Ws, TD | Ws, P | χ2 | ||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Days | Qcoat/ Ω−1∙cm−2∙sn | αcoat | Ω∙cm2 | F∙cm−2 | Qdl/ Ω−1∙cm−2∙sn | αdl | Ω∙cm2 | Ω∙cm2∙sP | s | ||
| 0 | 8.15 × 10−11 | 0.944 | 3.93 × 106 | 4.89 × 10−11 | 3.07 × 10−10 | 0.739 | 4.99 × 108 | 2.34 × 10−4 | |||
| 1 | 2.38 × 10−10 | 0.863 | 1.70 × 106 | 6.65 × 10−11 | 3.59 × 10−9 | 0.726 | 1.67 × 108 | 2.38 × 10−3 | |||
| 5 | 1.13 × 10−10 | 0.922 | 7.47 × 105 | 4.98 × 10−11 | 3.68 × 10−8 | 0.445 | 1.97 × 107 | 6.49 × 107 | 6.80 × 102 | 0.500 | 4.20 × 10−4 |
| 9 | 7.91 × 10−11 | 0.949 | 5.57 × 105 | 4.67 × 10−11 | 4.09 × 10−8 | 0.430 | 4.63 × 106 | 6.30 × 107 | 4.57 × 103 | 0.500 | 8.69 × 10−4 |
| 10 | 8.43 × 10−11 | 0.945 | 4.75 × 105 | 4.11 × 10−11 | 5.94 × 10−8 | 0.396 | 4.01 × 106 | 4.51 × 107 | 3.17 × 103 | 0.500 | 1.77 × 10−4 |
| 20 | 7.51 × 10−11 | 0.955 | 1.58 × 105 | 4.13 × 10−11 | 8.52 × 10−8 | 0.374 | 1.59 × 106 | 2.24 × 107 | 6.00 × 103 | 0.522 | 1.50 × 10−4 |
| 30 | 1.06 × 10−10 | 0.931 | 2.37 × 105 | 4.78 × 10−11 | 1.17 × 10−7 | 0.368 | 9.81 × 105 | 1.33 × 107 | 5.84 × 103 | 0.545 | 1.91 × 10−4 |
| 40 | 1.10 × 10−10 | 0.934 | 2.75 × 105 | 5.03 × 10−11 | 1.46 × 10−7 | 0.390 | 7.03 × 105 | 1.14 × 107 | 5.52 × 103 | 0.589 | 3.72 × 10−4 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, M.S.; Huang, J.; Zhang, H.; Noël, J.J.; Hedberg, Y.S.; Chen, J.; Eduok, U.; Barker, I.; Henderson, J.D.; Xian, C.; et al. Study on the Self-Repairing Effect of Nanoclay in Powder Coatings for Corrosion Protection. Coatings 2023, 13, 1220. https://doi.org/10.3390/coatings13071220
Yang MS, Huang J, Zhang H, Noël JJ, Hedberg YS, Chen J, Eduok U, Barker I, Henderson JD, Xian C, et al. Study on the Self-Repairing Effect of Nanoclay in Powder Coatings for Corrosion Protection. Coatings. 2023; 13(7):1220. https://doi.org/10.3390/coatings13071220
Chicago/Turabian StyleYang, Marshall Shuai, Jinbao Huang, Hui Zhang, James Joseph Noël, Yolanda Susanne Hedberg, Jian Chen, Ubong Eduok, Ivan Barker, Jeffrey Daniel Henderson, Chengqian Xian, and et al. 2023. "Study on the Self-Repairing Effect of Nanoclay in Powder Coatings for Corrosion Protection" Coatings 13, no. 7: 1220. https://doi.org/10.3390/coatings13071220