A New Coated Proppant for Packing Fractures in Oil Reservoirs
Abstract
:1. Introduction
- (1)
- (2)
- (3)
2. Experimental Section
2.1. Materials
2.2. Preparation of Tyzs-40-Coated Proppant
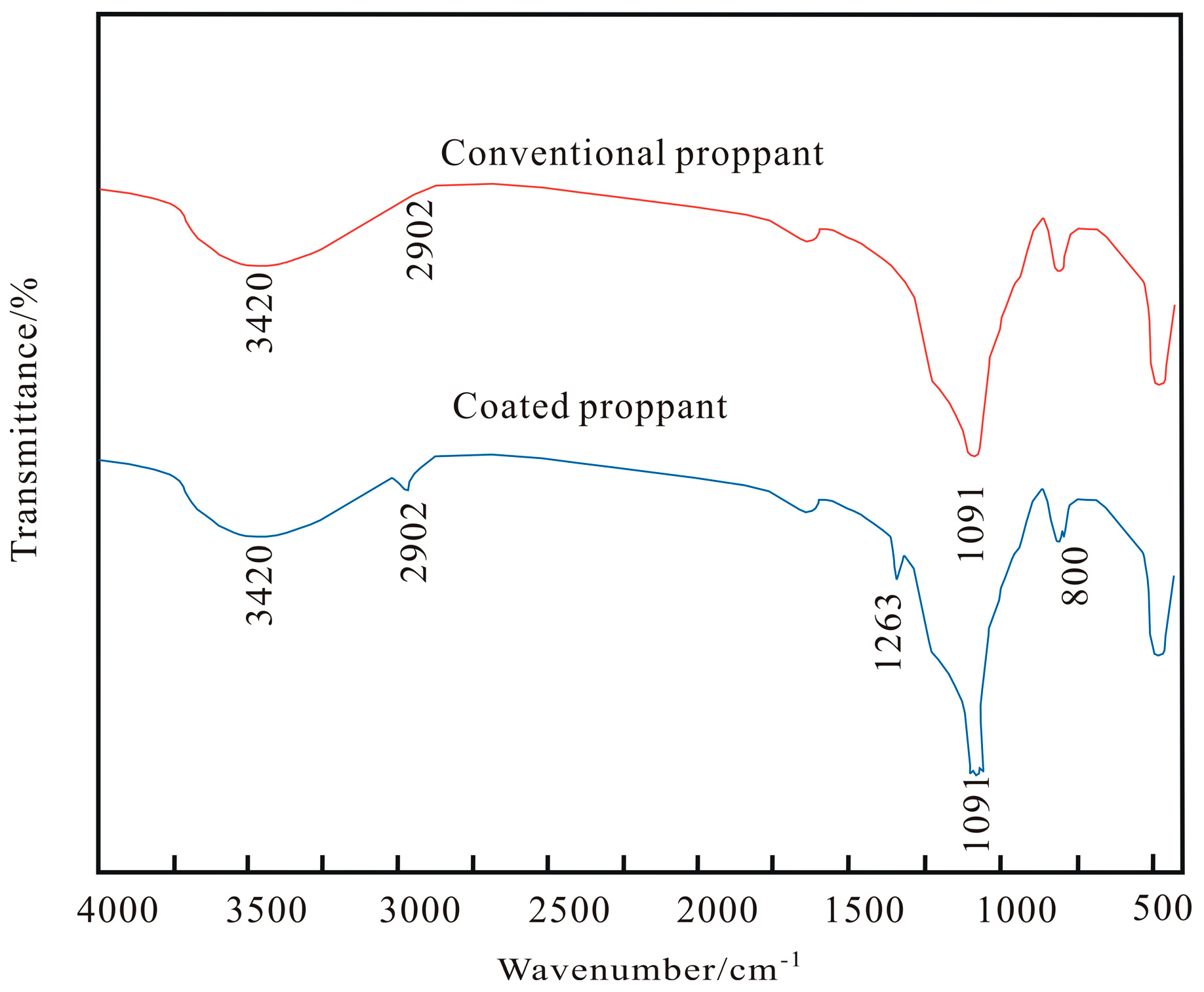
2.3. Tests of FT-IR Spectra
2.4. Tests of Compression Resistance, Temperature Resistance, Surface Wettability, and Water Plugging Capacity (WPC)
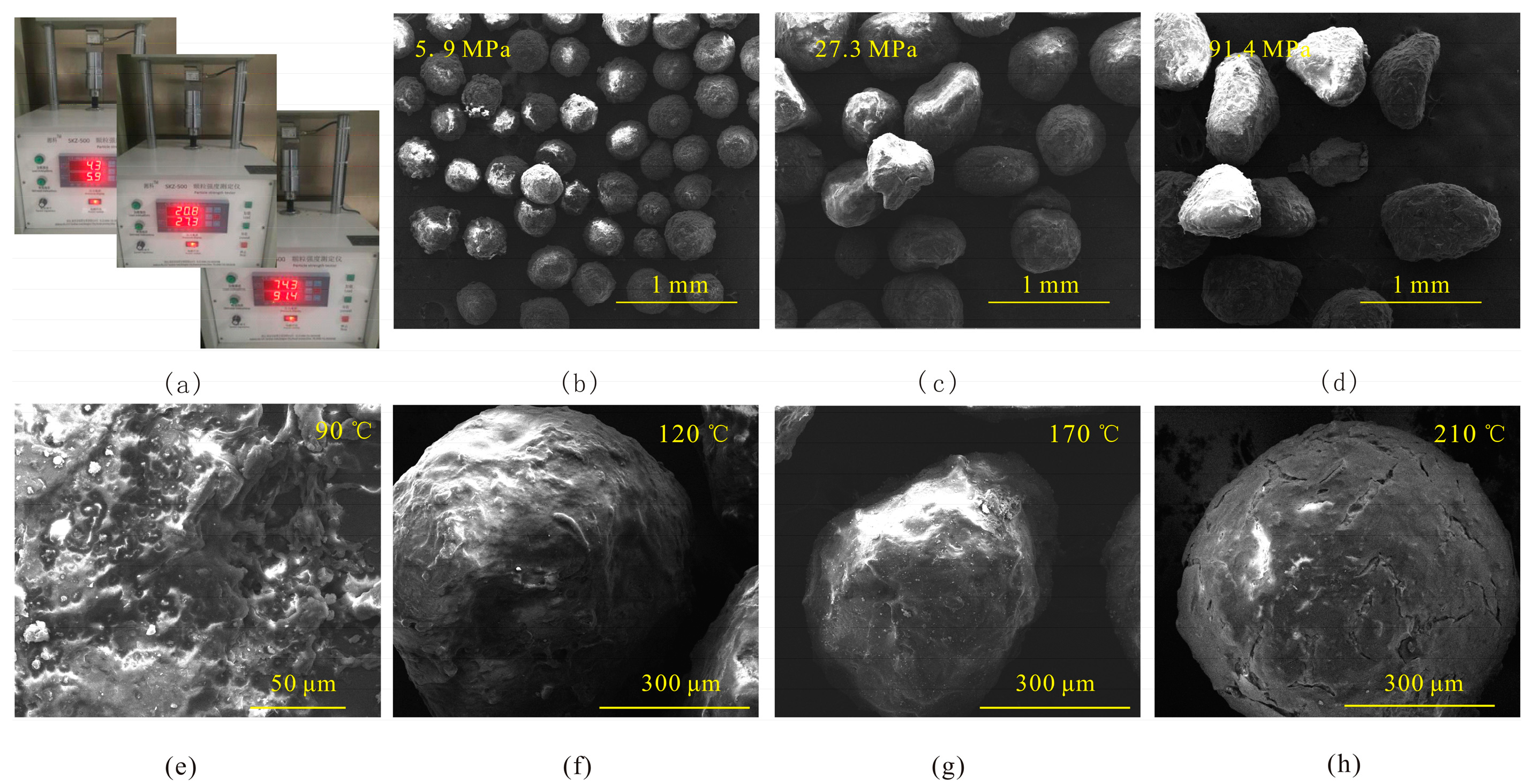
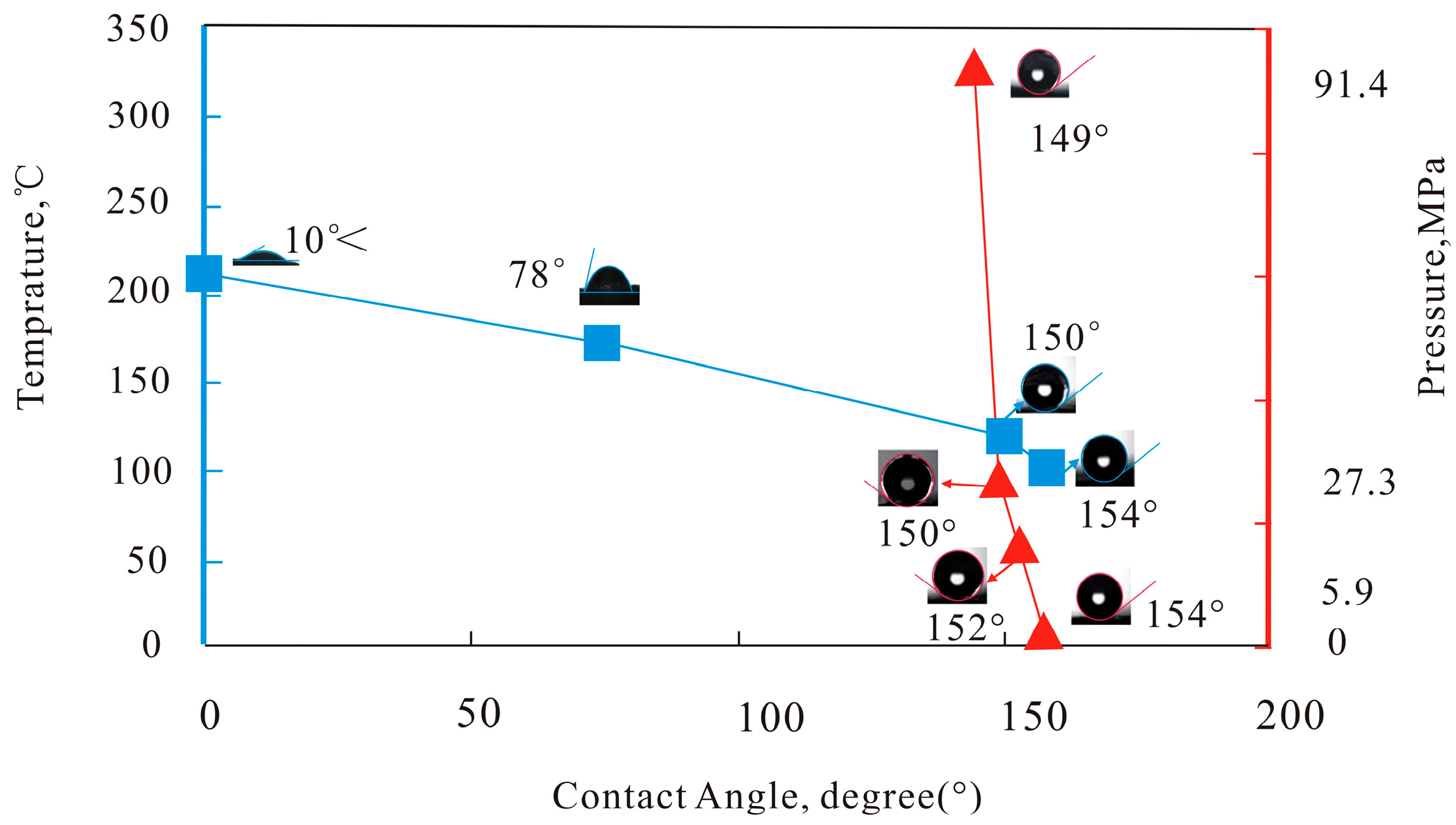
2.4.1. Test of Compression Resistance, Temperature Resistance, and Surface Wettability
2.4.2. WPC Test
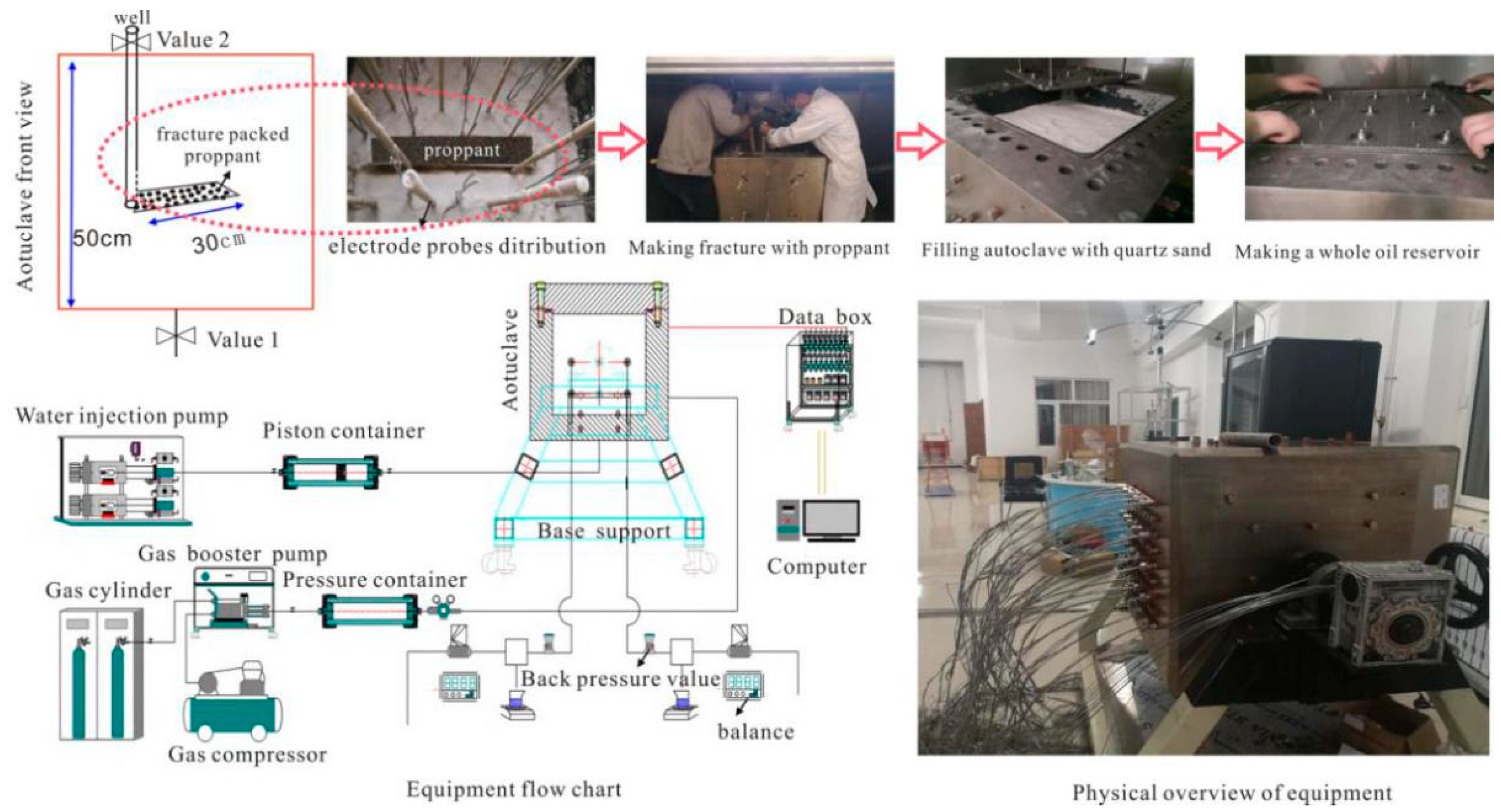
2.5. Test of Coated Proppant Application in 3D Oil Reservoir Development
3. Results and Discussion
3.1. FT-IR Spectrum
3.2. Morphology and Surface Wettability of Proppant Coating
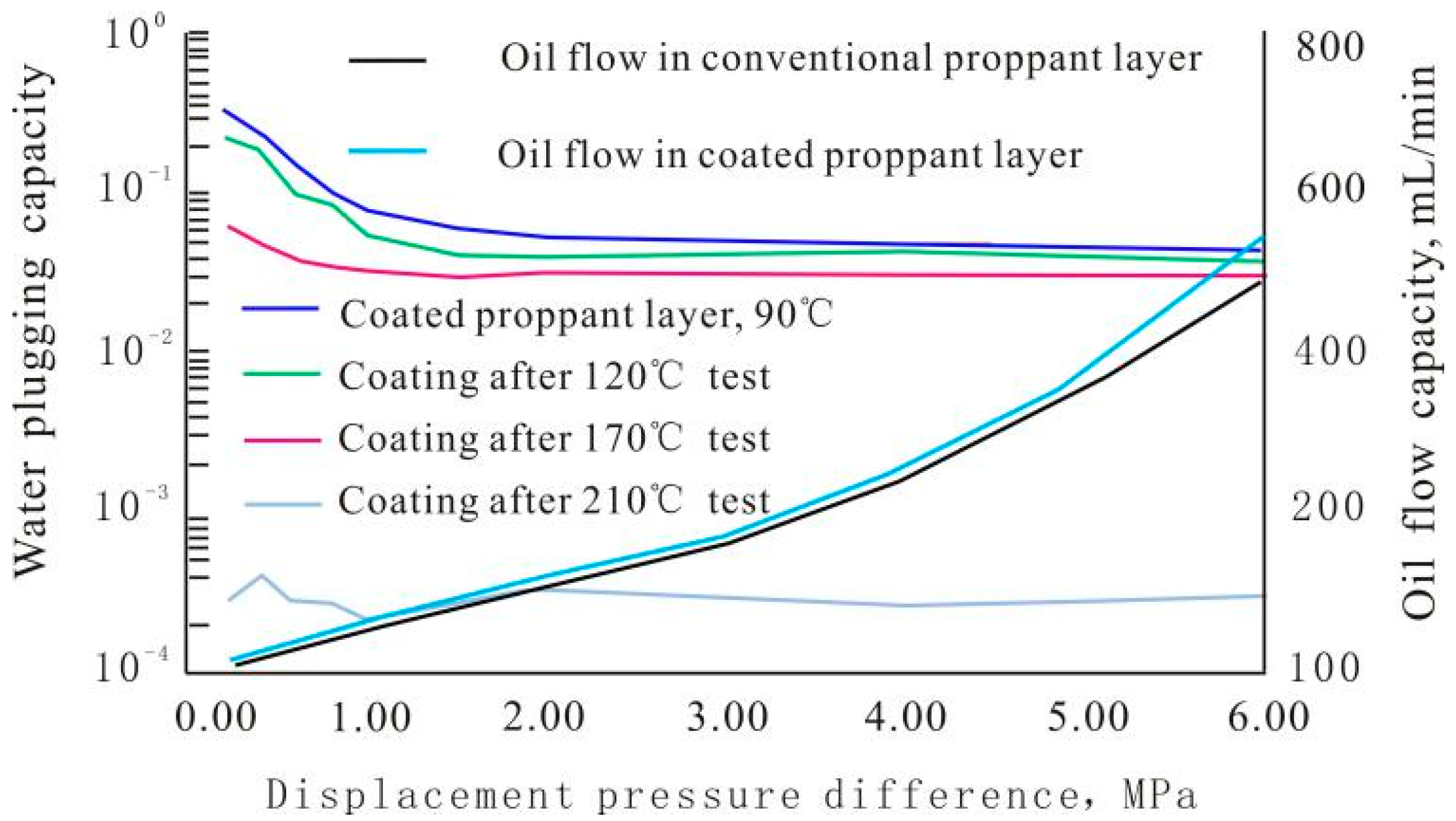
3.3. WPC of Coated Proppant Layer
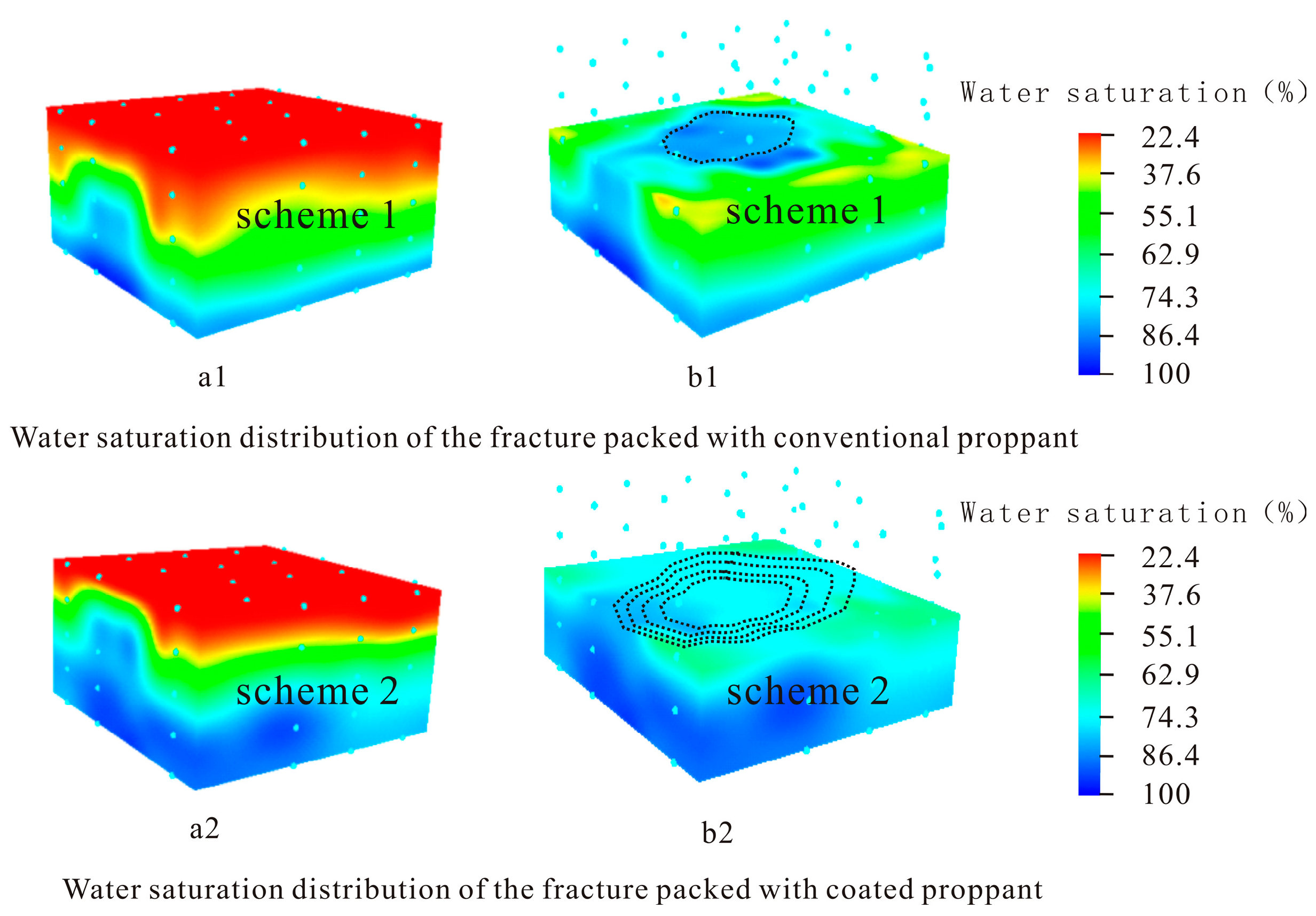
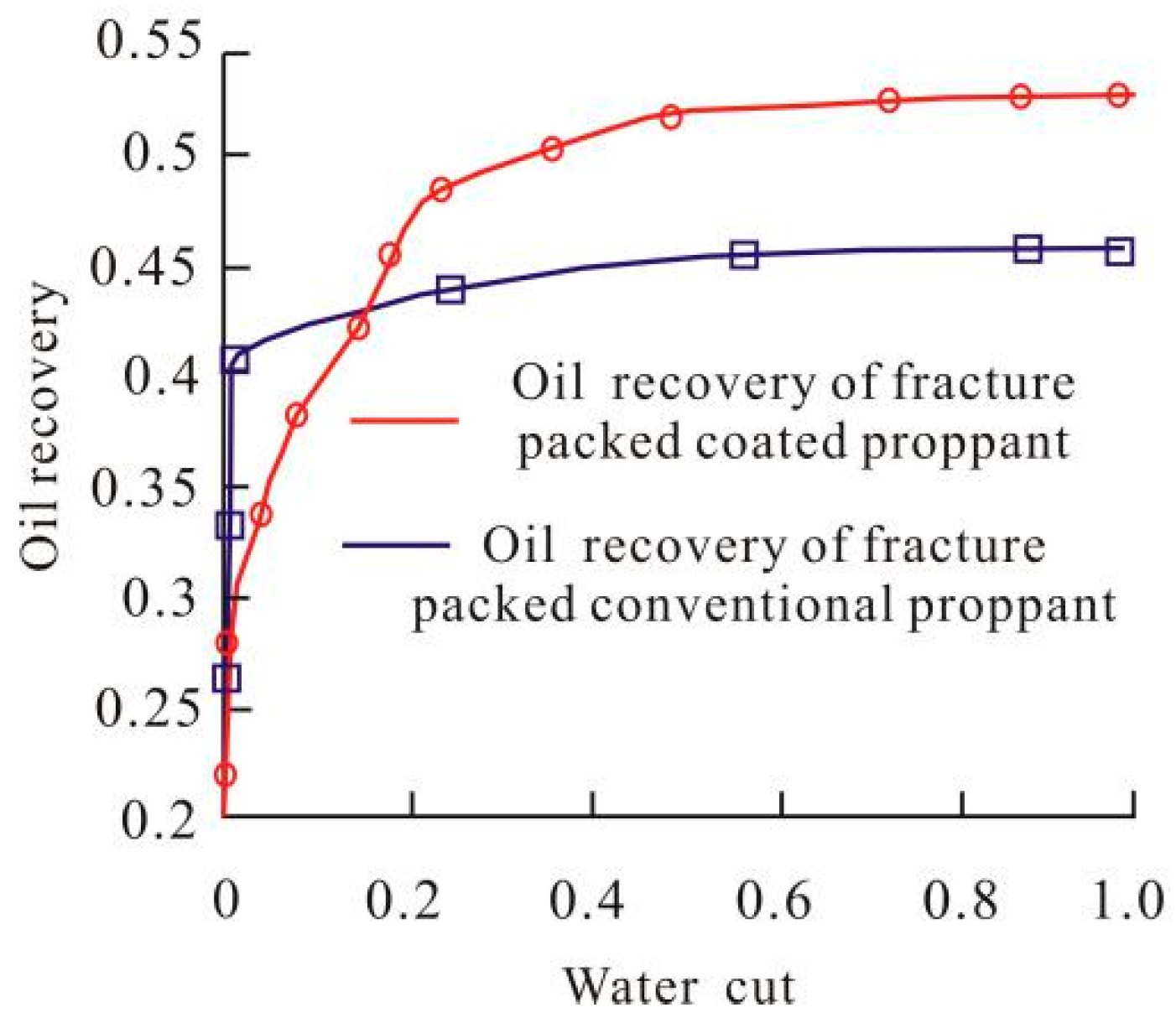
3.4. Coated Proppant Application in 3D Oil Reservoir Development
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ektefa, G.; Shahbazi, K. Hydraulic Fracturing Process in Tight Base Shale of Asmari Formation in Ahwaz Oilfield. J. Pet. Sci. Technol. 2020, 10, 49–55. [Google Scholar]
- Gao, D.; Liu, Y.; Ye, J.; Hu, Y.; Hou, S.; Li, Q.; Han, G. Performance Model for Vertical Wells with Multi-stage Horizontal Hydraulic Fractures in Water Flooded Multilayer Reservoirs. J. Pet. Sci. Technol. 2017, 7, 57–66. [Google Scholar]
- Hashemi, S.M.; Bashiri, G. utilizing conceptual modeling in the study of one the Iranian fractured carbonate reservoirs. J. Pet. Sci. Technol. 2013, 3, 65–76. [Google Scholar]
- Yildiz, T. Inflow Performance Relationship for Perforated Horizontal Wells. SPEJ 2004, 9, 265–279. [Google Scholar] [CrossRef]
- Wang, Z.; Wei, J.; Zhang, J.; Gong, B.; Yan, H. Optimization of perforation distribution for horizontal wells based on genetic algorithms. Pet. Sci. 2010, 7, 232–238. [Google Scholar] [CrossRef] [Green Version]
- Brekke, K.; Lien, S.C. New and Simple Completion Methods for Horizontal wells Improve Production Performance in High-Permeability, Thin Oil Zones. SPE Drill. Complet. 1994, 9, 205–209. [Google Scholar] [CrossRef]
- Li, M.; Yongxin, M.; Yi, X. A New Discovery about Inflow Control Devices in Controlling Water and Increasing Oil Recovery. J. Pet. Sci. Technol. 2017, 7, 32–40. [Google Scholar]
- Zernin, A.A.; Molokov, R.A.; Zharkova, K.V.; Plitkina, Y.A.; Sharipov, I.F.; Deryugin, I.I.; Nigmatullin, R.R.; Klassen, V.V. Design and prospects for the use of inflow control devices at the Tagulskoye oil-gas-condensate field (Russian). Neft. Khozyaystvo-Oil Ind. 2022, 11, 37–41. [Google Scholar]
- Konopczynski, M.; Moradi, M.; Krishnan, T.; Sandhu, H.; Lai, C.-L. Case Study: Oil Production Optimized With Autonomous Inflow Control Devices Offshore Malaysia. J. Pet. Technol. 2022, 74, 44–51. [Google Scholar] [CrossRef]
- Xie, K.; Lu, X.; Li, Q.; Jiang, W.; Yu, Q. Analysis of Reservoir Applicability of Hydrophobically Associating Polymer. SPE J. 2016, 21, 1–9. [Google Scholar] [CrossRef]
- Xie, K.; Lu, X.; Pan, H.; Han, D.; Hu, G.; Zhang, J.; Zhang, B.; Cao, B. Analysis of Dynamic Imbibition Effect of Surfactant in Microcracks of Reservoir at High Temperature and Low Permeability. Soc. Pet. Eng. 2018, 33, 596–606. [Google Scholar] [CrossRef]
- Shi, Y.; Niu, F.; Mohanty, K. Wettability Alteration with Weak Acid-Assisted Surfactant Flood. SPE J. 2023, 13, 1–15. [Google Scholar] [CrossRef]
- Mohammadian, E.; Junin, R.; Rahmani, O.; Idris, A.K. Effects of sonication radiation on oil recovery by ultrasonic waves stimulated water-flooding. Ultrasonics 2013, 53, 607–6014. [Google Scholar] [CrossRef] [PubMed]
- Chao, H.; Xue, Q.; Jing, Z.; Jian, Z.; Shun, T. Biomimetic superhydrophobic surfaces by combining mussel-inspired adhesion with lotus-inspired coating. Nanotechnology 2015, 26, 335602. [Google Scholar]
- Guo, Z.; Liu, W.; Su, B.-L. Superhydrophobic surfaces: From natural to biomimetic to functional. J. Colloid Interface Sci. 2011, 353, 335–355. [Google Scholar] [CrossRef]
- Luévano-Hipólito, R.E.; Torres-Martínez, L.M.; Cantú-Castro, L.V.F. Self-cleaning coatings based on fly ash and bismuth-photocatalysts: Bi2O3, Bi2O2CO3, BiOI, BiVO4, BiPO4. Constr. Build. Mater. 2019, 220, 206–213. [Google Scholar] [CrossRef]
- Shah, A.J.; Bhagchandani, G.B. Anti corrosive rubber coating. Int. J. Sci. Eng. Technol. 2013, 2, 443–447. [Google Scholar]
- Yang, J.; Tang, Y.; Xu, J.; Chen, B.; Tang, H.; Li, C. Durable superhydrophobic/superoleophilic epoxy/attapulgite nanocomposite coatings for oil/water separation. Surf. Coat. Technol. 2015, 272, 285–290. [Google Scholar] [CrossRef]
- Anokhina, T.S.; Ilyin, S.O.; Ignatenko, V.Y.; Bakhtin, D.S.; Kostyuk, A.V.; Antonov, S.V.; Volkov, A.V. Formation of Porous Films with Hydrophobic Surface from a Blend of Polymers. Polym. Sci. Ser. A 2019, 61, 619–626. [Google Scholar] [CrossRef]
- Paterlini, T.T.; Nogueira, L.F.B.; Tovani, C.B.; Cruz, M.A.E.; Derradi, R.; Ramos, A.P. The role played by modified bioinspired surfaces in interfacial properties of biomaterials. Biophys. Rev. 2017, 9, 683–698. [Google Scholar] [CrossRef] [Green Version]
- Park, I.C.; Kim, S.J. Electrochemical Characteristics in Seawater for Cold Thermal Spray-Coated Al-Mg Alloy Layer. Acta Metal. Sin. (Engl. Lett.) 2016, 29, 727–734. [Google Scholar] [CrossRef] [Green Version]
- Haider, A.J.; AL-Anbari, R.H.; Kadhim, G.R.; Salame, C.T. Exploring potential Environmental applications of TiO2 Nanoparticles. Energy Procedia 2017, 119, 332–345. [Google Scholar] [CrossRef]
- Schmitt, C.; Lebienvenu, M. Electrostatic painting of conductive composite materials. J. Mater. Process. Technol. 2003, 134, 303–309. [Google Scholar] [CrossRef]
- Jeong, S.; Park, S.; Cho, J. High-Performance, Layered, 3D-LiCoO2 Cathodes with a Nanoscale Co3O4 Coating via Chemical Etching. Adv. Energy Mater. 2011, 1, 368–372. [Google Scholar] [CrossRef]
- Liu, H. Preparation of Superhydrophobic Coatings on Zinc, Silicon, and Steel by a Solution-Immersion Technique. ACS Appl. Mater. Interfaces 2009, 1, 2086–2107. [Google Scholar] [CrossRef] [PubMed]
- Hu, S.; Cao, X.; Song, Y.; Li, C.; Xie, P.; Jiang, L. New responsive property of poly (e-caprolactone) as the thermal switch from superhydrophobic to superhydrophilic. Chem. Commun. 2008, 17, 2025–2027. [Google Scholar] [CrossRef]
- Xu, Z.; Zhao, Y.; Wang, H.; Zhou, H.; Qin, C.; Wang, X.; Lin, T. Fluorine-Free Superhydrophobic Coatings with pH-induced Wettability Transition for Controllable Oil-Water Separation. ACS Appl. Mater. Interfaces 2016, 8, 5661–5667. [Google Scholar] [CrossRef] [PubMed]
- Vreeburg, R.-J.; Roodhart, L.P.; Davies, D.R.; Penny, G.S. Proppant Backproduction During Hydraulic Fracturing-A New Failure Mechanism for Resin-Coated Proppants. J. Pet. Technol. 1994, 46, 884–889. [Google Scholar] [CrossRef]
- Shaoul, J.R.; Park, J.; Langford, M. The Effect of Resin-Coated Proppant and Proppant Production on Convergent-Flow Skin in Horizontal Wells With Transverse Fractures. SPE Prod. Oper. 2020, 35, 214–230. [Google Scholar] [CrossRef]
- Rassenfoss, S. Rethinking Proppant: From Liquid to Solid. J. Pet. Technol. 2015, 67, 58–59. [Google Scholar] [CrossRef]
- Shi, C.; Dai, J.; Li, C.; Shen, X.; Peng, L.; Zhang, P.; Wu, D.; Sun, D.; Zhao, J. A Modified Ceramic-Coating Separator with High-Temperature Stability for Lithium-Ion Battery. Polymers 2017, 9, 159–171. [Google Scholar] [CrossRef] [PubMed]
- Choi, J.-A.; Kimb, S.H.; Kima, D.-W. Enhancement of thermal stability and cycling performance in lithium-ion cells through the use ofceramic-coated separators. J. Power Sources 2010, 195, 6192–6196. [Google Scholar] [CrossRef]
- Panarin, V.Y.; Svavil’nyi, N.Y.; Khominich, A.I. An experimental study of the composite CNT/copper coating. Appl Nanosci. 2019, 9, 1111–1117. [Google Scholar] [CrossRef]



| PetroChina Category of Reservoir Layer Depth | Shallow Reservoir Layer | Average Reservoir Layer | Maximum Reservoir Layer |
|---|---|---|---|
| Reservoir Depth, m | ≈600 | 1800 ~ 2889 | 8937.77 |
| Average Fracture Closure Pressure, MPa | 5.9 | 23.7 | 91.4 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, H.; Kang, H.; Liu, J.; Wang, F.; Wang, C.; Yang, F.; Zhang, Y. A New Coated Proppant for Packing Fractures in Oil Reservoirs. Coatings 2023, 13, 1238. https://doi.org/10.3390/coatings13071238
Wang H, Kang H, Liu J, Wang F, Wang C, Yang F, Zhang Y. A New Coated Proppant for Packing Fractures in Oil Reservoirs. Coatings. 2023; 13(7):1238. https://doi.org/10.3390/coatings13071238
Chicago/Turabian StyleWang, Haidong, Haodong Kang, Jiaxin Liu, Fei Wang, Chunyao Wang, Fengyuan Yang, and Yunfeng Zhang. 2023. "A New Coated Proppant for Packing Fractures in Oil Reservoirs" Coatings 13, no. 7: 1238. https://doi.org/10.3390/coatings13071238





