Porous-Spherical Cr2O3-Cr(OH)3-Polypyrrole/Polypyrrole Nanocomposite Thin-Film Photodetector and Solar Cell Applications
Abstract
1. Introduction
2. Experimental Part
2.1. Materials
2.2. Ppy Thin-Film Preparation
2.3. Cr2O3-Cr(OH)3-Ppy/Ppy Nanocomposite Thin-Film Preparation
2.4. Characterization Process
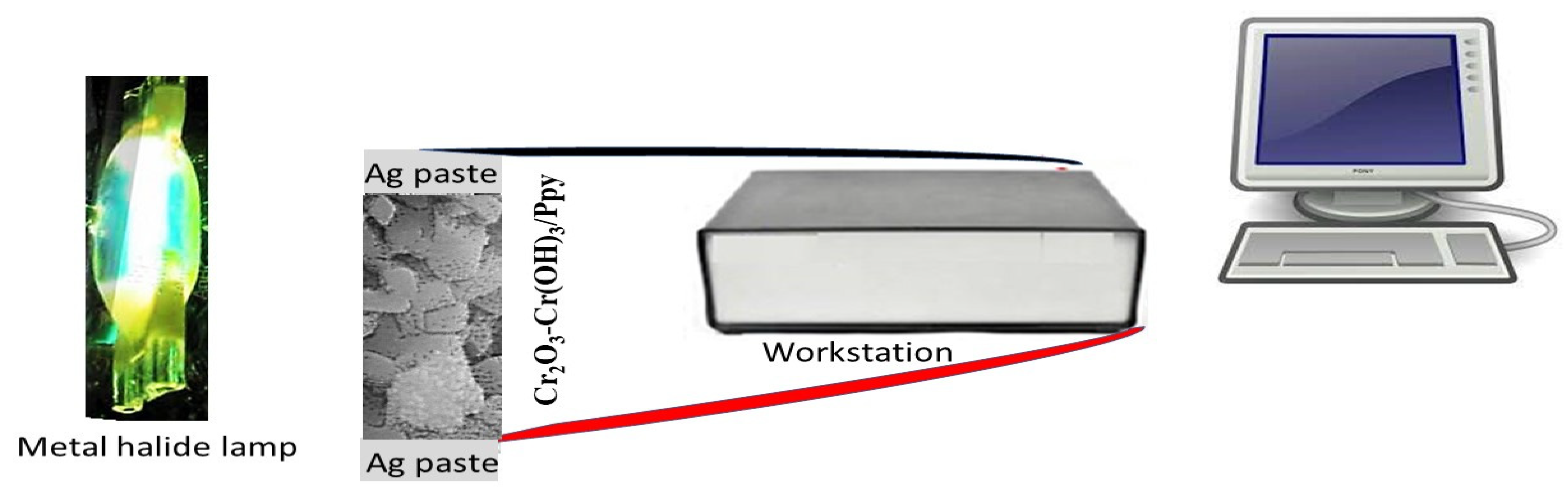
2.5. The Construction of Photodetector or Solar Cell Device and the Electrical Testing
3. Results and Discussion
3.1. Characterization and Analysis
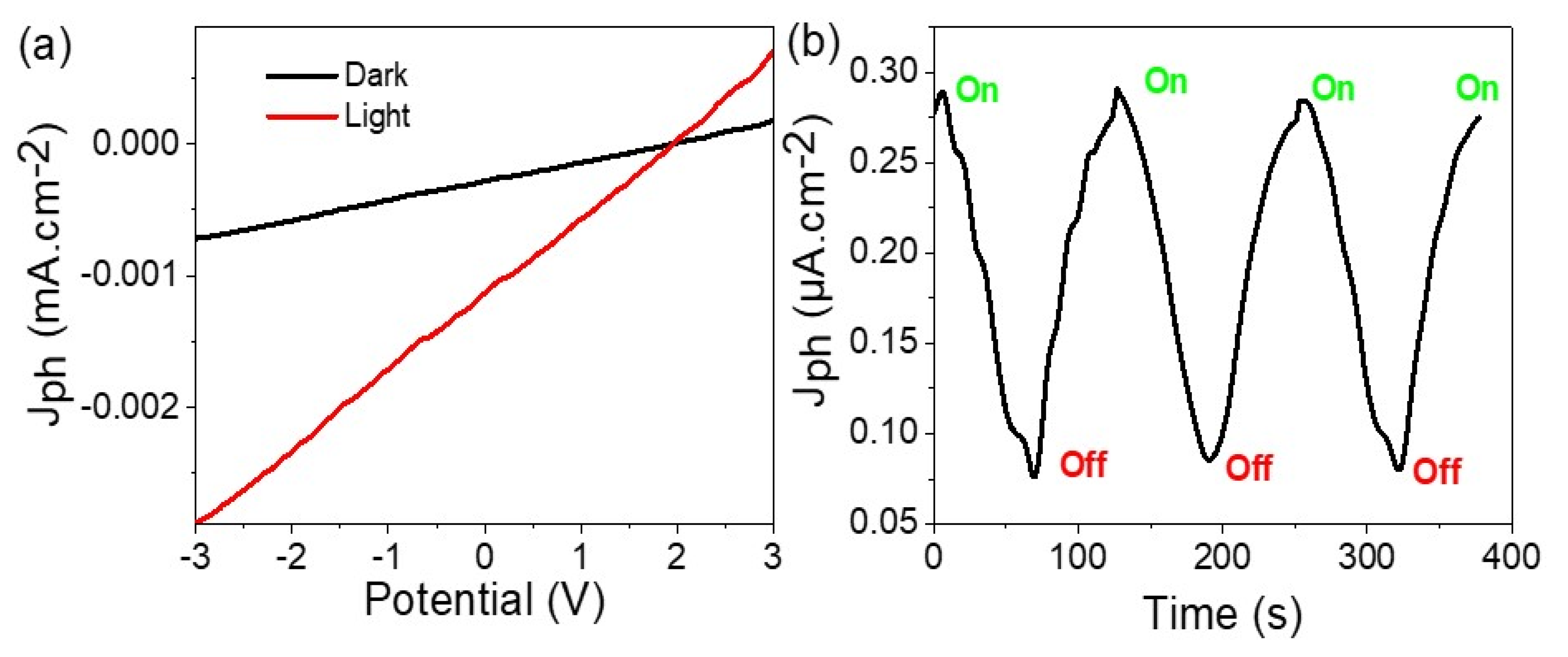
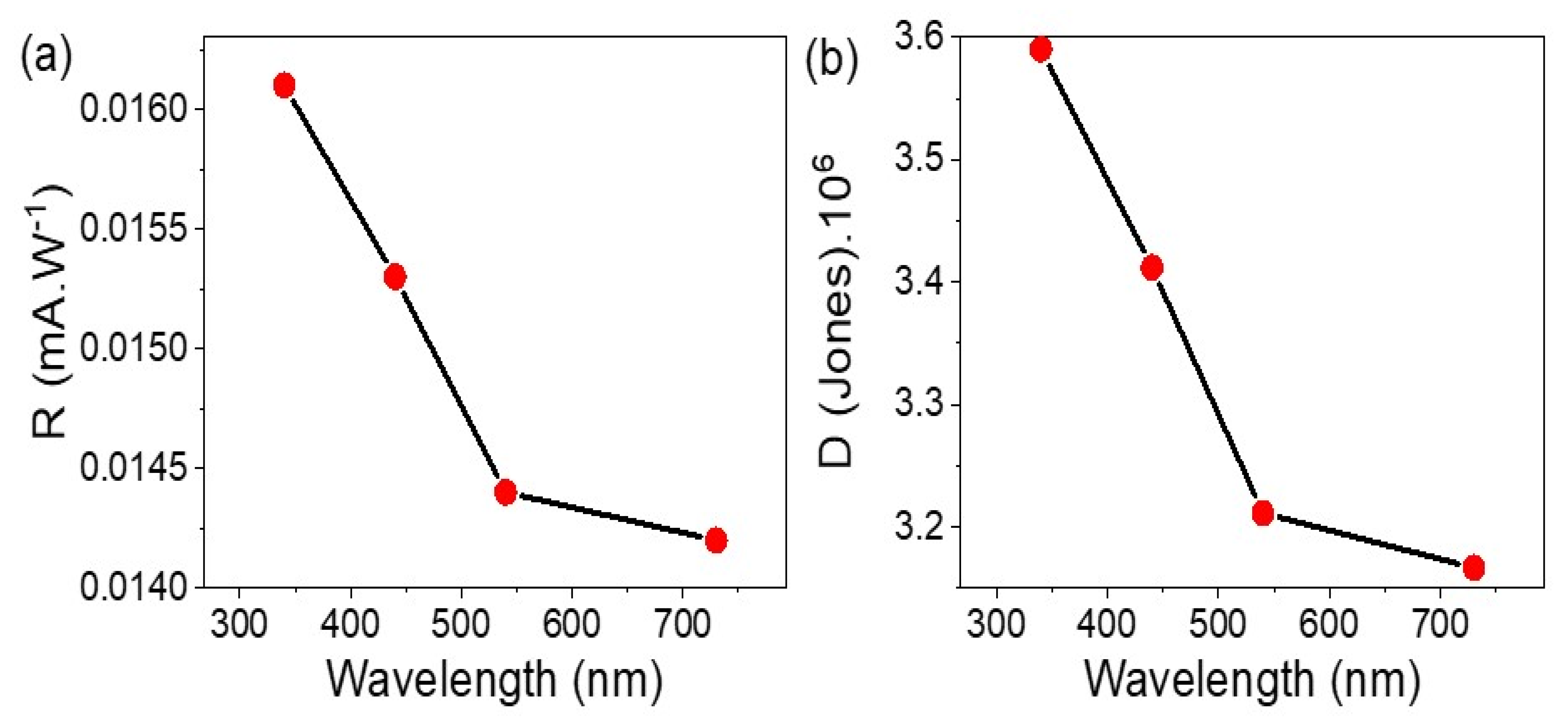
3.2. Electrical Testing
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Qin, B.; Cai, Y.; Wang, P.; Zou, Y.C.; Cao, J.; Qi, J. Crystalline Molybdenum Carbide-amorphous Molybdenum Oxide Heterostructures: In Situ Surface Reconfiguration and Electronic States Modulation for Li-S Batteries. Energy Storage Mater. 2022, 47, 345–353. [Google Scholar] [CrossRef]
- Qin, B.; Cai, Y.; Si, X.; Li, C.; Cao, J.; Fei, W.; Qi, J. Ultra-Lightweight Ion-Sieving Membranes for High-Rate Lithium Sulfur Batteries. Chem. Eng. J. 2022, 430, 132698. [Google Scholar] [CrossRef]
- Qin, B.; Wang, Q.; Yao, W.; Cai, Y.; Chen, Y.; Wang, P.; Zou, Y.; Zheng, X.; Cao, J.; Qi, J.; et al. Heterostructured Mn3O4-MnS Multi-Shelled Hollow Spheres for Enhanced Polysulfide Regulation in Lithium-Sulfur Batteries. Energy Environ. Mater. 2022, 1, e12475. [Google Scholar] [CrossRef]
- Shen, Z.; Gao, Q.; Zhu, X.; Guo, Z.; Guo, K.; Song, X.; Zhao, Y. In-Situ Free Radical Supplement Strategy for Improving the Redox Kinetics of Li-S Batteries. Energy Storage Mater. 2023, 57, 299–307. [Google Scholar] [CrossRef]
- Yan, Y.; Lin, J.; Xu, T.; Liu, B.; Huang, K.; Qiao, L.; Liu, S.; Cao, J.; Jun, S.C.; Yamauchi, Y.; et al. Atomic-Level Platinum Filling into Ni-Vacancies of Dual-Deficient NiO for Boosting Electrocatalytic Hydrogen Evolution. Adv. Energy Mater. 2022, 12, 2200434. [Google Scholar] [CrossRef]
- Qi, J.; Yan, Y.; Cai, Y.; Cao, J.; Feng, J. Nanoarchitectured Design of Vertical-Standing Arrays for Supercapacitors: Progress, Challenges, and Perspectives. Adv. Funct. Mater. 2021, 31, 2006030. [Google Scholar] [CrossRef]
- Yan, Y.; Lin, J.; Cao, J.; Guo, S.; Zheng, X.; Feng, J.; Qi, J. Activating and Optimizing the Activity of NiCoP Nanosheets for Electrocatalytic Alkaline Water Splitting through the V Doping Effect Enhanced by P Vacancies. J. Mater. Chem. A 2019, 7, 24486–24492. [Google Scholar] [CrossRef]
- Zhan, T.; Sun, J.; Feng, T.; Zhang, Y.; Zhou, B.; Zhang, B.; Wang, J.; Sarro, P.M.; Zhang, G.; Liu, Z.; et al. Electrical Characteristics and Photodetection Mechanism of TiO2/AlGaN/GaN Heterostructure-Based Ultraviolet Detectors with a Schottky Junction. J. Mater. Chem. C 2023, 11, 1704–1713. [Google Scholar] [CrossRef]
- Maldonado, F.; Rivera, R.; Stashans, A. Structure, Electronic and Magnetic Properties of Ca-Doped Chromium Oxide Studied by the DFT Method. Phys. B Condens. Matter 2012, 407, 1262–1267. [Google Scholar] [CrossRef]
- Gesheva, K.; Ivanova, T.; Bodurov, G.; Szilágyi, I.M.; Justh, N.; Kéri, O.; Boyadjiev, S.; Nagy, D.; Aleksandrova, M. Technologies for Deposition of Transition Metal Oxide Thin Films: Application as Functional Layers in “Smart Windows” and Photocatalytic Systems. J. Phys. Conf. Ser. 2016, 682, 012011. [Google Scholar] [CrossRef]
- Shaban, M.; Abukhadra, M.R.; Rabia, M.; Elkader, Y.A.; Abd El-Halim, M.R. Investigation the Adsorption Properties of Graphene Oxide and Polyaniline Nano/Micro Structures for Efficient Removal of Toxic Cr(VI) Contaminants from Aqueous Solutions; Kinetic and Equilibrium Studies. Rend. Lincei Sci. Fis. Nat. 2018, 29, 141–154. [Google Scholar] [CrossRef]
- Fathy, A.; Ahmed, A.M.; Ghanem, M.A.; Hamdy, H.; Shaban, M.; Mohammed, K.M.H.; Rabia, M. Supercapacitance Performance of Novel Core/Shell Poly(m-Toluidine)/(Co-Ni) Nanoplatelets/Nanoneedles Nanocomposite. J. Mol. Liq. 2023, 386, 122466. [Google Scholar] [CrossRef]
- Vu, N.M.; Luo, X.; Novakov, S.; Jin, W.; Nordlander, J.; Meisenheimer, P.B.; Trassin, M.; Zhao, L.; Heron, J.T. Bulk-like Dielectric and Magnetic Properties of Sub 100 Nm Thick Single Crystal Cr2O3 Films on an Epitaxial Oxide Electrode. Sci. Rep. 2020, 10, 14721. [Google Scholar] [CrossRef]
- Hashmi, A.; Nakanishi, K.; Farooq, M.U.; Ono, T. Ising Ferromagnetism and Robust Half-Metallicity in Two-Dimensional Honeycomb-Kagome Cr2O3 Layer. NPJ 2D Mater. Appl. 2020, 4, 39. [Google Scholar] [CrossRef]
- Auguste, R.; Chan, H.L.; Romanovskaia, E.; Qiu, J.; Schoell, R.; Liedke, M.O.; Butterling, M.; Hirschmann, E.; Attallah, A.G.; Wagner, A.; et al. A Multimodal Approach to Revisiting Oxidation Defects in Cr2O3. NPJ Mater. Degrad. 2022, 6, 61. [Google Scholar] [CrossRef]
- Sayyah, S.M.; Shaban, M.; Rabia, M. Electropolymerization of m-Toluidin on Platinum Electrode from Aqueous Acidic Solution and Character of the Obtained Polymer. Adv. Polym. Technol. 2018, 37, 126–136. [Google Scholar] [CrossRef]
- Sayyah, S.M.; Shaban, M.; Rabia, M. A Sensor of m-Toluidine/m-Cresol Polymer Film for Detection of Lead Ions by Potentiometric Methods. Sens. Lett. 2016, 14, 522–529. [Google Scholar] [CrossRef]
- Sayyah, S.M.; Shaban, M.; Rabia, M. A High-Sensitivity Potentiometric Mercuric Ion Sensor Based on m-Toluidine Films. IEEE Sens. J. 2016, 16, 1541–1548. [Google Scholar] [CrossRef]
- Besisa, D.H.A.; Mohamed, H.H.; Ewais, E.M.M.; Ahmed, Y.M.Z.; Amin, A.M.M. Synthesis of New Cr2O3/Fe2O3/Glass Composites from Industrial Wastes; from Undesired to Advanced Optical Products. Environ. Sci. Pollut. Res. 2022, 29, 84357–84365. [Google Scholar] [CrossRef]
- Inamdar, H.K.; Reddy, N.; Khader, S.A.; Naresh, P.; Ambika Prasad, M.V.N.; Durga Prasad, G.S. Polypyrrole/Cr2O3 Hybrid Nanocomposites (NCs) Prepared for Their Structural, Morphological, Optical and Conductivity Studies. Compos. Commun. 2019, 14, 21–28. [Google Scholar] [CrossRef]
- Abdelhamied, M.M.; Ghobashy, M.M.; Hadia, N.M.A.; Mohamed, W.S.; Sharshir, A.I.; Nady, N.; Mohamed, S.H.; Shaban, M.; Rabia, M. Chemical Deposition of Ag and Ag2O on Grafting Film of PET-COOH by Photografting Polymerization for Optoelectronic Application. J. Mater. Sci. Mater. Electron. 2023, 34, 41. [Google Scholar] [CrossRef]
- Hadia, N.M.A.; Khalafalla, M.A.H.; Abdel Salam, F.M.; Ahmed, A.M.; Shaban, M.; Almuqrin, A.H.; Hajjiah, A.; Hanafi, H.A.; Alruqi, M.; Mourad, A.-H.I.; et al. Conversion of Sewage Water into H2 Gas Fuel Using Hexagonal Nanosheets of the Polyaniline-Assisted Deposition of PbI2 as a Nanocomposite Photocathode with the Theoretical Qualitative Ab-Initio Calculation of the H2O Splitting. Polymers 2022, 14, 2148. [Google Scholar] [CrossRef] [PubMed]
- Abdelazeez, A.A.A.; Hadia, N.M.A.; Mourad, A.H.I.; El-Fatah, G.A.; Shaban, M.; Ahmed, A.M.; Alzaid, M.; Cherupurakal, N.; Rabia, M. Effect of Au Plasmonic Material on Poly m-Toluidine for Photoelectrochemical Hydrogen Generation from Sewage Water. Polymers 2022, 14, 768. [Google Scholar] [CrossRef] [PubMed]
- Bekhoukh, A.; Kiari, M.; Moulefera, I.; Sabantina, L.; Benyoucef, A. New Hybrid Adsorbents Based on Polyaniline and Polypyrrole with Silicon Dioxide: Synthesis, Characterization, Kinetics, Equilibrium, and Thermodynamic Studies for the Removal of 2,4-Dichlorophenol. Polymers 2023, 15, 2032. [Google Scholar] [CrossRef] [PubMed]
- Yamani, K.; Berenguer, R.; Benyoucef, A.; Morallón, E. Preparation of Polypyrrole (PPy)-Derived Polymer/ZrO2 Nanocomposites: Effects of Nanoparticles Interface and Polymer Structure. J. Therm. Anal. Calorim. 2019, 135, 2089–2100. [Google Scholar] [CrossRef]
- Wang, S.B.; Hsiao, C.H.; Chang, S.J.; Lam, K.T.; Wen, K.H.; Hung, S.C.; Young, S.J.; Huang, B.R. A CuO Nanowire Infrared Photodetector. Sens. Actuators A Phys. 2011, 171, 207–211. [Google Scholar] [CrossRef]
- Bai, Z.; Zhang, Y. Self-Powered UV-Visible Photodetectors Based on ZnO/Cu2O Nanowire/Electrolyte Heterojunctions. J. Alloys Compd. 2016, 675, 325–330. [Google Scholar] [CrossRef]
- Atta, A.; Negm, H.; Abdeltwab, E.; Rabia, M.; Abdelhamied, M.M. Facile Fabrication of Polypyrrole/NiOx Core-Shell Nanocomposites for Hydrogen Production from Wastewater. Polym. Adv. Technol. 2023, 34, 1633–1641. [Google Scholar] [CrossRef]
- Zaki, S.E.; Basyooni, M.A.; Shaban, M.; Rabia, M.; Eker, Y.R.; Attia, G.F.; Yilmaz, M.; Ahmed, A.M. Role of Oxygen Vacancies in Vanadium Oxide and Oxygen Functional Groups in Graphene Oxide for Room Temperature CO2 Gas Sensors. Sens. Actuators A Phys. 2019, 294, 17–24. [Google Scholar] [CrossRef]
- Turczyn, R.; Krukiewicz, K.; Katunin, A.; Sroka, J.; Sul, P. Fabrication and Application of Electrically Conducting Composites for Electromagnetic Interference Shielding of Remotely Piloted Aircraft Systems. Compos. Struct. 2020, 232, 111498. [Google Scholar] [CrossRef]
- Vishnuvardhan, T.K.; Kulkarni, V.R.; Basavaraja, C.; Raghavendra, S.C. Synthesis, Characterization and a.c. Conductivity of Polypyrrole/Y2O3 Composites. Bull. Mater. Sci. 2006, 29, 77–83. [Google Scholar] [CrossRef]
- Balayeva, O.O.; Azizov, A.A.; Muradov, M.B.; Alosmanov, R.M.; Eyvazova, G.M.; Mammadyarova, S.J. Cobalt Chromium-Layered Double Hydroxide, α- and β- Co(OH)2 and Amorphous Cr(OH)3: Synthesis, Modification and Characterization. Heliyon 2019, 5, e02725. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Yang, Y.; Li, F.; Song, G.; Shao, Y.; Ouyang, R.; Xu, L.; Li, W.; Miao, Y. A Newly Prepared Ni(OH)2@Cr(OH)3 Nanohybrid for Its Bioelectrocatalysis. Sens. Actuators B Chem. 2014, 198, 350–359. [Google Scholar] [CrossRef]
- Ahmed, A.A.; Rabia, M.; Shaban, M. The Structure and Photoelectrochemical Activity of Cr-Doped PbS Thin Films Grown by Chemical Bath Deposition. RSC Adv. 2020, 10, 14458–14470. [Google Scholar] [CrossRef] [PubMed]
- Sayyah, S.M.; Shaban, M.; Rabia, M. m-Toluidine Polymer Film Coated Platinum Electrode as a PH Sensor by Potentiometric Methods. Sens. Lett. 2015, 13, 961–966. [Google Scholar] [CrossRef]
- Xie, G.; Liu, X.; Li, Q.; Lin, H.; Li, Y.; Nie, M.; Qin, L. The Evolution of α-MnO2 from Hollow Cubes to Hollow Spheres and Their Electrochemical Performance for Supercapacitors. J. Mater. Sci. 2017, 52, 10915–10926. [Google Scholar] [CrossRef]
- Feng, M.; Lu, W.; Zhou, Y.; Zhen, R.; He, H.; Wang, Y.; Li, C. Synthesis of Polypyrrole/Nitrogen-Doped Porous Carbon Matrix Composite as the Electrode Material for Supercapacitors. Sci. Rep. 2020, 10, 15370. [Google Scholar] [CrossRef]
- Elsayed, A.M.; Rabia, M.; Shaban, M.; Aly, A.H.; Ahmed, A.M. Preparation of Hexagonal Nanoporous Al2O3/TiO2/TiN as a Novel Photodetector with High Efficiency. Sci. Rep. 2021, 11, 17572. [Google Scholar] [CrossRef]
- Mohamed, H.S.H.; Rabia, M.; Zhou, X.G.; Qin, X.S.; Khabiri, G.; Shaban, M.; Younus, H.A.; Taha, S.; Hu, Z.Y.; Liu, J.; et al. Phase-Junction Ag/TiO2 Nanocomposite as Photocathode for H2 Generation. J. Mater. Sci. Technol. 2021, 83, 179–187. [Google Scholar] [CrossRef]
- Mohamed, H.S.H.; Rabia, M.; Shaban, M.; Taha, S. Controlled Synthesis of CdS Nanoflowers Thin Films for H2 Electro-Generation. Mater. Sci. Semicond. Process. 2020, 120, 105307. [Google Scholar] [CrossRef]
- Mohamed, S.H.; Zhao, H.; Romanus, H.; El-Hossary, F.M.; Abo EL-Kassem, M.; Awad, M.A.; Rabia, M.; Lei, Y. Optical, Water Splitting and Wettability of Titanium Nitride/Titanium Oxynitride Bilayer Films for Hydrogen Generation and Solar Cells Applications. Mater. Sci. Semicond. Process. 2020, 105, 104704. [Google Scholar] [CrossRef]
- Liu, Z.; Li, F.; Li, S.; Hu, C.; Wang, W.; Wang, F.; Lin, F.; Wang, H. Fabrication of UV Photodetector on TiO2/Diamond Film. Sci. Rep. 2015, 5, 14420. [Google Scholar] [CrossRef]
- Mohamed, F.; Rabia, M.; Shaban, M. Synthesis and Characterization of Biogenic Iron Oxides of Different Nanomorphologies from Pomegranate Peels for Efficient Solar Hydrogen Production. J. Mater. Res. Technol. 2020, 9, 4255–4271. [Google Scholar] [CrossRef]
- Noothongkaew, S.; Thumthan, O.; An, K.S. Minimal Layer Graphene/TiO2 Nanotube Membranes Used for Enhancement of UV Photodetectors. Mater. Lett. 2018, 218, 274–279. [Google Scholar] [CrossRef]
- Tan, W.C.; Shih, W.H.; Chen, Y.F. A Highly Sensitive Graphene-Organic Hybrid Photodetector with a Piezoelectric Substrate. Adv. Funct. Mater. 2014, 24, 6818–6825. [Google Scholar] [CrossRef]
- Chen, Z.; Ci, H.; Tan, Z.; Dou, Z.; Chen, X.-D.; Liu, B.; Liu, R.; Lin, L.; Cui, L.; Gao, P.; et al. Growth of 12-Inch Uniform Monolayer Graphene Film on Molten Glass and Its Application in PbI2-Based Photodetector. Nano Res. 2019, 12, 1888–1893. [Google Scholar] [CrossRef]
- Zheng, L.; Yu, P.; Hu, K.; Teng, F.; Chen, H.; Fang, X. Scalable-Production, Self-Powered TiO2 Nanowell-Organic Hybrid UV Photodetectors with Tunable Performances. ACS Appl. Mater. Interfaces 2016, 8, 33924–33932. [Google Scholar] [CrossRef]
- Liu, K.; Sakurai, M.; Liao, M.; Aono, M. Giant Improvement of the Performance of ZnO Nanowire Photodetectors by Au Nanoparticles. J. Phys. Chem. C 2010, 114, 19835–19839. [Google Scholar] [CrossRef]
- Lan, T.; Fallatah, A.; Suiter, E.; Padalkar, S. Size Controlled Copper (I) Oxide Nanoparticles Influence Sensitivity of Glucose Biosensor. Sensors 2017, 17, 1944. [Google Scholar] [CrossRef]
- Kalra, A.; Vura, S.; Rathkanthiwar, S.; Muralidharan, R.; Raghavan, S.; Nath, D.N. Demonstration of High-Responsivity Epitaxial β-Ga2O3/GaN Metal-Heterojunction-Metal Broadband UV-A/UV-C Detector. Appl. Phys. Express 2018, 11, 064101. [Google Scholar] [CrossRef]
- Costas, A.; Florica, C.; Preda, N.; Apostol, N.; Kuncser, A.; Nitescu, A.; Enculescu, I. Radial Heterojunction Based on Single ZnO-CuxO Core-Shell Nanowire for Photodetector Applications. Sci. Rep. 2019, 9, 5553. [Google Scholar] [CrossRef] [PubMed]
- Ismail, R.A.; Mousa, A.M.; Shaker, S.S. Visible-Enhanced Silver-Doped PbI2 Nanostructure/Si Heterojunction Photodetector: Effect of Doping Concentration on Photodetector Parameters. Opt. Quantum Electron. 2019, 51, 362. [Google Scholar] [CrossRef]
- Zheng, L.; Hu, K.; Teng, F.; Fang, X. Novel UV-Visible Photodetector in Photovoltaic Mode with Fast Response and Ultrahigh Photosensitivity Employing Se/TiO2 Nanotubes Heterojunction. Small 2017, 13, 1602448. [Google Scholar] [CrossRef] [PubMed]
- Naldoni, A.; Guler, U.; Wang, Z.; Marelli, M.; Malara, F.; Meng, X.; Besteiro, L.V.; Govorov, A.O.; Kildishev, A.V.; Boltasseva, A.; et al. Broadband Hot-Electron Collection for Solar Water Splitting with Plasmonic Titanium Nitride. Adv. Opt. Mater. 2017, 5, 1601031. [Google Scholar] [CrossRef]
- Hong, Q.; Cao, Y.; Xu, J.; Lu, H.; He, J.; Sun, J.L. Self-Powered Ultrafast Broadband Photodetector Based on p-n Heterojunctions of CuO/Si Nanowire Array. ACS Appl. Mater. Interfaces 2014, 6, 20887–20894. [Google Scholar] [CrossRef]




| Materials/Band Position (cm−1) | Characteristic Group | |
|---|---|---|
| Ppy | Cr2O3-Cr(OH)3-Ppy | |
| 3402 | O-H [29] | |
| 1684 | 1632 | C=C quinoid |
| 1546 | Cr vibration inside the composite | |
| 1420 | 1466 | C=C benzene |
| 1312 | 1318 | C-N [30] |
| 1177 | 1151 | C-H |
| 1045, 910 and 798 | 1046 and 926 | Aromatic ring vibration |
| Structure | Wavelength (nm) | Bias (V) | R (mAW−1) |
|---|---|---|---|
| Graphene/P3HT [45] | 325 | 1 | NA |
| PbI2-graphene [46] | 550 | 2 | NA |
| TiO2-PANI [47] | 320 | 0 | 3 × 10−3 |
| ZnO/RGO [48] | 350 | 5 | 1.3 × 10−3 |
| GO/Cu2O [49] | 300 | 2 | 0.5 × 10−3 |
| Graphene/GaN [50] | 365 | 7 | 3 × 10−3 |
| PbI2-graphene [46] | 550 | 2 | NA |
| ZnO-CuO [51] | 405 | 1 | 3 × 10−3 |
| PbI2-5%Ag [52] | 532 | 6 | NA |
| CuO nanowires [26] | 390 | 5 | - |
| ZnO/Cu2O [27] | 350 | 2 | 4 × 10−3 |
| Se/TiO2 [53] | 450 | 1 | 5 × 10−3 |
| TiN/TiO2 [54] | 550 | 5 | - |
| CuO/Si Nanowire [55] | 405 | 0.2 | 3.8 × 10−3 |
| Cr2O3-Cr(OH)3-Ppy/Ppy nanocomposite thin film (this work) | 440 | 3 | 0.016 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rabia, M.; Trabelsi, A.B.G.; Elsayed, A.M.; Alkallas, F.H. Porous-Spherical Cr2O3-Cr(OH)3-Polypyrrole/Polypyrrole Nanocomposite Thin-Film Photodetector and Solar Cell Applications. Coatings 2023, 13, 1240. https://doi.org/10.3390/coatings13071240
Rabia M, Trabelsi ABG, Elsayed AM, Alkallas FH. Porous-Spherical Cr2O3-Cr(OH)3-Polypyrrole/Polypyrrole Nanocomposite Thin-Film Photodetector and Solar Cell Applications. Coatings. 2023; 13(7):1240. https://doi.org/10.3390/coatings13071240
Chicago/Turabian StyleRabia, Mohamed, Amira Ben Gouider Trabelsi, Asmaa M. Elsayed, and Fatemah H. Alkallas. 2023. "Porous-Spherical Cr2O3-Cr(OH)3-Polypyrrole/Polypyrrole Nanocomposite Thin-Film Photodetector and Solar Cell Applications" Coatings 13, no. 7: 1240. https://doi.org/10.3390/coatings13071240
APA StyleRabia, M., Trabelsi, A. B. G., Elsayed, A. M., & Alkallas, F. H. (2023). Porous-Spherical Cr2O3-Cr(OH)3-Polypyrrole/Polypyrrole Nanocomposite Thin-Film Photodetector and Solar Cell Applications. Coatings, 13(7), 1240. https://doi.org/10.3390/coatings13071240







