Structural and Thermal Stability of CrZrON Coatings Synthesized via Reactive Magnetron Sputtering
Abstract
:1. Introduction
2. Materials and Experimental Procedures
2.1. Deposition Conditions
2.2. Characterization of Coatings
3. Results and Discussion
4. Conclusions
- (1)
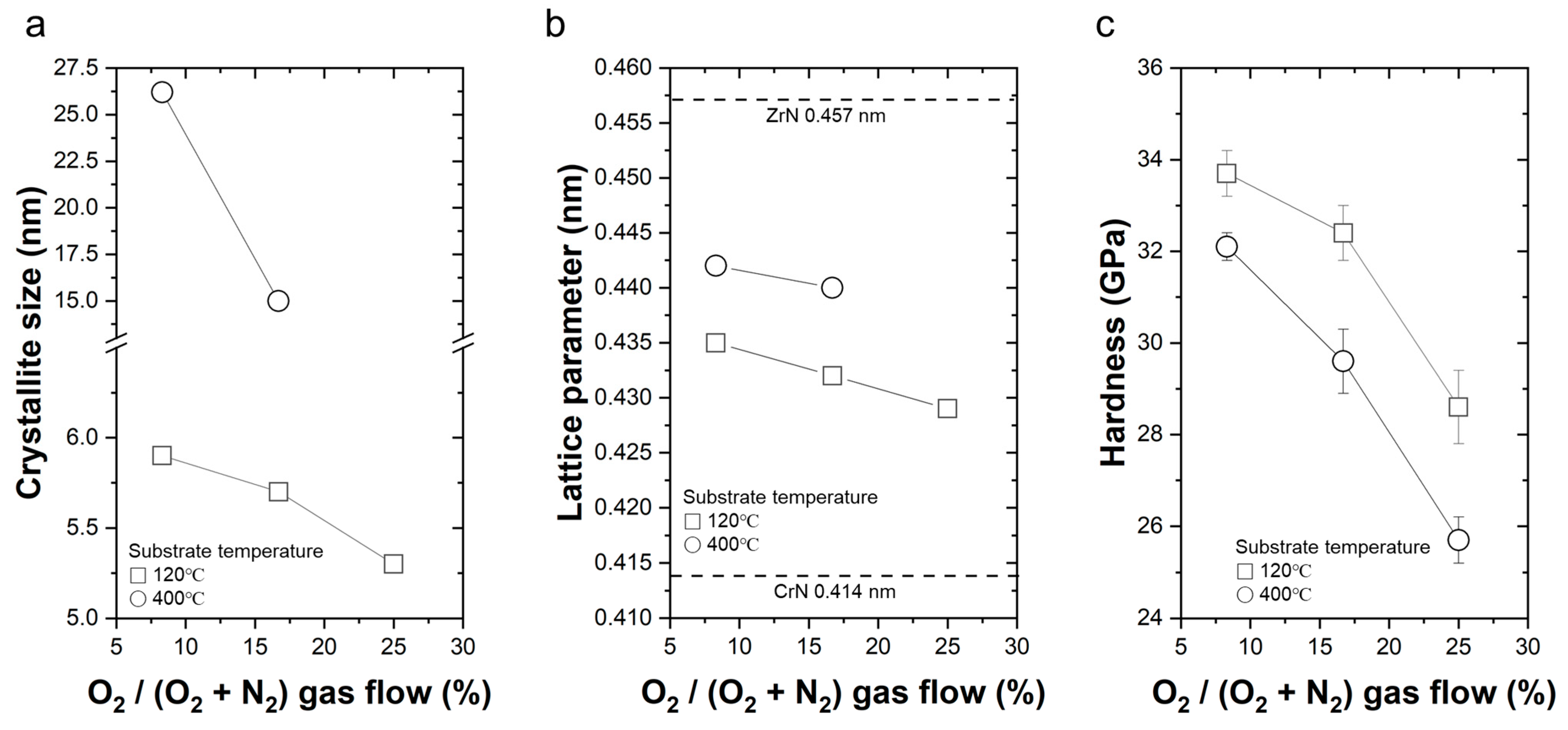
- Coatings deposited at lower temperatures exhibited a columnar structure, while those deposited at higher temperatures showed a transition towards a featureless or amorphous structure.
- (2)
- Increasing the O2/(O2 + N2) ratio led to a decrease in lattice parameter, suggesting the incorporation of oxygen and the formation of oxygen-rich compounds in the coatings.
- (3)
- Crystallite size exhibited different behaviors with respect to deposition temperature and O2/(O2 + N2) ratio, showing reductions due to enhanced nucleation, grain growth inhibition, and the presence of oxygen during deposition.
- (4)
- Hardness values decreased with increasing O2/(O2 + N2) ratio, attributed to oxygen incorporation and the formation of oxygen-rich compounds with lower hardness compared to nitride phases.
- (5)
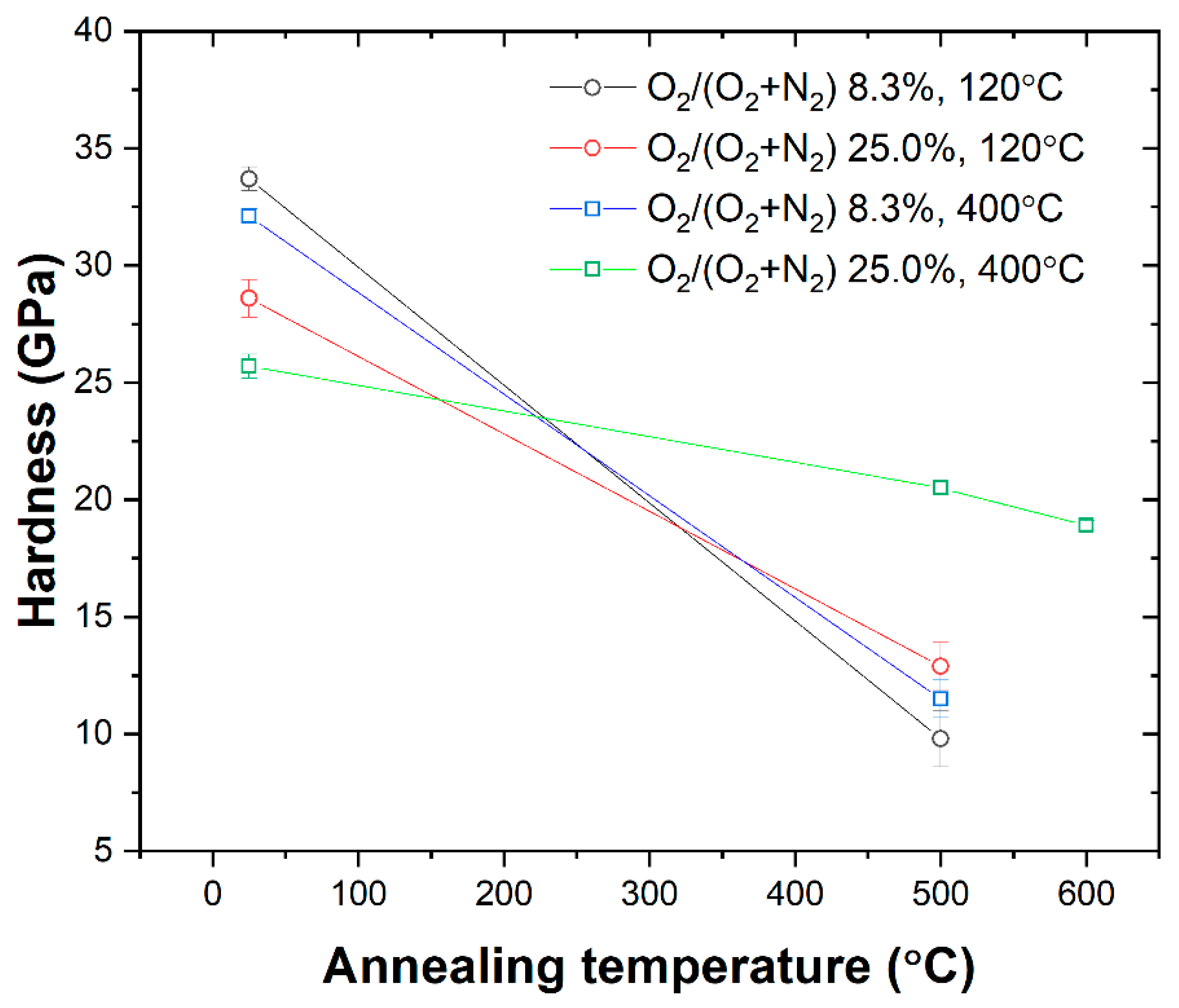
- Annealing experiments demonstrated that coatings with featureless or amorphous structures exhibited superior thermal stability, maintaining their structural integrity even at high temperatures.
- (6)
- The thermal stability of CrZrN coatings limited to 500 °C can be enhanced by incorporating oxygen, allowing CrZrON coatings to be utilized up to 600 °C, thus opening new avenues for their application in various industries.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Mayrhofer, P.H.; Mitterer, C.; Hultman, L.; Clemens, H. Microstructural design of hard coatings. Prog. Mater. Sci. 2006, 51, 1032–1114. [Google Scholar] [CrossRef]
- Polcar, T.; Parreira, N.M.G.; Novak, R. Friction and wear behaviour of CrN coating at temperatures up to 500 degrees C. Surf. Coat. Technol. 2007, 201, 5228–5235. [Google Scholar] [CrossRef]
- Lorenzo-Martin, C.; Ajayi, O.; Erdemir, A.; Fenske, G.R.; Wei, R. Effect of microstructure and thickness on the friction and wear behavior of CrN coatings. Wear 2013, 302, 963–971. [Google Scholar] [CrossRef]
- Kim, G.; Kim, B.; Lee, S.; Hahn, J. Structure and mechanical properties of Cr-Zr-N films synthesized by closed field unbalanced magnetron sputtering with vertical magnetron sources. Surf. Coat. Technol. 2005, 200, 1669–1675. [Google Scholar] [CrossRef]
- Li, W.Z.; Evaristo, M.; Cavaleiro, A. Influence of Al on the microstructure and mechanical properties of Cr-Zr-(Al-)N coatings with low and high Zr content. Surf. Coat. Technol. 2012, 206, 3764–3771. [Google Scholar] [CrossRef]
- Kim, S.M.; Kim, B.S.; Kim, G.S.; Lee, S.Y.; Lee, B.Y. Evaluation of the high temperature characteristics of the CrZrN coatings. Surf. Coat. Technol. 2008, 202, 5521–5525. [Google Scholar] [CrossRef]
- Kim, H.K.; Kim, S.M.; Lee, S.Y. Mechanical Properties and Thermal Stability of CrZrN/CrZrSiN Multilayer Coatings with Different Bilayer Periods. Coatings 2022, 12, 1025. [Google Scholar] [CrossRef]
- Peng, X.G.; Zhang, K.F.; Zheng, Y.G.; Zhou, H.; Wan, Z.H. Structure, morphologies and mechanical properties study of Cr-Zr-N films. Nucl. Instrum. Meth B 2018, 436, 112–118. [Google Scholar] [CrossRef]
- Khamseh, S.; Araghi, H. A study of the oxidation behavior of CrN and CrZrN ceramic thin films prepared in a magnetron sputtering system. Ceram. Int. 2016, 42, 9988–9994. [Google Scholar] [CrossRef] [Green Version]
- Kim, S.M.; Kim, G.S.; Lee, S.Y.; Lee, J.W.; Lee, J.Y.; Lee, B.Y. Mechanical Properties and Thermal Stability of CrSiN/AlN Multilayer Coatings Using a Closed-Field Unbalanced Magnetron Sputtering System. J. Korean Phys. Soc. 2009, 54, 1109–1114. [Google Scholar] [CrossRef]
- Kim, G.S.; Kim, S.M.; Lee, S.Y.; Lee, B.Y. Comparative studies on the thermal stability and corrosion resistance of CrN, CrSiN, and CrSiN/AlN coatings. J. Vac. Sci. Technol. A 2009, 27, 873–879. [Google Scholar] [CrossRef]
- Kim, D.J.; La, J.H.; Kim, K.S.; Kim, S.M.; Lee, S.Y. Tribological properties of CrZr-Si-N films synthesized using Cr-Zr-Si segment targets. Surf. Coat. Technol. 2014, 259, 71–76. [Google Scholar] [CrossRef]
- Kim, H.K.; Kim, S.M.; Lee, S.Y. Influence of Interlayer Materials on the Mechanical Properties and Thermal Stability of a CrAlN Coating on a Tungsten Carbide Substrate. Coatings 2022, 12, 1134. [Google Scholar] [CrossRef]
- Kim, D.; La, J.; Kim, S.M.; Lee, S.Y. The tribological performance of laser surface treated CrZrSiN thin films. Mater. Res. Bull. 2014, 58, 39–43. [Google Scholar] [CrossRef]
- Gan, M.D.; Chong, X.Y.; Yu, W.; Xiao, B.; Feng, J. Understanding the ultralow lattice thermal conductivity of monoclinic RETaO4 from acoustic-optical phonon anti-crossing property and a comparison with ZrO2. J. Am. Ceram. Soc. 2023, 106, 3103–3115. [Google Scholar] [CrossRef]
- Castaldi, L.; Kurapov, D.; Reiter, A.; Shkover, V.; Schwaller, P.; Patscheider, J. Effect of the oxygen content on the structure, morphology and oxidation resistance of Cr-O-N coatings. Surf. Coat. Technol. 2008, 203, 545–549. [Google Scholar] [CrossRef]
- Du, J.W.; Chen, L.; Chen, J.; Hu, C. Influence of oxygen addition on the structure, mechanical and thermal properties of CrN coating. Surf. Coat. Technol. 2021, 411, 126992. [Google Scholar] [CrossRef]
- Kim, D.J.; Kim, S.M.; La, J.H.; Lee, S.Y.; Hong, Y.S.; Lee, M.H. Synthesis and characterization of CrZrAlN films using unbalanced magnetron sputtering with segment targets. Met. Mater. Int. 2013, 19, 1295–1299. [Google Scholar] [CrossRef]
- Arreguin-Campos, M.; Gutierrez, Z.B.K.; Quinones-Galvan, J.G.; Santos-Cruz, J.; Mayen-Hernandez, S.A.; Zelaya-Angel, O.; Olvera, M.D.; Contreras-Puente, G.; de Moure-Flores, F. Fabrication of CdS/CdTe Heterostructures by Chemical Synthesis Using a p-Type CdTe Film Grown by Electrodeposition Employing EDTA as Strong Complexing Agent. J. Electron. Mater. 2019, 48, 3595–3602. [Google Scholar] [CrossRef]
- Khatibi, A.; Sjolen, J.; Greczynski, G.; Jensen, J.; Eklund, P.; Hultman, L. Structural and mechanical properties of Cr-Al-O-N thin films grown by cathodic arc deposition. Acta Mater. 2012, 60, 6494–6507. [Google Scholar] [CrossRef]
- Landalv, L.; Lu, J.; Spitz, S.; Leiste, H.; Ulrich, S.; Johansson-Joesaar, M.P.; Ahlgren, M.; Gothelid, E.; Alling, B.; Hultman, L.; et al. Structural evolution in reactive RF magnetron sputtered (Cr,Zr)(2)O-3 coatings during annealing. Acta Mater. 2017, 131, 543–552. [Google Scholar] [CrossRef] [Green Version]
- Kolobov, A.V.; Krbal, M.; Fons, P.; Tominaga, J.; Uruga, T. Distortion-triggered loss of long-range order in solids with bonding energy hierarchy. Nat. Chem. 2011, 3, 311–316. [Google Scholar] [CrossRef]
- Zhu, Y.H.; Zhou, Y.K.; Wang, A.M.; Li, H.; Fu, H.M.; Zhang, H.W.; Zhang, H.F.; Zhu, Z.W. Atomic-scale icosahedral short-range ordering in a rejuvenated Zr-based bulk metallic glass upon deep cryogenic treatment. Mat. Sci. Eng. Struct. 2022, 850, 143565. [Google Scholar] [CrossRef]
- Qiao, J.C.; Wang, Q.; Pelletier, J.M.; Kato, H.; Casalini, R.; Crespo, D.; Pineda, E.; Yao, Y.; Yang, Y. Structural heterogeneities and mechanical behavior of amorphous alloys. Prog. Mater. Sci. 2019, 104, 250–329. [Google Scholar] [CrossRef]
- Hu, Q.; Guo, S.; Wang, J.M.; Yan, Y.H.; Chen, S.S.; Lu, D.P.; Liu, K.M.; Zou, J.Z.; Zeng, X.R. Parametric Study of Amorphous High-Entropy Alloys formation from two New Perspectives: Atomic Radius Modification and Crystalline Structure of Alloying Elements. Sci. Rep. 2017, 7, 39917. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, T.; Inoue, J.; Saito, H.; Hirai, M.; Suematsu, H.; Jiang, W.H.; Yatsui, K. Influence of oxygen content on structure and hardness of Cr-N-O thin films prepared by pulsed laser deposition. Thin Solid Films 2006, 515, 2161–2166. [Google Scholar] [CrossRef]
- Bottger, P.H.M.; Lewin, E.; Patscheider, J.; Shklover, V.; Cahill, D.G.; Ghisleni, R.; Sobiech, M. Thermal conductivity of hard oxynitride coatings. Thin Solid Films 2013, 549, 232–238. [Google Scholar] [CrossRef]
- Smirniotis, P.G.; Boningari, T.; Damma, D.; Inturi, S.N.R. Single-step rapid aerosol synthesis of N-doped TiO2 for enhanced visible light photocatalytic activity. Catal. Commun. 2018, 113, 1–5. [Google Scholar] [CrossRef]
- Garg, P.; Rupert, T.J. Grain incompatibility determines the local structure of amorphous grain boundary complexions. Acta Mater. 2023, 244, 118599. [Google Scholar] [CrossRef]
- Lortaraprasert, C.; Shiomi, J. Robust combined modeling of crystalline and amorphous silicon grain boundary conductance by machine learning. NPJ Comput. Mater. 2022, 8, 219. [Google Scholar] [CrossRef]
- Yang, J.Q.; Shang, L.L.; Sun, J.Y.; Bai, S.Y.; Wang, S.Z.; Liu, J.K.; Yun, D.; Ma, D.Y. Restraining the Cr-Zr interdiffusion of Cr-coated Zr alloys in high temperature environment: A Cr/CrN/Cr coating approach. Corros. Sci. 2023, 214, 111015. [Google Scholar] [CrossRef]
- Yang, J.Q.; Bai, S.Y.; Sun, J.Y.; Wu, H.; Sun, S.H.; Wang, S.Z.; Li, Y.H.; Ma, W.H.; Tang, X.Y.; Xu, D.H. Microstructural understanding of the oxidation and inter-diffusion behavior of Cr-coated Alloy 800H in supercritical water. Corros. Sci. 2023, 211, 110910. [Google Scholar] [CrossRef]



| Deposition Temperature (°C) | O2/(O2 + N2) Gas Flow Ratio (%) | Chemical Composition (at.%) |
|---|---|---|
| 120 | 8.3 | Cr29.0 Zr14.0 O3.4 N53.6 |
| 16.7 | Cr27.4 Zr12.8 O6.7 N53.1 | |
| 25.0 | Cr26.5 Zr11.9 O11.1 N50.5 | |
| 400 | 8.3 | Cr24.9 Zr22.5 O14.1 N38.5 |
| 16.7 | Cr23.3 Zr20.8 O27.2 N28.7 | |
| 25.0 | Cr21.8 Zr19.3 O41.2 N17.9 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, S.-M.; Lee, S.-Y. Structural and Thermal Stability of CrZrON Coatings Synthesized via Reactive Magnetron Sputtering. Coatings 2023, 13, 1254. https://doi.org/10.3390/coatings13071254
Kim S-M, Lee S-Y. Structural and Thermal Stability of CrZrON Coatings Synthesized via Reactive Magnetron Sputtering. Coatings. 2023; 13(7):1254. https://doi.org/10.3390/coatings13071254
Chicago/Turabian StyleKim, Sung-Min, and Sang-Yul Lee. 2023. "Structural and Thermal Stability of CrZrON Coatings Synthesized via Reactive Magnetron Sputtering" Coatings 13, no. 7: 1254. https://doi.org/10.3390/coatings13071254





