Preparation of Molybdenum Coatings by Molten Salt Electrodeposition in Na3AlF6-NaF-Al2O3-MoO3 System
Abstract
:1. Introduction
2. Experimental
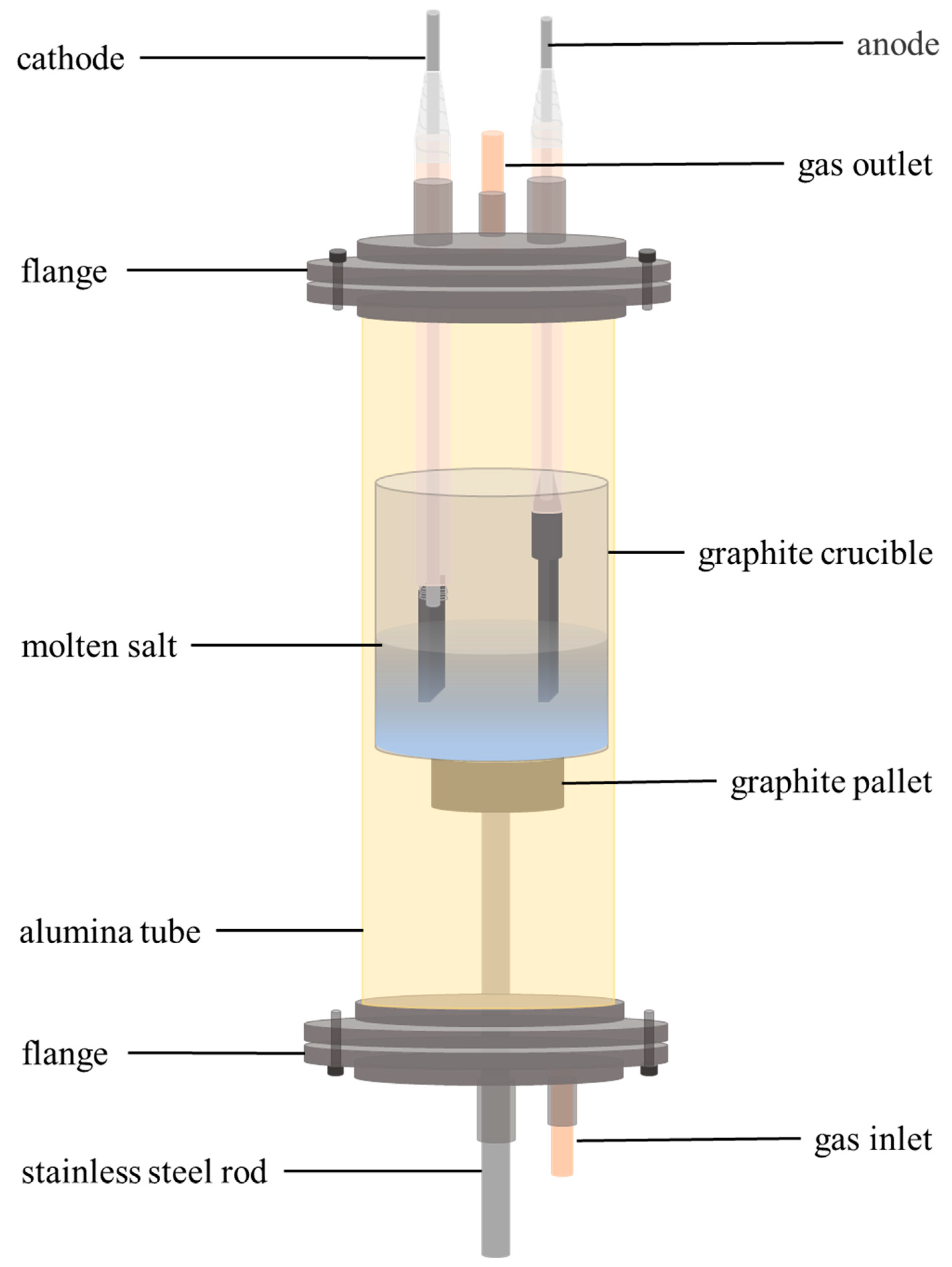
2.1. Raw Material and Experimental Setup
2.2. Coating Operation
2.3. Characterization
3. Results and Discussion
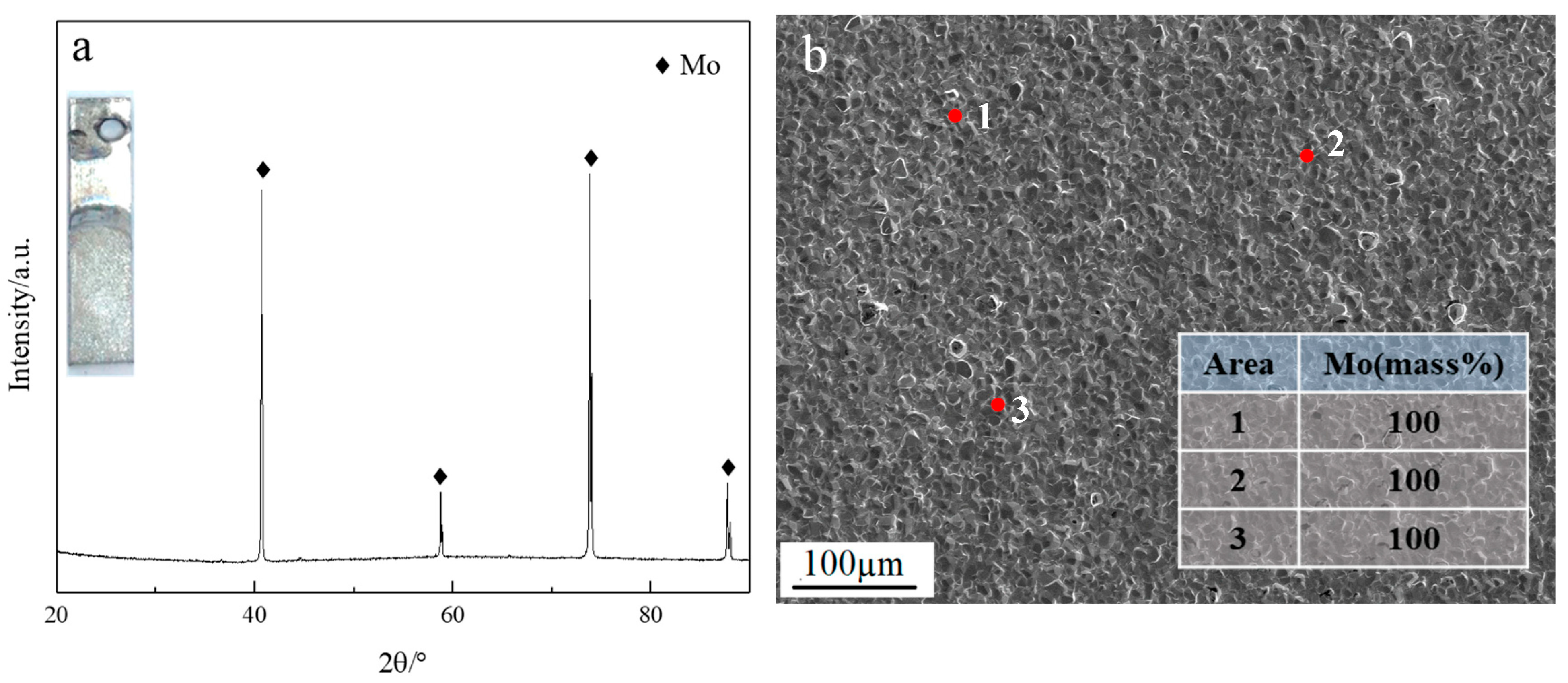
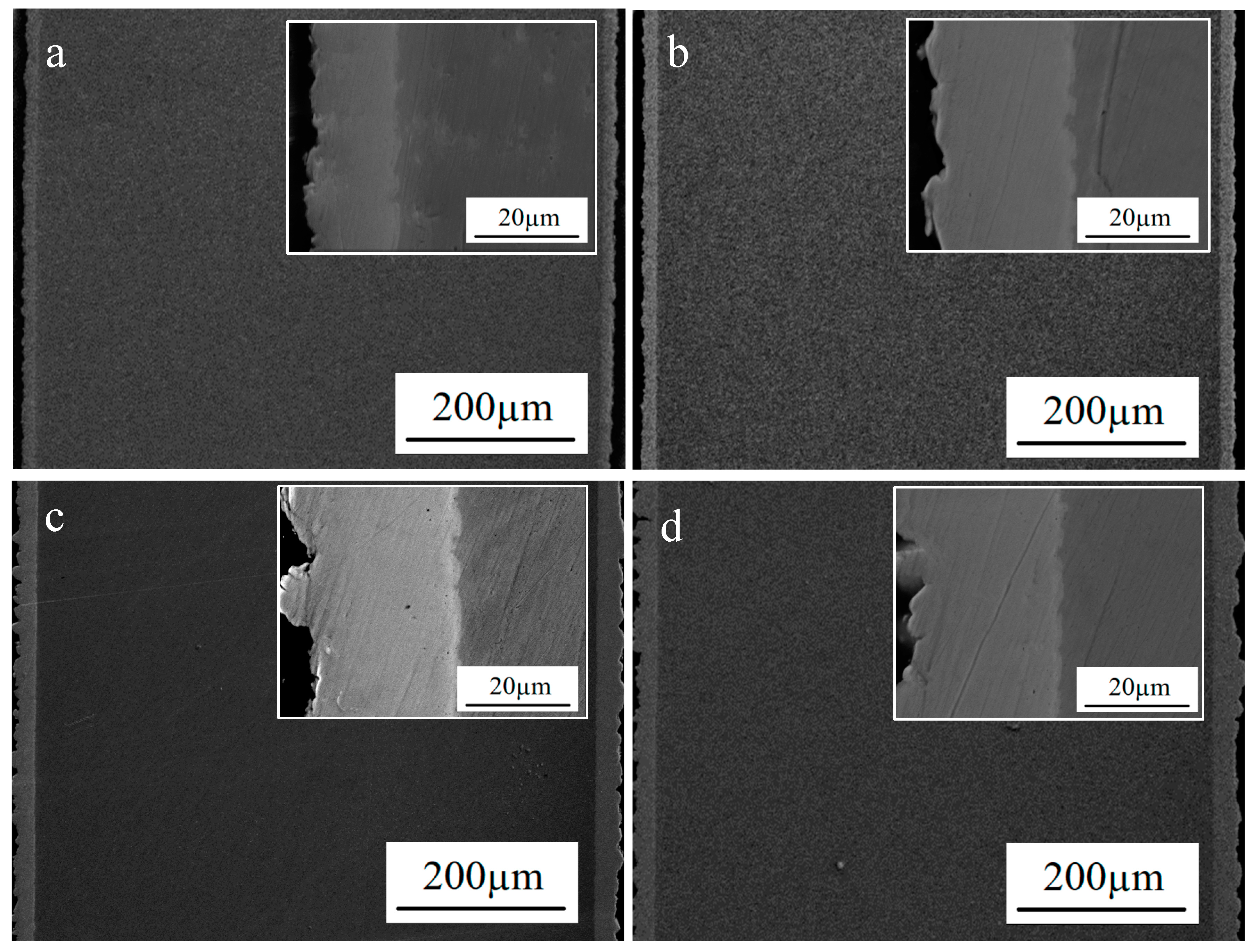
3.1. Effect of Current Densities on Molybdenum Coatings
3.2. Effect of Electrodeposition Time on Molybdenum Coating
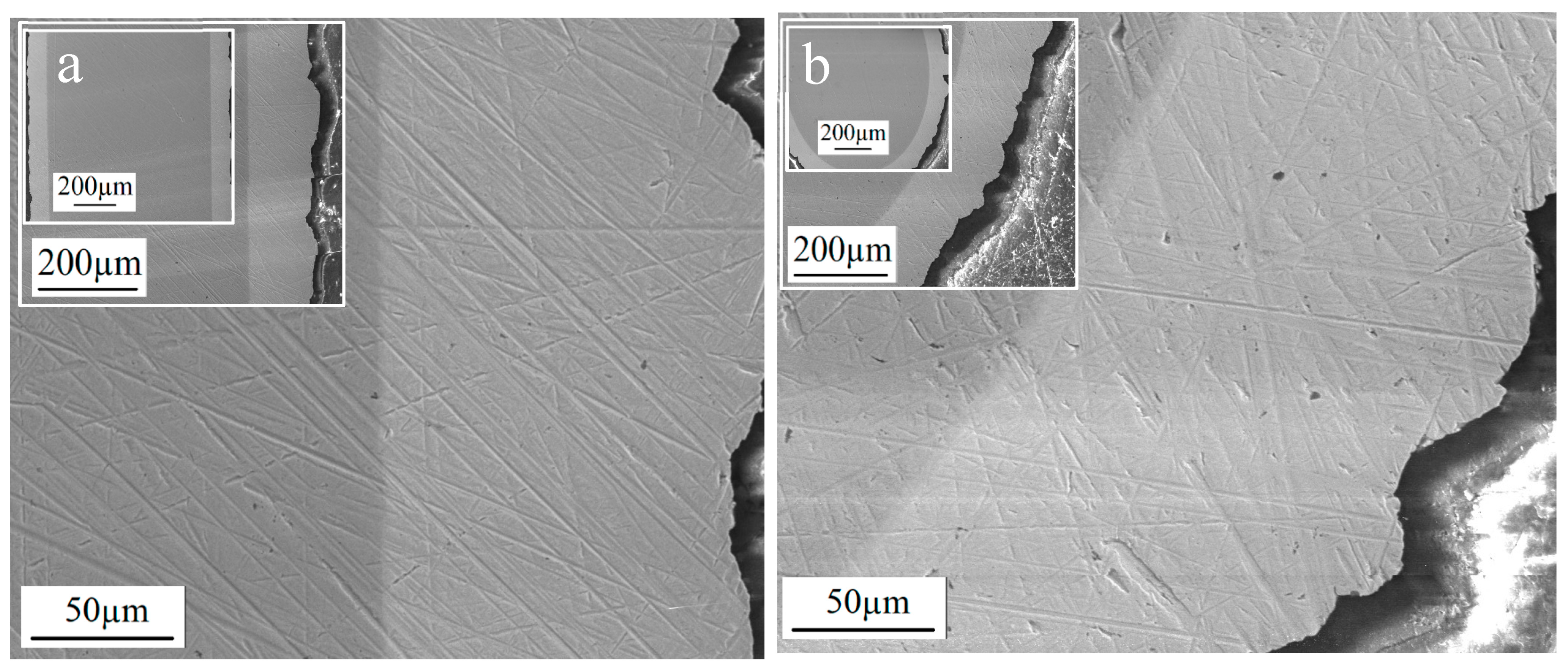
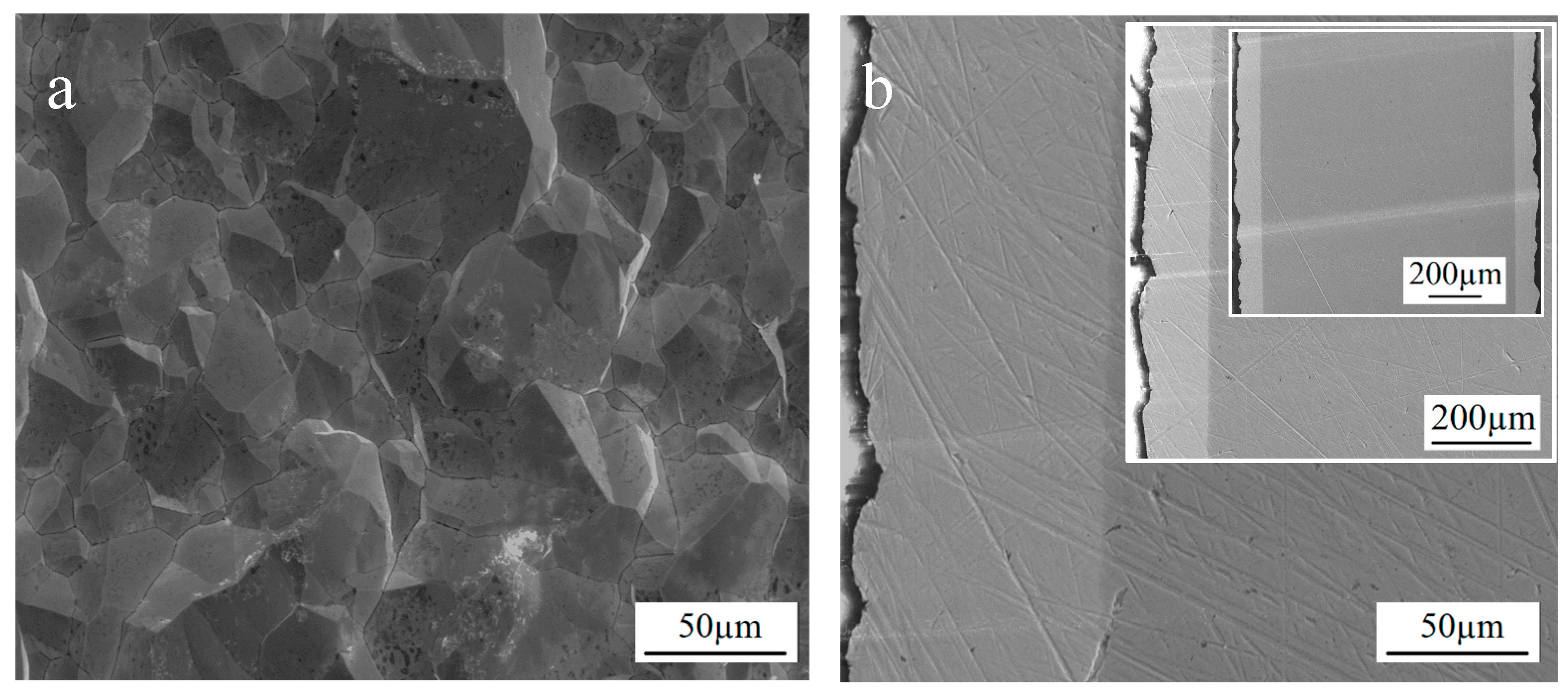
3.3. Preparation of Thick Molybdenum Coating
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Xiang, T.G. Molybdenum Metallurgy; Central South University Press: Changsha, China, 2002. [Google Scholar]
- Xu, K.D. Material Science and Engineering of Molybdenum; Metallurgical Industry Press: Beijing, China, 2014. [Google Scholar]
- Hwanga, B.; Jehoon, A.; Lee, S. Effects of blending elements on wear resistance of plasma-sprayed molybdenum blend coatings used for automotive synchronizer rings. Surf. Coat. Technol. 2005, 194, 256–264. [Google Scholar] [CrossRef]
- Koyama, K.; Hashimoto, Y.; Terawaki, K. Smooth electrodeposits of molybdenum from KF-K2B4O7-K2MoO4 fused salt melts. J. Less Common Met. 1987, 134, 141–151. [Google Scholar] [CrossRef]
- Koyama, K.; Hashimoto, Y.; Omori, S.; Terawaki, K. Electrodeposition of molybdenum in KF-Na2B4O7-K2MoO4 fused salts. Trans. Jpn. Inst. Met. 1984, 25, 265–275. [Google Scholar] [CrossRef] [Green Version]
- Babu, M.V.; Kumar, R.K.; Prabhakar, O.; Shankar, N.G. Simultaneous optimization of flame spraying process parameters for high quality Mo coatings using Taguchi methods. Surf. Coat. Technol. 1996, 79, 276–288. [Google Scholar] [CrossRef]
- Vaidya, A.; Streibl, T.; Li, L.; Sampath, S.; Kovarik, O.; Greenlaw, R. An integrated study of thermal spray process-structure-property correlations: A case study for plasma sprayed Mo coatings. MSEA 2005, 403, 191–204. [Google Scholar] [CrossRef]
- Jin, G.; Xu, B.S.; Wang, H.D.; Li, Q.F.; Wei, S.C. Tribological properties of molybdenum coatings sprayed by electro-thermal explosion directional spraying. Surf. Coat. Technol. 2007, 201, 6678–6680. [Google Scholar] [CrossRef]
- Khan, A.A.; Labbe, J.C.; Grimaud, A.; Fauchais, P. Molybdenum and tungsten coatings for x-ray targets obtained through the low-pressure plasma spraying process. J. Therm. Spray Technol. 1997, 6, 228–234. [Google Scholar] [CrossRef]
- Wlodek, S.T.; Wulff, J. The adhesion of vapor-deposited Mo coatings. J. Electrochem. Soc. 1960, 107, 565. [Google Scholar] [CrossRef]
- Fukutomi, M.; Okada, M.; Watanabe, R. Molybdenum coating of vanadium by chemical vapor deposition. J. Less Common Met. 1976, 48, 65–77. [Google Scholar] [CrossRef]
- Hasan, S.N.; Xu, M.; Asselin, E. Electrodeposition of metallic molybdenum and its alloys—A review. Can. Met. Q. 2019, 58, 1–18. [Google Scholar] [CrossRef]
- Senderoff, S.; Brenner, A. The electrolytic preparation of molybdenum from fused salts. I. Electrolytic studies. J. Electrochem. Soc. 1954, 101, 16. [Google Scholar] [CrossRef]
- Kipouros, G.J.; Sadoway, D.R. The electrodeposition of improved Mo coatings from molten salts by the use of electrolyte additives. J. Appl. Electrochem. 1988, 18, 823–830. [Google Scholar] [CrossRef]
- Ene, N.; Donath, C. Texture of electrolytic Mo deposition from molten alkali halide. J. Optoelectron. Adv. Mater. 2006, 8, 708. [Google Scholar]
- Senderoff, S.; Mellors, G.W. Electrodeposition of coherent deposits of refractory metals: V. Mechanism for the Deposition of Molybdenum from a Chloride Melt. J. Electrochem. Soc. 1967, 114, 556. [Google Scholar] [CrossRef]
- Babushkina, O.; Voyiatzis, G.; Østvold, T. Raman and infrared spectroscopic studies of (NaF-KF)-K2MoO2-B2O3 melts and the mechanism of electrodeposition of molybdenum. Anal. Chim. Acta 1999, 53, 320–328. [Google Scholar]
- Silný, A.; Daněk, V.; Chrenková, M. Mechanism of the molybdenum electrodeposition from molten salts. In Refractory Metals in Molten Salts: Their Chemistry, Electrochemistry and Technology; Springer Netherlands Press: Berlin, Germany, 1998; Volume 53, pp. 183–187. [Google Scholar]
- Terawaki, K.; Koyama, K.; Hashimoto, Y.; Omori, S. Electrodeposition of molybdenum in KF-B2O3-MoO3 fused salts. J. Jpn. Inst. Met. 1986, 50, 303–307. [Google Scholar] [CrossRef] [Green Version]
- Zatko, P.; Makyta, M.; Sykorova, J.; Silny, A. Electrodeposition of molybdenum from KF-K2MoO4-SiO2 Melts. Chem. Pap. 1994, 48, 10–14. [Google Scholar]
- McCawley, F.X.; Wyche, C.; Schlain, D. Electrodeposition of molybdenum coatings. J. Electrochem. Soc. 1969, 116, 1028. [Google Scholar] [CrossRef]
- Kolosov, V.N.; Karpenko, O.A.; Gel’, R.P.; Drobotenko, G.A.; Shevyrev, A.A. Electrodeposition of high-purity molybdenum coatings on nickel. Inorg. Mater. 2001, 37, 1263–1269. [Google Scholar] [CrossRef]
- Jin, W.; Ge, C.; Kou, Q.; Jiang, P.; Xiao, S. Electrochemical preparation of Mo coatings on nickel from KF-MoO3 melts. Int. J. Electrochem. Sci. 2021, 16, 210311. [Google Scholar] [CrossRef]
- Makyta, M.; Zatko, P.; Bezdicka, P. Electrodeposition of molybdenum from molten salts. Chem. Pap. 1993, 47, 28–31. [Google Scholar]
- Malyshev, V.V. Theoretical foundations and practical realization of molybdenum electrodeposition in ionic melts. Theor. Found. Chem. Eng. 2007, 41, 284–299. [Google Scholar] [CrossRef]
- Wu, X.Y.; Bai, S.X.; Wang, Z.; Hu, L.A.; Ye, Y.C.; Li, S.; Tang, Y. Research Progress of Molybdenum Coating Prepared by Electrodeposition. Rare Met. 2023, 51, 39–48. [Google Scholar]
- Yang, P.X.; An, M.Z.; Su, C.N.; Wang, F.P. Electrodeposition behaviors of pure cobalt in ionic liquid. Acta Phys.-Chim. Sin. 2008, 24, 2032–2036. [Google Scholar]
- Chung, C.K.; Chang, W.T. Effect of pulse frequency and current density on anomalous composition and nanomechanical property of electrodeposited Ni-Co films. Thin Solid Films 2009, 517, 4800–4804. [Google Scholar] [CrossRef]
- Jiang, F.; Zhang, Y.; Sun, N.; Liu, Z. Effect of direct current density on microstructure of tungsten coating electroplated from Na2WO4-WO3-NaPO3 system. Appl. Surf. Sci. 2014, 317, 867–874. [Google Scholar] [CrossRef]
- Abyaneh, M.Y. Calculation of overlap for nucleation and three-dimensional growth of centres. Electrochim. Acta 1982, 27, 1329–1334. [Google Scholar] [CrossRef]



| System | Current Density (mA·cm−2) | Cathode Current Efficiency (%) | Visual Evaluation |
|---|---|---|---|
| KF (85 mol %)-K2MoO4 (10 mol %)-SiO2 (5 mol %) [20] | 7.3 | 98.3 | Smooth deposit |
| 15.6 | 98.5 | Smooth deposit | |
| 29.3 | 92.5 | Smooth deposit | |
| 44 | 90.6 | Smooth deposit, dendrites | |
| 63.3 | 77 | Smooth deposit, Black powder | |
| 87.9 | 24.8 | Black powder | |
| 146.6 | 24.5 | Black powder | |
| KF-MoO3 (95:5 mol %) [23] | 11 | 92.5 | Smooth deposit |
| 33 | 91 | Smooth deposit | |
| 55 | 79 | Smooth deposit | |
| 77 | 73 | Smooth deposit, dendrites | |
| Na3AlF6-NaF (21:79 mol%)-Al2O3 (0.015 mol %)-MoO3 (5 mol %) | 10 | 97 | Smooth deposit |
| 30 | 97 | Smooth deposit | |
| 50 | 97 | Smooth deposit | |
| 70 | 97 | Smooth deposit |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kou, Q.; Jin, W.; Ge, C.; Pang, J.; Zhang, J.; Haarberg, G.M.; Xiao, S.; Wang, P. Preparation of Molybdenum Coatings by Molten Salt Electrodeposition in Na3AlF6-NaF-Al2O3-MoO3 System. Coatings 2023, 13, 1266. https://doi.org/10.3390/coatings13071266
Kou Q, Jin W, Ge C, Pang J, Zhang J, Haarberg GM, Xiao S, Wang P. Preparation of Molybdenum Coatings by Molten Salt Electrodeposition in Na3AlF6-NaF-Al2O3-MoO3 System. Coatings. 2023; 13(7):1266. https://doi.org/10.3390/coatings13071266
Chicago/Turabian StyleKou, Qian, Weiliang Jin, Chuntao Ge, Jie Pang, Jun Zhang, Geir Martin Haarberg, Saijun Xiao, and Ping Wang. 2023. "Preparation of Molybdenum Coatings by Molten Salt Electrodeposition in Na3AlF6-NaF-Al2O3-MoO3 System" Coatings 13, no. 7: 1266. https://doi.org/10.3390/coatings13071266




