Enhanced Electrochromic Properties of Nanocrystalline Molybdenum Oxide Films Modified by Dopamine
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of the Modified MoO3 Films
2.3. Structural and Compositional Characterizations
2.4. Electrochemical and EC Measurements
2.5. Fabrication and Characterization of the EC Device
3. Results and Discussion
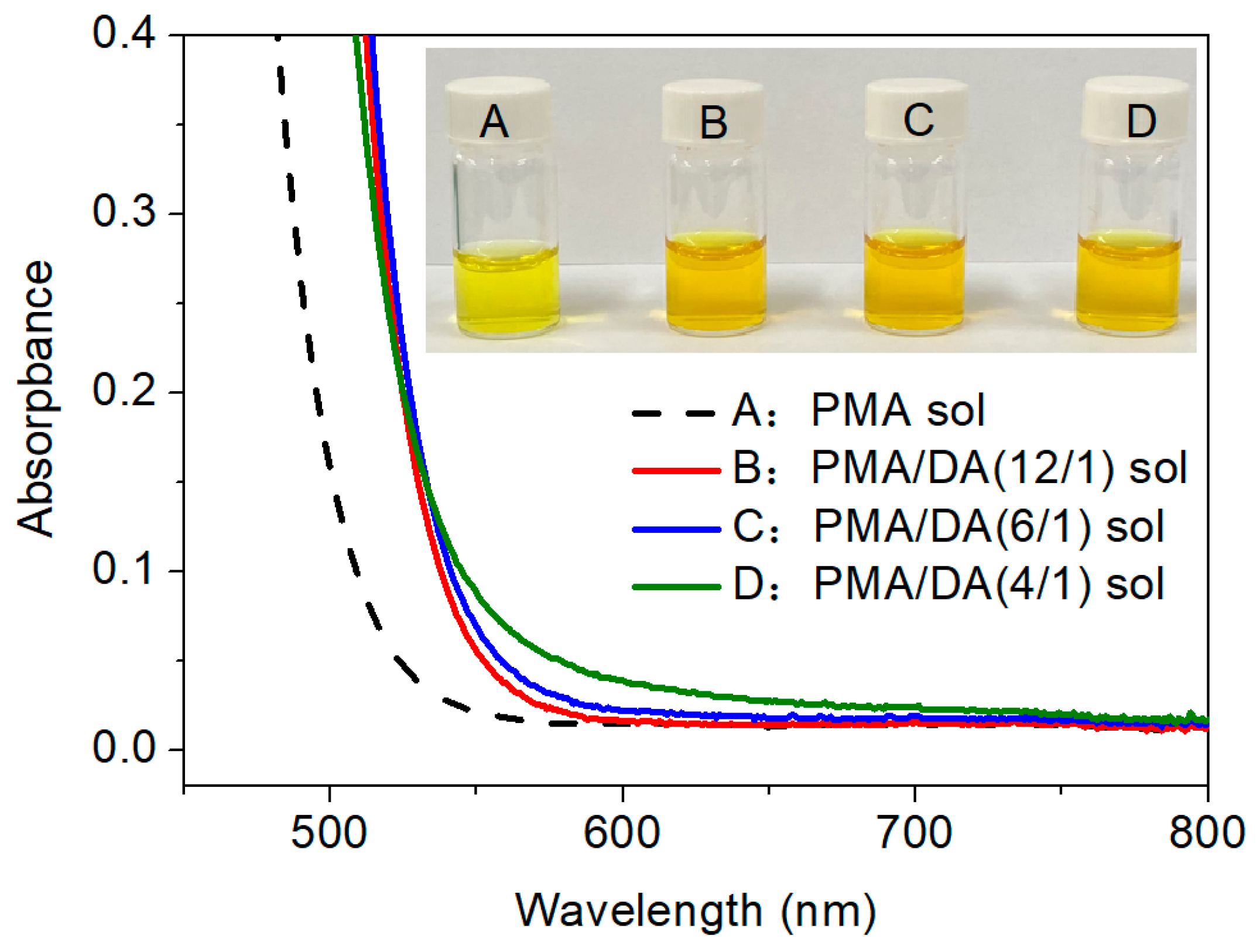
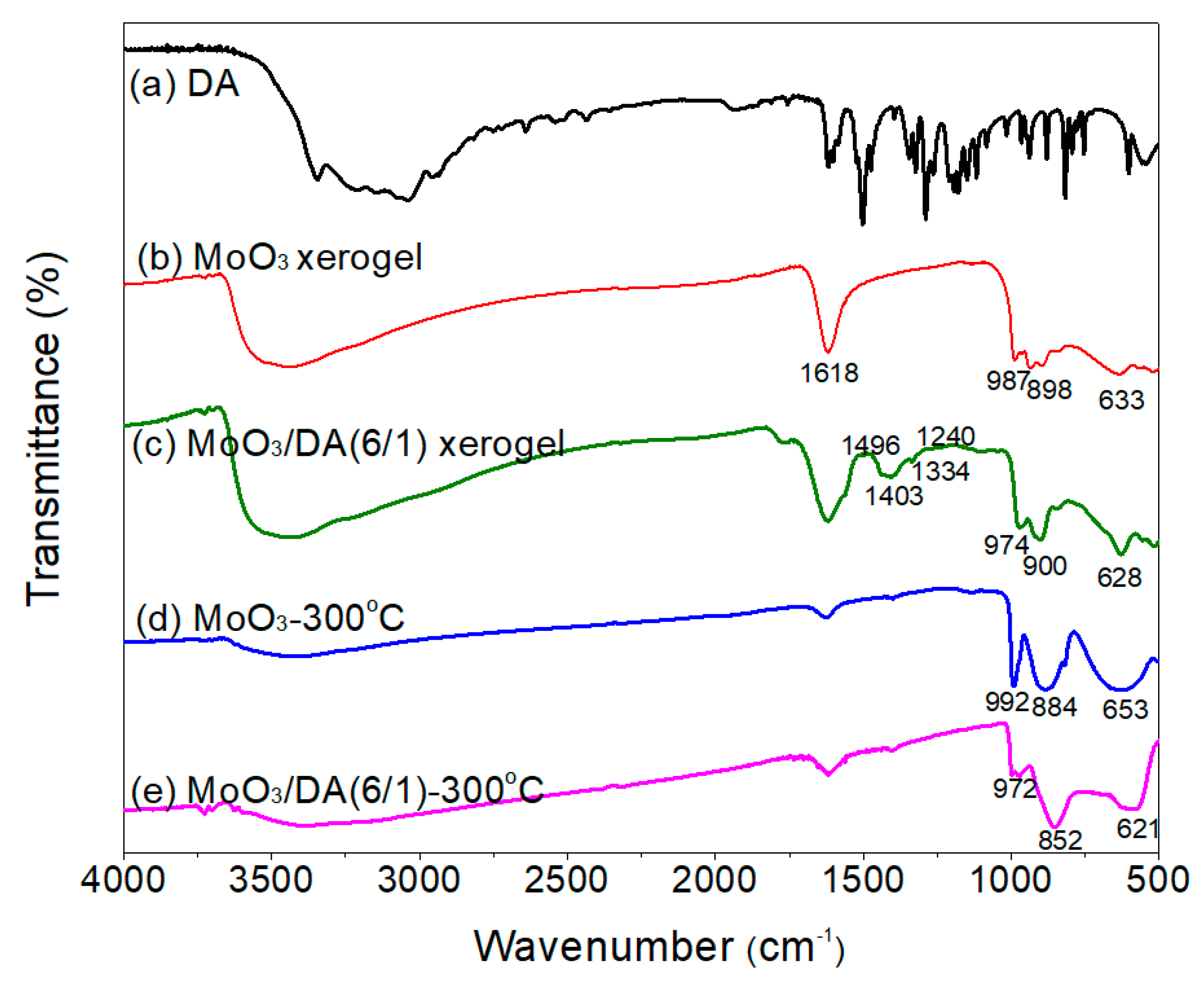
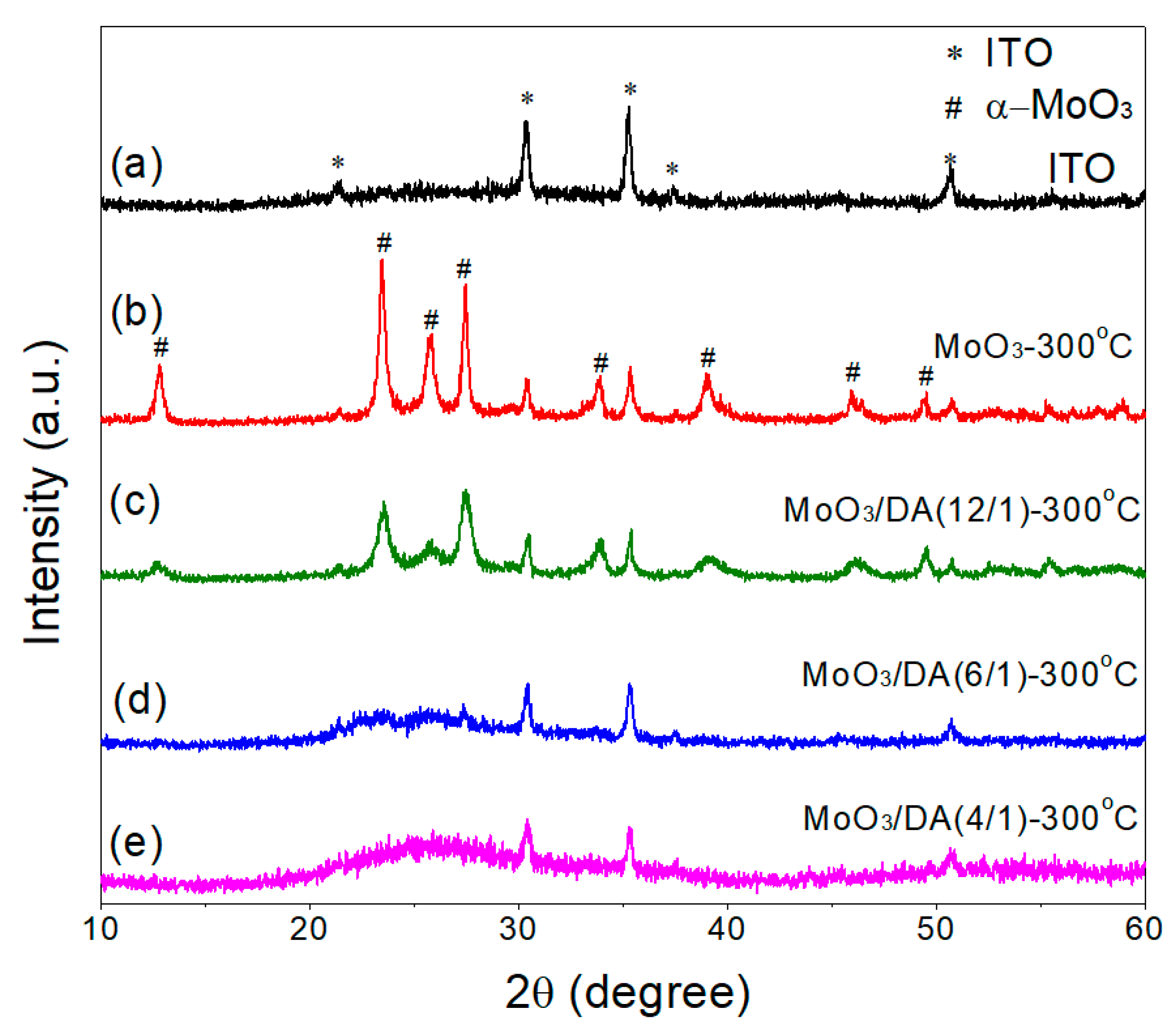
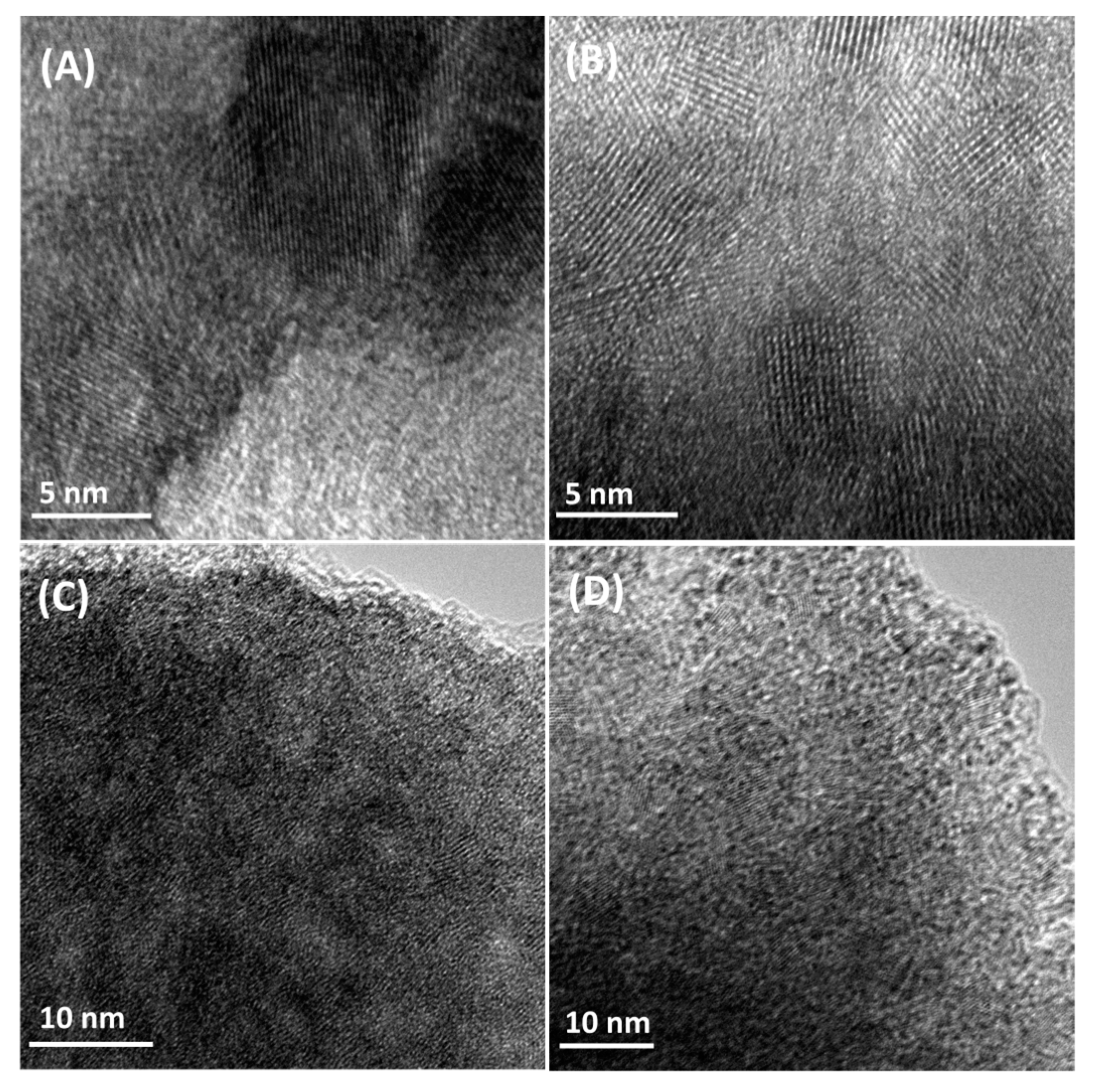
3.1. Preparation and Characterization of the Modified MoO3 Films
3.2. Electrochemical Properties of the Modified MoO3 Films
3.3. EC Properties of the Modified MoO3 Films
3.4. EC Properties of the Device with the Modified MoO3 Film as an Active Layer
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Huang, Y.; Wang, B.; Chen, F.; Han, Y.; Zhang, W.; Wu, X.; Li, R.; Jiang, Q.; Jia, X.; Zhang, R. Electrochromic materials based on ions insertion and extraction. Adv. Opt. Mater. 2021, 10, 2101783. [Google Scholar] [CrossRef]
- Zhang, S.; Peng, Y.; Zhao, J.; Fan, Z.; Ding, B.; Lee, J.Y.; Zhang, X.; Xuan, Y. Amorphous and porous tungsten oxide films for fast-switching dual-band electrochromic smart windows. Adv. Opt. Mater. 2022, 11, 2202115. [Google Scholar] [CrossRef]
- Wu, C.-L.; Wang, C.-K.; Lin, C.-K.; Wang, S.-C.; Huang, J.-L. Electrochromic properties of nanostructured tungsten oxide films prepared by surfactant-assisted sol-gel process. Surf. Coat. Technol. 2013, 231, 403–407. [Google Scholar] [CrossRef]
- Kim, J.; Lee, K.H.; Lee, S.; Park, S.; Chen, H.; Kim, S.K.; Yim, S.; Song, W.; Lee, S.S.; Yoon, D.H.; et al. Minimized optical scattering of MXene-derived 2D V2O5 nanosheet-based electrochromic device with high multicolor contrast and accuracy. Chem. Eng. J. 2023, 453, 139973. [Google Scholar] [CrossRef]
- Hsu, S.-C.; Chao, S.-H.; Wu, N.-J.; Huang, J.-H.; Kang, J.-L.; Weng, H.C.; Liu, T.-Y. Facile synthesis of hybrid WO3/MoOx electrochromic films for application in complementary electrochromic devices. J. Alloys Compd. 2023, 945, 169256. [Google Scholar] [CrossRef]
- Duan, Y.; Wang, C.; Hao, J.; Jiao, Y.; Xu, Y.; Wang, J. Electrochromic performance and capacitor performance of α-MoO3 nanorods fabricated by a one-step procedure. Coatings 2021, 11, 783. [Google Scholar] [CrossRef]
- Wang, J.; Hou, L.; Ma, D. Molybdenum oxide electrochromic materials and devices. J. Inorg. Mater. 2021, 36, 461–470. [Google Scholar] [CrossRef]
- Sivakumar, R.; Gopalakrishnan, R.; Jayachandran, M.; Sanjeeviraja, C. Characterization on electron beam evaporated α-MoO3 thin films by the influence of substrate temperature. Curr. Appl. Phys. 2007, 7, 51–59. [Google Scholar] [CrossRef]
- Mohamed, S.H.; Kappertz, O.; Ngaruiya, J.M.; Leervad Pedersen, T.P.; Drese, R.; Wuttig, M. Correlation between structure, stress and optical properties in direct current sputtered molybdenum oxide films. Thin Solid Film. 2003, 429, 135–143. [Google Scholar] [CrossRef]
- Gesheva, K.; Szekeres, A.; Ivanova, T. Optical properties of chemical vapor deposited thin films of molybdenum and tungsten based metal oxides. Sol. Energy Mater. Sol. Cells 2003, 76, 563–576. [Google Scholar] [CrossRef]
- Ivanova, T.; Gesheva, K.A.; Popkirov, G.; Ganchev, M.; Tzvetkova, E. Electrochromic properties of atmospheric CVD MoO3 and MoO3–WO3 films and their application in electrochromic devices. Mater. Sci. Eng. B 2005, 119, 232–239. [Google Scholar] [CrossRef]
- Lin, F.; Cheng, J.; Engtrakul, C.; Dillon, A.C.; Nordlund, D.; Moore, R.G.; Weng, T.-C.; Williams, S.K.R.; Richards, R.M. In situ crystallization of high performing WO3-based electrochromic materials and the importance for durability and switching kinetics. J. Mater. Chem. 2012, 22, 16817–16823. [Google Scholar] [CrossRef]
- Wei, C.-C.; Wu, T.-H.; Huang, J.-W.; Young, B.-L.; Jian, W.-B.; Lin, Y.-L.; Chen, J.-T.; Hsu, C.-S.; Ma, Y.-R.; Tsukagoshi, K. Nanoparticulate films of WO3 and MoO3 composites for enhancing UV light electrochromic transmittance variation and energy storage applications. Electrochim. Acta 2023, 442, 141897. [Google Scholar] [CrossRef]
- Lin, S.-Y.; Wang, C.-M.; Kao, K.-S.; Chen, Y.-C.; Liu, C.-C. Electrochromic properties of MoO3 thin films derived by a sol–gel process. J. Sol-Gel Sci. Technol. 2009, 53, 51–58. [Google Scholar] [CrossRef]
- Taylor, D.J.; Cronin, J.P.; Allard, L.F.; Birnie, D.P. Microstructure of laser-fired, sol-gel-derived tungsten oxide films. Chem. Mater. 1996, 8, 1396–1401. [Google Scholar] [CrossRef]
- Novak, T.G.; Kim, J.; Tiwari, A.P.; Kim, J.; Lee, S.; Lee, J.; Jeon, S. 2D MoO3 nanosheets synthesized by exfoliation and oxidation of MoS2 for high contrast and fast response time electrochromic devices. ACS Sustain. Chem. Eng. 2020, 8, 11276–11282. [Google Scholar] [CrossRef]
- Ou, J.Z.; Balendhran, S.; Field, M.R.; McCulloch, D.G.; Zoolfakar, A.S.; Rani, R.A.; Zhuiykov, S.; O’Mullane, A.P.; Kalantar-zadeh, K. The anodized crystalline WO3 nanoporous network with enhanced electrochromic properties. Nanoscale 2012, 4, 5980–5988. [Google Scholar] [CrossRef] [PubMed]
- Dhanasankar, M.; Purushothaman, K.K.; Muralidharan, G. Enhanced electrochromism in cerium doped molybdenum oxide thin films. Mater. Res. Bull. 2010, 45, 1969–1972. [Google Scholar] [CrossRef]
- Layegh, M.; Ghodsi, F.E.; Hadipour, H. Experimental and theoretical study of Fe doping as a modifying factor in electrochemical behavior of mixed-phase molybdenum oxide thin films. Appl. Phys. A 2019, 126, 14. [Google Scholar] [CrossRef]
- Jittiarporn, P.; Sikong, L.; Kooptarnond, K.; Taweepreda, W.; Stoenescu, S.; Badilescu, S.; Truong, V.-V. Electrochromic properties of MoO3-WO3 thin films prepared by a sol-gel method, in the presence of a triblock copolymer template. Surf. Coat. Technol. 2017, 327, 66–74. [Google Scholar] [CrossRef]
- Cholant, C.M.; Westphal, T.M.; Balboni, R.D.C.; Moura, E.A.; Gündel, A.; Flores, W.H.; Pawlicka, A.; Avellaneda, C.O. Thin films of V2O5/MoO3 and their applications in electrochromism. J. Solid State Electrochem. 2017, 21, 1509–1515. [Google Scholar] [CrossRef]
- Deepa, M.; Saxena, T.K.; Singh, D.P.; Sood, K.N.; Agnihotry, S.A. Spin coated versus dip coated electrochromic tungsten oxide films: Structure, morphology, optical and electrochemical properties. Electrochim. Acta 2006, 51, 1974–1989. [Google Scholar] [CrossRef]
- Fang, Y.; Sun, X.; Cao, H. Influence of PEG additive and annealing temperature on structural and electrochromic properties of sol-gel derived WO3 films. J. Sol-Gel Sci. Technol. 2011, 59, 145–152. [Google Scholar] [CrossRef]
- Shi, Y.; Sun, M.; Zhang, Y.; Cui, J.; Wang, Y.; Shu, X.; Qin, Y.; Tan, H.H.; Liu, J.; Wu, Y. Structure modulated amorphous/crystalline WO3 nanoporous arrays with superior electrochromic energy storage performance. Sol. Energy Mater. Sol. Cells 2020, 212, 110579. [Google Scholar] [CrossRef]
- Zhou, D.; Che, B.; Kong, J.; Lu, X. A nanocrystalline tungsten oxide electrochromic coating with excellent cycling stability prepared via a complexation-assisted sol–gel method. J. Mater. Chem. C 2016, 4, 8041–8051. [Google Scholar] [CrossRef]
- Zhou, D.; Tong, Z.; Xie, H.; Sun, J.; Chen, F. Effects of additives on electrochromic properties of nanocrystalline tungsten oxide films prepared by complexation-assisted sol-gel method. Materials 2023, 16, 2681. [Google Scholar] [CrossRef]
- Zhou, D.; Zhou, R.; Chen, C.; Yee, W.-A.; Kong, J.; Ding, G.; Lu, X. Non-volatile polymer electrolyte based on poly(propylene carbonate), ionic liquid, and lithium perchlorate for electrochromic devices. J. Phys. Chem. B 2013, 117, 7783–7789. [Google Scholar] [CrossRef]
- Xiong, S.; Phua, S.L.; Dunn, B.S.; Ma, J.; Lu, X. Covalently bonded polyaniline-TiO2 hybrids: A facile approach to highly stable anodic electrochromic materials with low oxidation potentials. Chem. Mater. 2010, 22, 255–260. [Google Scholar] [CrossRef]
- Wang, Z.; Zou, Y.; Li, Y.; Cheng, Y. Metal-containing polydopamine nanomaterials: Catalysis, energy, and theranostics. Small 2020, 16, 1907042. [Google Scholar] [CrossRef] [PubMed]
- Griffith, W.P.; Pumphrey, C.A.; Rainey, T.A. Catecholato complexes of ruthenium, iridium, rhenium, molybdenum, and tungsten. J. Chem. Soc. -Dalton Trans. 1986, 6, 1125–1128. [Google Scholar] [CrossRef]
- Elhendawy, A.M.; Griffith, W.P.; Pumphrey, C.A. Complexes of osmium, uranium, molybdenum, and tungsten with the catechol amines adrenaline, noradrenaline, dopamine, dopa, and isoproterenol. J. Chem. Soc. -Dalton Trans. 1988, 7, 1817–1821. [Google Scholar] [CrossRef]
- Urdaneta, I.; Keller, A.; Atabek, O.; Palma, J.L.; Finkelstein-Shapiro, D.; Tarakeshwar, P.; Mujica, V.; Calatayud, M. Dopamine adsorption on TiO2 anatase surfaces. J. Phys. Chem. C 2014, 118, 20688–20693. [Google Scholar] [CrossRef]
- Kleiman-Shwarsctein, A.; Laursen, A.B.; Cavalca, F.; Tang, W.; Dahl, S.; Chorkendorff, I. A general route for RuO2 deposition on metal oxides from RuO4. Chem. Commun. 2012, 48, 967–969. [Google Scholar] [CrossRef] [PubMed]
- Valverde-Aguilar, G.; Prado-Prone, G.; Vergara-Aragon, P.; Garcia-Macedo, J.; Santiago, P.; Rendon, L. Photoconductivity studies on nanoporous TiO2/dopamine films prepared by sol-gel method. Appl. Phys. A-Mater. Sci. Process. 2014, 116, 1075–1084. [Google Scholar] [CrossRef]
- Dalavi, D.S.; Devan, R.S.; Patil, R.A.; Patil, R.S.; Ma, Y.-R.; Sadale, S.B.; Kim, I.; Kim, J.-H.; Patil, P.S. Efficient electrochromic performance of nanoparticulate WO3 thin films. J. Mater. Chem. C 2013, 1, 3722–3728. [Google Scholar] [CrossRef]
- Cheng, P.; Shi, L.; Li, W.; Fang, X.; Cao, D.; Zhao, Y.; Cao, P.; Liu, D.; He, D. Efficient Regulation of Polysulfides by MoS(2) /MoO(3) Heterostructures for High-Performance Li-S Batteries. Small 2023, 19, e2206083. [Google Scholar] [CrossRef] [PubMed]
- Wen-Cheun Au, B.; Chan, K.-Y.; Knipp, D. Effect of film thickness on electrochromic performance of sol-gel deposited tungsten oxide (WO3). Opt. Mater. 2019, 94, 387–392. [Google Scholar] [CrossRef]
- Quy, V.H.V.; Jo, I.-R.; Kang, S.-H.; Ahn, K.-S. Amorphous-crystalline dual phase WO3 synthesized by pulsed-voltage electrodeposition and its application to electrochromic devices. J. Ind. Eng. Chem. 2021, 94, 264–271. [Google Scholar] [CrossRef]
- Huang, H.; Tian, J.; Zhang, W.K.; Gan, Y.P.; Tao, X.Y.; Xia, X.H.; Tu, J.P. Electrochromic properties of porous NiO thin film as a counter electrode for NiO/WO3 complementary electrochromic window. Electrochim. Acta 2011, 56, 4281–4286. [Google Scholar] [CrossRef]
- Cong, S.; Tian, Y.; Li, Q.; Zhao, Z.; Geng, F. Single-Crystalline Tungsten Oxide Quantum Dots for Fast Pseudocapacitor and Electrochromic Applications. Adv. Mater. 2014, 26, 4260–4267. [Google Scholar] [CrossRef]
- Kondalkar, V.V.; Mali, S.S.; Kharade, R.R.; Khot, K.V.; Patil, P.B.; Mane, R.M.; Choudhury, S.; Patil, P.S.; Hong, C.K.; Kim, J.H.; et al. High performing smart electrochromic device based on honeycomb nanostructured h-WO3 thin films: Hydrothermal assisted synthesis. Dalton Trans. 2015, 44, 2788–2800. [Google Scholar] [CrossRef] [PubMed]
- Romero, R.; Dalchiele, E.A.; Martin, F.; Leinen, D.; Ramos-Barrado, J.R. Electrochromic behaviour of Nb2O5 thin films with different morphologies obtained by spray pyrolysis. Sol. Energy Mater. Sol. Cells 2009, 93, 222–229. [Google Scholar] [CrossRef]
- Lee, S.H.; Deshpande, R.; Parilla, P.A.; Jones, K.M.; To, B.; Mahan, A.H.; Dillon, A.C. Crystalline WO3 nanoparticles for highly improved electrochromic applications. Adv. Mater. 2006, 18, 763–766. [Google Scholar] [CrossRef]
- Rai, V.; Singh, R.S.; Blackwood, D.J.; Zhili, D. A review on recent advances in electrochromic devices: A material approach. Adv. Eng. Mater. 2020, 22, 2000082. [Google Scholar] [CrossRef]
- Deepa, M.; Srivastava, A.K.; Sood, K.N.; Murugan, A.V. Nanostructured tungsten oxide-poly(3,4-ethylenedioxythiophene): Poly(styrenesulfonate) hybrid films: Synthesis, electrochromic response, and durability characteristics. J. Electrochem. Soc. 2008, 155, D703–D710. [Google Scholar] [CrossRef]

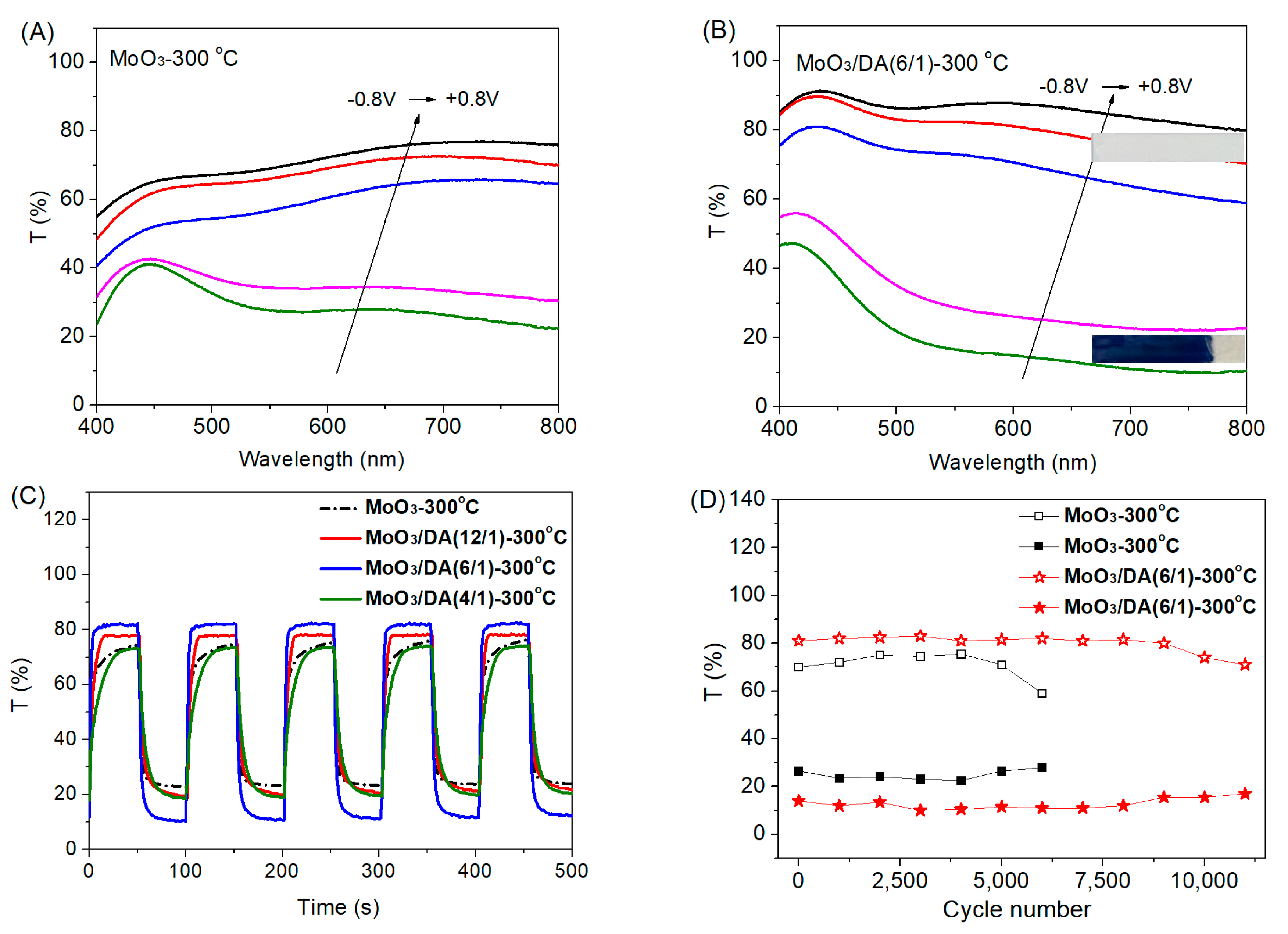
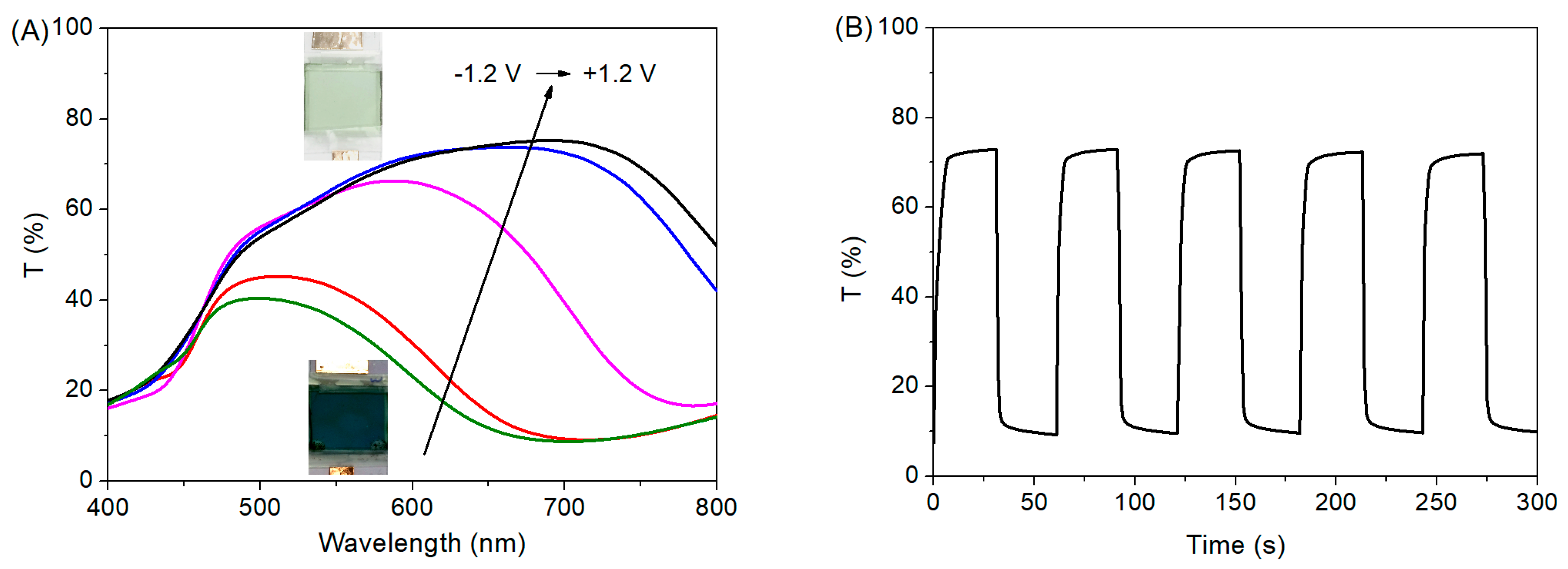
| EC Films or Device | ∆T (%) | CE (cm2 C−1) | Bleaching Time (s) | Coloration Time (s) |
|---|---|---|---|---|
| MoO3-300 °C Film | 51 | 65 | 14 | 8 |
| MoO3/DA(12/1)-300 °C Film | 59 | 74 | 8 | 9 |
| MoO3/DA(6/1)-300 °C Film | 71 | 88 | 5 | 6 |
| MoO3/DA(4/1)-300 °C Film | 54 | 69 | 19 | 15 |
| Dual Active Layer Device | 63 | 148 | 4 | 3 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhou, D.; Yang, L. Enhanced Electrochromic Properties of Nanocrystalline Molybdenum Oxide Films Modified by Dopamine. Coatings 2023, 13, 1292. https://doi.org/10.3390/coatings13071292
Zhou D, Yang L. Enhanced Electrochromic Properties of Nanocrystalline Molybdenum Oxide Films Modified by Dopamine. Coatings. 2023; 13(7):1292. https://doi.org/10.3390/coatings13071292
Chicago/Turabian StyleZhou, Dan, and Liping Yang. 2023. "Enhanced Electrochromic Properties of Nanocrystalline Molybdenum Oxide Films Modified by Dopamine" Coatings 13, no. 7: 1292. https://doi.org/10.3390/coatings13071292
APA StyleZhou, D., & Yang, L. (2023). Enhanced Electrochromic Properties of Nanocrystalline Molybdenum Oxide Films Modified by Dopamine. Coatings, 13(7), 1292. https://doi.org/10.3390/coatings13071292






