Determining the Annealing Temperature Dependency of Wetting and Mechanical Features on Fe3Si Films
Abstract
:1. Introduction
2. Materials and Methods
2.1. Production of Fe3Si Films
2.2. Post Annealing of Fe3Si Films
2.3. Investigation of the Properties of Fe3Si Films
3. Results and Discussion
3.1. XRD Results of Fe3Si Films
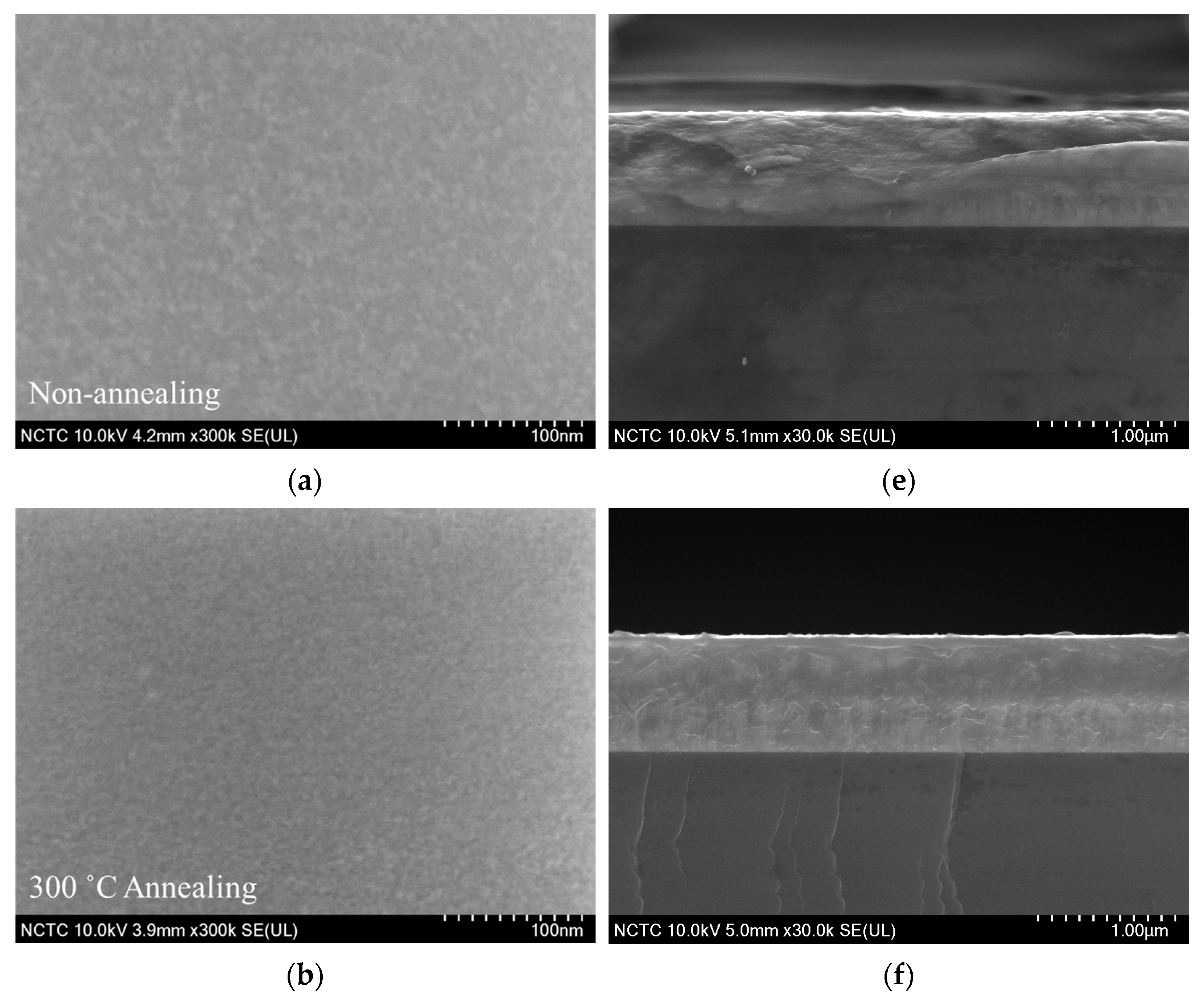
3.2. FESEM Results of Fe3Si films
3.3. AFM Results of Fe3Si Films
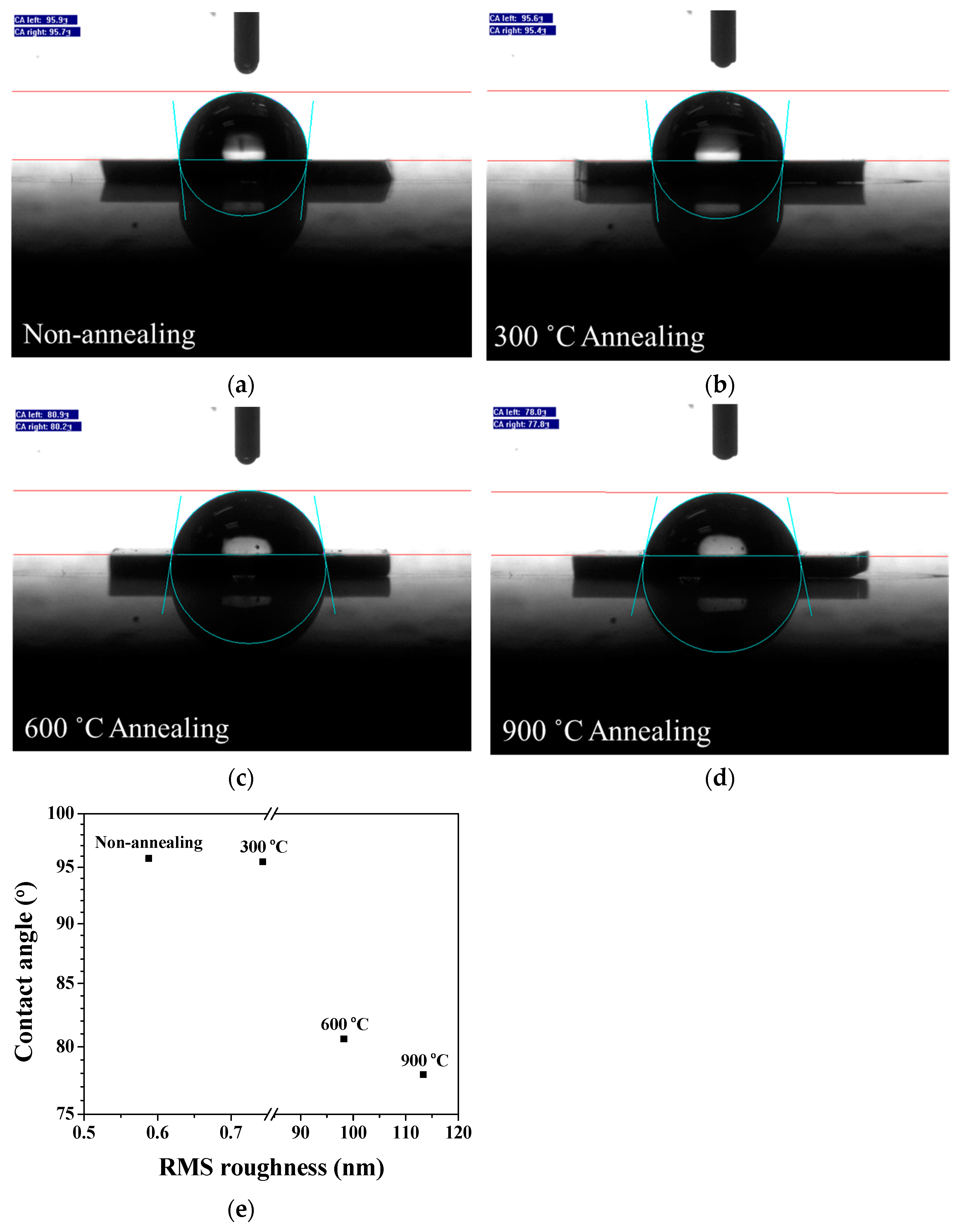
3.4. Contact Angles of Fe3Si Films
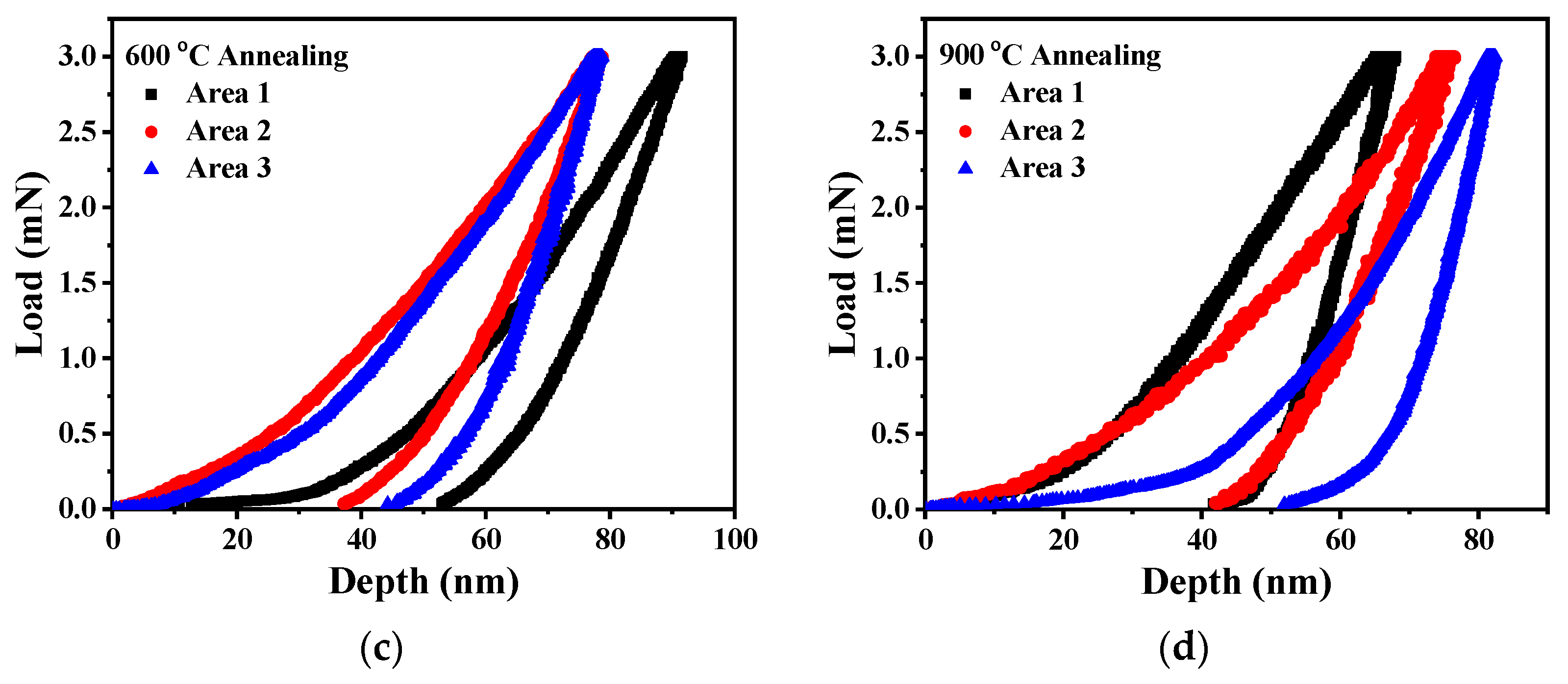
3.5. Nanoindentation Results of Fe3Si Films
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Borwornpornmetee, N.; Charoenyuenyao, P.; Chaleawpong, R.; Paosawatyanyong, B.; Phatthanakun, R.; Sittimart, P.; Aramaki, K.; Hamasaki, T.; Yoshitake, T.; Promros, N. Physical Properties of Fe3Si Films Coated through Facing Targets Sputtering after Microwave Plasma Treatment. Coatings 2021, 11, 923. [Google Scholar] [CrossRef]
- Yoshitake, T.; Nakagauchi, D.; Ogawa, T.; Itakura, M.; Kuwano, N.; Tomokiyo, Y.; Kajiwara, T.; Nagayama, K. Room-Temperature Epitaxial Growth of Ferromagnetic Fe3Si Films on Si(111) by Facing Target Direct-Current Sputtering. Appl. Phys. Lett. 2005, 86, 262505. [Google Scholar] [CrossRef]
- Nakagauchi, D.; Yoshitake, T.; Nagayama, K. Fabrication of Ferromagnetic Fe3Si Thin Films by Pulsed Laser Deposition Using an Fe3Si Target. Vacuum 2004, 74, 653–657. [Google Scholar] [CrossRef]
- Sakai, K.; Noda, Y.; Tsumagari, D.; Deguchi, H.; Takeda, K.; Yoshitake, T. Temperature-Dependent Interlayer Coupling in Fe3Si/FeSi2 Artificial Lattices. Phys. Status Solidi (A) Appl. Mater. Sci. 2014, 211, 323–328. [Google Scholar] [CrossRef]
- Starke, U.; Schardt, J.; Weiss, W.; Meier, W.; Polop, C.; de Andres, P.L.; Heinz, K. Structural and Compositional Reversible Phase Transitions on Low-Index Fe3Si Surfaces. Europhys. Lett. 2001, 56, 822. [Google Scholar] [CrossRef] [Green Version]
- Gude, A.; Mehrer, H. Diffusion in the D03-Type Intermetallic Phase Fe3Si. Philos. Mag. A 1997, 76, 1–29. [Google Scholar] [CrossRef]
- Sadoh, T.; Takeuchi, H.; Ueda, K.; Kenjo, A.; Miyao, M. Epitaxial Growth of Ferromagnetic Silicide Fe3Si on Si(111) Substrate. Jpn. J. Appl. Phys. 2006, 45, 3598. [Google Scholar] [CrossRef]
- Hamaya, K.; Ueda, K.; Kishi, Y.; Ando, Y.; Sadoh, T.; Miyao, M. Epitaxial Ferromagnetic Fe3Si/Si(111) Structures with High-Quality Heterointerfaces. Appl. Phys. Lett. 2008, 93, 132117. [Google Scholar] [CrossRef] [Green Version]
- Herfort, J.; Schönherr, H.-P.; Ploog, K.H. Epitaxial Growth of Fe3Si/GaAs(001) Hybrid Structures. Appl. Phys. Lett. 2003, 83, 3912–3914. [Google Scholar] [CrossRef]
- Tang, C.; Wen, F.; Chen, H.; Liu, J.; Tao, G.; Xu, N.; Xue, J. Corrosion Characteristics of Fe3Si Intermetallic Coatings Prepared by Molten Salt Infiltration in Sulfuric Acid Solution. J. Alloys Compd. 2019, 778, 972–981. [Google Scholar] [CrossRef]
- Chang-bin, T.; Hong-xia, C.; Fu-rong, W.; Ni-jun, X.; Wei, H.; Juan-qin, X. Facile Preparation and Tribological Property of Alloyed Fe3Si Coatings on Stainless Steels Surface by Molten-Salt Infiltration Method. Surf. Coat. Technol. 2020, 397, 126049. [Google Scholar] [CrossRef]
- Schneeweiss, O.; Pizúrová, N.; Jirásková, Y.; Žák, T.; Cornut, B. Fe3Si surface coating on SiFe steel. J. Magn. Magn. Mater. 2000, 215, 115–117. [Google Scholar] [CrossRef]
- Lee, M.; Park, Y.; Kim, K.; Hong, J. Influence of Sputtering Conditions on the Properties of Aluminum-Doped Zinc Oxide Thin Film Fabricated Using a Facing Target Sputtering System. Thin Solid Film. 2020, 703, 137980. [Google Scholar] [CrossRef]
- Tominaga, K.; Ueda, T.; Ao, T.; Kataoka, M.; Mori, I. ITO Films Prepared by Facing Target Sputtering System. Thin Solid Films 1996, 281–282, 194–197. [Google Scholar] [CrossRef]
- Hossain, M.F.; Biswas, S.; Takahashi, T.; Kubota, Y.; Fujishima, A. Influence of Direct Current Power on the Photocatalytic Activity of Facing Target Sputtered TiO2 Thin Films. Thin Solid Film. 2008, 517, 1091–1095. [Google Scholar] [CrossRef]
- Jeong, J.A.; Shin, H.S.; Choi, K.H.; Kim, H.K. Flexible Al-Doped ZnO Films Grown on PET Substrates Using Linear Facing Target Sputtering for Flexible OLEDs. J. Phys. D Appl. Phys. 2010, 43, 465403. [Google Scholar] [CrossRef]
- Xie, J.; Xie, Q.; Ma, R.; Huang, J.; Zhang, C.; Liu, D. Annealing Temperature Dependent Structures and Properties of Ferromagnetic Fe3Si Films Fabricated by Resistive Thermal Evaporation. J. Mater. Sci. Mater. Electron. 2018, 29, 1369–1376. [Google Scholar] [CrossRef]
- Ng, Z.-N.; Chan, K.-Y.; Tohsophon, T. Effects of Annealing Temperature on ZnO and AZO Films Prepared by Sol–Gel Technique. Appl. Surf. Sci. 2012, 258, 9604–9609. [Google Scholar] [CrossRef]
- Raj, C.A.; Muneeswaran, M.; Jegatheesan, P.; Giridharan, N.V.; Sivakumar, V.J.; Senguttuvan, G. Effect of Annealing Time in the Low-Temperature Growth of BFO Thin Films Spin Coated on Glass Substrates. J. Mater. Sci. Mater. Electron. 2013, 24, 4148–4154. [Google Scholar] [CrossRef]
- Bazhan, Z.; Ghodsi, F.E.; Mazloom, J. Effect of Phase Transition Induced by Annealing Temperature on Wettability, Optical and Photocatalytic Properties of Nanostructured Iron Oxide Thin Film. J. Mater. Sci. Mater. Electron. 2018, 29, 11489–11497. [Google Scholar] [CrossRef]
- Gojda, F.; Loulakis, M.; Papoutsakis, L.; Tzortzakis, S.; Chrissopoulou, K.; Anastasiadis, S.H. Altering the Surface Properties of Metal Alloys Utilizing Facile and Ecological Methods. Langmuir 2022, 38, 4826–4838. [Google Scholar] [CrossRef]
- Terai, Y.; Yoneda, K.; Noda, K.; Miura, N.; Fujiwara, Y. Effect of Residual Impurities on Transport Properties of β-FeSi2 Epitaxial Films Grown by Molecular Beam Epitaxy. J. Appl. Phys. 2012, 112, 13702. [Google Scholar] [CrossRef]
- Tode, M.; Takigawa, Y.; Ohmukai, M.; Kurosawa, K.; Muroya, M. Epitaxially Growth of β-FeSi2 Thin Films on Si(100) Substrates from ε-FeSi Targets with ArF Excimer Laser Deposition. J. Phys. Conf. Ser. 2007, 59, 376–379. [Google Scholar] [CrossRef]
- Shimura, K.; Katsumata, T.; Yamaguchi, K.; Yamamoto, H.; Hojou, K. The Role of Sputter Etching and Annealing Processes on the Formation of β-FeSi2 Thin Films. Thin Solid Films 2004, 461, 22–27. [Google Scholar] [CrossRef]
- Nishikawa, Y.; Hiehata, K.; Nakamura, K. Reduction of Surface Cracks in β-FeSi2 Films by Substrate Heating during RF-Sputtering Deposition. Trans. Mat. Res. Soc. Jpn. 2013, 38, 243–247. [Google Scholar] [CrossRef]
- Lakshantha, W.J.; Dhoubhadel, M.S.; Reinert, T.; McDaniel, F.D.; Rout, B. Investigation of Various Phases of Fe–Si Structures Formed in Si by Low Energy Fe Ion Implantation. Nucl. Instrum. Methods Phys. Res. B 2015, 365, 114–119. [Google Scholar] [CrossRef]
- Baldwin, N.R.; Ivey, D.G. Iron Slicide Formation in Bulk Iron-Silicon Diffusion Couples. J. Phase Equilibria 1995, 16, 300–307. [Google Scholar] [CrossRef]
- Li, M.; Chen, X.; Guan, J.; Wang, J.; Liang, C. Thermally Induced Phase Transition and Magnetic Properties of Fe–FeSi2 with Core–Shell Structure. Phys. Status Solidi (A) 2013, 210, 2710–2715. [Google Scholar] [CrossRef]
- Tian, Y.; Bento Montes, A.R.; Vančo, Ľ.; Čaplovičová, M.; Vogrinčič, P.; Šutta, P.; Satrapinskyy, L.; Zeman, M.; Isabella, O. Toward BaSi2/Si Heterojunction Thin-Film Solar Cells: Insights into Heterointerface Investigation, Barium Depletion, and Silicide-Mediated Silicon Crystallization. Adv. Mater. Interfaces 2020, 7, 2000887. [Google Scholar] [CrossRef]
- Isobe, T.; Nakashima, H.; Hashimoto, K. Diffusion Coefficient of Interstitial Iron in Silicon. Jpn. J. Appl. Phys. 1989, 28, 1282. [Google Scholar] [CrossRef]
- Mehrer, H. Diffusion in Intermetallics. Mater. Trans. JIM 1996, 37, 1259–1280. [Google Scholar] [CrossRef] [Green Version]
- Béjina, F.; Jaoul, O. Silicon Self-Diffusion in Quartz and Diopside Measured by Nuclear Micro-Analysis Methods. Phys. Earth Planet. Inter. 1996, 97, 145–162. [Google Scholar] [CrossRef]
- Ramappa, D.A.; Henley, W.B. Diffusion of Iron in Silicon Dioxide. J. Electrochem. Soc. 1999, 146, 3773. [Google Scholar] [CrossRef]
- Inaba, T.; Saito, Y.; Kominami, H.; Nakanishi, Y.; Murakami, K.; Matsuyama, T.; Tatsuoka, H. Growth of SiOx Nanofibers Using FeSi Substrates with Ga Droplets. Jpn. J. Appl. Phys. 2006, 45, L1320. [Google Scholar] [CrossRef]
- Drelich, J.; Chibowski, E.; Meng, D.D.; Terpilowski, K. Hydrophilic and Superhydrophilic Surfaces and Materials. Soft Matter 2011, 7, 9804–9828. [Google Scholar] [CrossRef]
- Krasowska, M.; Zawala, J.; Malysa, K. Air at Hydrophobic Surfaces and Kinetics of Three Phase Contact Formation. Adv. Colloid Interface Sci. 2009, 147–148, 155–169. [Google Scholar] [CrossRef]
- Mohamed, A.M.A.; Abdullah, A.M.; Younan, N.A. Corrosion Behavior of Superhydrophobic Surfaces: A Review. Arab. J. Chem. 2015, 8, 749–765. [Google Scholar] [CrossRef] [Green Version]
- Cassie, A.B.D.; Baxter, S. Wettability of Porous Surfaces. Trans. Faraday Soc. 1944, 40, 546–551. [Google Scholar] [CrossRef]
- Lafuma, A.; Quéré, D. Superhydrophobic States. Nat. Mater. 2003, 2, 457–460. [Google Scholar] [CrossRef] [PubMed]
- Wenzel, R.N. Surface Roughness and Contact Angle. J. Phys. Colloid Chem. 1949, 53, 1466–1467. [Google Scholar] [CrossRef]
- Quéré, D. Wetting and Roughness. Annu. Rev. Mater. Res 2008, 38, 71–99. [Google Scholar] [CrossRef]
- Beegan, D.; Chowdhury, S.; Laugier, M.T. Comparison between Nanoindentation and Scratch Test Hardness (Scratch Hardness) Values of Copper Thin Films on Oxidised Silicon Substrates. Surf. Coat. Technol. 2007, 201, 5804–5808. [Google Scholar] [CrossRef]
- Fischer-Cripps, A.C. A Review of Analysis Methods for Sub-Micron Indentation Testing. Vacuum 2000, 58, 569–585. [Google Scholar] [CrossRef]
- Torrents, A.; Yang, H.; Mohamed, F.A. Effect of Annealing on Hardness and the Modulus of Elasticity in Bulk Nanocrystalline Nickel. Metall. Mater. Trans. A 2010, 41, 621–630. [Google Scholar] [CrossRef] [Green Version]
- Swygenhoven, H. Van Grain Boundaries and Dislocations. Science (1979) 2002, 296, 66–67. [Google Scholar] [CrossRef]
- Chen, J.; Wang, W.; Qian, L.H.; Lu, K. Critical Shear Stress for Onset of Plasticity in a Nanocrystalline Cu Determined by Using Nanoindentation. Scr. Mater. 2003, 49, 645–650. [Google Scholar] [CrossRef]
- Chen, L.; Ahadi, A.; Zhou, J.; Ståhl, J.-E. Modeling Effect of Surface Roughness on Nanoindentation Tests. Procedia CIRP 2013, 8, 334–339. [Google Scholar] [CrossRef] [Green Version]
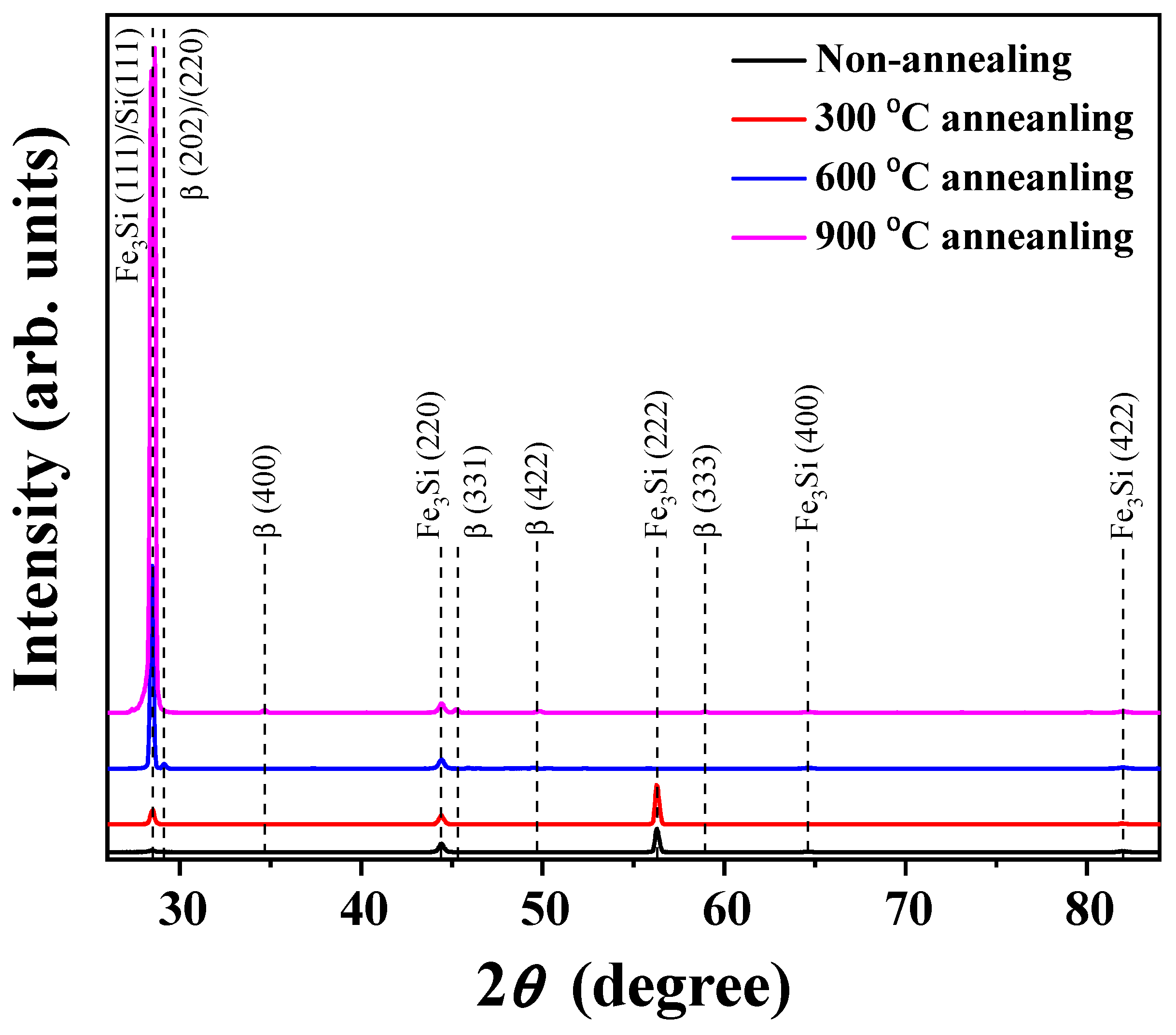




| Angle | Orientation | Reference |
|---|---|---|
| 28.50 | Fe3Si (111) | [8] |
| 29.12 | β (202)/(220) | [19,20,21,22] |
| 34.72 | β (400) | [19] |
| 44.38 | Fe3Si (220) | [8] |
| 45.20 | β (331) | [19] |
| 49.80 | β (422) | [26] |
| 56.28 | Fe3Si (222) | [2] |
| 58.90 | β (333) | [26] |
| 64.72 | Fe3Si (400) | [8] |
| 82.06 | Fe3Si (422) | [8] |
| Annealing Temperature (°C) | RMS Roughness (nm) |
|---|---|
| Non-annealing | 0.588 |
| 300 | 0.743 |
| 600 | 98.235 |
| 900 | 113.361 |
| Annealing Temperature (°C) | θ CA (°) | Wetting State |
|---|---|---|
| Non-annealed | 95.8 | Hydrophobic |
| 300 | 95.5 | Hydrophobic |
| 600 | 80.6 | Hydrophilic |
| 900 | 77.9 | Hydrophilic |
| Annealing Temperature (°C) | H (GPa) | Er (GPa) |
|---|---|---|
| Without annealing | 8.741 ± 0.111 | 184.899 ± 1.343 |
| 300 | 9.363 ± 0.081 | 204.803 ± 1.347 |
| 600 | 14.543 ± 0.983 | 271.261 ± 34.495 |
| 900 | 15.341 ± 1.777 | 323.187 ± 89.125 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Borwornpornmetee, N.; Achirawongwat, C.; Traiprom, T.; Saekow, B.; Porntheeraphat, S.; Paosawatyanyong, B.; Yoshitake, T.; Promros, N. Determining the Annealing Temperature Dependency of Wetting and Mechanical Features on Fe3Si Films. Coatings 2023, 13, 1328. https://doi.org/10.3390/coatings13081328
Borwornpornmetee N, Achirawongwat C, Traiprom T, Saekow B, Porntheeraphat S, Paosawatyanyong B, Yoshitake T, Promros N. Determining the Annealing Temperature Dependency of Wetting and Mechanical Features on Fe3Si Films. Coatings. 2023; 13(8):1328. https://doi.org/10.3390/coatings13081328
Chicago/Turabian StyleBorwornpornmetee, Nattakorn, Chawapon Achirawongwat, Thawichai Traiprom, Bunpot Saekow, Supanit Porntheeraphat, Boonchoat Paosawatyanyong, Tsuyoshi Yoshitake, and Nathaporn Promros. 2023. "Determining the Annealing Temperature Dependency of Wetting and Mechanical Features on Fe3Si Films" Coatings 13, no. 8: 1328. https://doi.org/10.3390/coatings13081328
APA StyleBorwornpornmetee, N., Achirawongwat, C., Traiprom, T., Saekow, B., Porntheeraphat, S., Paosawatyanyong, B., Yoshitake, T., & Promros, N. (2023). Determining the Annealing Temperature Dependency of Wetting and Mechanical Features on Fe3Si Films. Coatings, 13(8), 1328. https://doi.org/10.3390/coatings13081328






