Enhanced Performance of Nickel–Cobalt Oxides as Selective Coatings for Flat-Plate Solar Thermal Collector Applications
Abstract
1. Introduction
| Composition | Method of Fabrication | Substrate | Solar Absorptance (α) | Thermal Emittance (ε) | Scale-Up Test | Year/Ref. |
|---|---|---|---|---|---|---|
| NixCo3−xO4 | Dip-coating method | Stainless Steel | 0.92 | 0.14 | No | 2019 [28] |
| Ni0.9Fe0.1Co2Oy | Sol–gel, spraying | Stainless Steel | 0.93 | 0.11 | No | 2021 [30] |
| Cu0.5Cr1.1Mn1.4O4 | Hydrothermal technique, co-precipitation, spray coating | Haynes 230 | 0.97 | 0.88 | No | 2019 [31] |
| Co–Cr, SiO2 | Electroplating, dip coating | Stainless Steel | 0.96 | 0.12 | No | 2022 [32] |
| Nickel/black Cobalt | Electrodeposition | Stainless Steel | 0.95 | 0.07 | Yes | 2020 [33] |
| CuCrxMn2−xO4 | Sol–gel combustion/spray-coating technique | Aluminum | 0.92–0.93 | 0.22–0.31 | No | 2012 [34] |
| CuMnNiOx, SiO2 and MgF2 | Sol–gel, solvothermal technique, dip coating | Stainless Steel | 0.94 | 0.14 | No | 2022 [10] |
| Co:Ni:Mn = 2:1:1 | Dip coating | Stainless Steel | 0.91 | 0.13 | No | 2023 [35] |
| Nickel–Black Nickel | Electrodeposition | Copper | 0.90 | 0.08 | Yes | 2019 [36] |
| Cr/Cr2O3 | Electrodeposition | Stainless Steel | 0.90 | NA | No | 2018 [37] |
| Black Nickel | Electrodeposition | Copper | 0.85 | 0.11 | Yes | 2022 [38] |
| C/Ni/NiO | Sol–gel, spin coating | Copper | 0.80 | 0.11 | No | 2019 [39] |
2. Materials and Methods
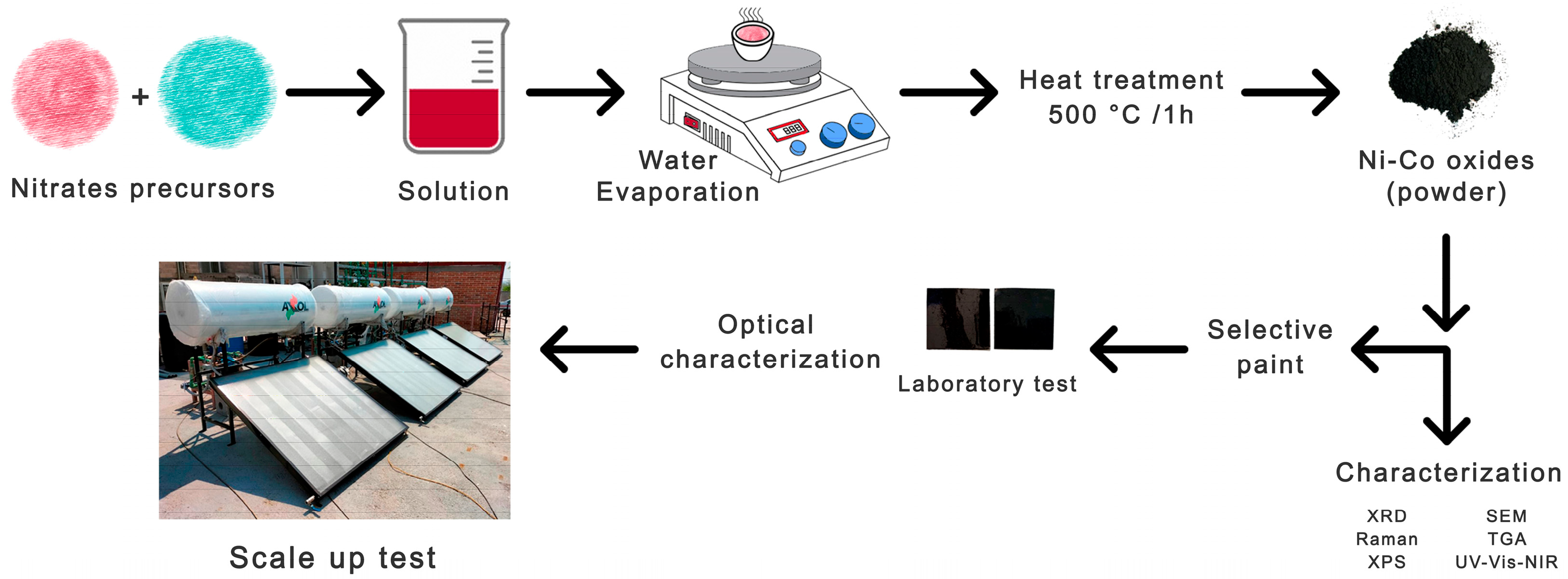
2.1. Synthesis of Powder Metal Oxides
2.2. Characterization of Powder Metal Oxides
2.3. Use of Powders to Formulate Selective Paints
2.4. Application of Selective Paints
2.5. Optical Characterization
2.6. Contact Angle Measurements
2.7. Scale-Up
3. Results and Discussion
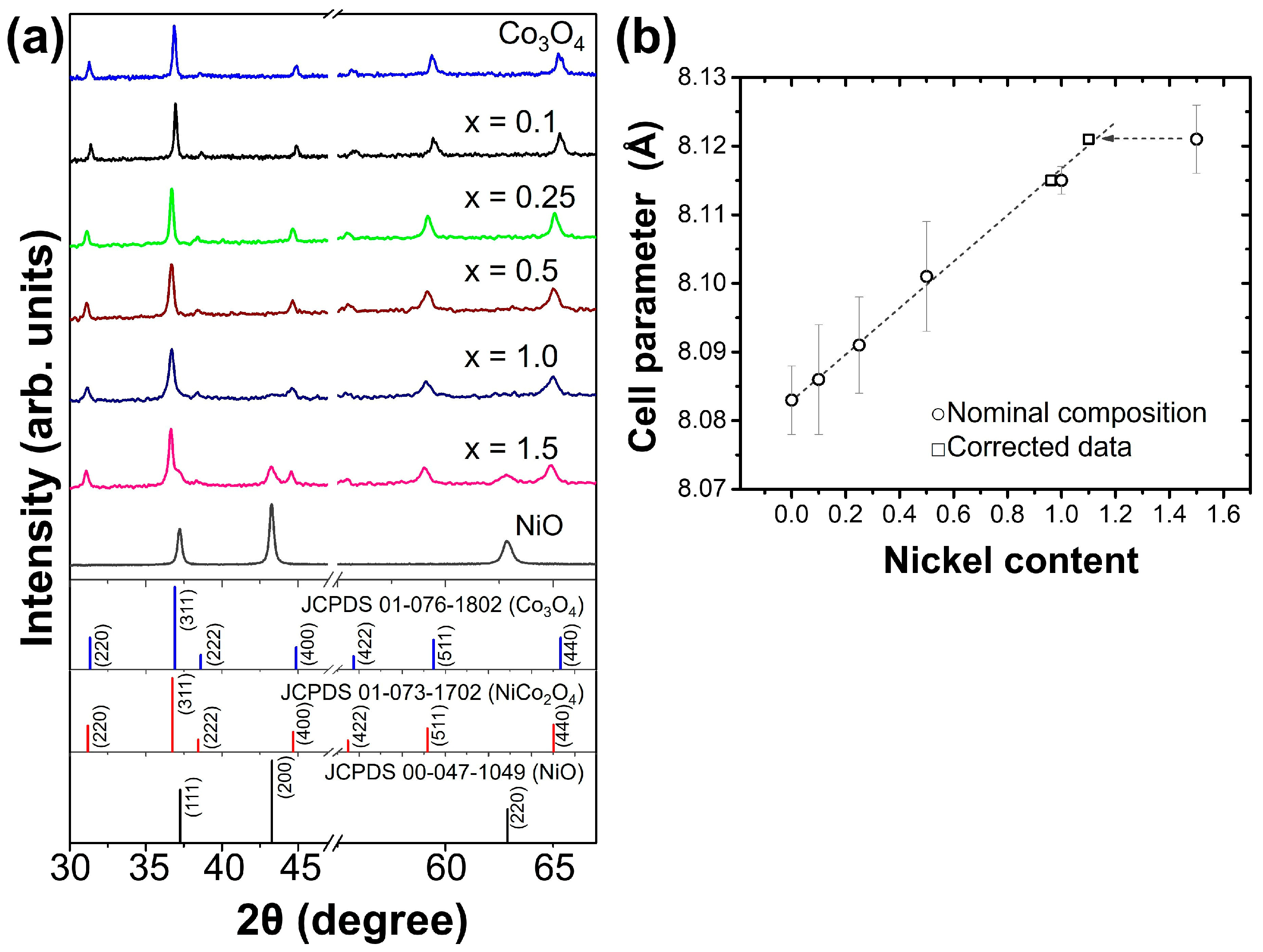
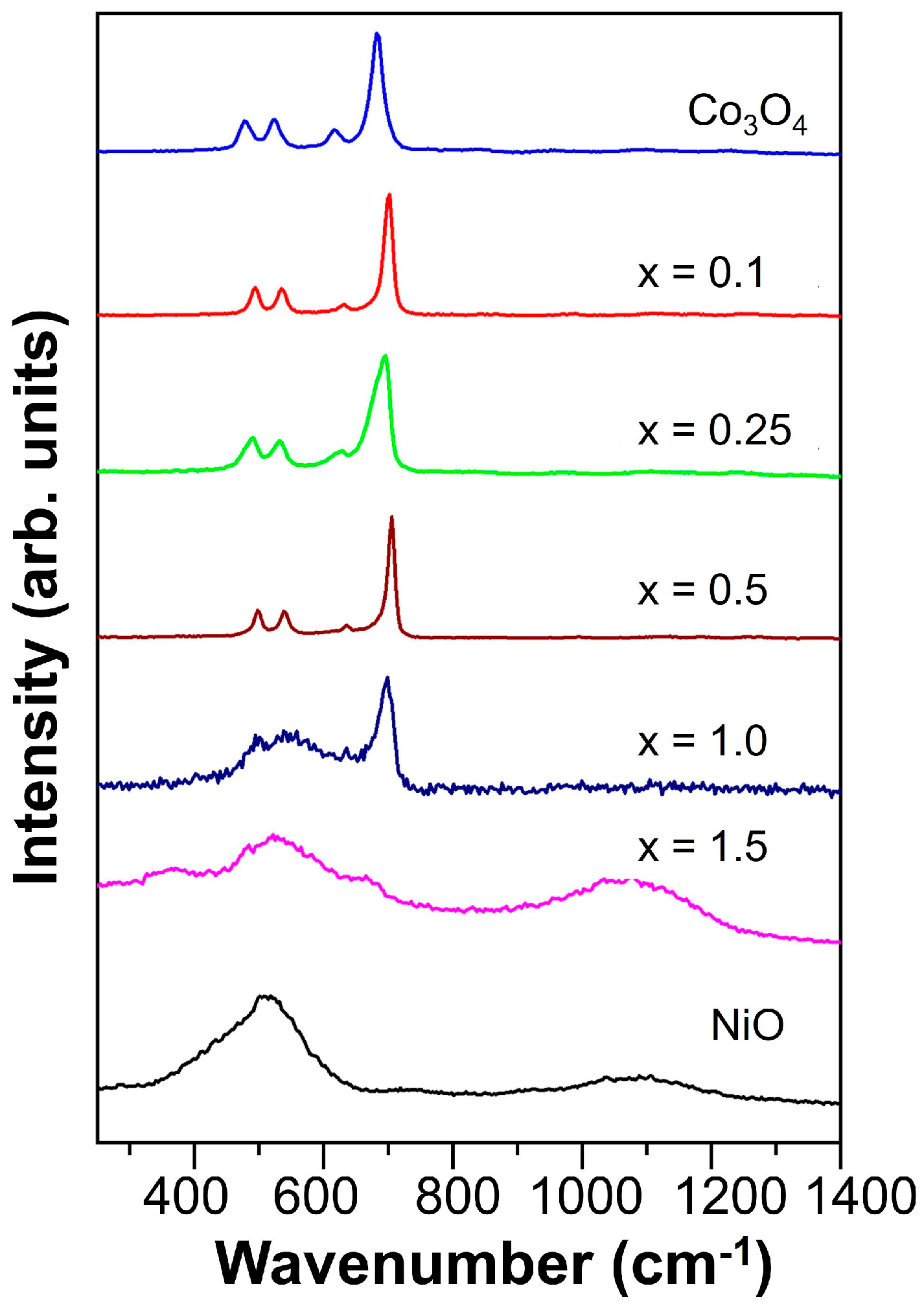
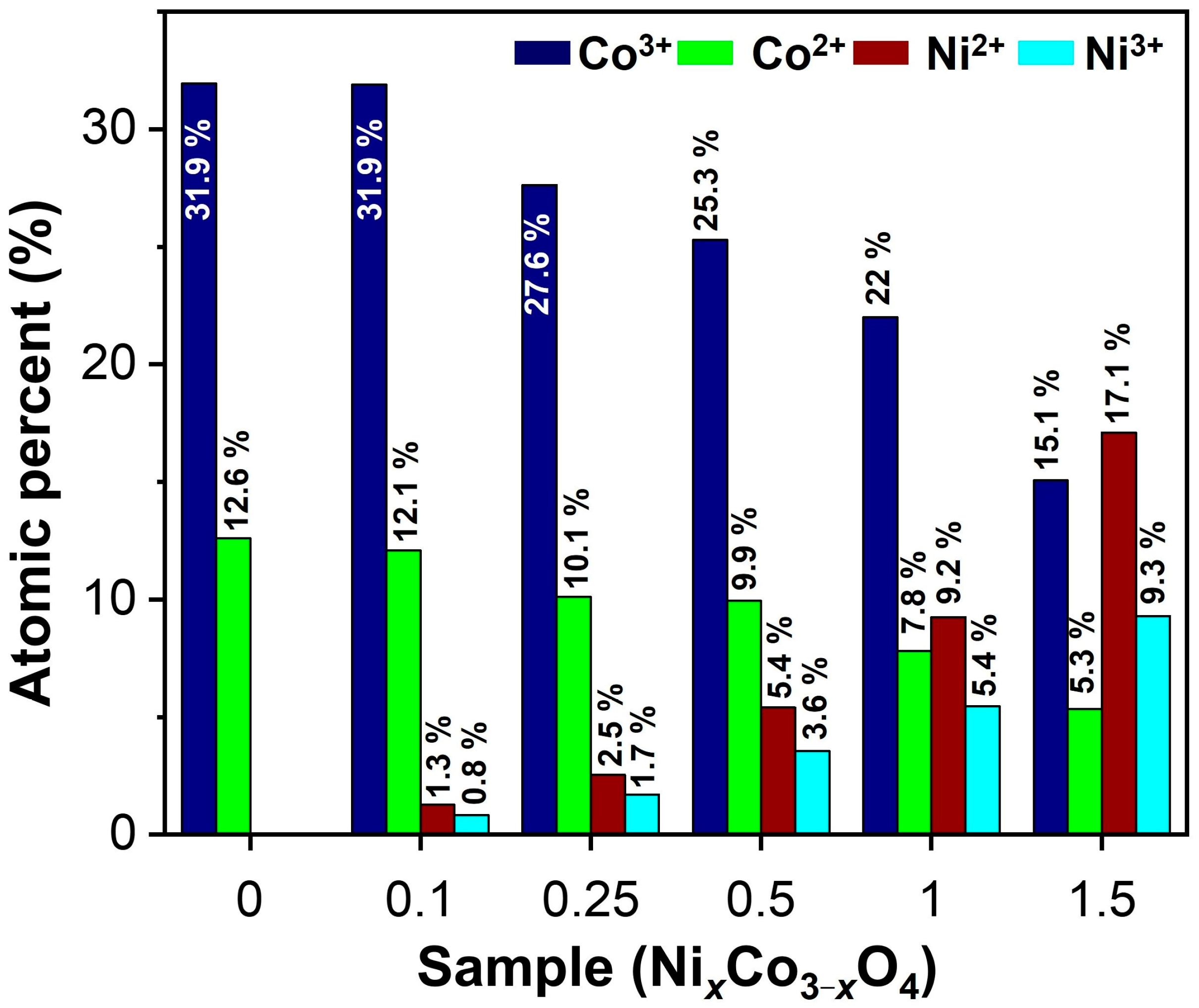
3.1. Physicochemical Analysis of Synthesized Powders
3.2. Evaluation of Selective Paints
3.3. Scale-Up
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Rabaia, M.K.H.; Abdelkareem, M.A.; Sayed, E.T.; Elsaid, K.; Chae, K.J.; Wilberforce, T.; Olabi, A.G. Environmental Impacts of Solar Energy Systems: A Review. Sci. Total Environ. 2021, 754, 141989. [Google Scholar] [CrossRef]
- Sharif, A.; Meo, M.S.; Chowdhury, M.A.F.; Sohag, K. Role of Solar Energy in Reducing Ecological Footprints: An Empirical Analysis. J. Clean. Prod. 2021, 292, 126028. [Google Scholar] [CrossRef]
- Hosseini, S.E.; Wahid, M.A. Hydrogen from Solar Energy, a Clean Energy Carrier from a Sustainable Source of Energy. Int. J. Energy Res. 2020, 44, 4110–4131. [Google Scholar] [CrossRef]
- Wu, Z.; Wang, J.; Liu, Y.; Hou, S.; Liu, X.; Zhang, Q.; Cao, F. A Review of Spectral Controlling for Renewable Energy Harvesting and Conserving. Mater. Today Phys. 2021, 18, 100388. [Google Scholar] [CrossRef]
- Chen, X.; Zhang, D.; Wang, Y.; Ling, X.; Jin, X. The Role of Sensible Heat in a Concentrated Solar Power Plant with Thermochemical Energy Storage. Energy Convers. Manag. 2019, 190, 42–53. [Google Scholar] [CrossRef]
- Gong, J.; Li, C.; Wasielewski, M.R. Advances in Solar Energy Conversion. Chem. Soc. Rev. 2019, 48, 1862–1864. [Google Scholar] [CrossRef]
- Madhukeshwara, N.; Prakash, E.S. An Investigation on the Performance Characteristics of Solar Flat Plate Collector with Different Selective Surface Coatings. Int. J. Energy Environ. 2012, 3, 99–108. Available online: https://www.ijee.ieefoundation.org/vol3/issue1/IJEE_10_v3n1.pdf (accessed on 7 February 2022).
- Zhang, J.; Wang, C.; Shi, J.; Wei, D.; Zhao, H.; Ma, C. Solar Selective Absorber for Emerging Sustainable Applications. Adv. Energy Sustain. Res. 2022, 3, 2100195. [Google Scholar] [CrossRef]
- Sun, B.; Wang, L.; Sun, Y.; Ren, J.; Yang, Y.; Liu, H.; Liang, D.; Li, A.; Wang, C. Optical Performance, Thermal Stability, and Failure Analysis of the WNx-Si3N4 Multilayer Solar Selective Absorbing Coatings. ACS Appl. Energy Mater. 2022, 5, 1883–1893. [Google Scholar] [CrossRef]
- Phani Kumar, K.K.; Mallick, S.; Sakthivel, S. Nanoparticles Based Single and Tandem Stable Solar Selective Absorber Coatings with Wide Angular Solar Absorptance. Sol. Energy Mater. Sol. Cells 2022, 242, 111758. [Google Scholar] [CrossRef]
- Selikhov, Y.; Klemeš, J.J.; Kapustenko, P.O.; Arsenyeva, O. The Study of Flat Plate Solar Collector with Absorbing Elements from a Polymer Material. Energy 2022, 256, 124677. [Google Scholar] [CrossRef]
- Liu, B.; Wang, C.; Bazri, S.; Badruddin, I.A.; Orooji, Y.; Saeidi, S.; Wongwises, S.; Mahian, O. Optical Properties and Thermal Stability Evaluation of Solar Absorbers Enhanced by Nanostructured Selective Coating Films. Powder Technol. 2021, 377, 939–957. [Google Scholar] [CrossRef]
- Ma, P.; Geng, Q.; Liu, G. Photothermal Conversion Applications of the Transition Metal (Cu, Mn, Co, Cr, and Fe) Oxides with Spinel Structure. In Magnetic Spinels—Synthesis, Properties and Applications, 1st ed.; Seehra, M.S., Ed.; Intech Publisher: London, UK, 2017; Volume 12, pp. 273–284. [Google Scholar] [CrossRef]
- Zaki, A.; Carrasco, J.; Bielsa, D.; Faik, A. Tunable Redox Temperature of a Co3−xMnxO4 (0 ≤ x ≤ 3) Continuous Solid Solution for Thermochemical Energy Storage. ACS Appl. Mater. Interfaces 2020, 12, 7010–7020. [Google Scholar] [CrossRef] [PubMed]
- Ho, C.T.; Weng, T.H.; Wang, C.Y.; Yen, S.J.; Yew, T.R. Tunable Band Gaps of Co3−xCuxO4 Nanorods with Various Cu Doping Concentrations. RSC Adv. 2014, 4, 20053–20057. [Google Scholar] [CrossRef]
- Krishnan, S.G.; Arulraj, A.; Khalid, M.; Reddy, M.V.; Jose, R. Energy Storage in Metal Cobaltite Electrodes: Opportunities & Challenges in Magnesium Cobalt Oxide. Renew. Sustain. Energy Rev. 2021, 141, 110798. [Google Scholar] [CrossRef]
- Moore, R.J.; White, J. Equilibrium relationships in the system NiO-CoO-O2. J. Mater. Sci. 1974, 9, 1393–1400. [Google Scholar] [CrossRef]
- Han, L.; Meng, Q.; Wang, D.; Zhu, Y.; Wang, J.; Du, X.; Stach, E.A.; Xin, H.L. Interrogation of bimetallic particle oxidation in three dimensions at the nanoscale. Nat. Commun. 2016, 7, 13335. [Google Scholar] [CrossRef]
- Nikolov, I.; Darkaoui, R.; Zhecheva, E.; Stoyanova, R.; Dimitrov, N.; Vitanov, T. Electrocatalytic activity of spinel related cobalties MxCo3−xO4 (M = Li, Ni, Cu) in the oxygen evolution reaction. J. Electroanal. Chem. 1997, 429, 157–168. [Google Scholar] [CrossRef]
- Guan, X.; Luo, P.; Yu, Y.; Li, X.; Chen, D. Solvent-Tuned Synthesis of Mesoporous Nickel Cobaltite Nanostructures and Their Catalytic Properties. Appl. Sci. 2019, 9, 1100. [Google Scholar] [CrossRef]
- Zhu, C.; Fu, S.; Du, D.; Lin, Y. Facilely Tuning Porous NiCo2O4 Nanosheets with Metal Valence-State Alteration and Abundant Oxygen Vacancies as Robust Electrocatalysts Towards Water Splitting. Chem. Eur. J. 2016, 22, 4000–4007. [Google Scholar] [CrossRef]
- Eskandari, M.; Malekfar, R.; Buceta, D.; Taboada, P. NiCo2O4-Based Nanostructured Composites for High-Performance Pseudocapacitor Electrodes. Colloids Surf. A Physicochem. Eng. Asp. 2020, 584, 124039. [Google Scholar] [CrossRef]
- Li, Y.; Han, X.; Yi, T.F.; He, Y.; Li, X. Review and Prospect of NiCo2O4-Based Composite Materials for Supercapacitor Electrodes. J. Energy Chem. 2019, 31, 54–78. [Google Scholar] [CrossRef]
- Bacelis-Martínez, R.D.; Oskam, G.; Rodriguez Gattorno, G.; Ruiz-Gómez, M.A. Inkjet Printing as High-Throughput Technique for the Fabrication of NiCo2O4 Film. Adv. Mater. Sci. Eng. 2017, 2017, 9647458. [Google Scholar] [CrossRef]
- Portilla-Nieto, Y.; Zaki, A.; Vidal, K.; Hernaiz, M.; Aranzabe, E.; Doppiu, S.; Faik, A. Development of Co3−xNixO4 Materials for Thermochemical Energy Storage at Lower Red-Ox Temperature. Sol. Energy Mater. Sol. Cells 2021, 230, 111194. [Google Scholar] [CrossRef]
- Shi, H.; Zhao, G. Water Oxidation on Spinel NiCo2O4 Nanoneedles Anode: Microstructures, Specific Surface Character, and the Enhanced Electrocatalytic Performance. J. Phys. Chem. C 2014, 118, 25939–25946. [Google Scholar] [CrossRef]
- Macias, J.D.; Bacelis-Martinez, R.D.; Ruiz-Gomez, M.A.; Bante-Guerra, J.; Villafan-Vidales, H.I.; Rodriguez-Gattorno, G.; Romero-Paredes, H.; Alvarado-Gil, J.J. Thermophysical and Optical Properties of NiCo2O4@ZrO2: A Potential Composite for Thermochemical Processes. Int. J. Hydrogen Energy 2021, 46, 10632–10641. [Google Scholar] [CrossRef]
- Atchuta, S.R.; Sakthivel, S.; Barshilia, H.C. Nickel doped cobaltite spinel as a solar selective absorber coating for efficient photothermal conversion with a low thermal radiative loss at high operating temperatures. Sol. Energy Mat. Sol. C 2019, 200, 109917. [Google Scholar] [CrossRef]
- Abidat, I.; Morais, C.; Comminges, C.; Canaff, C.; Rousseau, J.; Guignard, N.; Napporn, T.W.; Habrioux, A.; Kokoh, K.B. Three Dimensionally Ordered Mesoporous Hydroxylated NixCo3−xO4 Spinels for the Oxygen Evolution Reaction: On the Hydroxyl-Induced Surface Restructuring Effect. J. Mater. Chem. A 2017, 5, 7173–7183. [Google Scholar] [CrossRef]
- Yang, Z.; Tan, S.; Yang, J.; Li, Y.; Yang, X.; Deng, J. Study on the Solar Selectivity and Air Thermal Stability of Cobalt–Nickel–Iron Oxide Coating Fabricated by Spraying Method. Opt. Mater. 2021, 111, 110573. [Google Scholar] [CrossRef]
- Rubin, E.B.; Chen, Y.; Chen, R. Optical Properties and Thermal Stability of Cu Spinel Oxide Nanoparticle Solar Absorber Coatings. Sol. Energy Mater. Sol. Cells 2019, 195, 81–88. [Google Scholar] [CrossRef]
- Zäll, E.; Nordenström, A.; Järn, M.; Mossegård, J.; Wågberg, T. Environmentally Sustainable Electroplating of Selective Cobalt-Chromium Coating on Stainless Steel for Efficient Solar Collectors. Sol. Energy Mater. Sol. Cells 2022, 245, 111821. [Google Scholar] [CrossRef]
- Herrera-Zamora, D.M.; Lizama-Tzec, F.I.; Santos-González, I.; Rodríguez-Carvajal, R.A.; García-Valladares, O.; Arés-Muzio, O.; Oskam, G. Electrodeposited Black Cobalt Selective Coatings for Application in Solar Thermal Collectors: Fabrication, Characterization, and Stability. Sol. Energy 2020, 207, 1132–1145. [Google Scholar] [CrossRef]
- Geng, Q.; Zhao, X.; Gao, X.; Yu, H.; Yang, S.; Liu, G. Optimization Design of CuCrxMn2−xO4-Based Paint Coatings Used for Solar Selective Applications. Sol. Energy Mater. Sol. Cells 2012, 105, 293–301. [Google Scholar] [CrossRef]
- Kumar, K.K.P.; Mallick, S.; Sakthivel, S. Cobalt-Rich Spinel Oxide-Based Wide Angular Spectral Selective Absorber Coatings for Solar Thermal Conversion Applications. Renew. Energy 2023, 203, 334–344. [Google Scholar] [CrossRef]
- Lizama-Tzec, F.I.; Herrera-Zamora, D.M.; Arés-Muzio, O.; Gómez-Espinoza, V.H.; Santos-González, I.; Cetina-Dorantes, M.; Vega-Poot, A.G.; García-Valladares, O.; Oskam, G. Electrodeposition of Selective Coatings Based on Black Nickel for Flat-Plate Solar Water Heaters. Sol. Energy 2019, 194, 302–310. [Google Scholar] [CrossRef]
- Nunes, R.A.X.; Costa, V.C.; Sade, W.; Araújo, F.R.; Silva, G.M. Selective Surfaces of Black Chromium for Use in Solar Absorbers. Mater. Res. 2018, 21, e20170556. [Google Scholar] [CrossRef]
- Kafle, B.P.; Basnet, B.; Timalsina, B.; Deo, A.; Malla, T.N.; Acharya, N.; Adhikari, A. Optical, Structural and Thermal Performances of Black Nickel Selective Coatings for Solar Thermal Collectors. Sol. Energy 2022, 234, 262–274. [Google Scholar] [CrossRef]
- Khan, S.; Wu, Z.; ul haq, M.; Yuan, G.; Khan, M.; Song, C.; Han, G.; Liu, Y. Study of Annealing Effect Upon the Structural and Solar-Selective Properties of C/Ni/NiO Composite Coatings Prepared by Sol–Gel Method. J. Sol-Gel Sci. Technol. 2018, 89, 120–127. [Google Scholar] [CrossRef]
- Kuboon, S.; Hu, Y.H. Study of NiO−CoO and Co3O4−Ni3O4 Solid Solutions in Multiphase Ni−Co−O Systems. Ind. Eng. Chem. Res. 2011, 50, 2015–2020. [Google Scholar] [CrossRef]
- Shannon, R.D.; Prewitt, C.T. Effective Ionic Radii in Oxides and Fluorides. Acta Cryst. 1969, B25, 925–946. [Google Scholar] [CrossRef]
- Le Bail, A.; Duroy, H.; Fourquet, J.L. Ab-initio structure determination of LiSbWO6 by X-ray powder diffraction. Mat. Res. Bull. 1988, 23, 447–452. [Google Scholar] [CrossRef]
- Rodríguez-Carvajal, J. FullProf Suite; Institute Leon Brillouin: Saclay, France, 2009. [Google Scholar]
- Rashad, M.; Rüsing, M.; Berth, G.; Lischka, K.; Pawlis, A. CuO and Co3O4 Nanoparticles: Synthesis, Characterizations, and Raman Spectroscopy. J. Nanomater. 2013, 2013, 82. [Google Scholar] [CrossRef]
- Hadjiev, V.G.; Iliev, M.N.; Vergilov, I.V. The Raman Spectra of Co3O4. J. Phys. C Solid State Phys. 1988, 21, L199–L201. [Google Scholar] [CrossRef]
- Sasmal, A.; Nayak, A.K. Morphology-Dependent Solvothermal Synthesis of Spinel NiCo2O4 Nanostructures for Enhanced Energy Storage Device Application. J. Energy Storage 2023, 58, 106342. [Google Scholar] [CrossRef]
- Umeshbabu, E.; Rajeshkhanna, G.; Justin, P.; Rao, G.R. Magnetic, Optical and Electrocatalytic Properties of Urchin and Sheaf-Like NiCo2O4 Nanostructures. Mater. Chem. Phys. 2015, 165, 235–244. [Google Scholar] [CrossRef]
- Gupta, J.; Ahmed, A.S. Interfacial Exchange Coupling and Defects Driven Magnetic and Optical Properties of Co3O4-NiO Nanocomposites. Phys. B 2020, 599, 412383. [Google Scholar] [CrossRef]
- Mironova-Ulmane, N.; Kuzmin, A.; Steins, I.; Grabis, J.; Sildos, I.; Pärs, M. Raman Scattering in Nanosized Nickel Oxide NiO. J. Phys: Conf. Ser. 2007, 93, 012039. [Google Scholar] [CrossRef]
- Dupin, J.C.; Gonbeau, D.; Vinatier, P.; Levasseur, A. Systematic XPS Studies of Metal Oxides, Hydroxides and Peroxides. Phys. Chem. Chem. Phys. 2000, 2, 1319–1324. [Google Scholar] [CrossRef]
- Carrillo, A.J.; Moya, J.; Bayón, A.; Jana, P.; de la Peña O’Shea, V.A.; Romero, M.; González-Aguilar, J.; Serrano, D.P.; Pizarro, P.; Coronado, J.M. Thermochemical Energy Storage at High Temperature Via Redox Cycles of Mn and Co Oxides: Pure Oxides Versus Mixed Ones. Sol. Energy Mater. Sol. Cells 2014, 123, 47–57. [Google Scholar] [CrossRef]
- Tescari, S.; Agrafiotis, C.; Breuer, S.; de Oliveira, L.; Puttkamer, M.N.; Roeb, M.; Sattler, C. Thermochemical Solar Energy Storage Via Redox Oxides: Materials and Reactor/Heat Exchanger Concepts. Energy Procedia 2014, 49, 1034–1043. [Google Scholar] [CrossRef]
- Agrafiotis, C.; Roeb, M.; Schmücker, M.; Sattler, C. Exploitation of Thermochemical Cycles Based on Solid Oxide Redox Systems for Thermochemical Storage of Solar Heat. Part 1: Testing of Cobalt Oxide-Based Powders. Sol. Energy 2014, 102, 189–211. [Google Scholar] [CrossRef]
- Shi, X.; Bernasek, S.L.; Selloni, A. Formation, Electronic Structure, and Defects of Ni Substituted Spinel Cobalt Oxide: A DFT+U Study. J. Phys. Chem. C 2016, 120, 14892–14898. [Google Scholar] [CrossRef]
- Ogawa, K.; Harada, J.; Fujiwara, T.; Takahashi, H. UV–vis Absorption Spectra of Powdered Materials: Direct Measurements by Optical Waveguide Spectroscopy. Chem. Lett. 2004, 33, 1446–1447. [Google Scholar] [CrossRef]
- Mahavar, S.; Saquib Khan, M.; Yadav, T. Synthesis, Characterization and Testing of Black Metal Oxide Nanoparticles as Solar Concentrator Receiver Material. Mater. Today-Proc. 2019, 8, 22–27. [Google Scholar] [CrossRef]
- Samos-Puerto, A.; Rodríguez-Gattorno, G.; Ruiz-Gómez, M.A. Fine tuning of inkjet printability parameters for NiO nanofilms fabrication. Colloids Surf. A 2019, 583, 123959. [Google Scholar] [CrossRef]
- Hong, S.-J.; Mun, H.-J.; Kim, B.-J.; Kim, Y.-S. Characterization of Nickel Oxide Nanoparticles Synthesized under Low Temperature. Micromachines 2021, 12, 1168. [Google Scholar] [CrossRef]

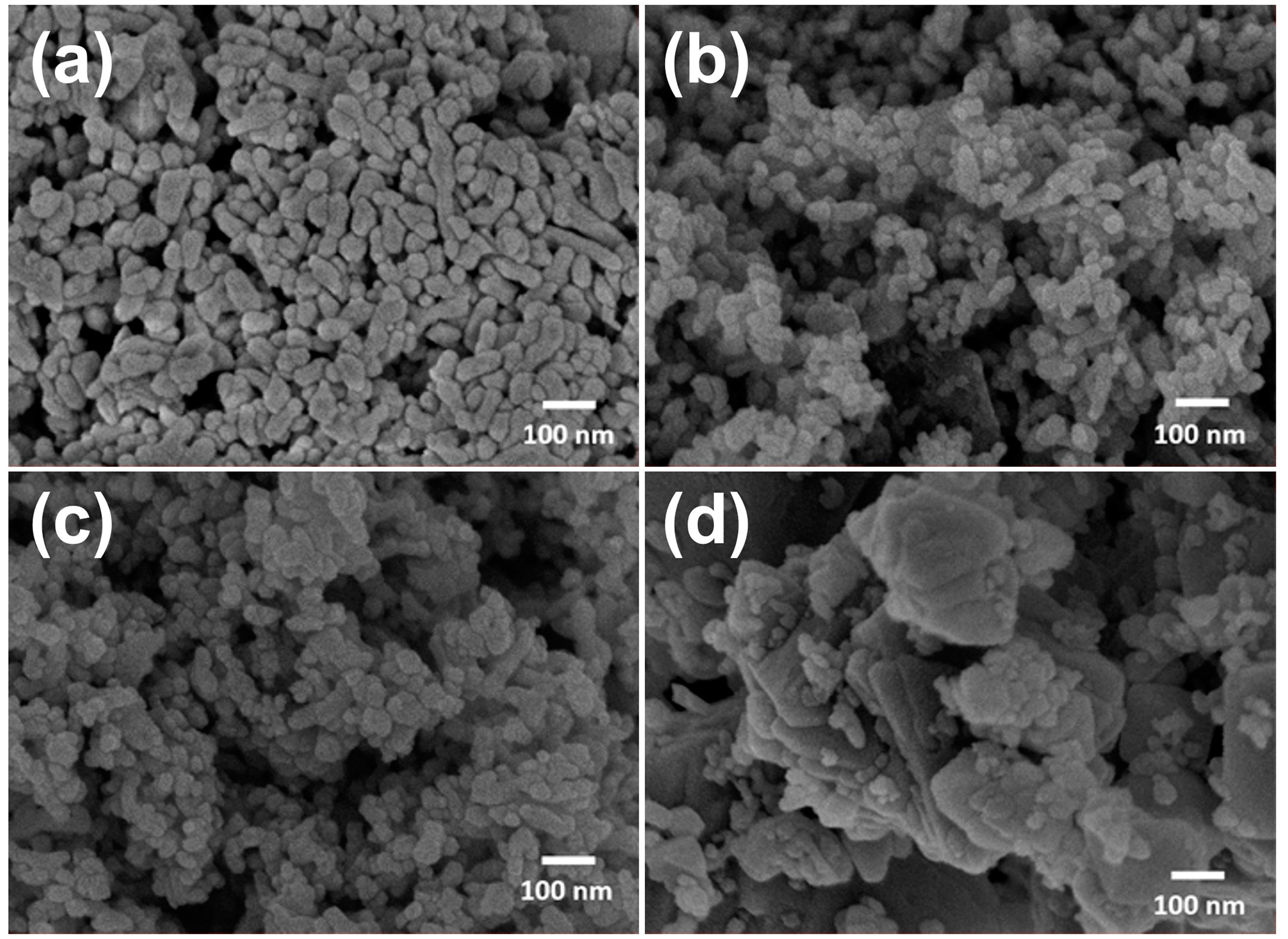

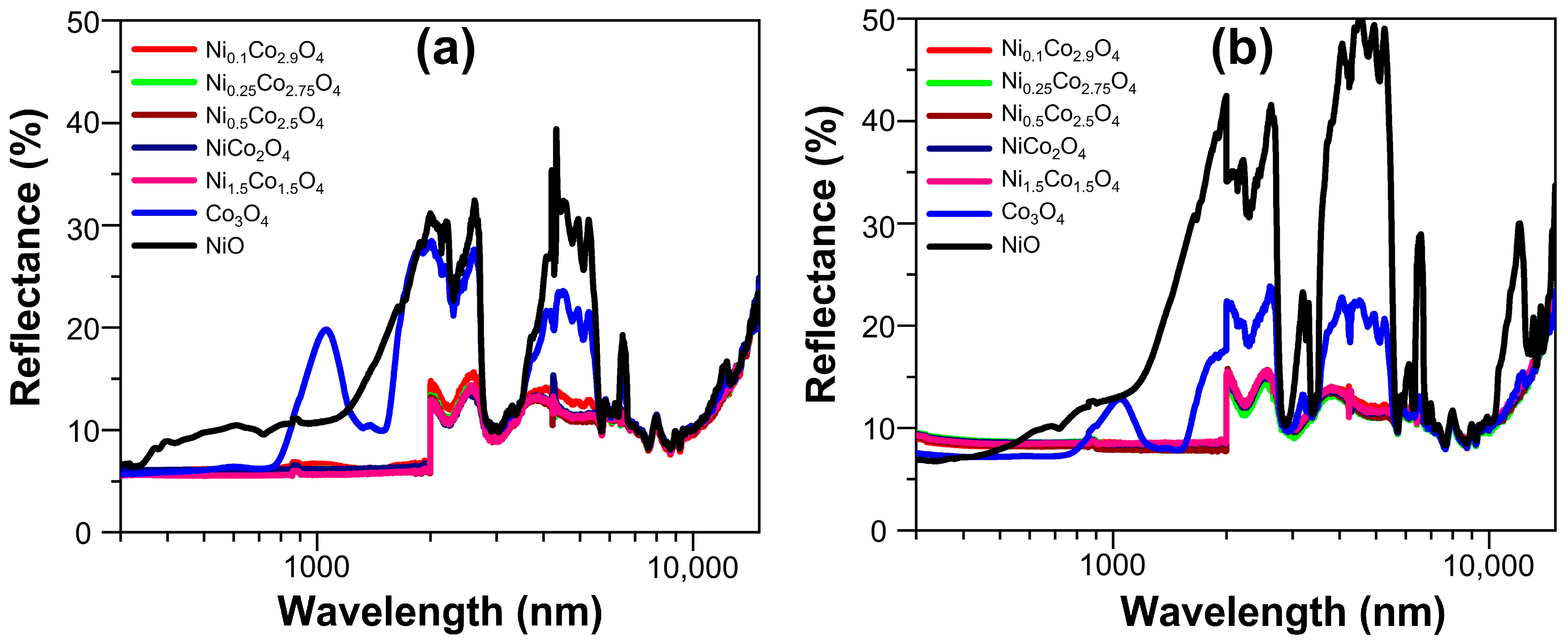



| At.% | Co3O4 | Ni0.1Co2.9O4 | Ni0.25Co2.75O4 | Ni0.5Co2.5O4 | NiCo2O4 | Ni1.5Co1.5O4 | NiO |
|---|---|---|---|---|---|---|---|
| Co | 41.16 | 43.38 | 36.84 | 35.12 | 28.53 | 24.77 | 0 |
| Ni | 0 | Not detected | 3.61 | 7.27 | 15.82 | 27.48 | 44.58 |
| O | 58.84 | 56.62 | 59.55 | 57.61 | 55.65 | 47.75 | 55.42 |
| Sample | α (%) |
|---|---|
| Co3O4 | 84 ± 1 |
| Ni0.1Co2.9O4 | 85 ± 1 |
| Ni0.25Co2.75O4 | 86 ± 1 |
| Ni0.5Co2.5O4 | 87 ± 1 |
| NiCo2O4 | 86 ± 1 |
| Ni1.5Co1.5O4 | 87 ± 1 |
| NiO | 78 ± 1 |
| Sample | Brush-Painted without Thermal Treatment | Brush-Painted and Thermally Treated at 200 °C for 24 h | ||
|---|---|---|---|---|
| α (%) | ε (%) | α (%) | ε (%) | |
| Co3O4 | 93 | 87 | 92 | 87 |
| Ni0.1Co2.9O4 | 94 | 88 | 92 | 88 |
| Ni0.25Co2.75O4 | 94 | 88 | 91 | 88 |
| Ni0.5Co2.5O4 | 94 | 88 | 92 | 88 |
| NiCo2O4 | 94 | 88 | 91 | 88 |
| Ni1.5Co1.5O4 | 94 | 88 | 91 | 88 |
| NiO | 90 | 86 | 90 | 82 |
| Sample | Spray-Painted without Thermal Treatment | Spray-Painted and Thermally Treated at 200 °C for 24 h | ||
|---|---|---|---|---|
| α (%) | ε (%) | α (%) | ε (%) | |
| NiCo2O4 | 96 | 69 | 95 | 67 |
| HVA commercial | 91 | 84 | 89 | 84 |
| Solar Collectors | Useful Heat per 8 h Day (MJ) | Useful Heat per 24 h Day (MJ) | Useful Heat per Year in 8 h (MJ) | Useful Heat per Year in 24 h (MJ) |
|---|---|---|---|---|
| NiCo2O4 | 12.6 | 9.0 | 4592 | 3280 |
| HVA commercial | 13.4 | 9.7 | 4890 | 3552 |
| Minimum limit | 12.5 | 8.7 | 4550 | 3170 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bacelis-Martínez, R.D.; Herrera-Zamora, D.M.; Ávila Santos, M.; García-Valladares, O.; Franco-Bacca, A.P.; Rodríguez-Gattorno, G.; Ruiz-Gómez, M.Á. Enhanced Performance of Nickel–Cobalt Oxides as Selective Coatings for Flat-Plate Solar Thermal Collector Applications. Coatings 2023, 13, 1329. https://doi.org/10.3390/coatings13081329
Bacelis-Martínez RD, Herrera-Zamora DM, Ávila Santos M, García-Valladares O, Franco-Bacca AP, Rodríguez-Gattorno G, Ruiz-Gómez MÁ. Enhanced Performance of Nickel–Cobalt Oxides as Selective Coatings for Flat-Plate Solar Thermal Collector Applications. Coatings. 2023; 13(8):1329. https://doi.org/10.3390/coatings13081329
Chicago/Turabian StyleBacelis-Martínez, Reyna Dianela, Dallely Melissa Herrera-Zamora, Manuel Ávila Santos, Octavio García-Valladares, Adriana Paola Franco-Bacca, Geonel Rodríguez-Gattorno, and Miguel Ángel Ruiz-Gómez. 2023. "Enhanced Performance of Nickel–Cobalt Oxides as Selective Coatings for Flat-Plate Solar Thermal Collector Applications" Coatings 13, no. 8: 1329. https://doi.org/10.3390/coatings13081329
APA StyleBacelis-Martínez, R. D., Herrera-Zamora, D. M., Ávila Santos, M., García-Valladares, O., Franco-Bacca, A. P., Rodríguez-Gattorno, G., & Ruiz-Gómez, M. Á. (2023). Enhanced Performance of Nickel–Cobalt Oxides as Selective Coatings for Flat-Plate Solar Thermal Collector Applications. Coatings, 13(8), 1329. https://doi.org/10.3390/coatings13081329










