On the Electrochemically Active Surface Area Determination of Electrodeposited Porous Cu 3D Nanostructures
Abstract
:1. Introduction
2. Materials and Methods
2.1. Sample Preparation
2.2. Electrochemical Measurements
2.3. Morphological Characterisation
3. Results
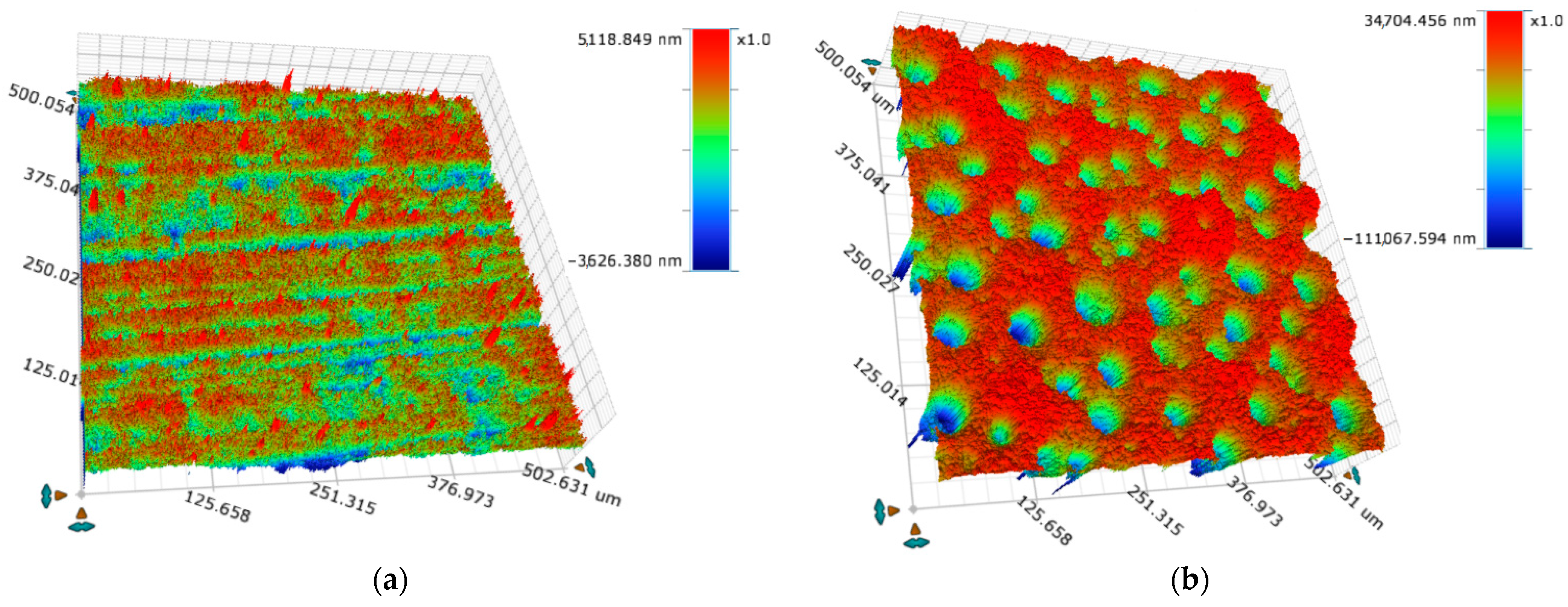
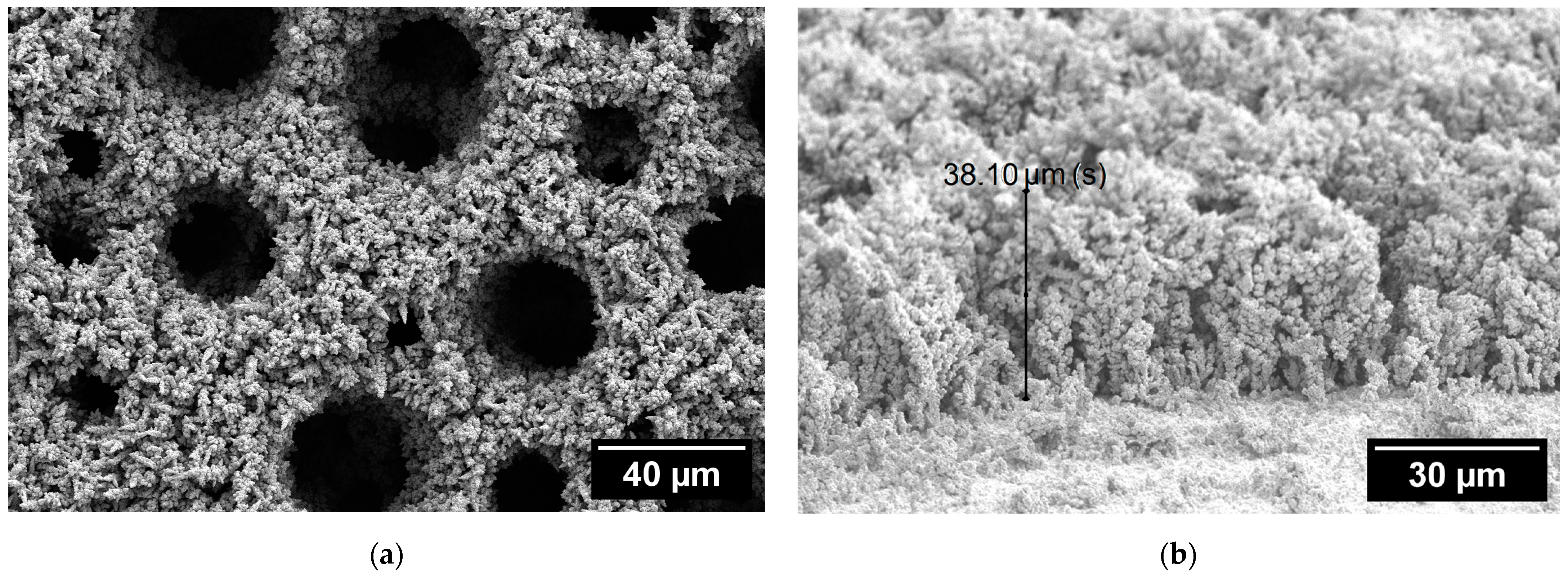
3.1. SEM and Optical Profilometry
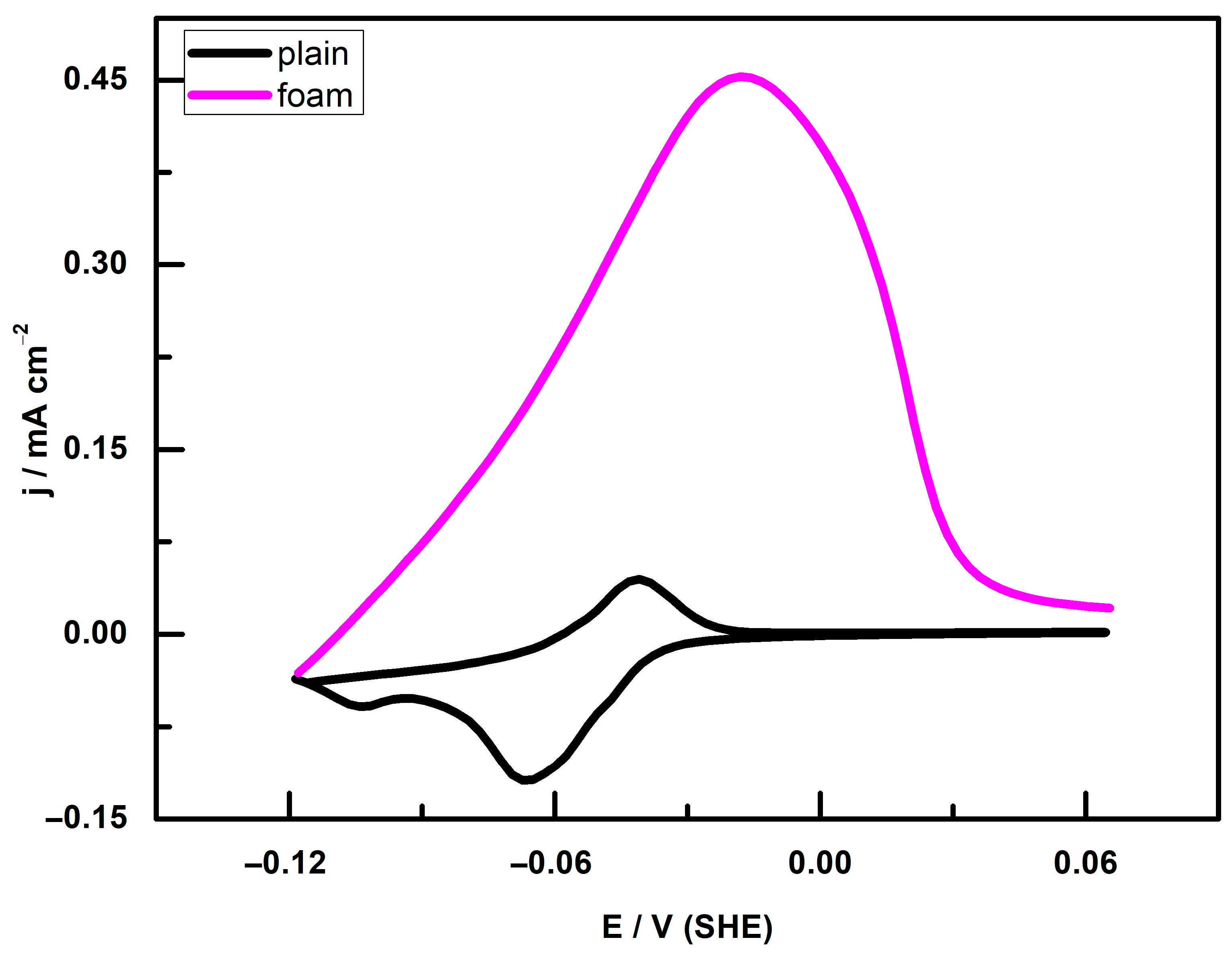
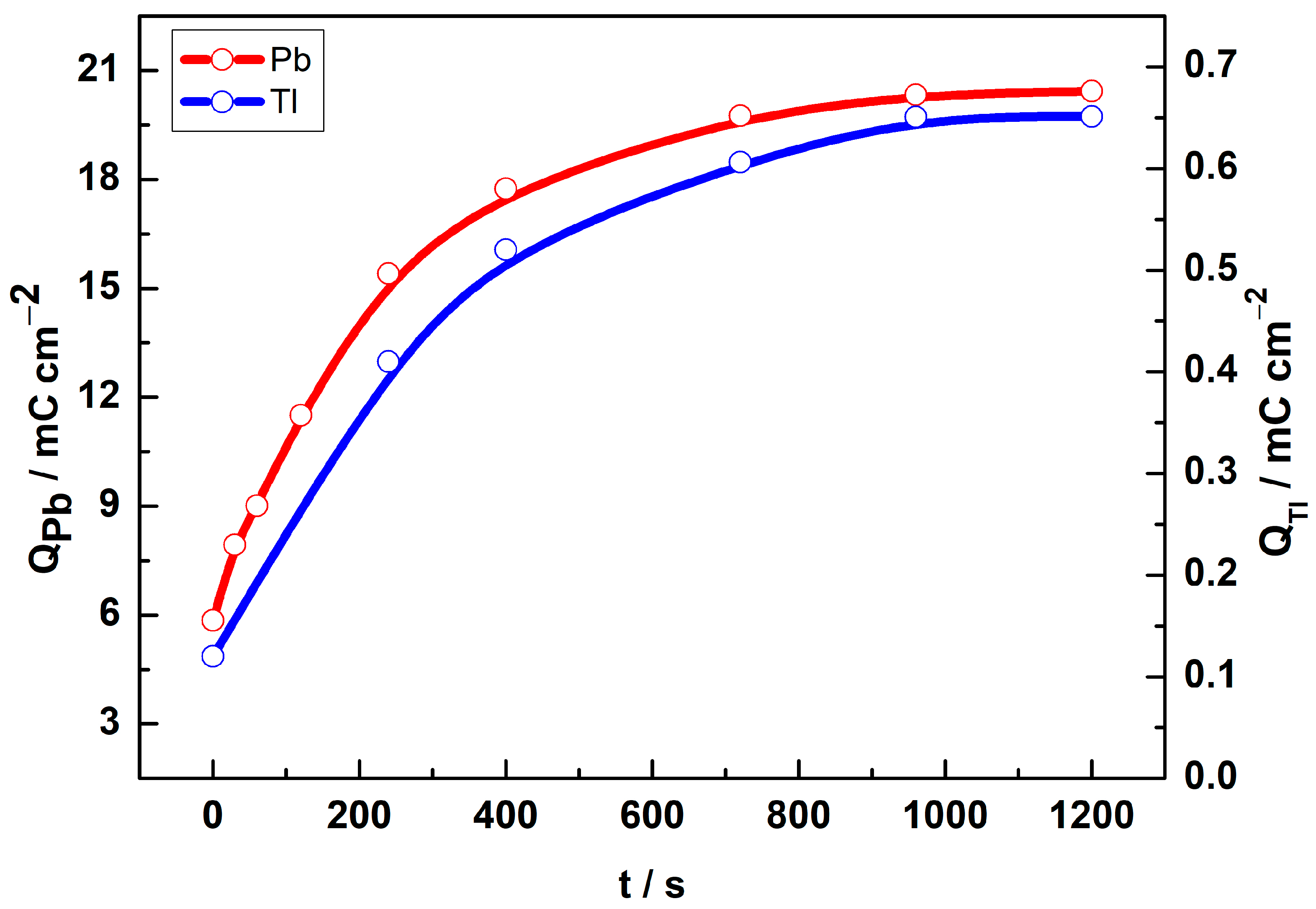
3.2. UPD Measurements
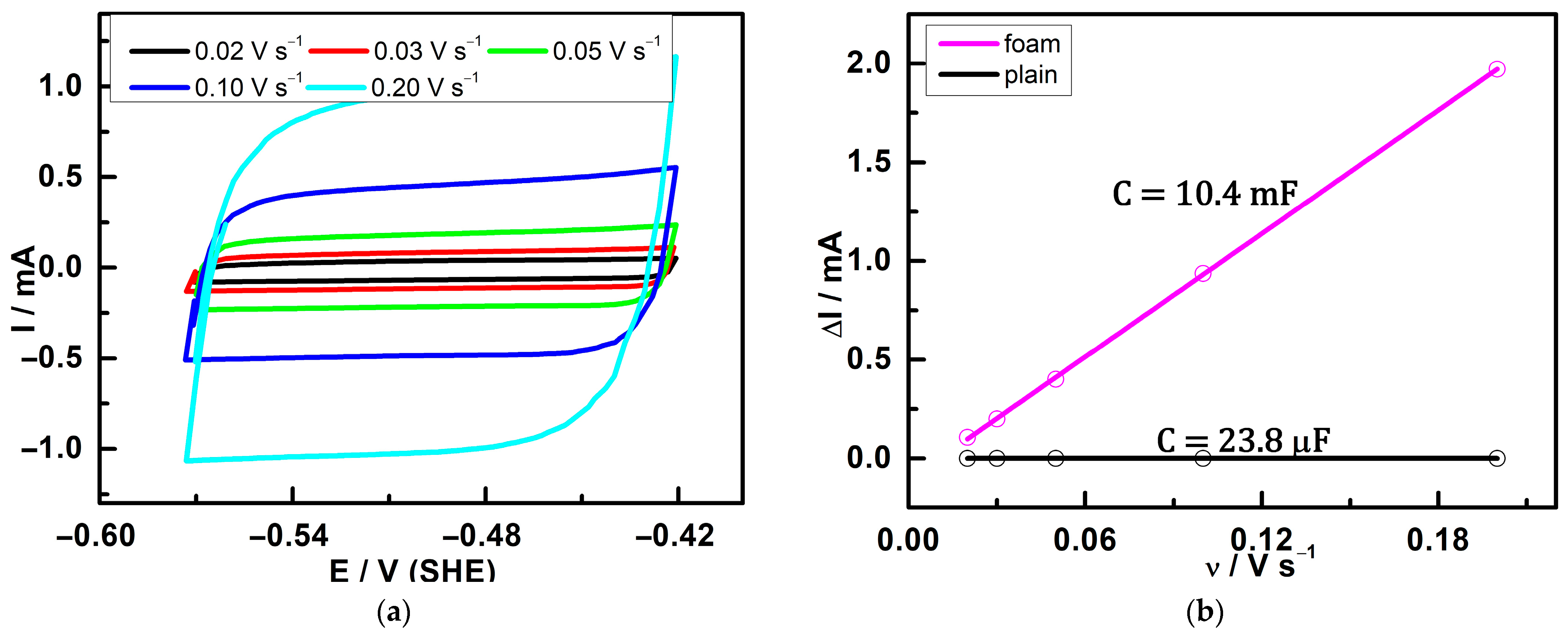
3.3. Double-Layer Capacitance
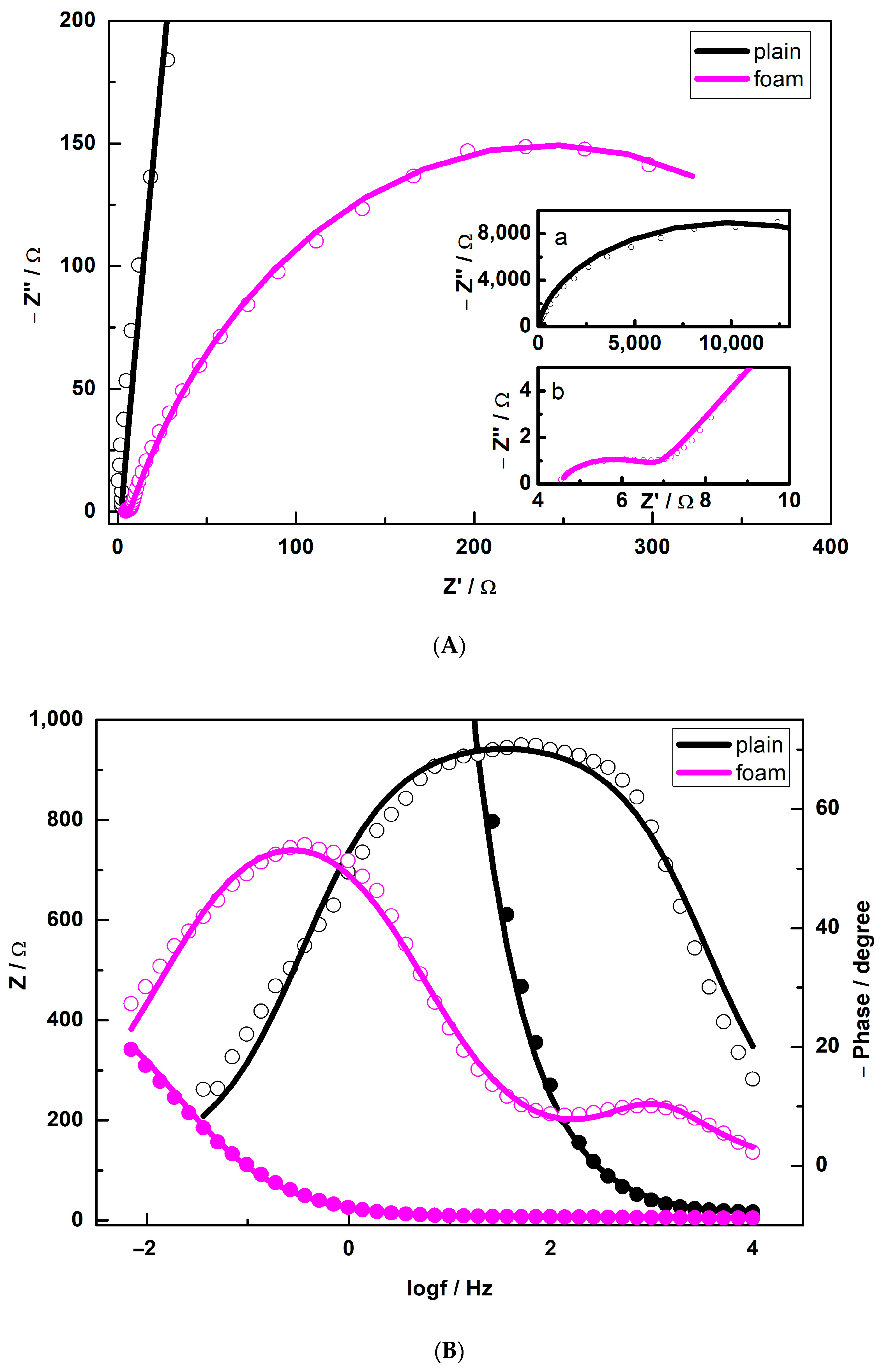
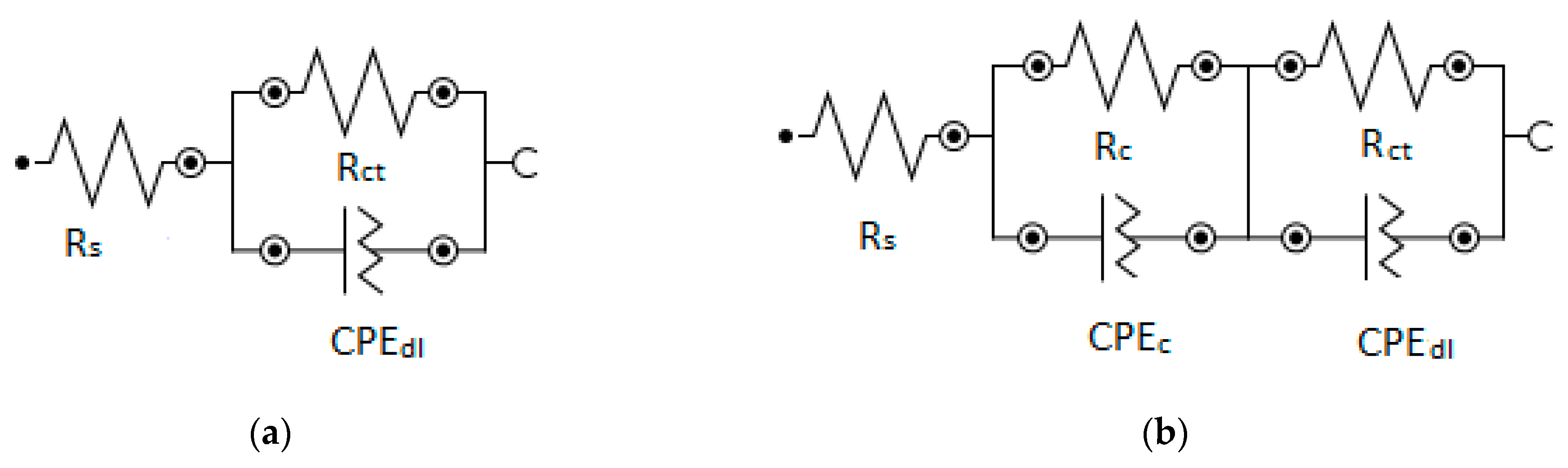
3.4. EIS Study
3.5. Suitability of ESr Determination Methods for Cu 3D Structures
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Xie, H.; Wang, T.; Liang, J.; Li, Q.; Sun, S. Cu-Based Nanocatalysts for Electrochemical Reduction of CO2. Nano Today 2018, 21, 41–54. [Google Scholar] [CrossRef]
- Xu, M.; Chen, Y.; Liang, J.; Mo, D.; Lyu, S. Electrodeposition Patterned Copper Foam with Micro/Nanostructures for Reducing Supercooling in Water-Based Cool Storage Phase-Change Materials. Appl. Sci. 2020, 10, 4202. [Google Scholar] [CrossRef]
- Zhu, P.; Zhao, Y. Effects of Electrochemical Reaction and Surface Morphology on Electroactive Surface Area of Porous Copper Manufactured by Lost Carbonate Sintering. RSC Adv. 2017, 7, 26392–26400. [Google Scholar] [CrossRef] [Green Version]
- Diao, K.K.; Xiao, Z.; Zhao, Y.Y. Specific Surface Areas of Porous Cu Manufactured by Lost Carbonate Sintering: Measurements by Quantitative Stereology and Cyclic Voltammetry. Mater. Chem. Phys. 2015, 162, 571–579. [Google Scholar] [CrossRef] [Green Version]
- Giri, S.D.; Sarkar, A. Estimating Surface Area of Copper Powder: A Comparison between Electrochemical, Microscopy and Laser Diffraction Methods. Adv. Powder Technol. 2018, 29, 3520–3526. [Google Scholar] [CrossRef]
- Nitopi, S.; Bertheussen, E.; Scott, S.B.; Liu, X.; Engstfeld, A.K.; Horch, S.; Seger, B.; Stephens, I.E.L.; Chan, K.; Hahn, C.; et al. Progress and Perspectives of Electrochemical CO2 Reduction on Copper in Aqueous Electrolyte. Chem. Rev. 2019, 119, 7610–7672. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Trasatti, S.; Petrii, O.A. Real Surface Area Measurements in Electrochemistry. Pure Appl. Chem. 1991, 63, 711–734. [Google Scholar] [CrossRef]
- Reyter, D.; Chamoulaud, G.; Bélanger, D.; Roué, L. Electrocatalytic Reduction of Nitrate on Copper Electrodes Prepared by High-Energy Ball Milling. J. Electroanal. Chem. 2006, 596, 13–24. [Google Scholar] [CrossRef]
- Oviedo, O.A.; Reinaudi, L.; Garcia, S.; Leiva, E.P.M. Underpotential Deposition from Fundamentals and Theory to Applications at the Nanoscale; Springer: Berlin/Heidelberg, Germany, 2016; Volume 2, ISBN 978-3-319-24392-4. [Google Scholar]
- Kowalik, R. Analysis of the Underpotential Deposition of Cadmium on Copper. Arch. Metall. Mater. 2015, 60, 1629–1632. [Google Scholar] [CrossRef] [Green Version]
- Nasirpouri, F. On the Electrodeposition Mechanism of Pb on Copper Substrate from a Perchlorate Solution Studied by Electrochemical Quartz Crystal Microbalance. Ionics 2011, 17, 331–337. [Google Scholar] [CrossRef]
- Plieth, W. Ad-Atoms and Underpotential Deposition. In Electrochemistry for Materials Science; Elsevier: Amsterdam, The Netherlands, 2008; pp. 101–142. [Google Scholar] [CrossRef]
- Sudha, V.; Sangaranarayanan, M.V. Underpotential Deposition of Metals: Structural and Thermodynamic Considerations. J. Phys. Chem. B 2002, 106, 2699–2707. [Google Scholar] [CrossRef]
- Juodkazyte, J.; Šebeka, B.; Savickaja, I.; Selskis, A.; Jasulaitiene, V.; Kalinauskas, P. Evaluation of Electrochemically Active Surface Area of Photosensitive Copper Oxide Nanostructures with Extremely High Surface Roughness. Electrochim. Acta 2013, 98, 109–115. [Google Scholar] [CrossRef]
- Norkus, E.; Vaškelis, A.; Jačiauskiene, J.; Stalnioniene, I.; Stalnionis, G. Obtaining of High Surface Roughness Copper Deposits by Electroless Plating Technique. Electrochim. Acta 2006, 51, 3495–3499. [Google Scholar] [CrossRef]
- Ramanauskas, R.; Stulgys, D. Comparison of Methods of Determining Cu Electrodes Real Surface. Chemija 1990, 1, 64. [Google Scholar]
- Sebastián-Pascual, P.; Escudero-Escribano, M. Surface Characterization of Copper Electrocatalysts by Lead Underpotential Deposition. J. Electroanal. Chem. 2021, 896, 115446. [Google Scholar] [CrossRef]
- Brisard, G.M.; Zenati, E.; Gasteiger, H.A.; Marković, N.M.; Ross, P.N. Ex Situ Low-Energy Electron Diffraction and Auger Electron Spectroscopy and Electrochemical Studies of the Underpotential Deposition of Lead on Cu(100) and Cu(111). ACS Symp. Ser. 1997, 656, 142–155. [Google Scholar] [CrossRef]
- Rudd, J.A.; Hernandez-Aldave, S.; Kazimierska, E.; Hamdy, L.B.; Bain, O.J.E.; Barron, A.R.; Andreoli, E. Investigation into the Re-Arrangement of Copper Foams Pre-and Post-CO2 Electrocatalysis. Chemistry 2021, 3, 687–703. [Google Scholar] [CrossRef]
- Zeng, J.; Bejtka, K.; Ju, W.; Castellino, M.; Chiodoni, A.; Sacco, A.; Farkhondehfal, M.A.; Hernández, S.; Rentsch, D.; Battaglia, C.; et al. Advanced Cu-Sn Foam for Selectively Converting CO2 to CO in Aqueous Solution. Appl. Catal. B Environ. 2018, 236, 475–482. [Google Scholar] [CrossRef]
- Giri, S.D.; Mahajani, S.M.; Suresh, A.K.; Sarkar, A. Electrochemical Reduction of CO2 on Activated Copper: Influence of Surface Area. Mater. Res. Bull. 2020, 123, 110702. [Google Scholar] [CrossRef]
- Boukamp, B.A. A Linear Kronig-Kramers Transform Test for Immittance Data Validation. J. Electrochem. Soc. 1995, 142, 1885–1894. [Google Scholar] [CrossRef]
- Shin, H.C.; Liu, M. Copper Foam Structures with Highly Porous Nanostructured Walls. Chem. Mater. 2004, 16, 5460–5464. [Google Scholar] [CrossRef]
- Sen, S.; Liu, D.; Palmore, G.T.R. Electrochemical Reduction of CO2 at Copper Nanofoams. ACS Catal. 2014, 4, 3091–3095. [Google Scholar] [CrossRef]
- Lin, C.H.; Won, Y. Pressure-Dependent Thermal Characterization of Biporous Copper Structures. IEEE Trans. Compon. Packag. Manuf. Technol. 2020, 10, 568–576. [Google Scholar] [CrossRef]
- Xu, H.; Feng, J.X.; Tong, Y.X.; Li, G.R. Cu2O-Cu Hybrid Foams as High-Performance Electrocatalysts for Oxygen Evolution Reaction in Alkaline Media. ACS Catal. 2017, 7, 986–991. [Google Scholar] [CrossRef]
- Jüttner, K. Electrochemical Impedance Spectroscopy (EIS) of Corrosion Processes on Inhomogeneous Surfaces. Electrochim. Acta 1990, 35, 1501–1508. [Google Scholar] [CrossRef]
- Goncalves, M.R.; Gomes, A.; Condecao, J.; Fernandes, T.R.C.; Pardal, T.; Sequeira, C.A.C.; Branco, J.B. Electrochemical conversion of CO2 to C2 hydrocarbons using different ex situ copper electrodeposits. Electrochim. Acta 2013, 102, 388–392. [Google Scholar] [CrossRef]

| Cu Layer | Electrolyte | Current Density | Deposition Time |
|---|---|---|---|
| Plain | 0.5 M Cu2SO4 0.5 M H2SO4 1 M C2H5OH | 0.02 A∙cm−2 | 18 min |
| Foam | 0.2 M Cu2SO4 1.5 M H2SO4 | 3.0 A∙cm−2 | 20 s |
| Method | Surface | Surface Roughness Factor |
|---|---|---|
| Pb UPD | Plain | 2.5 ± 0.3 |
| Foam | 80 ± 2 | |
| Tl UPD | Plain | 1.8 ± 0.1 |
| Foam | 116 ± 2 | |
| Double-layer capacitanceCyclic voltammetry | Plain | 1.8 ± 0.1 |
| Foam | 814 ± 29 | |
| Double-layer capacitanceImpedance | Plain | 2.1 ± 0.2 |
| Foam | 986 ± 36 | |
| Optical profiler | Plain | 2.2 |
| Sample | Rs, Ω | Rc, Ω | Y0(CPEc)/ 10−6 Ω−1 sn | n (CPEc) | Rct, Ω | Y0(CPEdl)/ 10−3 Ω−1 sn | n (CPEdl) |
|---|---|---|---|---|---|---|---|
| Plain | 13 | - | - | - | 23,000 | 22.4 × 10−3 | 0.8 |
| Foam | 5 | 2.1 | 214 | 0.89 | 471 | 12 | 0.79 |
| Deposition Duration, s | Average Pore Size, µm | Average Pore Density, cm−2 | fR, by Double-Layer Capacitance | fR, by Tl UPD |
|---|---|---|---|---|
| 5 | 7.3 | 3.2 × 107 | 90 ± 12 | 24 ± 4 |
| 10 | 16.5 | 1.2 × 107 | 290 ± 19 | 40 ± 9 |
| 15 | 21.5 | 8.0 × 106 | 600 ± 26 | 76 ± 10 |
| 20 | 25.3 | 4.0 × 106 | 814 ± 29 | 116 ± 12 |
| 25 | 28.8 | 3.0 × 106 | 900 ± 38 | 126 ± 13 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Serapinienė, B.; Gudavičiūtė, L.; Tutlienė, S.; Grigucevičienė, A.; Selskis, A.; Juodkazytė, J.; Ramanauskas, R. On the Electrochemically Active Surface Area Determination of Electrodeposited Porous Cu 3D Nanostructures. Coatings 2023, 13, 1335. https://doi.org/10.3390/coatings13081335
Serapinienė B, Gudavičiūtė L, Tutlienė S, Grigucevičienė A, Selskis A, Juodkazytė J, Ramanauskas R. On the Electrochemically Active Surface Area Determination of Electrodeposited Porous Cu 3D Nanostructures. Coatings. 2023; 13(8):1335. https://doi.org/10.3390/coatings13081335
Chicago/Turabian StyleSerapinienė, Birutė, Laima Gudavičiūtė, Skirmantė Tutlienė, Asta Grigucevičienė, Algirdas Selskis, Jurga Juodkazytė, and Rimantas Ramanauskas. 2023. "On the Electrochemically Active Surface Area Determination of Electrodeposited Porous Cu 3D Nanostructures" Coatings 13, no. 8: 1335. https://doi.org/10.3390/coatings13081335








