Advances of Sulfonated Hyaluronic Acid in Biomaterials and Coatings—A Review
Abstract
1. Introduction
1.1. Hyaluronidase Action
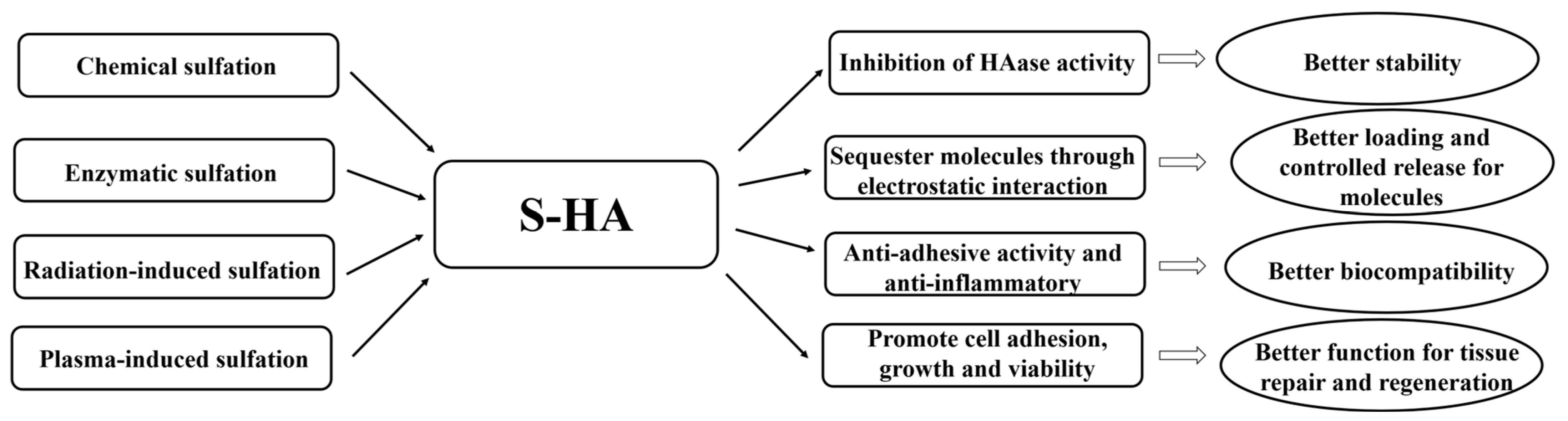
1.2. Effect of S-HA on Hyaluronidase Activity and S-HA Synthetic Techniques
2. Selective Binding Characteristics of S-HA
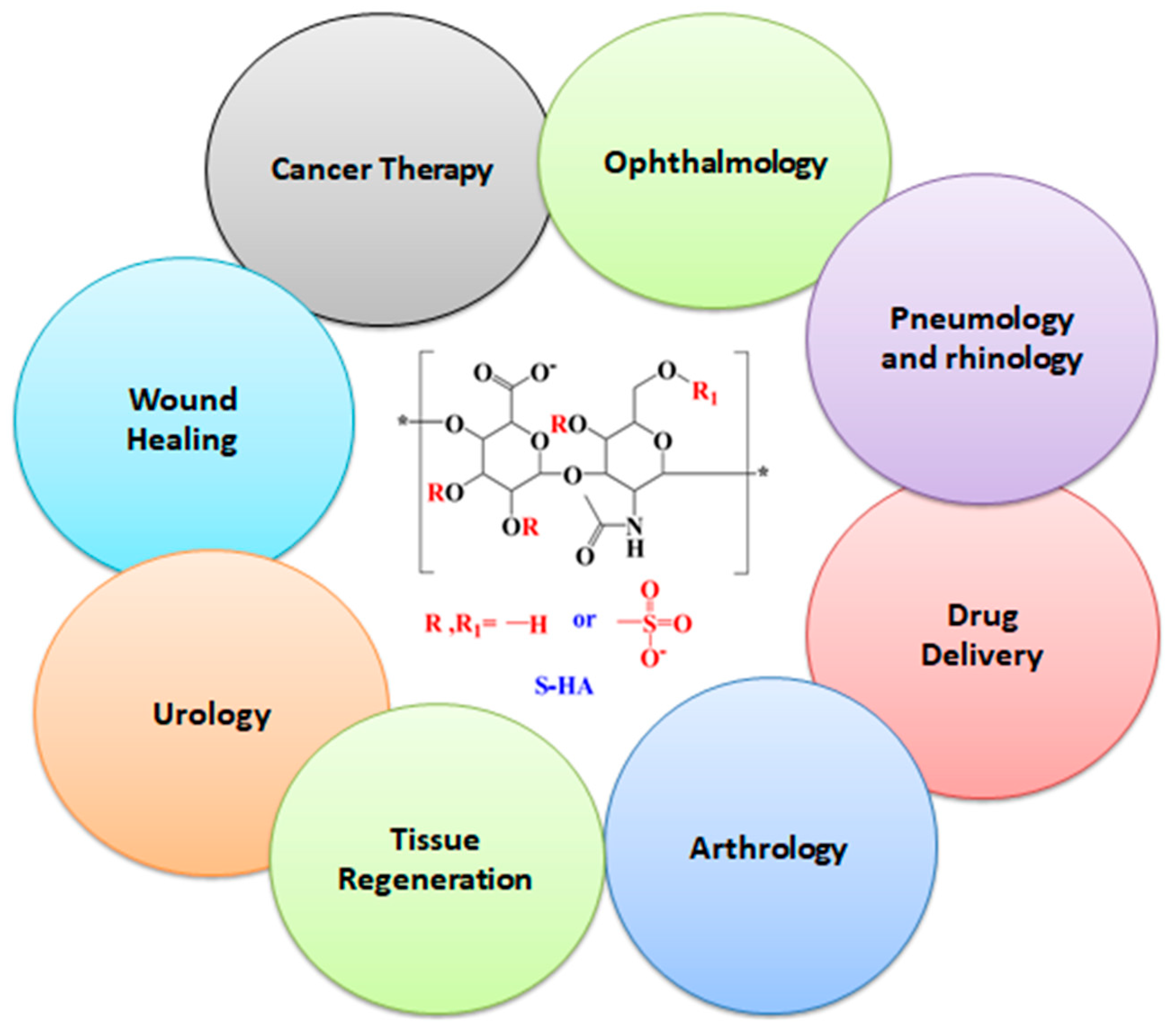
3. Applications of Sulfonated Hyaluronic Acid (S-HA)
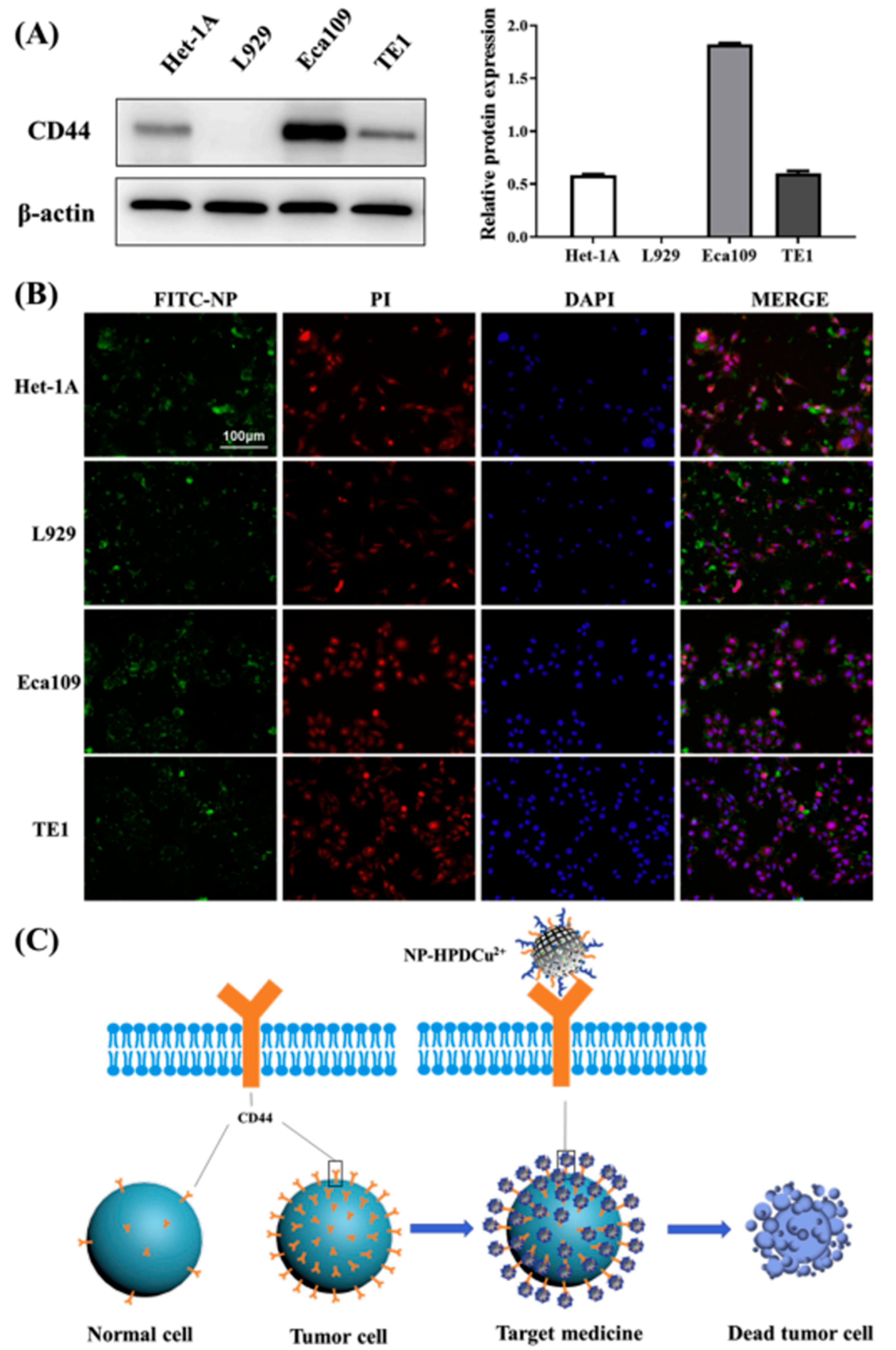
3.1. Drug Delivery
3.2. Tissue Engineering
3.3. Treatment for Osteoarthritis
3.4. Treatment for Inflammatory Diseases
3.5. Wound Healing
4. Summary and Perspective
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Larrañeta, E.; Henry, M.; Irwin, N.J.; Trotter, J.; Perminova, A.A.; Donnelly, R.F. Synthesis and characterization of hyaluronic acid hydrogels crosslinked using a solvent-free process for potential biomedical applications. Carbohydr. Polym. 2018, 181, 1194–1205. [Google Scholar] [CrossRef] [PubMed]
- Angello, J.C.; Hauschka, S.D. Hyaluronic acid synthesis and turnover by myotubes in culture. Dev. Biol. 1979, 73, 322–337. [Google Scholar] [CrossRef] [PubMed]
- Feng, Q.; Lin, S.; Zhang, K.; Dong, C.; Wu, T.; Huang, H.; Bian, L. Sulfated hyaluronic acid hydrogels with retarded degradation and enhanced growth factor retention promote hMSC chondrogenesis and articular cartilage integrity with reduced hypertrophy. Acta Biomater. 2017, 53, 329–342. [Google Scholar] [CrossRef] [PubMed]
- Schuurmans, C.C.L.; Mihajlovic, M.; Hiemstra, C.; Ito, K.; Hennink, W.E.; Vermonden, T. Hyaluronic acid and chondroitin sulfate (meth)acrylate-based hydrogels for tissue engineering: Synthesis, characteristics and pre-clinical evaluation. Biomaterials 2021, 268, 120602. [Google Scholar] [CrossRef]
- Della Sala, F.; Fabozzi, A.; di Gennaro, M.; Nuzzo, S.; Makvandi, P.; Solimando, N.; Borzacchiello, A. Advances in Hyaluronic-Acid-Based (Nano)Devices for Cancer Therapy. Macromol. Biosci. 2022, 22, 2100304. [Google Scholar] [CrossRef]
- Ogawa, D.; Shikata, K.; Matsuda, M.; Akima, K.; Iwahashi, M.; Okada, S.; Makino, H. Sulfated hyaluronic acid, a potential selectin inhibitor, ameliorates experimentally induced crescentic glomerulonephritis. Nephron Exp. Nephrol. 2005, 99, 26–32. [Google Scholar] [CrossRef]
- Li, J.; Wang, S.; Sheng, Y.; Liu, C.; Xue, Z.; Tong, P.; Guan, S. Designing HA/PEI nanoparticle composite coating on biodegradable Mg–Zn–Y-Nd alloy to direct cardiovascular cells fate. Smart Mater. Med. 2021, 2, 124–133. [Google Scholar] [CrossRef]
- Magnani, A.; Lamponi, S.; Consumi, M.; Barbucci, R. Biological performance of two materials based on sulfated hyaluronic acid and polyurethane. J. Mater. Chem. 1999, 9, 2393–2398. [Google Scholar] [CrossRef]
- Yu, Y.; Zhu, S.J.; Dong, H.T.; Zhang, X.Q.; Li, J.; Guan, S.K. A novel MgF2/PDA/S-HA coating on the bio-degradable ZE21B alloy for better multi-functions on cardiovascular application. J. Magnes. Alloy. 2023, 11, 480–492. [Google Scholar] [CrossRef]
- Bhattacharya, D.S.; Svechkarev, D.; Bapat, A.; Patil, P.; Hollingsworth, M.A.; Mohs, A.M. Sulfation Modulates the Targeting Properties of Hyaluronic Acid to P-Selectin and CD44. ACS Biomater. Sci. Eng. 2020, 6, 3585–3598. [Google Scholar] [CrossRef]
- Benitez, A.; Yates, T.J.; Lopez, L.E.; Cerwinka, W.H.; Bakkar, A.; Lokeshwar, V.B. Targeting hyaluronidase for cancer therapy: Antitumor activity of sulfated hyaluronic acid in prostate cancer cells. Cancer Res. 2011, 71, 4085–4095. [Google Scholar] [CrossRef]
- Buhren, B.A.; Schrumpf, H.; Bölke, E.; Kammers, K.; Gerber, P.A. Standardized in vitro analysis of the degradability of hyaluronic acid fillers by hyaluronidase. Eur. J. Med. Res. 2018, 23, 37. [Google Scholar] [CrossRef] [PubMed]
- Sturabotti, E.; Consalvi, S.; Tucciarone, L.; Macrì, E.; Di Lisio, V.; Francolini, I.; Martinelli, A. Synthesis of Novel Hyaluronic Acid Sulfonated Hydrogels Using Safe Reactants: A Chemical and Biological Characterization. Gels 2022, 8, 480. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.; Zhu, S.; Hou, Y.; Li, J.; Guan, S. Sulfur Contents in Sulfonated Hyaluronic Acid Direct the Cardiovascular Cells Fate. ACS Appl. Mater. Interfaces 2020, 12, 46827–46836. [Google Scholar] [CrossRef]
- Miura, T.; Yuasa, N.; Ota, H.; Habu, M.; Kawano, M.; Nakayama, F.; Nishihara, S. Highly sulfated hyaluronic acid maintains human induced pluripotent stem cells under feeder-free and bFGF-free conditions. Biochem. Biophys. Res. Commun. 2019, 518, 506–512. [Google Scholar] [CrossRef]
- Feng, M.; Tang, B.; Liang, S.H.; Jiang, X. Sulfur Containing Scaffolds in Drugs: Synthesis and Application in Medicinal Chemistry. Curr Top. Med. Chem. 2016, 16, 1200–1216. [Google Scholar] [CrossRef]
- Olivito, F.; Amodio, N.; Di Gioia, M.L.; Nardi, M.; Oliverio, M.; Juli, G.; Tassone, P.; Procopio, A. Synthesis and preliminary evaluation of the anti-cancer activity on A549 lung cancer cells of a series of unsaturated disulfides. MedChemComm 2018, 10, 116–119. [Google Scholar] [CrossRef]
- Rzany, B.; Becker-Wegerich, P.; Bachmann, F.; Erdmann, R.; Wollina, U. Hyaluronidase in the correction of hyaluronic acid-based fillers: A review and a recommendation for use. J. Cosmet. Dermatol. 2009, 8, 317–323. [Google Scholar] [CrossRef] [PubMed]
- Stern, R.; Jedrzejas, M.J. Hyaluronidases: Their genomics, structures, and mechanisms of action. Chem. Rev. 2006, 106, 818–839. [Google Scholar] [CrossRef] [PubMed]
- Cen, L.; Neoh, K.G.; Li, Y.; Kang, E.T. Assessment of in vitro bioactivity of hyaluronic acid and sulfated hyaluronic acid functionalized electroactive polymer. Biomacromolecules 2004, 5, 2238–2246. [Google Scholar] [CrossRef]
- Jones, D.; Tezel, A.; Borrell, M. In vitro resistance to degradation of hyaluronic acid dermal fillers by ovine testicular hyaluronidase. Dermatol. Surg. 2010, 36, 804–809. [Google Scholar] [CrossRef]
- Hintze, V.; Miron, A.; Moeller, S.; Schnabelrauch, M.; Wiesmann, H.P.; Worch, H.; Scharnweber, D. Sulfated hyaluronan and chondroitin sulfate derivatives interact differently with human transforming growth factor-β1 (TGF-β1). Acta Biomater. 2012, 8, 2144–2152. [Google Scholar] [CrossRef] [PubMed]
- Cavallini, M.; Gazzola, R.; Metalla, M.; Vaienti, L. The role of hyaluronidase in the treatment of complications from hyaluronic acid dermal fillers. Aesthetic Surg. J. 2013, 33, 1167–1174. [Google Scholar] [CrossRef]
- Chung, C.; Beecham, M.; Mauck, R.L.; Burdick, J.A. The influence of degradation characteristics of hyaluronic acid hydrogels on in vitro neocartilage formation by mesenchymal stem cells. Biomaterials 2009, 30, 4287–4296. [Google Scholar] [CrossRef]
- Isoyama, T.; Thwaites, D.M.; Selzer, G.; Carey, R.I.; Barbucci, R.; Lokeshwar, V.B. Differential selectivity of hyaluronidase inhibitors toward acidic and basic hyaluronidases. Glycobiology 2006, 16, 11–21. [Google Scholar] [CrossRef] [PubMed]
- Schulz, M.C.; Korn, P.; Stadlinger, B.; Range, U.; Möller, S.; Becher, J.; Hintze, V. Coating with artificial matrices from collagen and sulfated hyaluronan influences the osseointegration of dental implants. J. Mater. Sci. Mater. Med. 2014, 25, 247–258. [Google Scholar] [CrossRef]
- Jordan, A.R.; Lokeshwar, S.D.; Lopez, L.E.; Hennig, M.; Chipollini, J.; Yates, T.; Lokeshwar, V.B. Antitumor activity of sulfated hyaluronic acid fragments in pre-clinical models of bladder cancer. Oncotarget 2017, 8, 24262–24274. [Google Scholar] [CrossRef][Green Version]
- Kunze, R.; Rösler, M.; Möller, S.; Schnabelrauch, M.; Riemer, T.; Hempel, U.; Dieter, P. Sulfated hyaluronan derivatives reduce the proliferation rate of primary rat calvarial osteoblasts. Glycoconj. J. 2010, 27, 151–158. [Google Scholar] [CrossRef]
- Bayer, I.S. Hyaluronic acid and controlled release: A review. Molecules 2020, 25, 2649. [Google Scholar] [CrossRef]
- Kim, D.W.; Yoon, E.S.; Ji, Y.H.; Park, S.H.; Il Lee, B.; Dhong, E.S. Vascular complications of hyaluronic acid fillers and the role of hyaluronidase in management. J. Plast. Reconstr. Aesthetic Surg. 2011, 64, 1590–1595. [Google Scholar] [CrossRef]
- Li, J.; Qiao, M.; Ji, Y.; Lin, L.; Zhang, X.; Linhardt, R.J. Chemical, enzymatic and biological synthesis of hyaluronic acids. Int. J. Biol. Macromol. 2020, 152, 199–206. [Google Scholar] [CrossRef] [PubMed]
- Miura, T.; Kawano, M.; Takahashi, K.; Yuasa, N.; Habu, M.; Kimura, F.; Nakayama, F. High-Sulfated Hyaluronic Acid Ameliorates Radiation-Induced Intestinal Damage Without Blood Anticoagulation. Adv. Radiat. Oncol. 2022, 7, 100900. [Google Scholar] [CrossRef] [PubMed]
- McCarty, M.F.; Russell, A.L.; Seed, M.P. Sulfated glycosaminsglycan and glucosamine may synergize in promoting synovial hyaluronic acid synthesis. Med. Hypotheses 2000, 54, 798–802. [Google Scholar] [CrossRef]
- Lim, D.K.; Wylie, R.G.; Langer, R.; Kohane, D.S. Selective binding of C-6 OH sulfated hyaluronic acid to the angiogenic isoform of VEGF165. Biomaterials 2016, 77, 130–138. [Google Scholar] [CrossRef]
- Matsuda, M.; Shikata, K.; Shimizu, F.; Suzuki, Y.; Miyasaka, M.; Kawachi, H.; Makino, H. Therapeutic effect of sulphated hyaluronic acid, a potential selectin-blocking agent, on experimental progressive mesangial proliferative glomerulonephritis. J. Pathol. 2002, 198, 407–414. [Google Scholar] [CrossRef]
- Vilela, C.A.; Correia, C.; Oliveira, J.M.; Sousa, R.A.; Espregueira-Mendes, J.; Reis, R.L. Cartilage Repair Using Hydrogels: A Critical Review of in Vivo Experimental Designs. ACS Biomater. Sci. Eng. 2015, 1, 726–739. [Google Scholar] [CrossRef]
- Muzzarelli, R.A.; Greco, A.F.; Busilacchi, A.; Sollazzo, V.; Gigante, A. Chitosan, hyaluronan and chondroitin sulfate in tissue engineering for cartilage regeneration: A review. Carbohydr. Polym. 2012, 89, 723–739. [Google Scholar] [CrossRef]
- Henson, F.M.D.; Getgood, A.M.J.; Caborn, D.M.; Wayne McIlwraith, C.W.; Rushton, N. Effect of a solution of hyaluronic acid-chondroitin sulfate-N-acetyl glucosamine on the repair response of cartilage to single-impact load damage. Am. J. Vet. Res. 2012, 73, 306–312. [Google Scholar] [CrossRef] [PubMed]
- Subramaniam, S.; Fang, Y.H.; Sivasubramanian, S.; Lin, F.H.; Lin, C.P. Hydroxyapatite-calcium sulfate-hyaluronic acid composite encapsulated with collagenase as bone substitute for alveolar bone regeneration. Biomaterials 2016, 74, 99–108. [Google Scholar] [CrossRef]
- Hou, Y.; Zhang, X.; Li, J.; Wang, L.; Guan, S. A multi-functional MgF2/polydopamine/hyaluronan-astaxanthin coating on the biodegradable ZE21B alloy with better corrosion resistance and biocompatibility for cardiovascular application. J. Magnes. Alloy. 2022, in press. [CrossRef]
- Xue, Z.; Sun, X.; Li, H.; Iqbal, M.; Hou, Y.; Jin, Z.; Li, J. Response of cardiovascular environment to sulfonated hyaluronic acid with higher sulfur content. Colloids Surf. B Biointerfaces 2023, 22, 113046. [Google Scholar] [CrossRef] [PubMed]
- Möller, S.; Schmidtke, M.; Weiss, D.; Schiller, J.; Pawlik, K.; Wutzler, P.; Schnabelrauch, M. Synthesis and antiherpetic activity of carboxymethylated and sulfated hyaluronan derivatives. Carbohydr. Polym. 2012, 90, 608–615. [Google Scholar] [CrossRef] [PubMed]
- Albanese, A.; Lamponi, S.; Barbucci, R. Blood-interaction performance of differently sulphated hyaluronic acids. Thromb. Res. 1996, 81, 383–395. [Google Scholar]
- Yeom, J.; Bhang, S.H.; Kim, B.S.; Seo, M.S.; Hwang, E.J.; Cho, I.H.; Hahn, S.K. Effect of cross-linking reagents for hyaluronic acid hydrogel dermal fillers on tissue augmentation and regeneration. Bioconjug. Chem. 2010, 21, 240–247. [Google Scholar] [CrossRef] [PubMed]
- Sall, I.; Férard, G. Comparison of the sensitivity of 11 crosslinked hyaluronic acid gels to bovine testis hyaluronidase. Polym. Degrad. Stab. 2007, 92, 915–919. [Google Scholar] [CrossRef]
- Payan, E.; Jouzeau, J.Y.; Lapicque, F.; Muller, N.; Netter, P. Hyaluronidase degradation of hyaluronic acid from different sources: Influence of the hydrolysis conditions on the production and the relative proportions of tetra- and hexasaccharide produced. Int. J. Biochem. 1993, 25, 325–329. [Google Scholar] [CrossRef]
- Yao, W.; Chen, M.; Dou, X.; Jin, H.; Zhang, X.; Zhu, Y.; Li, Z. Unravel a neuroactive sHA sulfation pattern with neurogenesis activity by a library of defined oligosaccharides. Eur. J. Med. Chem. 2019, 163, 583–596. [Google Scholar] [CrossRef]
- Vogel, S.; Ullm, F.; Müller, C.D.; Pompe, T.; Hempel, U. Impact of binding mode of low-sulfated hyaluronan to 3D collagen matrices on its osteoinductive effect for human bone marrow stromal cells. Biol. Chem. 2021, 402, 1465–1478. [Google Scholar] [CrossRef]
- Zerbinati, N.; Mocchi, R.; Galadari, H.; Maccario, C.; Maggi, M.; Rauso, R.; Sommatis, S. In vitro evaluation of the biological availability of hyaluronic acid polyethylene glycols-cross-linked hydrogels to bovine testes hyaluronidase. Biomed Res. Int. 2019, 2019, 3196723. [Google Scholar] [CrossRef]
- Felz, S.; Neu, T.R.; van Loosdrecht, M.C.M.; Lin, Y. Aerobic granular sludge contains Hyaluronic acid-like and sulfated glycosaminoglycans-like polymers. Water Res. 2020, 169, 115291. [Google Scholar] [CrossRef]
- Hamilton, D.W.; Riehle, M.O.; Rappuoli, R.; Monaghan, W.; Barbucci, R.; Curtis, A.S.G. The response of primary articular chondrocytes to micrometric surface topography and sulphated hyaluronic acid-based matrices. Cell Biol. Int. 2005, 29, 605–615. [Google Scholar] [CrossRef] [PubMed]
- Magnani, A.; Lamponi, S.; Rappuoli, R.; Barbucci, R. Sulphated Hyaluronic Acids: A Chemical and Biological Characterisation. Polym. Int. 1998, 46, 225–240. [Google Scholar] [CrossRef]
- Koehler, L.; Ruiz-Gómez, G.; Balamurugan, K.; Rother, S.; Freyse, J.; Möller, S.; Hintze, V. Dual Action of Sulfated Hyaluronan on Angiogenic Processes in Relation to Vascular Endothelial Growth Factor-A. Sci. Rep. 2019, 9, 18143. [Google Scholar] [CrossRef] [PubMed]
- Satish, L.; Santra, S.; Tsurkan, M.V.; Werner, C.; Jana, M.; Sahoo, H. Conformational changes of GDNF-derived peptide induced by heparin, heparan sulfate, and sulfated hyaluronic acid—Analysis by circular dichroism spectroscopy and molecular dynamics simulation. Int. J. Biol. Macromol. 2021, 82, 2144–2150. [Google Scholar] [CrossRef] [PubMed]
- Burdick, J.A.; Prestwich, G.D. Hyaluronic acid hydrogels for biomedical applications. Adv. Mater. 2011, 23, 41–56. [Google Scholar] [CrossRef]
- Yasin, A.; Ren, Y.; Li, J.; Sheng, Y.; Cao, C.; Zhang, K. Advances in Hyaluronic Acid for Biomedical Applications. Front. Bioeng. Biotechnol. 2022, 10, 910290. [Google Scholar] [CrossRef]
- Manfredi, C.; Spirito, L.; Calace, F.P.; Balsamo, R.; Terribile, M.; Stizzo, M.; Arcaniolo, D. Oral Preparation of Hyaluronic Acid, Chondroitin Sulfate, Curcumin, and Quercetin (Ialuril® Soft Gels) for the Prevention of LUTS after Intravesical Chemotherapy. Pathophysiology 2022, 29, 365–373. [Google Scholar] [CrossRef]
- Kurczewska, J. Recent Reports on Polysaccharide-Based Materials for Drug Delivery. Polymers 2022, 14, 4189. [Google Scholar] [CrossRef]
- Schuiringa, G.H.; Mihajlovic, M.; van Donkelaar, C.C.; Vermonden, T.; Ito, K. Creating a Functional Biomimetic Cartilage Implant Using Hydrogels Based on Methacrylated Chondroitin Sulfate and Hyaluronic Acid. Gels 2022, 8, 7. [Google Scholar] [CrossRef]
- Vázquez, I.; Rodríguez-Amado, J.A.; Montemayor, M.I.; Fraguas, J.; Del González, M.P.; Murado, M.A. Chondroitin sulfate, hyaluronic acid and chitin/chitosan production using marine waste sources: Characteristics, applications and eco-friendly processes: A review. Mar. Drugs 2013, 11, 747–774. [Google Scholar] [CrossRef]
- Nagira, T.; Nagahata-Ishiguro, M.; Tsuchiya, T. Effects of sulfated hyaluronan on keratinocyte differentiation and Wnt and Notch gene expression. Biomaterials 2007, 28, 844–850. [Google Scholar] [CrossRef]
- Amhare, A.F.; Lei, J.; Deng, H.; Lv, Y.; Han, J.; Zhang, L. Biomedical application of chondroitin sulfate with nanoparticles in drug delivery systems: Systematic review. J. Drug Target. 2021, 29, 259–268. [Google Scholar] [CrossRef]
- Cui, L.; Li, J.K.; Guan, S.; Zhang, K.X.; Zhang, K.; Li, J.A. Injectable Multifunctional CMC/HA-DA Hydrogel for Repairing Skin Injury. Mater. Today Bio 2022, 14, 100257. [Google Scholar]
- Wei, S.; Li, J.; He, H.; Shu, C.; Dardik, A.; Bai, H. A three-layered hydrogel patch with hierarchy releasing of PLGA nanoparticle drugs decrease neointimal hyperplasia. Smart Mater. Med. 2022, 3, 139–147. [Google Scholar] [CrossRef]
- Lei, C.; Liu, X.R.; Chen, Q.B.; Li, Y.; Zhou, J.L.; Zhou, L.Y.; Zou, T. Hyaluronic acid and albumin based nanoparticles for drug delivery. J. Control. Release 2021, 331, 416–433. [Google Scholar] [CrossRef]
- Xu, R.; Zhang, K.; Liang, J.; Gao, F.; Li, J.; Guan, F. Hyaluronic acid/polyethyleneimine nanoparticles loaded with copper ion and disulfiram for esophageal cancer. Carbohydr. Polym. 2021, 261, 117846. [Google Scholar]
- Rother, S.; Galiazzo, V.D.; Kilian, D.; Fiebig, K.M.; Becher, J.; Moeller, S.; Hintze, V. Hyaluronan/Collagen Hydrogels with Sulfated Hyaluronan for Improved Repair of Vascularized Tissue Tune the Binding of Proteins and Promote Endothelial Cell Growth. Macromol. Biosci. 2017, 17, 1700154. [Google Scholar]
- Schmidt, J.R.; Vogel, S.; Moeller, S.; Kalkhof, S.; Schubert, K.; von Bergen, M.; Hempel, U. Sulfated hyaluronic acid and dexamethasone possess a synergistic potential in the differentiation of osteoblasts from human bone marrow stromal cells. J. Cell. Biochem. 2019, 120, 8706–8722. [Google Scholar]
- Vogel, S.; Arnoldini, S.; Möller, S.; Schnabelrauch, M.; Hempel, U. Sulfated hyaluronan alters fibronectin matrix assembly and promotes osteogenic differentiation of human bone marrow stromal cells. Sci. Rep. 2016, 6, 36418. [Google Scholar]
- Yao, S.; Cui, J.; Chen, S.; Zhou, X.; Li, J.; Zhang, K. Extracellular matrix coatings on cardiovascular materials—A review. Coatings 2022, 12, 1039. [Google Scholar]
- Zou, D.; Li, J.; Kou, F.; Luo, X.; Yang, P. Reveal crucial subtype of natural chondroitin sulfate on the functionalized coatings for cardiovascular implants. J. Mater. Sci. Technol. 2021, 91, 67–77. [Google Scholar] [CrossRef]
- Li, J.; Li, W.; Zou, D.; Kou, F.; Hou, Y.; Yasin, A.; Zhang, K. Comparison of conjugating chondroitin sulfate A and B on amine-rich surface: For deeper understanding on directing cardiovascular cells fate. Compos. Part B Eng. 2022, 228, 109430. [Google Scholar] [CrossRef]
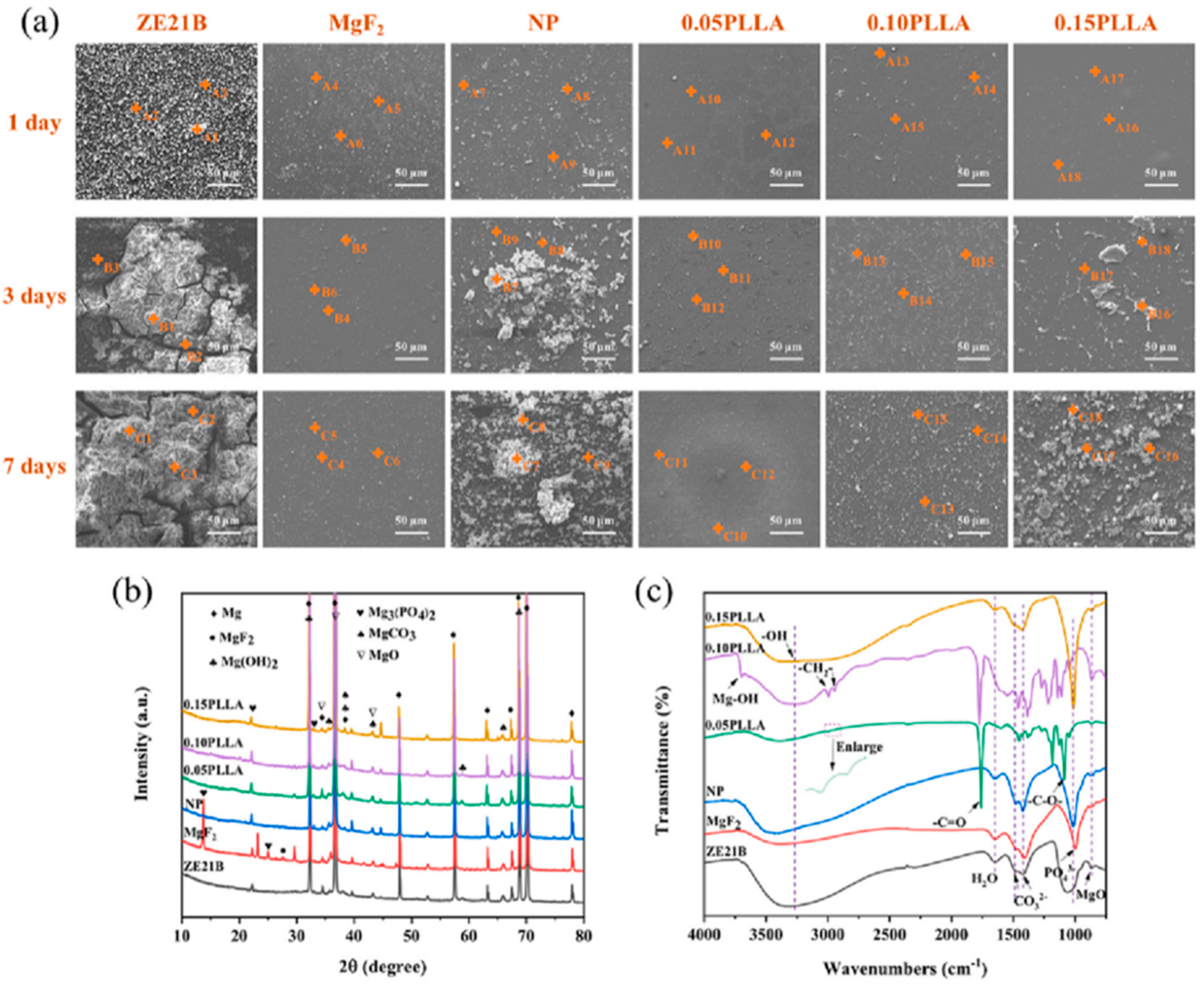
- Tong, P.; Chen, L.; Sun, X.; Li, H.; Feng, Y.; Li, J.; Guan, S. Surface modification of biodegradable magnesium alloy with Poly (L-lactic acid) and Sulfonated hyaluronic acid nanoparticles for cardiovascular application. Int. J. Biol. Macromol. 2023, 237, 124191. [Google Scholar] [CrossRef]
- Chai, Y.; Feng, Y.; Zhang, K.; Li, J. Preparation of fluorescent carbon dots composites and their potential applications in biomedicine and drug delivery—A review. Pharmaceutics 2022, 14, 2482. [Google Scholar] [CrossRef]
- Cohen, M.M.; Altman, R.D.; Hollstrom, R.; Hollstrom, C.; Sun, C.; Gipson, B. Safety and efficacy of intra-articular sodium hyaluronate (Hyalgan®) in a randomized, double-blind study for osteoarthritis of the ankle. Foot Ankle Int. 2008, 29, 657–663. [Google Scholar] [CrossRef]
- Chen, G.; Ito, Y.; Imanishi, Y.; Magnani, A.; Lamponi, S.; Barbucci, R. Photoimmobilization of sulfated hyaluronic acid for antithrombogenicity. Bioconjug. Chem. 1997, 8, 730–734. [Google Scholar] [CrossRef]
- Shankland, W.E. The effects of glucosamine and chondroitin sulfate on osteoarthritis of the TMJ: A preliminary report of 50 patients. Cranio—J. Craniomandib. Sleep Pract. 1998, 16, 230–235. [Google Scholar] [CrossRef]
- Hempel, U.; Möller, S.; Noack, C.; Hintze, V.; Scharnweber, D.; Schnabelrauch, M.; Dieter, P. Sulfated hyaluronan/collagen I matrices enhance the osteogenic differentiation of human mesenchymal stromal cells in vitro even in the absence of dexamethasone. Acta Biomater. 2012, 8, 4064–4072. [Google Scholar] [CrossRef]
- Vangsness, C.T.; Spiker, W.; Erickson, J. A Review of Evidence-Based Medicine for Glucosamine and Chondroitin Sulfate Use in Knee Osteoarthritis. Arthrosc.—J. Arthrosc. Relat. Surg. 2009, 25, 86–94. [Google Scholar] [CrossRef]
- Zhang, Z.Q.; Yang, Y.X.; Li, J.A.; Zeng, R.C.; Guan, S.K. Advances in coatings on magnesium alloys for cardiovascular stents—A review. Bioact. Mater. 2021, 6, 4729–4757. [Google Scholar] [CrossRef]
- Hou, Y.C.; Cui, X.; Qin, Z.; Su, C.; Zhang, G.; Tang, J.N.; Li, J.A.; Zhang, J.Y. Three-dimensional bioprinting of artificial blood vessel: Process, bio-inks and challenges. Int. J. Bioprinting 2023, 9, 740. [Google Scholar]
- Xie, M.; Su, J.; Zhou, S.; Li, J.; Zhang, K. Application of Hydrogels as Three-dimensional Bioprinting ink for Tissue engineering. Gels 2023, 9, 88. [Google Scholar] [CrossRef] [PubMed]
- Abi Zeid Daou, C.; Bassim, M. Hyaluronic acid in otology: Its uses, advantages and drawbacks—A review. Am. J. Otolaryngol.—Head Neck Med. Surg. 2020, 41, 102375. [Google Scholar] [CrossRef] [PubMed]
- Hong, C.I.; Jung, E.G.; Han, K.I.; Kim, Y.H.; Lee, S.H.; Lee, H.S.; Han, M.D. Structural Characteristics and Anti-inflammatory Activities of Chemically Sulfated-hyaluronic Acid from Streptococcus dysgalactiae. J. Life Sci. 2016, 26, 545–554. [Google Scholar] [CrossRef]
- Lebaudy, E.; Fournel, S.; Lavalle, P.; Vrana, N.E.; Gribova, V. Recent Advances in Antiinflammatory Material Design. Adv. Healthc. Mater. 2021, 10, 2001373. [Google Scholar] [CrossRef]
- Graça, M.F.P.; Miguel, S.P.; Cabral, C.S.D.; Correia, I.J. Hyaluronic acid—Based wound dressings: A review. Carbohydr. Polym. 2020, 241, 116364. [Google Scholar] [CrossRef]
- Nishikawa, H.; Mori, I.; Umemoto, J. Influences of sulfated glycosaminoglycans on biosynthesis of hyaluronic acid in rabbit knee synovial membrane. Arch. Biochem. Biophys. 1985, 240, 146–153. [Google Scholar] [CrossRef]
- Oe, M.; Tashiro, T.; Yoshida, H.; Nishiyama, H.; Masuda, Y.; Maruyama, K.; Fukui, N. Oral hyaluronan relieves knee pain: A review. Nutr. J. 2016, 15, 11. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Iqbal, M.; Yasin, A.; Akram, A.; Li, J.-A.; Zhang, K. Advances of Sulfonated Hyaluronic Acid in Biomaterials and Coatings—A Review. Coatings 2023, 13, 1345. https://doi.org/10.3390/coatings13081345
Iqbal M, Yasin A, Akram A, Li J-A, Zhang K. Advances of Sulfonated Hyaluronic Acid in Biomaterials and Coatings—A Review. Coatings. 2023; 13(8):1345. https://doi.org/10.3390/coatings13081345
Chicago/Turabian StyleIqbal, Mujahid, Aqeela Yasin, Ambreen Akram, Jing-An Li, and Kun Zhang. 2023. "Advances of Sulfonated Hyaluronic Acid in Biomaterials and Coatings—A Review" Coatings 13, no. 8: 1345. https://doi.org/10.3390/coatings13081345
APA StyleIqbal, M., Yasin, A., Akram, A., Li, J.-A., & Zhang, K. (2023). Advances of Sulfonated Hyaluronic Acid in Biomaterials and Coatings—A Review. Coatings, 13(8), 1345. https://doi.org/10.3390/coatings13081345






