Examination of Non-Modified Carbon Fibre Bundle as an Electrode for Electrochemical Sensing
Abstract
:1. Introduction
2. Experimental
2.1. Materials
2.2. Instrumentation
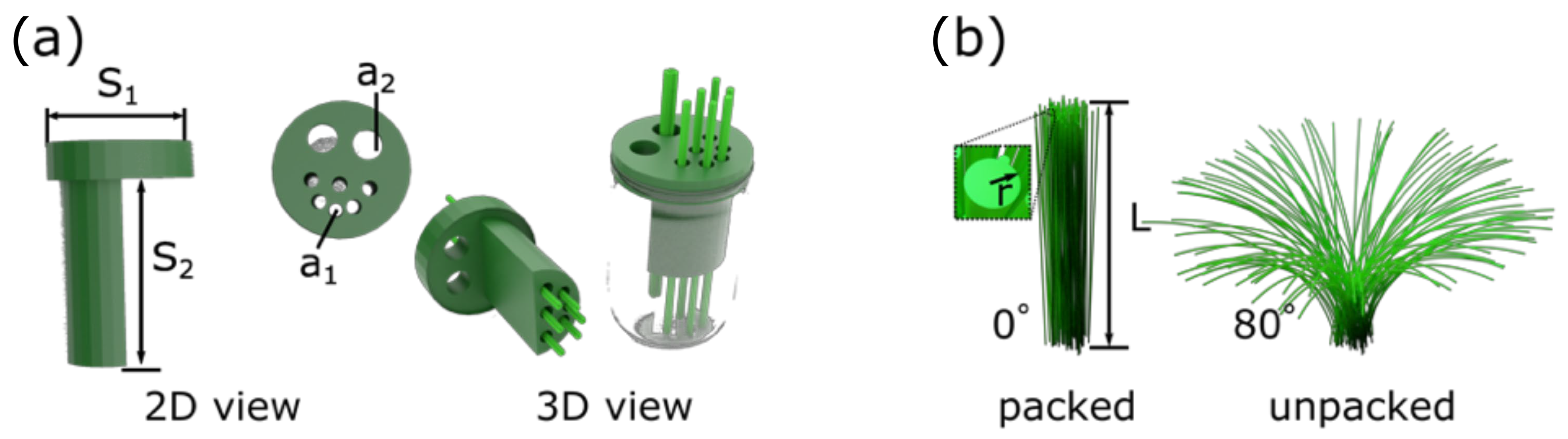
2.3. Optimisation of 3D-Printed Electrochemical Cell and Electrode Design
3. Results and Discussion
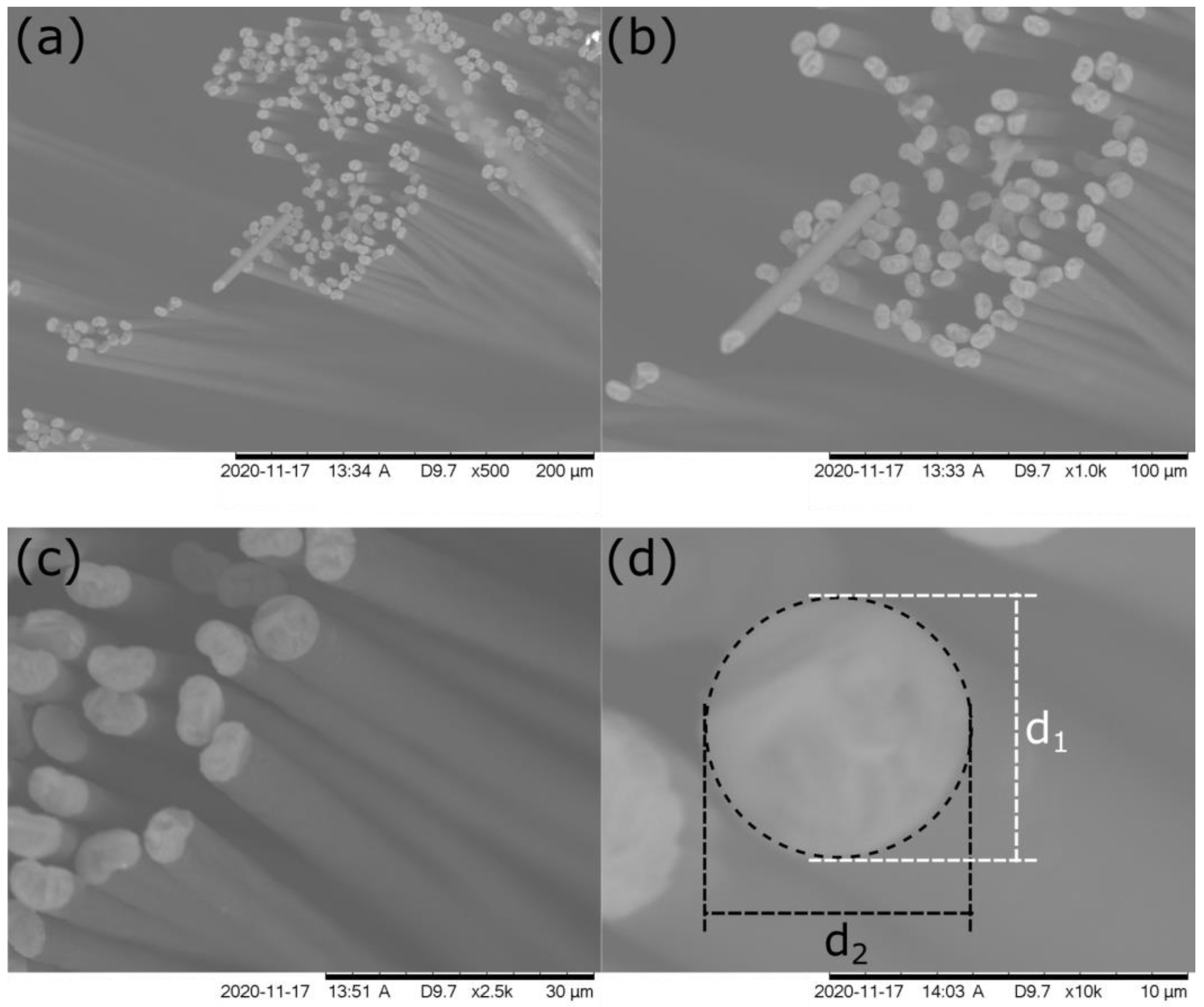
3.1. Characterisation of the CFMB Electrode Geometrical Area
3.2. Characterisation of CFMB Electrode in Phosphate Buffer
3.3. Characterisation of CFMB Electrode Using Ferrocene
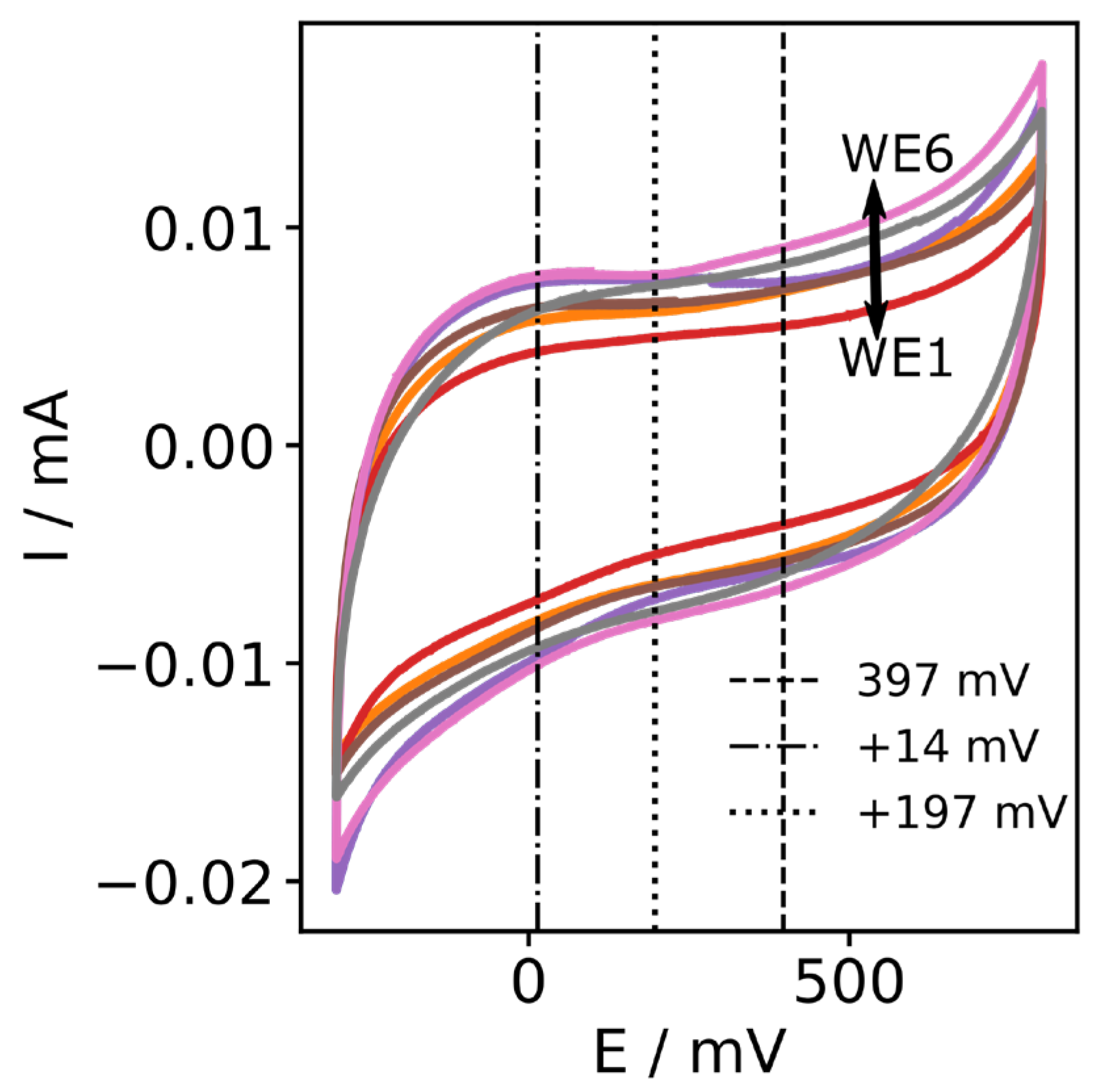
3.4. Stability and Reproducibility of Carbon Fibre Bundle Electrodes
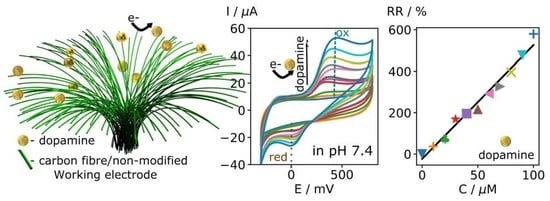
3.5. Testing of Carbon Fibre Bundle Electrodes for Dopamine Detection
3.6. Testing of Carbon Fibre Bundle Electrodes for Dopamine Selectivity
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Baradoke, A.; Hein, R.; Li, X.; Davis, J.J.J. Reagentless Redox Capacitive Assaying of C-Reactive Protein at a Polyaniline Interface. Anal. Chem. 2020, 92, 3508–3511. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Baradoke, A.; Pastoriza-Santos, I.; González-Romero, E. Screen-printed GPH electrode modified with Ru nanoplates and PoPD polymer film for NADH sensing: Design and characterization. Electrochim. Acta 2019, 300, 316–323. [Google Scholar] [CrossRef]
- Baradoke, A.; Jose, B.; Pauliukaite, R.; Forster, R.J. Properties of Anti-CA125 antibody layers on screen-printed carbon electrodes modified by gold and platinum nanostructures. Electrochim. Acta 2019, 306, 299–306. [Google Scholar] [CrossRef]
- Baradoke, A.; Santos, A.; Bueno, P.R.; Davis, J.J. Introducing polymer conductance in diagnostically relevant transduction. Biosens. Bioelectron. 2020, 172, 112705. [Google Scholar] [CrossRef]
- Tiwari, J.N.; Vij, V.; Kemp, K.C.; Kim, K.S. Engineered carbon-nanomaterial-based electrochemical sensors for biomolecules. ACS Nano 2016, 10, 46–80. [Google Scholar] [CrossRef] [Green Version]
- Drobysh, M.; Ramanavicius, A.; Baradoke, A. Science of the Total Environment Polyaniline-based electrochemical immunosensor for the determination of antibodies against SARS-CoV-2 spike protein. Sci. Total Environ. 2023, 862, 160700. [Google Scholar] [CrossRef]
- Schröder, P.; Schröder, P.; Schröder, P.; Aguiló-Aguayo, N.; Auer, A.; Grießer, C.; Kunze-Liebhaüser, J.; Ma, Y.; Hummel, M.; Obendorf, D.; et al. Activation of carbon tow electrodes for use in iron aqueous redox systems for electrochemical applications. J. Mater. Chem. C 2020, 8, 7755–7764. [Google Scholar] [CrossRef]
- Eyckens, D.J.; Arnold, C.L.; Simon, Ž.; Gengenbach, T.R.; Pinson, J.; Wickramasingha, Y.A.; Henderson, L.C. Covalent sizing surface modification as a route to improved interfacial adhesion in carbon fibre-epoxy composites. Compos. Part A Appl. Sci. Manuf. 2021, 140, 106147. [Google Scholar] [CrossRef]
- Castañeda, L.F.; Walsh, F.C.; Nava, J.L.; de León, C.P. Graphite felt as a versatile electrode material: Properties, reaction environment, performance and applications. Electrochim. Acta 2017, 258, 1115–1139. [Google Scholar] [CrossRef]
- Li, H.; Liebscher, M.; Ranjbarian, M.; Hempel, S.; Tzounis, L.; Schröfl, C.; Mechtcherine, V. Electrochemical modification of carbon fiber yarns in cementitious pore solution for an enhanced interaction towards concrete matrices. Appl. Surf. Sci. 2019, 487, 52–58. [Google Scholar] [CrossRef]
- Gajalakshmi, K.; Senthilkumar, N.; Mohan, B.; Anbuchezhiyan, G. An investigation on microstructure and mechanical behaviour of copper-nickel coated carbon fibre reinforced aluminium composites. Mater. Res. Express 2020, 7, 115701. [Google Scholar] [CrossRef]
- Budiyantoro, C.; Rochardjo, H.S.B.; Nugroho, G. Effects of processing variables of extrusion–pultrusion method on the impregnation quality of thermoplastic composite filaments. Polymers 2020, 12, 2833. [Google Scholar] [CrossRef] [PubMed]
- Xie, S.; Liu, S.; Cheng, P.F.; Lu, X. Recent Advances toward Achieving High-Performance Carbon-Fiber Materials for Supercapacitors. ChemElectroChem 2018, 5, 571–582. [Google Scholar] [CrossRef]
- Jamal, M.; Sarac, A.S.; Magner, E. Conductive copolymer-modified carbon fibre microelectrodes: Electrode characterisation and electrochemical detection of p-aminophenol. Sens. Actuators B Chem. 2004, 97, 59–66. [Google Scholar] [CrossRef]
- Sugawara, K.; Yugami, A.; Kojima, A. Voltammetric detection of biological molecules using chopped carbon fiber. Anal. Sci. 2010, 26, 1059–1063. [Google Scholar] [CrossRef] [PubMed]
- Ngamchuea, K.; Lin, C.; Batchelor-Mcauley, C.; Compton, R.G. Supported Microwires for Electroanalysis: Sensitive Amperometric Detection of Reduced Glutathione. Anal. Chem. 2017, 89, 3780–3786. [Google Scholar] [CrossRef] [PubMed]
- Vara, H.; Collazos-Castro, J.E. Biofunctionalized Conducting Polymer/Carbon Microfiber Electrodes for Ultrasensitive Neural Recordings. ACS Appl. Mater. Interfaces 2015, 7, 27016–27026. [Google Scholar] [CrossRef]
- Moosburger-Will, J.; Bauer, M.; Schubert, F.; Kunzmann, C.; Lachner, E.; Zeininger, H.; Maleika, M.; Hönisch, B.; Küpfer, J.; Zschoerper, N.; et al. Methyltrimethoxysilane plasma polymerization coating of carbon fiber surfaces. Surf. Coat. Technol. 2017, 311, 223–230. [Google Scholar] [CrossRef]
- Sharp, D.; Gladstone, P.; Smith, R.B.; Forsythe, S.; Davis, J. Approaching intelligent infection diagnostics: Carbon fibre sensor for electrochemical pyocyanin detection. Bioelectrochemistry 2010, 77, 114–119. [Google Scholar] [CrossRef]
- Anderson, A.; Phair, J.; Benson, J.; Meenan, B.; Davis, J. Investigating the use of endogenous quinoid moieties on carbon fibre as means of developing micro pH sensors. Mater. Sci. Eng. C 2014, 43, 533–537. [Google Scholar] [CrossRef]
- Ezekiel, H.B.; Sharp, D.; Villalba, M.M.; Davis, J. Laser-anodised carbon fibre: Coupled activation and patterning of sensor substrates. J. Phys. Chem. Solids 2008, 69, 2932–2935. [Google Scholar] [CrossRef] [Green Version]
- Li, G.; Wu, J.; Qi, X.; Wan, X.; Liu, Y.; Chen, Y.; Xu, L. Molecularly imprinted polypyrrole film-coated poly(3,4-ethylenedioxythiophene):polystyrene sulfonate-functionalized black phosphorene for the selective and robust detection of norfloxacin. Mater. Today Chem. 2022, 26, 101043. [Google Scholar] [CrossRef]
- Xia, Y.; Li, G.; Zhu, Y.; He, Q.; Hu, C. Facile preparation of metal-free graphitic-like carbon nitride / graphene oxide composite for simultaneous determination of uric acid and dopamine. Microchem. J. 2023, 190, 108726. [Google Scholar] [CrossRef]
- Bismarck, A.; Lee, A.F.; Saraç, A.S.; Schulz, E.; Wilson, K. Electrocoating of carbon fibres: A route for interface control in carbon fibre reinforced poly methylmethacrylate? Compos. Sci. Technol. 2005, 65, 1564–1573. [Google Scholar] [CrossRef]
- Yang, Z.; Peng, H.; Wang, W.; Liu, T. Crystallization behavior of poly(ε-caprolactone)/layered double hydroxide nanocomposites. J. Appl. Polym. Sci. 2010, 116, 2658–2667. [Google Scholar] [CrossRef]
- Ates, M.; Uludag, N.; Sarac, A.S. Electrochemical impedance of poly(9-tosyl-9H-carbazole-co-pyrrole) electrocoated carbon fiber. Mater. Chem. Phys. 2011, 127, 120–127. [Google Scholar] [CrossRef]
- Ates, M.; Yilmaz, K.; Shahryari, A.; Omanovic, S.; Sarac, A.S. A study of the electrochemical behavior of poly [N-Vinyl carbazole] formed on carbon-fiber microelectrodes and its response to dopamine. IEEE Sens. J. 2008, 8, 1628–1639. [Google Scholar] [CrossRef]
- Gerhardt, G.A.; Oke, A.F.; Nagy, G.; Moghaddam, B.; Adams, R.N. Nafion-coated electrodes with high selectivity for CNS electrochemistry. Brain Res. 1984, 290, 390–395. [Google Scholar] [CrossRef] [PubMed]
- Sarac, A.S.; Sezgin, S.; Ates, M.; Turhan, C.M. Electrochemical impedance spectroscopy and morphological analyses of pyrrole, phenylpyrrole and methoxyphenylpyrrole on carbon fiber microelectrodes. Surf. Coat. Technol. 2008, 202, 3997–4005. [Google Scholar] [CrossRef]
- Cumba, L.R.; Camisasca, A.; Giordani, S.; Forster, R.J. Electrochemical Properties of Screen-Printed Carbon Nano-Onion Electrodes. Molecules 2020, 25, 3884. [Google Scholar] [CrossRef]
- Wang, Y.; Tong, Q.; Ma, S.R.; Zhao, Z.X.; Pan, L.B.; Cong, L.; Han, P.; Peng, R.; Yu, H.; Lin, Y.; et al. Oral berberine improves brain dopa/dopamine levels to ameliorate Parkinson’s disease by regulating gut microbiota. Signal Transduct. Target. Ther. 2021, 6, 77. [Google Scholar] [CrossRef]
- Elton, A.; Faulkner, M.L.; Robinson, D.L.; Boettiger, C.A. Acute depletion of dopamine precursors in the human brain: Effects on functional connectivity and alcohol attentional bias. Neuropsychopharmacology 2021, 46, 1421–1431. [Google Scholar] [CrossRef] [PubMed]
- Ferlazzo, A.; Espro, C.; Iannazzo, D.; Bonavita, A.; Neri, G. Yttria-zirconia electrochemical sensor for the detection of tyrosine. Mater. Today Commun. 2023, 35, 106036. [Google Scholar] [CrossRef]
- Xu, Z.; Qiao, X.; Tao, R.; Li, Y.; Zhao, S.; Cai, Y.; Luo, X. Biosensors and Bioelectronics A wearable sensor based on multifunctional conductive hydrogel for simultaneous accurate pH and tyrosine monitoring in sweat. Biosens. Bioelectron. 2023, 234, 115360. [Google Scholar] [CrossRef] [PubMed]
- Gan, A.W. Ion transfer voltammetry of amino acids with an all-solid-state ion-selective electrode for non-destructive phenylalanine sensing. Electroanalysis 2023, 35, e202200501. [Google Scholar] [CrossRef]
- Ferlazzo, A.; Espro, C.; Iannazzo, D.; Neri, G. Determination of Phenylalanine by a Novel. IEEE Trans. Instrum. Meas. 2023, 72, 1–8. [Google Scholar] [CrossRef]
- Maria, A.; Martins, G.; Wilkins, M.D.; Ligler, F.S.; Daniele, M.A.; Freytes, D.O. Microphysiological System for High-Throughput Computer Vision Measurement of Microtissue Contraction. ACS Sens. 2021, 6, 985–994. [Google Scholar] [CrossRef]
- Baradoke, A.; Juodkazyte, J.; Masilionis, I.; Selskis, A.; Pauliukaite, R.; Valiokas, R. Combined soft lithographic and electrochemical fabrication of nanostructured platinum microelectrode arrays for miniaturized sensor applications. Microelectron. Eng. 2019, 208, 39–46. [Google Scholar] [CrossRef]
- Cėpla, V.; Rakickas, T.; Stankevičienė, G.; Mazėtytė-Godienė, A.; Baradokė, A.; Ruželė, Ž.; Valiokas, R. Photografting and Patterning of Poly(ethylene glycol) Methacrylate Hydrogel on Glass for Biochip Applications. ACS Appl. Mater. Interfaces 2020, 12, 32233–32246. [Google Scholar] [CrossRef]
- Šljukić, B.; Banks, C.E.; Compton, R.G. An overview of the electrochemical reduction of oxygen at carbon-based modified electrodes. J. Iran. Chem. Soc. 2005, 2, 1–25. [Google Scholar] [CrossRef]
- Dhakate, S.R.; Bahl, O.P. Effect of carbon fiber surface functional groups on the mechanical properties of carbon-carbon composites with HTT. Carbon 2003, 41, 1193–1203. [Google Scholar] [CrossRef]
- Santos Miranda, M.E.; Marcolla, C.; Rodriguez, C.A.; Wilhelm, H.M.; Sierakowski, M.R.; Belle Bresolin, T.M.; de Freitas, R.A. Chitosan and N-carboxymethylchitosan: I. The role of N-carboxymethylation of chitosan in the thermal stability and dynamic mechanical properties of its films. Polym. Int. 2006, 55, 961–969. [Google Scholar] [CrossRef]
- Nicholson, R.S. Theory and Application of Cyclic Voltammetry for Measurement of Electrode Reaction Kinetics. Anal. Chem. 1965, 37, 1351–1355. [Google Scholar] [CrossRef]
- Randviir, E.P. A cross examination of electron transfer rate constants for carbon screen-printed electrodes using Electrochemical Impedance Spectroscopy and cyclic voltammetry. Electrochim. Acta 2018, 286, 179–186. [Google Scholar] [CrossRef]
- Siraj, N.; Grampp, G.; Landgraf, S.; Punyain, K. Cyclic Voltammetric Study of Heterogeneous Electron Transfer Rate Constants of Various Organic Compounds in Ionic liquids: Measurements at Room Temperature. Z. Für Phys. Chem. 2013, 227, 105–120. [Google Scholar] [CrossRef]
- Lavagnini, I.; Antiochia, R.; Magno, F. An Extended Method for the Practical Evaluation of the Standard Rate Constant from Cyclic Voltammetric Data. Electroanalysis 2004, 16, 505–506. [Google Scholar] [CrossRef]
- Fanjul-Bolado, P.; Hernández-Santos, D.; Lamas-Ardisana, P.J.; Martín-Pernía, A.; Costa-García, A. Electrochemical characterization of screen-printed and conventional carbon paste electrodes. Electrochim. Acta 2008, 53, 3635–3642. [Google Scholar] [CrossRef]
- Yi, Y.; Weinberg, G.; Prenzel, M.; Greiner, M.; Heumann, S.; Becker, S.; Schlögl, R. Electrochemical corrosion of a glassy carbon electrode. Catal. Today 2017, 295, 32–40. [Google Scholar] [CrossRef]
- Khan, A.F.; Brownson, D.A.C.; Randviir, E.P.; Smith, G.C.; Banks, C.E. 2D Hexagonal Boron Nitride (2D-hBN) Explored for the Electrochemical Sensing of Dopamine. Anal. Chem. 2016, 88, 9729–9737. [Google Scholar] [CrossRef] [Green Version]
- Asif, A.; Heiskanen, A.; Emnéus, J.; Keller, S.S. Pyrolytic Carbon Nanograss Electrodes for Electrochemical Detection of Dopamine. Electrochim. Acta 2021, 379, 138122. [Google Scholar] [CrossRef]
- Shrivastava, A.; Gupta, V. Methods for the determination of limit of detection and limit of quantitation of the analytical methods. Chron. Young Sci. 2011, 2, 21. [Google Scholar] [CrossRef]
- Richter, E.M.; Rocha, D.P.; Cardoso, R.M.; Keefe, E.M.; Foster, C.W.; Munoz, R.A.A.; Banks, C.E. Complete Additively Manufactured (3D-Printed) Electrochemical Sensing Platform. Anal. Chem. 2019, 91, 12844–12851. [Google Scholar] [CrossRef] [PubMed]
- How, G.T.S.; Pandikumar, A.; Ming, H.N.; Ngee, L.H. Highly exposed {001} facets of titanium dioxide modified with reduced graphene oxide for dopamine sensing. Sci. Rep. 2014, 4, 2–9. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Lin, X. Covalent modification of glassy carbon electrodes with glycine for voltammetric separation of dopamine and ascorbic acid. Anal. Bioanal. Chem. 2001, 370, 956–962. [Google Scholar] [CrossRef] [PubMed]
- Emadoddin, M.; Mozaffari, S.A.; Ebrahimi, F. An antifouling impedimetric sensor based on zinc oxide embedded polyvinyl alcohol nanoplatelets for wide range dopamine determination in the presence of high concentration ascorbic acid. J. Pharm. Biomed. Anal. 2021, 205, 114278. [Google Scholar] [CrossRef]
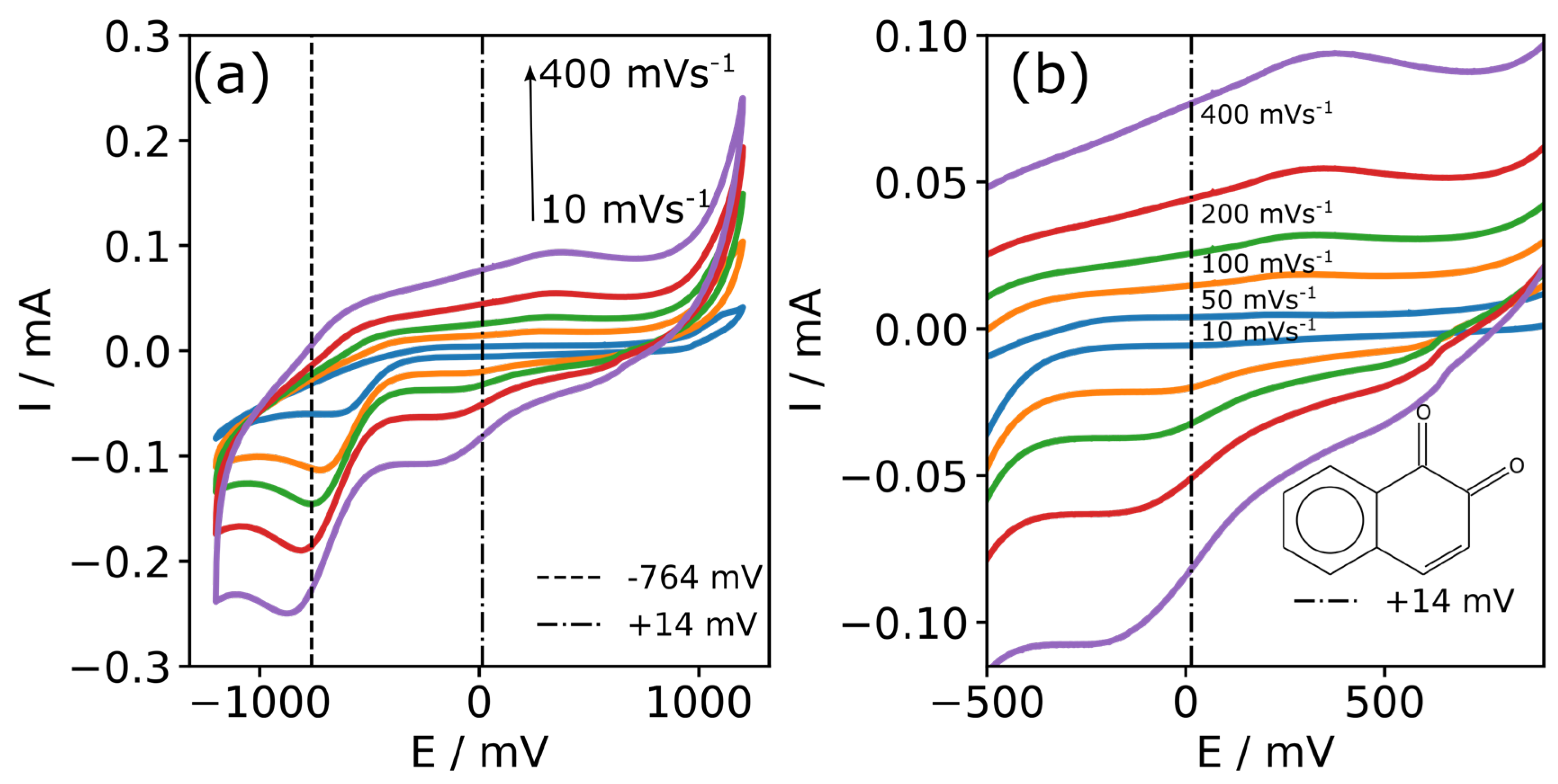

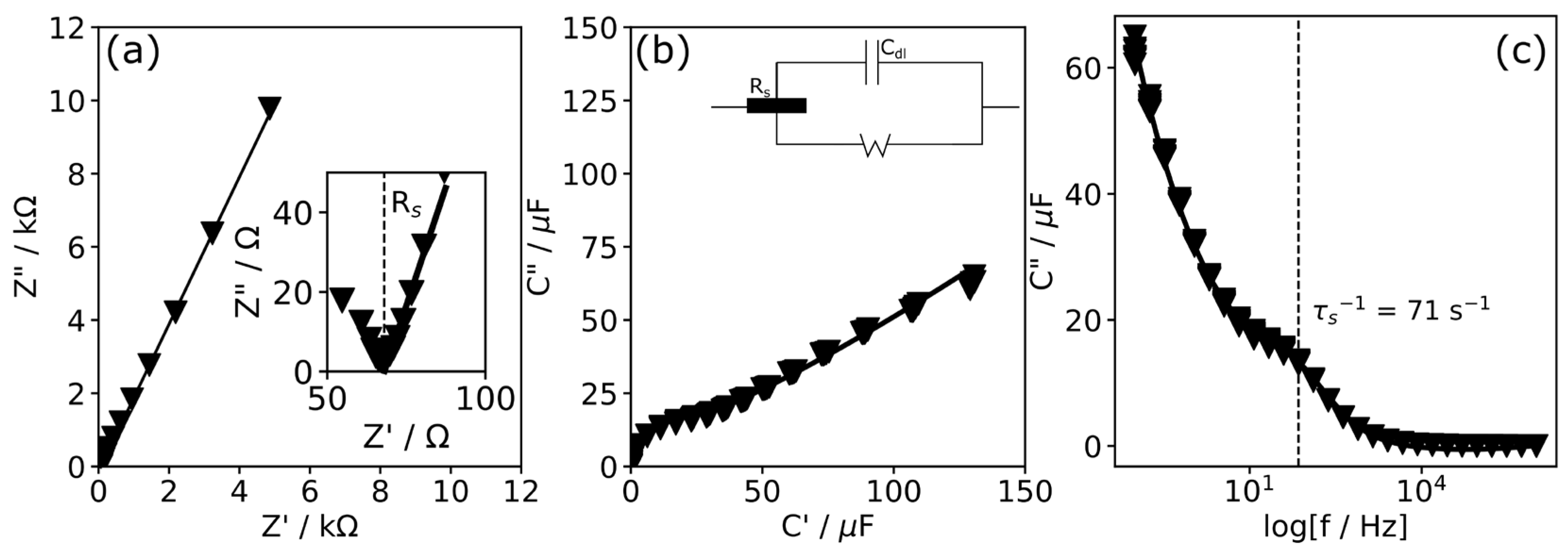
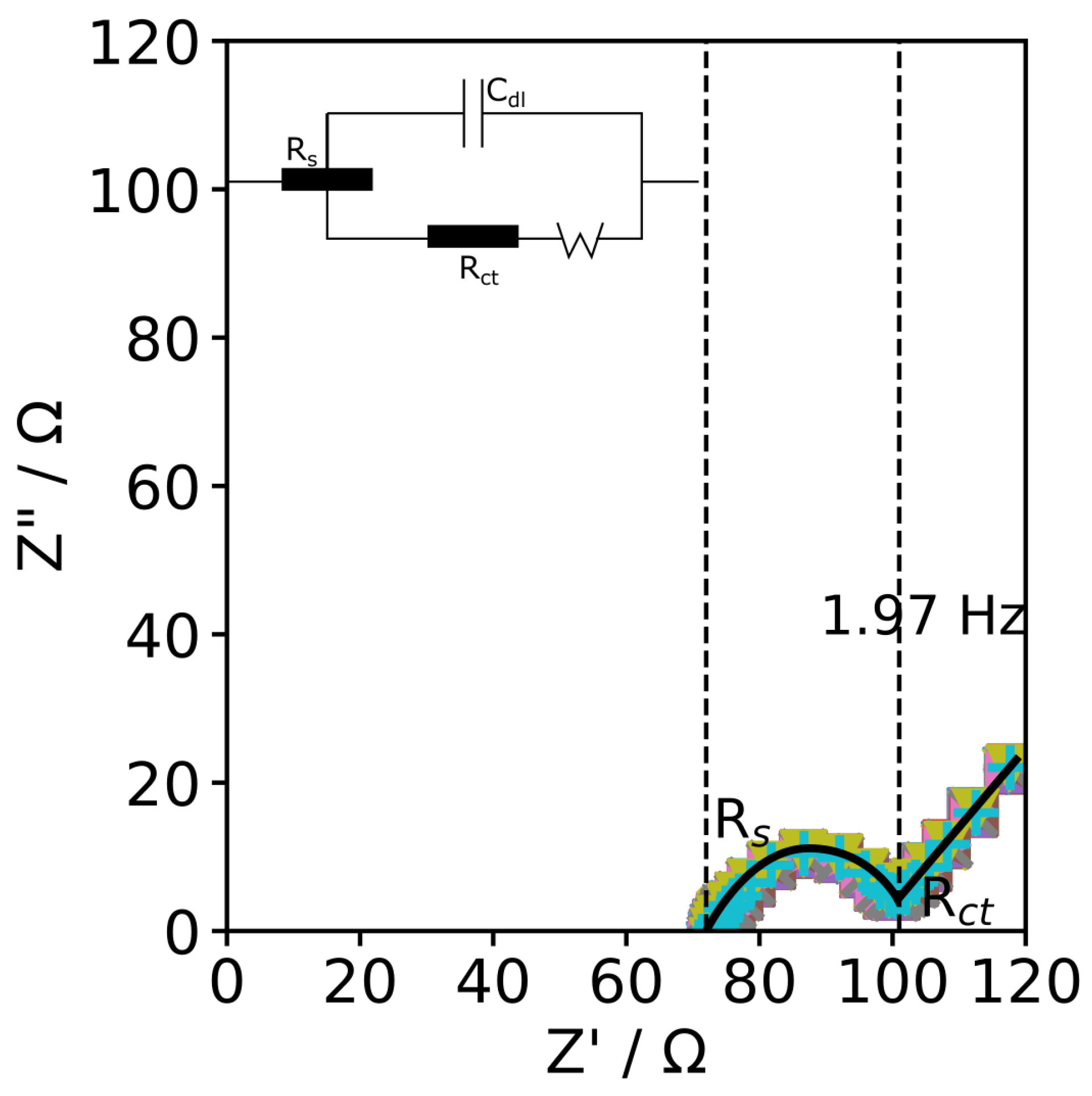
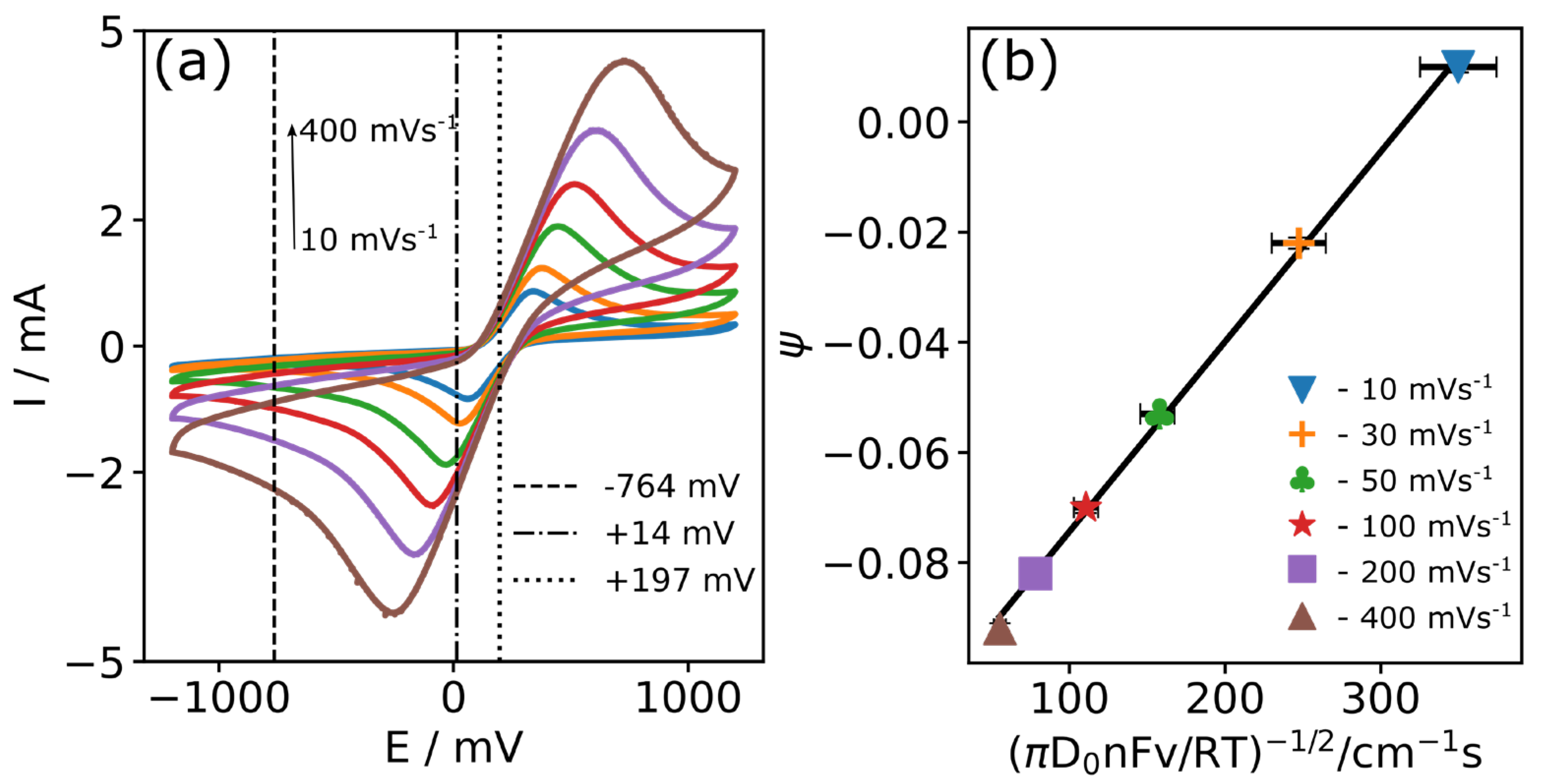
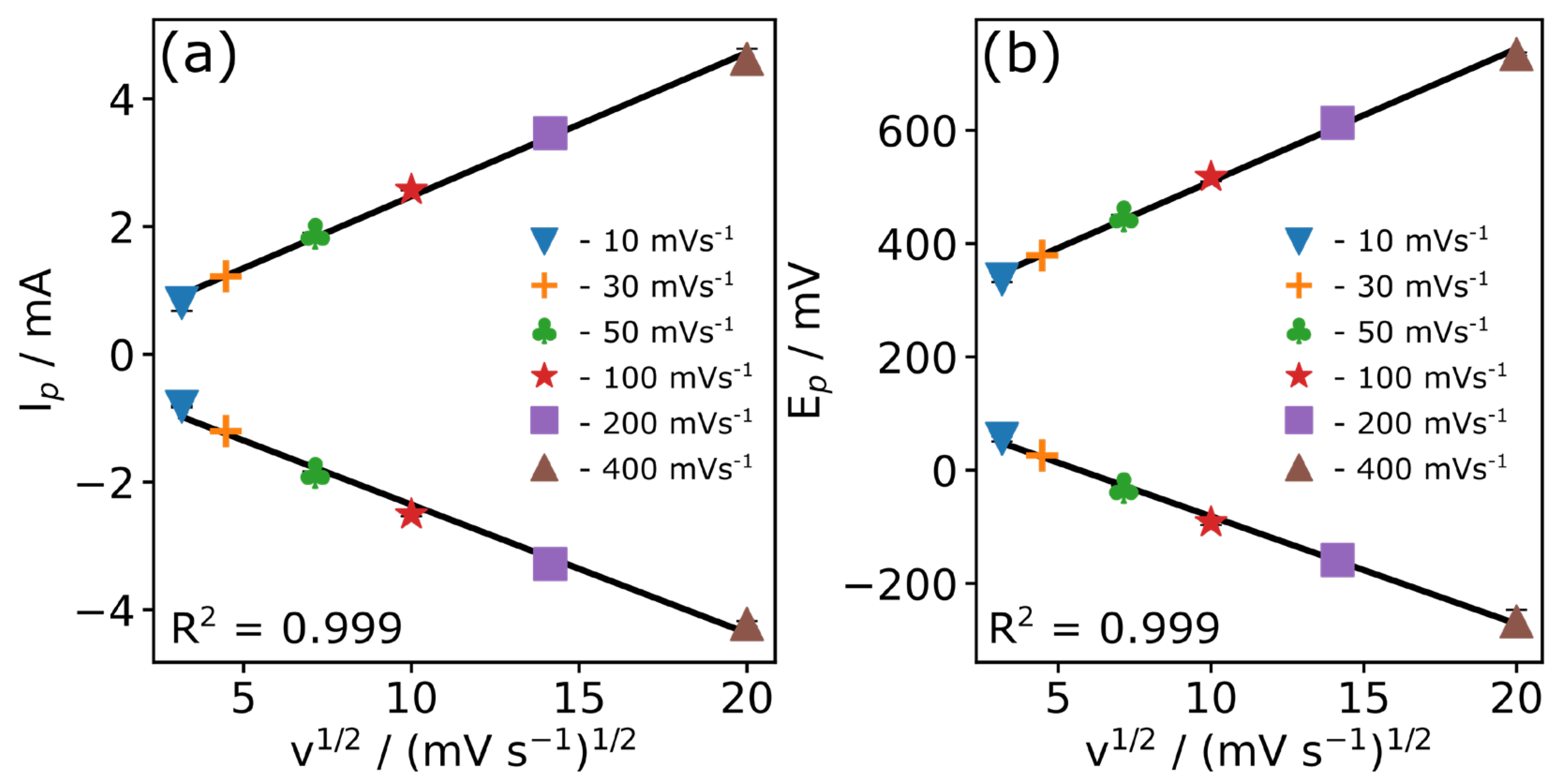

| v (mV s−1) | Epa (mV) | Epc (mV) | Ipa (mA) | Ipc (mA) |
|---|---|---|---|---|
| 10 | 55.2 ± 4.6 | 336.9 ± 5.1 | −0.821 ± 0.015 | 0.803 ± 0.125 |
| 20 | 25.9 ± 0.1 | 378.9 ± 1.6 | −1.205 ± 0.021 | 1.221 ± 0.023 |
| 50 | −31.7 ± 4.0 | 447.9 ± 2.7 | −1.858 ± 0.025 | 1.887 ± 0.016 |
| 100 | −91.9 ± 5.0 | 517.7 ± 7.9 | −2.508 ± 0.028 | 2.576 ± 0.010 |
| 200 | −158.7 ± 11.4 | 613.3 ± 16.2 | −3.283 ± 0.028 | 3.463 ± 0.052 |
| 400 | −267.1 ± 20.5 | 734.4 ± 3.0 | −4.222 ± 0.044 | 4.627 ± 0.157 |
| v (mV s−1) | ΔEp (mV) | E0 (mV) | −Ipc/Ipa | (πD0nFv/RT)−1/2 | Ψ |
|---|---|---|---|---|---|
| 10 | 281.7 ± 2.1 | 196.1 ± 4.8 | 0.976 ± 0.138 | 349.845 ± 24.560 | 0.010 ± 0.001 |
| 20 | 353.0 ± 1.7 | 202.4 ± 0.8 | 1.013 ± 0.004 | 247.378 ± 17.367 | −0.022 ± 0.001 |
| 50 | 479.6 ± 1.7 | 208.1 ± 3.3 | 1.016 ± 0.005 | 156.455 ± 10.984 | −0.053 ± 0.001 |
| 100 | 609.6 ± 12.7 | 212.9 ± 2.0 | 1.027 ± 0.015 | 110.631 ± 7.767 | −0.070 ± 0.001 |
| 200 | 772.1 ± 25.7 | 227.3 ± 5.7 | 1.055 ± 0.025 | 78.228 ± 5.492 | −0.082 ± 0.002 |
| 400 | 1001.5 ± 19.8 | 233.7 ± 10.8 | 1.096 ± 0.048 | 55.315 ± 3.883 | −0.092 ± 0.001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Elsakova, A.; Merzlikin, M.; Jafarov, A.; Zilinskaite, N.; Sulciute, A.; Baradoke, A. Examination of Non-Modified Carbon Fibre Bundle as an Electrode for Electrochemical Sensing. Coatings 2023, 13, 1372. https://doi.org/10.3390/coatings13081372
Elsakova A, Merzlikin M, Jafarov A, Zilinskaite N, Sulciute A, Baradoke A. Examination of Non-Modified Carbon Fibre Bundle as an Electrode for Electrochemical Sensing. Coatings. 2023; 13(8):1372. https://doi.org/10.3390/coatings13081372
Chicago/Turabian StyleElsakova, Alexandra, Mark Merzlikin, Ali Jafarov, Nemira Zilinskaite, Agne Sulciute, and Ausra Baradoke. 2023. "Examination of Non-Modified Carbon Fibre Bundle as an Electrode for Electrochemical Sensing" Coatings 13, no. 8: 1372. https://doi.org/10.3390/coatings13081372