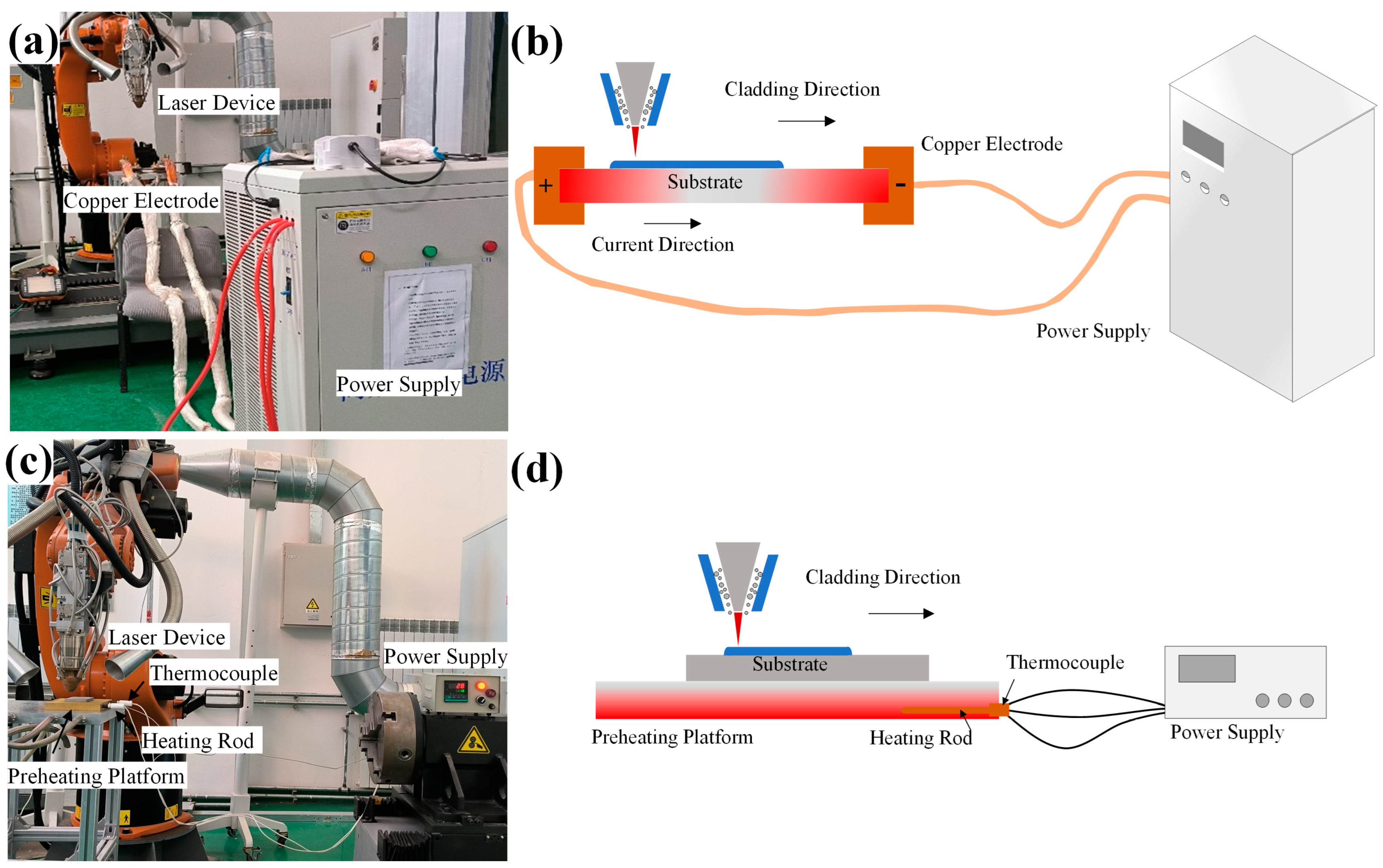
3.1. Phase Analysis
The XRD diffraction patterns of the Co-based coatings under different conditions are displayed in
Figure 2. It can be seen from
Figure 2a that the coating in the absence of treatment mainly comprised γ-Co, α-Ti, COTi
2 solid solution, TiC, and Cr
7C
3 hard phases, and it was similar to the composition of the coating prepared with the pulsed current, as shown in
Figure 2a. The coating with EAPH and CPH led to no significant differences, as shown in
Figure 2b, indicating that the pulsed current did not significantly affect the phase composition of the coating. Nevertheless, the formation of high-strength hard phases directly governs the diverse mechanical properties of the coating. Two strong diffraction peaks were observed at 37~40° in the XRD diffraction pattern, indicating the presence of more α-Ti and γ-Co in the coating. This could be attributed to the dilution of the substrate, which allowed Ti to enter the coating and form solid solutions and enhanced phases with C and Co, while the unreacted Ti remains in the coating.
At room temperature, pure Co exists as ε-Co, which has a hexagonal close-packed (hcp) structure. Upon reaching 417 °C, ε-Co transforms isomerically into γ-Co, which has a face-centered cubic (fcc) structure that is stable at a high temperature. ε-Co transforms isomerically into a face-centered cubic (fcc) γ-Co at 417 °C; however, the rapid and non-equilibrium melting and solidification during laser cladding hinder the transformation into the γ-Co. Moreover, the presence of elements such as Ni and Cr can lower the phase transition temperature and stabilize the fcc structure of Co. As a result, a significant amount of sub-stable γ-Co phase was retained in the coating at room temperature [
20]. Additionally, the similar atomic radii of Cr and Co serve to stabilize the γ-Co cubic dot structure. However, pure titanium also undergoes allotropic transformation, from the hcp structured α-Ti to the body-centered cubic (bcc) structured β-Ti phase at elevated temperatures up to 882.5 °C. In contrast to Co, the precipitation of β-Ti out of the α-phase does not properly proceed during rapid solidification. This is due to the fact that the crystal structure transformation of β-Ti is less affected by the cooling rate, making it difficult to suppress. The allotropic transformation of Ti still occurred in the coatings, generating martensite, i.e., a supersaturated solid solution α′-Ti of the hcp structure, which was the α-Ti detected during XRD diffraction.
3.2. Microstructure Analysis
Figure 3 shows the cross-section morphology and EDS line sweep distribution of coatings under various parameters. The macroscopic morphology of the coatings reveal that the coatings were divided into two areas: a corrosion-resistant area with a lower degree of lining; and a less corrosion-resistant white bright zone located at the bottom of the bonding area, which indicated that the coating and substrate achieved good metallurgical bonding [
21]. Meanwhile, the number of unmelted particles on the surface of the coating with EAPH increased, and a similar phenomenon was observed in the CPH coating, as shown in
Figure 3c. This might be attributed to the powder feeding process, where powder splashed onto the previously scanned area in the residual high-temperature region and micro-melting of powder particles occurred. Additionally, preheating increased the surface temperature of the coating, leading to an increase in the volume of melted powder particles that more easily adhered to the coating surface.
The EDS line scan results in
Figure 3 demonstrate that the coating mainly consisted of Ti, Co, Cr, Si, C, and W. The Ti content is significantly higher than that of Co and Cr in all regions of the coating, indicating the strong convection generated in the molten pool during the laser cladding process that carried the Ti element from the CP-Ti substrate to the coatings. Moreover, the coatings exhibited significant elemental inhomogeneity, with Ti showing large fluctuations and a sharp increase in the bonding region. The distribution of other elements was similar to that of Ti, but their trend of change in the bonding region was opposite. There is a small peak of the C element content in the binding zone, indicating that the C element is mainly distributed at the bottom of the coatings. The strong dilution effect caused the high content of Ti elements at the bottom of the coating, which has a good affinity for C, to facilitate the formation of TiC [
22]. The solid–liquid interface in the combined area advanced rapidly, and the generated TiC was quickly crystallized and deposited. Generally, the degree of element fluctuation is related to the grain size. Smaller grains result in a more uniform distribution of elements and a lower degree of compositional segregation. The elemental distributions of the coatings are compared in
Figure 3, and it was found that EAPH significantly reduced elemental fluctuations, particularly for Ti, compared to both the unassisted and CPH coating. Additionally,
Figure 3b,c demonstrate that the elemental fluctuation of the coating with EAPH was significantly smaller than that of the coating with CPH at the same temperature, which illustrated the non-thermal effect of the current on the refinement of the organization.
Figure 4 illustrates the microstructure of the unassisted coatings. In
Figure 4, the unassisted coating exhibited mainly upward-growing columnar dendrites at the bottom, with the growth direction perpendicular to the bonding line, along with a few cellular and equiaxed crystals interspersed between the dendrites. The middle region was mostly composed of disordered columnar and cellular crystals, with a few equiaxed crystals. The proportion of equiaxed crystals increased towards the top, with grains significantly finer than those in the middle region. Moreover, a significant number of dendritic precipitates resulting from the convergence of dotted spheres were distributed on the substrate. The solidification structure is determined by the temperature gradient (G) and the solidification rate (R) at the solid–liquid interface front, as proposed by the component subcooling theory [
23]. During the non-equilibrium solidification process of laser cladding, the bonding zone was in contact with the lower-temperature substrate, leading to a large heat loss through heat conduction, resulting in significant subcooling at the bottom of the molten pool. The solidification structure at the fusion interface was rapidly nucleated and grew in the opposite direction of the heat flow. As solidification progressed, R increased while G decreased, which reduced the G/R value. Compared to the bonding interface, the limited heat transfer rate in molten pool resulted in the development of coarse columnar and cellular crystals. The upper surface and both sides of the cladding layer were in contact with air, while the bottom mainly relied on heat transfer for dissipation. As a result, temperature gradients of varying sizes existed in different directions within the middle. The growth of grains competed under different constraints, leading to a significant variation in orientations and crystal morphology. Upon reaching the top, the molten pool experienced enhanced heat dissipation due to thermal radiation and air. This led to a large solidification rate and free growth of the interface, resulting in an equiaxed crystal.
As shown in
Figure 5, the microstructure of the Co-based coating with EAPH is significantly different from that of the unassisted coating. In
Figure 5(a1), the columnar dendrites, which had originally been coarse and highly directional, grew in different directions, with the dendrite secondary arm being broken and fractured at the bottom. Furthermore, a greater number of small equiaxed grains appeared at the growth front of the dendrites. The morphology of the grains in the middle and upper parts was similar, but a small amount of disordered morphology and a whisker-like structure could be observed. The area of the whisker-like structure increased with the higher current and preheating temperature, as shown in
Figure 5. At 960 A, a considerable amount of a whisker-like structure appeared beneath the dendrites in the bonding zone, exhibiting a dense microstructure that enabled a smooth transition and metallurgical bonding, in contrast to the coarser dendrites. The tentacles in the middle lower regions of the coating became significantly finer, and the intergranular gap became narrower. At the top, equiaxed crystal bands with a grain size of about 1 μm were observed. Nonetheless, the increased preheating temperature resulted in a higher heat accumulation, which led to slower heat dissipation in the melt pool and crystal solidification speed, resulting in a lower distribution of fine grains.
The current of EAPH forced the thermal growth pattern within the molten pool, resulting in the formation of finer columnar dendrites and cellular crystals with dispersed orientation, distinct from the unassisted coating. Electromagnetic stirring induced by the pulsed current only affected liquid, which had a limited stirring effect during the brief duration of the molten pool, while preheating increased the temperature of the molten pool and extended nucleation. The broken dendrite arms at the bottom and the melt at the top continued to grow, thus mitigating the tissue refinement effect, resulting in the presence of coarse cellular crystals. Although there were more equiaxed grains in the middle and top, improving the microstructure uniformity of the coating, the high-frequency electric field had a less significant effect on the upper middle of the coating compared to the dendrite breaking at the bottom of the coating. This was mainly due to the distribution characteristics of the electric field. While the skin effect of the pulsed current allowed for the concentration of the current at the surface of the conductor, the small coating area relative to the cross-section of substrate limited the flow of the current and weakened the electromagnetic stirring effect. Moreover, for metals with similar compositions, the current runs preferentially through the solid due to the higher resistivity of the liquid [
17]. Consequently, the current density increased at the bottom of the molten pool, where solidification occurred faster.
Figure 6 illustrates the microstructure of Co-based coatings under CPH. At 110 °C and 160 °C, the structure of each part of the coating exhibited little difference from that of the unassisted Co-based coating, with a transition from columnar dendrites to both cellular and equiaxed crystals from bottom to top. The increase in the preheating temperature led to longer columnar dendrites growing at the bottom. This was attributed to the prolonged cooling time of the molten pool caused by preheating. As a consequence, the advancement of the solid–liquid interface at the bottom of the coating slowed down, which extended the epitaxial growth time of the grains. Additionally, the number of fine equiaxed grains at the bottom of the coating gradually increased. Some dendrites were also observed in the middle of the coating (
Figure 6(c2,d2)). The distribution of grains at the top gradually became uneven, with coarser cellular grains. Comparing
Figure 5 and
Figure 6, we see that there was an increase in fine equiaxed grains at the top of the coatings with EAPH. While the structure was somewhat disorganized in the middle of the coating with CPH, there was no whisker-like organization, similar to that found in current-assisted coatings. Additionally, numerous dendritic precipitates were distributed throughout the coatings, and the size of precipitates increased as the preheating temperature increased, particularly in the central region of the coatings. However, the dendritic structure is smaller at EAPH than at the same temperature of CPH. Meanwhile, in the whisker-like organization produced by the current assistance, the increased number of grain boundaries made it difficult for the point spherical precipitates to come together, which mostly manifest as thin strips or granules.
Table 4 presents the average grain size of the top and middle of each coating, measured using the linear intercept method. The grain size exhibited a U-shaped trend with the increasing current, decreasing first and then increasing. Additionally, increasing the preheating temperature led to an increase in grain size. This indicates that the high-frequency current has a positive effect on grain refinement, but the Joule heat generated by the current can coarsen the grains. Therefore, an optimal external current exists.
Figure 7 shows the results of the distribution of alloy elements in a typical dendritic structure via an EDS map analysis. As illustrated in
Figure 7, Ti, Co, Cr, Si, W, and C elements were the primary elements distributed throughout the zone. The Ti element was diffusely distributed in the micro-zone and enriched in the dendritic structure. The coatings were diluted with matrix elements due to the convection caused by heat transfer during laser cladding, which led to the detection of Ti throughout the coating. The SEM image at high magnification in
Figure 7 reveals that the dendritic precipitates were composed of ellipsoidal particles, which were mainly distributed along the grain boundaries and linearly precipitated along them. Based on the XRD and the distribution of Ti and C elements, the dendritic precipitates were identified as TiC. Furthermore, the SEM images reveals a diffuse distribution of extensive in situ synthesized TiC within the coatings. TiC could deform plastically at a high temperature. The greater presence of TiC in the coatings near the interface effectively prevented fatigue cracks, enhanced the bonding strength between the substrate and the coating, and improved the workpiece’s fatigue resistance. The uniform distribution of TiC was crucial for improving the microhardness and wear resistance of the coatings. Each equiaxed and cellular crystal was enriched with Co, Cr, Si, and W elements. However, the Si and W elements were scarce at the grain boundaries. Outside the TiC dendrites, Co, Cr, and Ti elements are uniformly distributed, with relatively fewer C elements. The grain boundaries were mainly composed of γ-Co and α-Ti, whereas the grains were predominantly composed of CoTi
2 solid solutions and carbides such as Cr
3C
7.
3.3. Current Refinement Mechanism
The schematic diagram in
Figure 8a illustrates nucleation growth at the bottom of the molten pool in laser cladding, where greater subcooling of the larger components drove the formation of more columnar dendrites. The introduction of a pulsed current in laser cladding induced a periodic variation of the pulsed current that generated a magnetic field. The interaction between the induced magnetic field and the current led to the distribution of the Lorentz force in the molten pool, and the alternating electromagnetic force aggravated the Marangoni convection at the bottom, which lead to the stress concentrations at the roots and secondary arms of growing dendrites, resulting in fragmentation [
24], as illustrated in
Figure 8b. Then, the independent fractured columnar dendrites were transported by forced convection to the inside of the molten pool, resulting in the finer grains being displayed in the lower and middle regions, as illustrated in
Figure 8c. Meanwhile, convection carried the high-temperature melt from the upper part of the molten pool to the bottom, causing the dendrite tip and root to remelt, hindering the growth of columnar dendrites and leading to the fracture of dendrites [
25]. Additionally, the front end of the columnar dendrites concentrated the Joule heat effect, which made it easy to break in the collision of the dendrite, and then underwent remelting and recrystallization [
26], playing a role in grain refinement. Because of the magnitude of the electromagnetic force and the limited action time, some thick primary dendrites were not broken, while only the root of the secondary dendrite arm fractured, as shown in
Figure 4(a1).
The periodic high-frequency pulsed current induced electromagnetic stirring during the laser cladding process, which affected the microstructure in the molten pool. The Lorentz force caused the melts to collide with each other, leading to the shearing of melts and fragmentation of columnar crystals and secondary dendrites at the bottom of the molten pool. The broken fine crystals were then dispersed into the molten pool due to strong convection and re-nucleated, increasing the nucleation rate and refining the grains [
27]. Preheating increased the molten pool temperature, which intensified the convection. This effect was further enhanced by electromagnetic forces, and then it altered the unidirectional heat and slow mass transfer during solidification. In the rapid diffusion of the solute, the temperature of the molten pool became more homogeneous, leading to a decrease in the temperature gradient [
28]. As a result, constitutional supercooling in the solution increased. According to the nucleation theory, increasing the constitutional supercooling enhances nucleation drive. Fractured fine dendrites entered the molten pool and formed new nuclei, thus increasing the rate of non-homogeneous nucleation within the molten pool [
29].
Besides the effect of mechanical scouring induced by the electromagnetic force on the nucleation rate, the current density in the molten pool is also believed to influence it. The uniform nucleation rate in the coatings can be expressed by the electric density as follows [
17,
24]:
where
Ie is the nucleation rate,
I0 is the nucleation rate factor, Δ
G is the Gibbs free energy,
A and
B are parameters related to materials,
J is electric density,
k is the Boltzmann constant,
T is the absolute temperature, and
t is electrifying time. Formula (1) indicates that when the electric density,
J, exceeds
, the nucleation rate is
Ie >
I0. Therefore, an appropriate electric density can enhance the nucleation rate during the solidification process in the molten pool and refine the grain size. In addition, the solid phase that precipitated first at the bottom created a thermoelectric potential difference with the liquid phase [
30], leading to a greater electric density in the bottom of the coating where the molten pool solidified faster. Therefore, the effect of fine crystallization at the bottom of the coating was significant.
J.D. Hunt proposed the critical condition for the transformation of columnar crystals into equiaxed crystals [
31].
where
G is the temperature gradient,
N0 is the non-homogeneous nucleation rate, Δ
TN is the undercooling at the heterogeneous nucleation temperature, and Δ
TC is the undercooling of columnar front. From the previous analysis, it was observed that the application of pulsed current significantly increased
N0, decreased
G, and increased Δ
TC, which effectively promoted the transformation of columnar crystals to equiaxed crystals, refined the grain size, and improved the coating’s mechanical properties. In contrast, the unassisted coating exhibited the rapid growth of columnar crystals at the bottom due to the large temperature gradient along the solidification front. The transition from columnar to isometric crystals did not occur until at the middle or top of the coating.
3.4. Microhardness
The microhardness distribution curves for each coating cross-section are presented in
Figure 9. The hardness profile can be divided into three zones: the harder cladding zone, the bonding zone with a decreasing trend, and the substrate zone. As shown in
Figure 9, The microhardness of each coating was significantly higher than that of the substrate, with the unassisted coating having the highest hardness of 948.51 HV
1, which was approximately 5.6 times greater than the substrate’s microhardness of 170 HV
1. The hardness of the coating initially increased and then decreased from the surface to the substrate. The low surface microhardness was attributed to the complex thermal convection occurring inside the molten pool during the cladding process. Impurities rose to the surface of the coatings and formed a relatively loose microstructure. However, the thickness of the affected area was small, and it can be eliminated by mechanical processing before usage, without impacting the performance of the coatings. Excessive dilution near the bonding zone caused a reduction in hardness. While Ti, Co, C, and other elements can form hard reinforcements, the liquid Ti in the bonding zone solidified before it could fully react, resulting in a reduced degree of strengthening by mechanisms such as solid solution and dispersion strengthening. Consequently, the central region of the clad layer usually exhibits a good cladding effect with high hardness.
Figure 9a shows that the coating’s highest hardness increased as the pulsed current was applied and the region of high hardness expanded, reaching a maximum hardness of 1032.68 HV
1 at 720 A. However, as the current increased further, the coating’s hardness decreased. The microstructure and phase composition have a significant impact on the hardness of the laser clad coating [
32]. The main hard phases, Cr
7C
3 and TiC, have hardness values of 1580–1800 HV [
33] and 3200 HV, respectively. The microstructure analysis showed that while the grain refinement in the middle of the coating increased with the current, the segregation of TiC at the grain boundaries intensified, causing TiC particles to become larger and resulting in a decrease in the hardness of the coatings due to decreased dispersion strengthening effect. The microhardness of the coatings after CPH ranged from 800 to 1000 HV
1. At 200 °C, the preheated coating had reduced hardness in the top due to large cracks and weakened diffusion of the Ti element.
Figure 9a,b show no significant difference in hardness between the top regions of CPH and EAPH coatings. This is because the external environment affected the solidification microstructure on the surface of the coating, while each auxiliary condition had only a negligible impact on the surface grains, resulting in insignificant differences in microhardness. However, the hardness at the bottom of the 600 A and 720 A current-assisted coatings was considerably higher due to the presence of more equiaxed crystals and hard phases at the bottom. Finer grains were predicted to have fewer intragranular defects and dislocations, making the microstructure less susceptible to dislocation plugging and crosscutting under external loads. This required greater force to move the dislocations, indicating an improvement in hardness. The results and analysis above demonstrated the effect of the current in fragmenting the dendrites and refining the grains.
3.5. Wear Resistance
The friction coefficient curves for CP-Ti substrate and the coatings obtained for each test parameter during dry friction wear testing, as well as the amount of wear, are displayed in
Figure 10. The coefficient of friction of the coating is lower than that of the CP-Ti substrate, with or without auxiliary treatment, as shown by the friction curve in
Figure 10.
Figure 10 demonstrates that both the substrate and the coatings for each parameter experienced a wear-in phase and a stable wear phase, with varying fluctuations in the early stages of wear that eventually level off. The CP-Ti substrate had a longer and more fluctuating wear time. This is because CP-Ti had an oxide film on its surface during the early stage of wear. The friction coefficient was low initially but increased as the wear progressed. As the wear progressed, the oxide film flaked off and formed abrasive particles. The abrasive particles slid along the side walls of the grooves formed during wear until they reached the contact point. The pressure of the Si
3N
4 friction pair on the substrate cut the surface and produced plough grooves. This resulted in a rough substrate surface and an increase in the coefficient of friction. As the width of the abrasion marks increased, abrasive chips were more easily released to the sides, weakening abrasive wear and smoothing the friction coefficient. The average coefficient of friction for CP-Ti was approximately 0.406, while that of the untreated Co-based coating was approximately 0.389, slightly lower than that of the substrate. This was mainly due to in situ reactions in the coating that generated hard phases, such as TiC and Cr
7C
3, which supported the frictional load and allowed the coating to resist pressure from abrasive particles and pairs [
34]. As a result, the abrasion mark narrowed, the contact area between the specimens and abrasive pairs reduced, the resistance to sliding of the friction pair diminished, movement became easier, and the coefficient of friction decreased [
35], leading to an increase in abrasion resistance; thereby, the coating was protected.
Figure 10b,c shows that the friction coefficients of the coatings were reduced by EAPH and CPH, and the friction coefficient during the steady wear stage was consistently around 0.3–0.35. The average friction coefficient of the coatings decreased with the increase of the current and reached the minimum value of approximately 0.320 at 720 A. At a lower preheating temperature, the friction coefficient of the coatings remained relatively stable during the wear process and gradually increased. This phenomenon can be explained as follows: during the early stage of wear, the surface roughness of the wear specimen was small due to pretreatment, and the increase in hardness compared to the unassisted coating resulted in greater resistance to the friction partner and a lower coefficient of friction. As the friction process proceeded, some abrasive debris was eliminated as the friction pair slides, but high-hardness debris, such as TiC, could become trapped between the friction pair and the coating, causing an increase in the coating’s friction coefficient due to their abrasive action [
36]. The friction coefficient of coatings obtained at 250 °C preheating experienced sharp fluctuations, which may be attributed to the deterioration of the coating quality at the higher preheating temperature and the increased probability of microcrack formation between internal reinforcing phases and the matrix due to coarse grains [
9]. The coatings chipped under a higher load, leading to fluctuations in the coefficient of friction due to larger metal fragments.
As shown in
Figure 10d, the unassisted coating had a wear weight loss of 7.13 mg, which was approximately 39.4% less than that of CP-Ti. With the assistance of EAPH, the wear weight loss of the coating was further reduced by approximately 80% at 720 A compared to the unassisted coating. However, as the current increased, the wear also increased, and the wear performance deteriorated. The observation was consistent with the friction coefficient results that specimens with a lower friction coefficient also exhibited a lower wear loss [
37]. Moreover, CPH also led to different degrees of reduction in the coatings, following a similar trend as EAPH. At 960 A and a corresponding preheating temperature of 250 °C, the average friction coefficient and wear loss of the coatings were comparable to those of the untreated coating, indicating that an appropriate preheating temperature can effectively improve the coating quality and frictional wear performance. The results also suggested that the Joule heating effect had a greater impact on wear performance to other current effects.
Figure 11 shows the wear morphology of the Co-based coating with different auxiliary treatment. The wear profile of the Co-based coating without assistance is presented in
Figure 11a. The wear surface exhibited significant fragmentation and spalling, with shallow and discontinuous furrows, which suggested the presence of microcutting. On the one hand, a discrepancy in thermal properties between the Co-based powder and CP-Ti led to the formation of cracking defects within the lap coating, which expanded under the applied load, ultimately resulting in extensive cracking and spalling. On the other hand, the TiC and Cr
7C
3 precipitated in the coatings were slightly convex to the substrates. They were subjected to greater forces, resulting in crushing and the formation of abrasive grains. Although they were hard, when dislodged abrasive grains scratched them, it was difficult to produce noticeable grooves. The large area of flaking provided space for abrasive grains, and the flaking particles were discharged into the flaking pits during the abrasion process. This could only cause the surface layer to have intermittent light scratches.
Figure 11b,e show that the current treatment resulted in abrasive wear and adhesive wear, with numerous furrows on the wear surface and a small adhesive layer, indicating improved wear resistance [
38]. The coating prepared at 720 A exhibited smooth wear marks, with minimal flaking and plastic deformation, and signs of microcutting were much less compared to the unassisted coating. The coating possessed optimal lubricity and wear reduction, suggesting a transition from abrasive wear assisted by oxidation to polished wear [
39]. The refinement and uniform distribution of carbides in the coating effectively inhibited the dislocation slip [
40]. The diffuse distribution of fine TiC acted as a hard-supporting skeleton, preventing abrasive particles from embedding in the substrate or changing direction and avoiding plastic deformation. The increased microhardness of the coating reduced surface furrowing, while the refinement grain improved toughness and prevented crack propagation, ultimately eliminating spalling [
41].
With further increases in the current, the furrows deepened again at 840 A. At 960 A, the wear profile was analogous to that of an unassisted Co-based coating, with numerous spalling areas and a large adhesive layer in the unspalled areas, which indicated deterioration in wear performance [
42]. This phenomenon could be attributed to the coarsening of the grains. On the one hand, the decrease in the number of grain boundaries led to the extension of microcracks in the carbide and substrate formation. On the other hand, coarse grains resulted in a decline in the hardness of the coatings, weakening their resistance to abrasive grains. The surface of the coating was subjected to intensified microcutting, resulting in severe plastic deformation and leading to the work hardening effect. Under the prolonged periods of alternating stress, the coating ultimately underwent surface fragmentation and flaking [
43].
Figure 11f–i indicate that the wear profile of the Co-based coating at each CPH temperature was not considerably different from that of the EAPH coatings, implying that the temperature of EAPH primarily influenced the wear performance. Moreover, the wear properties of the coatings declined at 960 A and 250 °C relative to a lower current and preheating temperature, demonstrating that an excessive preheat temperature could negatively affect the wear properties of the coatings.