The Effect of Yttrium Addition on Microstructure and Mechanical Properties of Refractory TiTaZrHfW High-Entropy Films
Abstract
:1. Introduction
2. Experimental Details
2.1. Deposition Process
2.2. X-ray Diffraction (XRD)
2.3. Scanning Electron Microscopy (SEM)
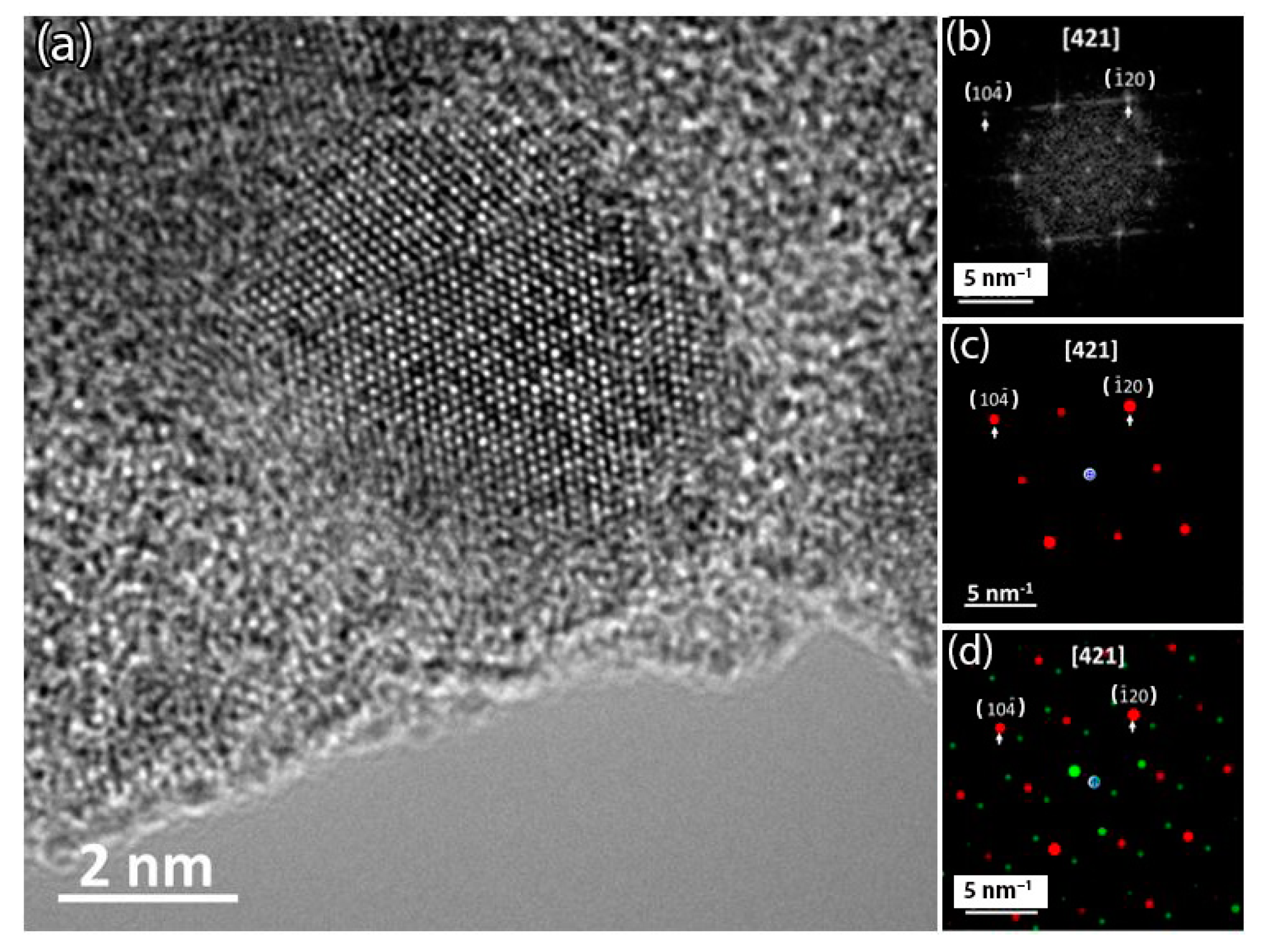
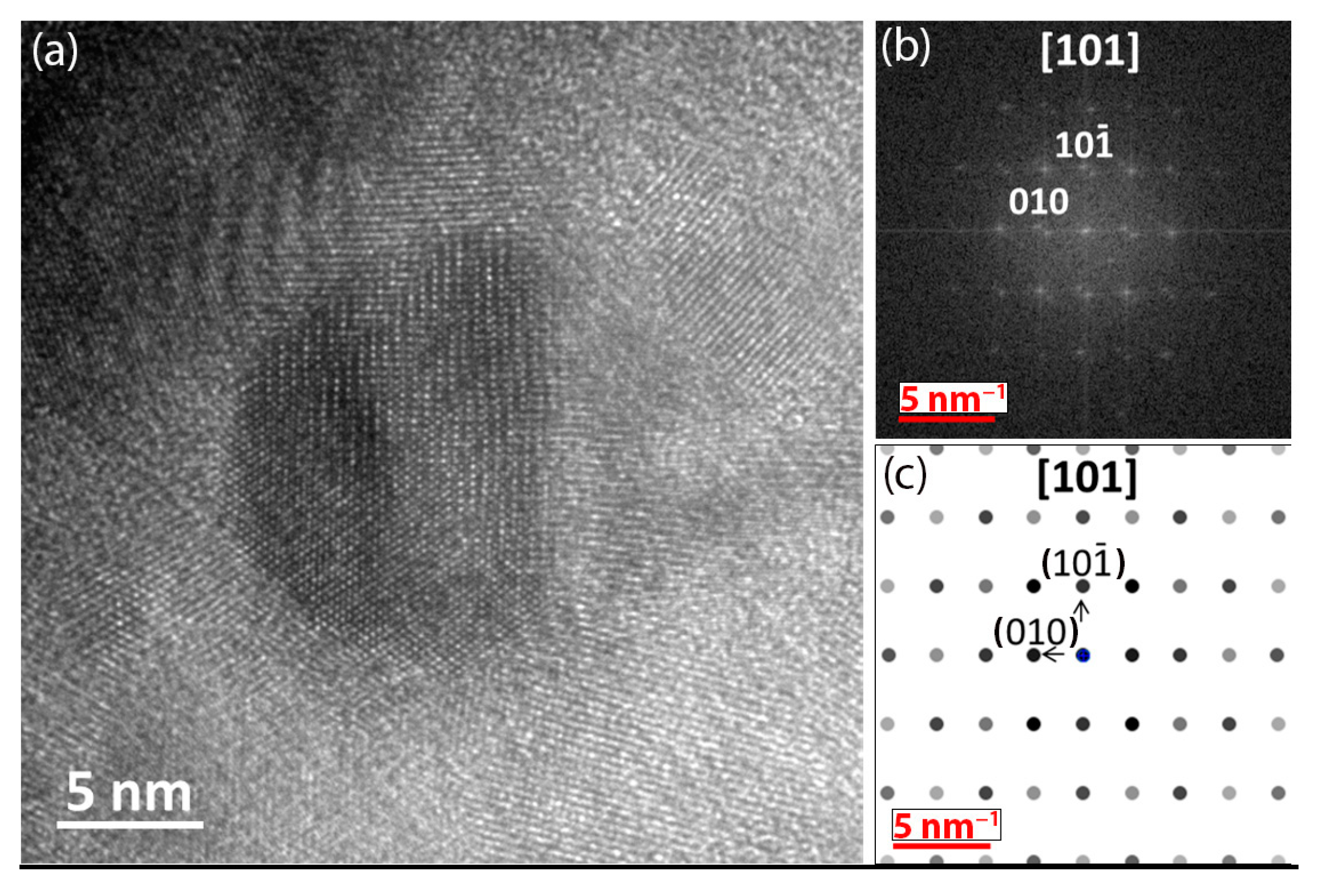
2.4. Transmission Electron Microscopy (TEM)
2.5. Nano-Indentation
3. Results and Discussion
3.1. Sputtering Yield, Deposition Rate, and Chemcial Composition
3.2. Strucure of (TiTaZrHfW)100−xYx
3.3. Microstrucure of (TiTaZrHfW)100−xYx
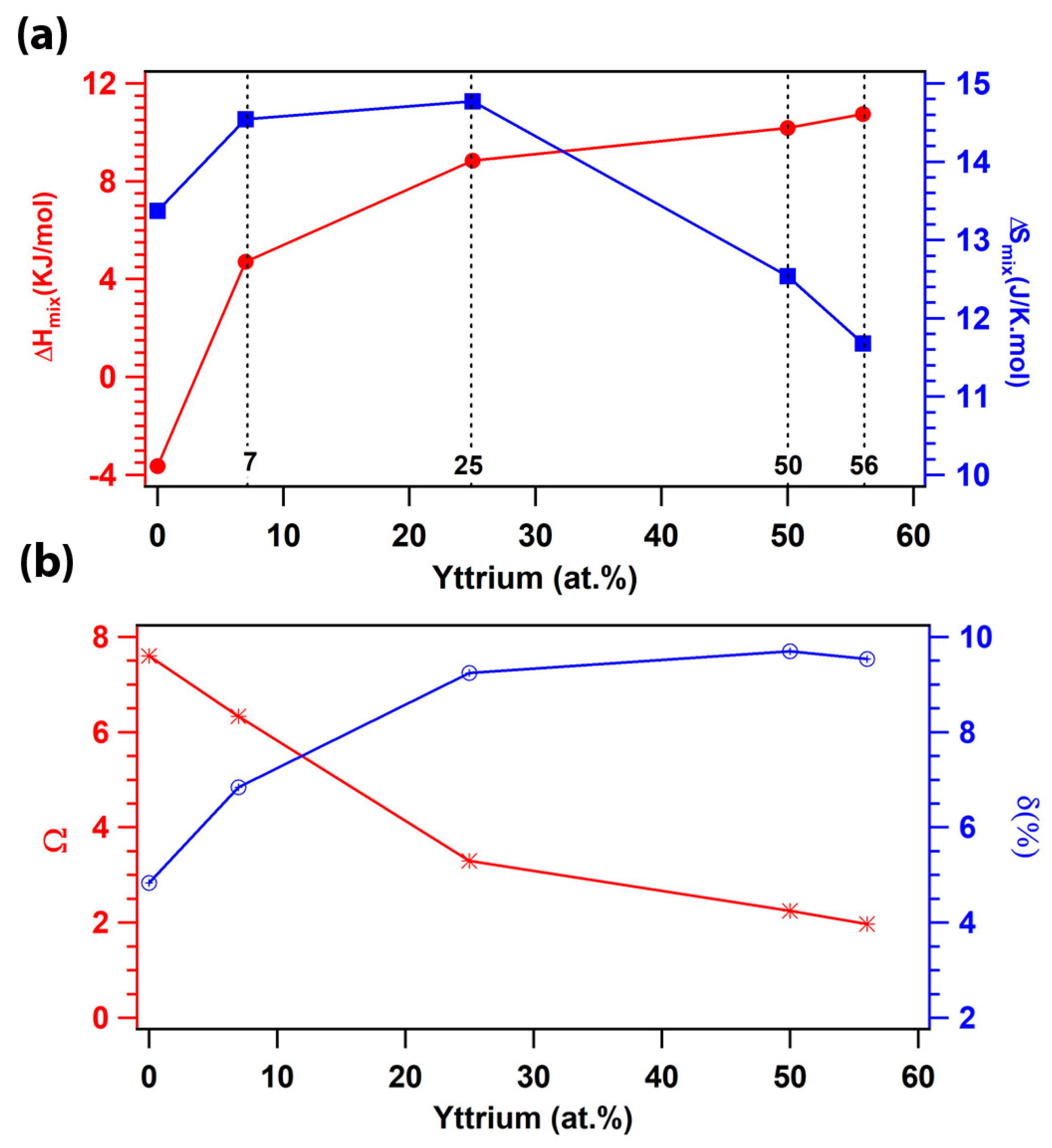
3.4. Thermodynamic Criteria
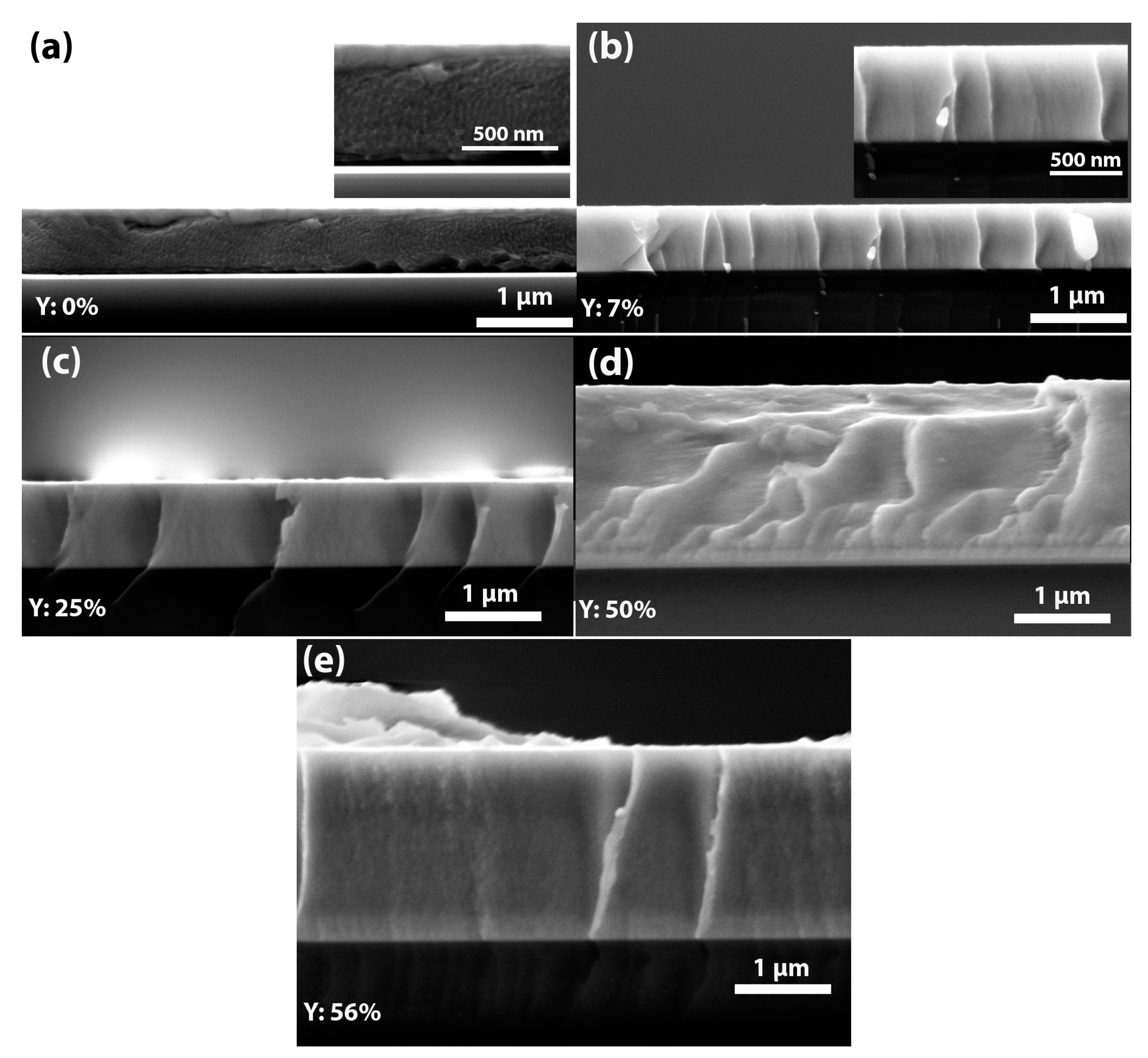
3.5. Morphology of (TiTaZrHfW)100−xYx
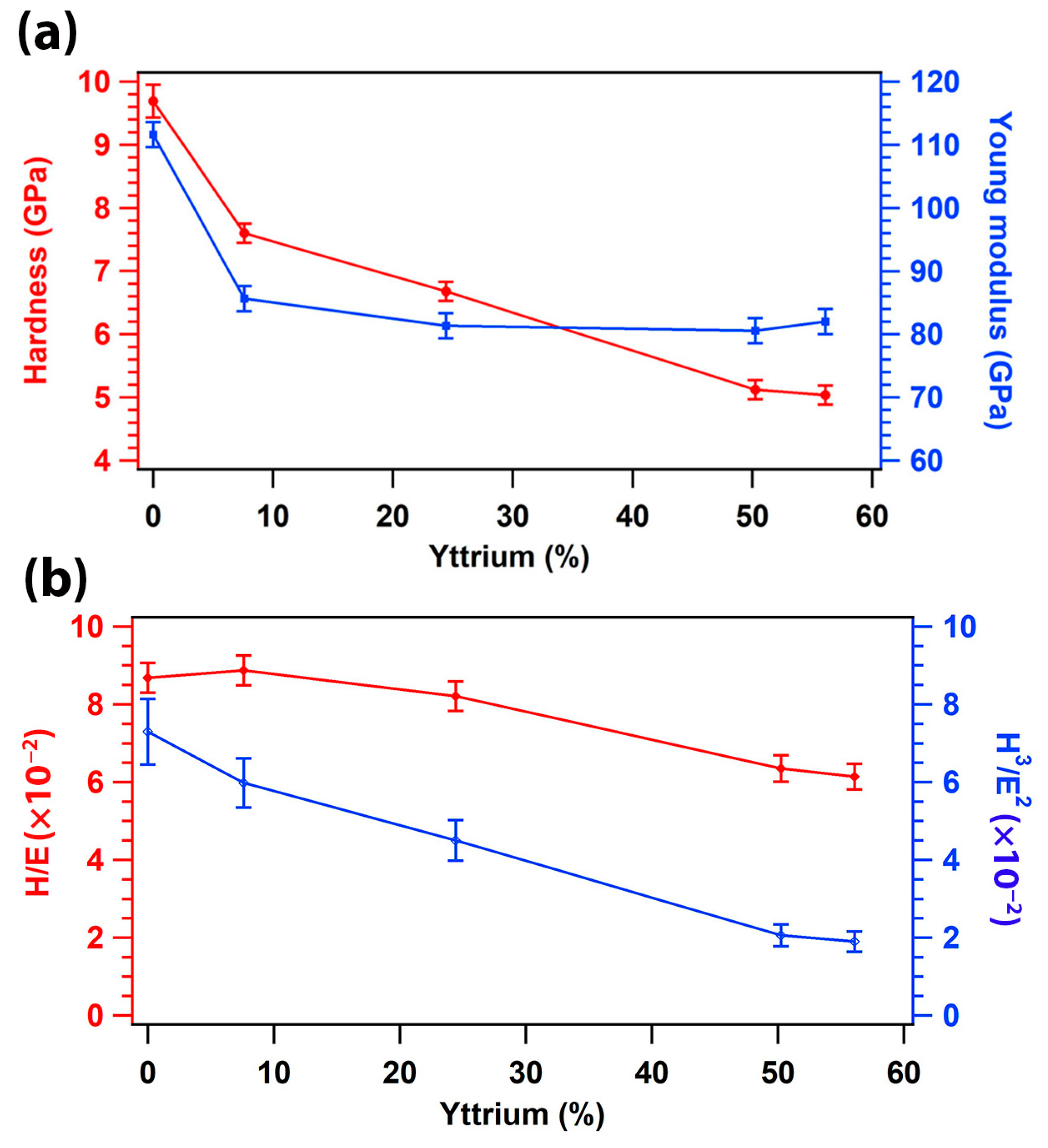
3.6. Mechanical Properties of (TiTaZrHfW)100−xYx
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Yeh, J.-W.; Chen, S.-K.; Lin, S.-J.; Gan, J.-Y.; Chin, T.-S.; Shun, T.-T.; Tsau, C.-H.; Chang, S.-Y. Nanostructured High-Entropy Alloys with Multiple Principal Elements: Novel Alloy Design Concepts and Outcomes. Adv. Eng. Mater. 2004, 6, 299–303. [Google Scholar] [CrossRef]
- Cantor, B.; Chang, I.T.H.; Knight, P.; Vincent, A.J.B. Microstructural Development in Equiatomic Multicomponent Alloys. Mater. Sci. Eng. A 2004, 375–377, 213–218. [Google Scholar] [CrossRef]
- Zhang, W.; Liaw, P.K.; Zhang, Y. Science and Technology in High-Entropy Alloys. Sci. China Mater. 2018, 61, 2–22. [Google Scholar] [CrossRef]
- Zhang, F.; Zhang, C.; Chen, S.-L.; Zhu, J.; Cao, W.-S.; Kattner, U.R. An Understanding of High Entropy Alloys from Phase Diagram Calculations. Calphad 2014, 45, 1–10. [Google Scholar] [CrossRef]
- Gludovatz, B.; Hohenwarter, A.; Catoor, D.; Chang, E.H.; George, E.P.; Ritchie, R.O. A Fracture-Resistant High-Entropy Alloy for Cryogenic Applications. Science 2014, 345, 1153–1158. [Google Scholar] [CrossRef] [PubMed]
- Tsai, D.-C.; Chang, Z.-C.; Kuo, B.-H.; Chang, S.-Y.; Shieu, F.-S. Effects of Silicon Content on the Structure and Properties of (AlCrMoTaTi)N Coatings by Reactive Magnetron Sputtering. J. Alloys Compd. 2014, 616, 646–651. [Google Scholar] [CrossRef]
- Wang, W.-R.; Wang, W.-L.; Wang, S.-C.; Tsai, Y.-C.; Lai, C.-H.; Yeh, J.-W. Effects of Al Addition on the Microstructure and Mechanical Property of AlxCoCrFeNi High-Entropy Alloys. Intermetallics 2012, 26, 44–51. [Google Scholar] [CrossRef]
- Chang, Z.-C. Structure and Properties of Duodenary (TiVCrZrNbMoHfTaWAlSi)N Coatings by Reactive Magnetron Sputtering. Mater. Chem. Phys. 2018, 220, 98–110. [Google Scholar] [CrossRef]
- Cheng, J.B.; Liang, X.B.; Xu, B.S. Effect of Nb Addition on the Structure and Mechanical Behaviors of CoCrCuFeNi High-Entropy Alloy Coatings. Surf. Coat. Technol. 2014, 240, 184–190. [Google Scholar] [CrossRef]
- Liang, H.; Miao, J.; Gao, B.; Deng, D.; Wang, T.; Lu, Y.; Cao, Z.; Jiang, H.; Li, T.; Kang, H. Microstructure and Tribological Properties of AlCrFe2Ni2W0.2Mo0.75 High-Entropy Alloy Coating Prepared by Laser Cladding in Seawater, NaCl Solution and Deionized Water. Surf. Coat. Technol. 2020, 400, 126214. [Google Scholar] [CrossRef]
- Jadhav, M.S.; Sahane, D.; Verma, A.; Singh, S. Thermal Stability and Thermal Expansion Behavior of FeCoCrNi2Al High Entropy Alloy. Adv. Powder Technol. 2021, 32, 378–384. [Google Scholar] [CrossRef]
- Chen, S.; Cai, Z.; Lu, Z.; Pu, J.; Chen, R.; Zheng, S.; Mao, C.; Chen, S. Tribo-Corrosion Behavior of VAlTiCrCu High-Entropy Alloy Film. Mater. Charact. 2019, 157, 109887. [Google Scholar] [CrossRef]
- Yan, Y.; McGarrity, K.A.; Delia, D.J.; Fekety, C.; Wang, K. The Oxidation-Resistance Mechanism of WTaNbTiAl Refractory High Entropy Alloy. Corros. Sci. 2022, 204, 110377. [Google Scholar] [CrossRef]
- Luo, J.; Sun, W.; Duan, R.; Yang, W.; Chan, K.C.; Ren, F.; Yang, X.-S. Laser Surface Treatment-Introduced Gradient Nanostructured TiZrHfTaNb Refractory High-Entropy Alloy with Significantly Enhanced Wear Resistance. J. Mater. Sci. Technol. 2022, 110, 43–56. [Google Scholar] [CrossRef]
- Wang, Z.; Yan, Y.; Wu, Y.; Huang, X.; Zhang, Y.; Su, Y.; Qiao, L. Corrosion and Tribocorrosion Behavior of Equiatomic Refractory Medium Entropy TiZr (Hf, Ta, Nb) Alloys in Chloride Solutions. Corros. Sci. 2022, 199, 110166. [Google Scholar] [CrossRef]
- Cao, B.X.; Yang, T.; Fan, L.; Luan, J.H.; Jiao, Z.B.; Liu, C.T. Refractory Alloying Additions on the Thermal Stability and Mechanical Properties of High-Entropy Alloys. Mater. Sci. Eng. A 2020, 797, 140020. [Google Scholar] [CrossRef]
- Senkov, O.N.; Wilks, G.B.; Scott, J.M.; Miracle, D.B. Mechanical Properties of Nb25Mo25Ta25W25 and V20Nb20Mo20Ta20W20 Refractory High Entropy Alloys. Intermetallics 2011, 19, 698–706. [Google Scholar] [CrossRef]
- Huang, C.; Zhang, Y.; Shen, J.; Vilar, R. Thermal Stability and Oxidation Resistance of Laser Clad TiVCrAlSi High Entropy Alloy Coatings on Ti–6Al–4V Alloy. Surf. Coat. Technol. 2011, 206, 1389–1395. [Google Scholar] [CrossRef]
- Zhang, H.; Pan, Y.; He, Y.-Z. Synthesis and Characterization of FeCoNiCrCu High-Entropy Alloy Coating by Laser Cladding. Mater. Des. 2011, 32, 1910–1915. [Google Scholar] [CrossRef]
- Qiu, X.-W.; Liu, C.-G. Microstructure and Properties of Al2CrFeCoCuTiNi High-Entropy Alloys Prepared by Laser Cladding. J. Alloys Compd. 2013, 553, 216–220. [Google Scholar] [CrossRef]
- Chen, T.K.; Shun, T.T.; Yeh, J.W.; Wong, M.S. Nanostructured Nitride Films of Multi-Element High-Entropy Alloys by Reactive DC Sputtering. Surf. Coat. Technol. 2004, 188–189, 193–200. [Google Scholar] [CrossRef]
- Achache, S.; Lamri, S.; Arab Pour Yazdi, M.; Billard, A.; François, M.; Sanchette, F. Ni-Free Superelastic Binary Ti–Nb Coatings Obtained by DC Magnetron Co-Sputtering. Surf. Coat. Technol. 2015, 275, 283–288. [Google Scholar] [CrossRef]
- Shen, W.-J.; Tsai, M.-H.; Chang, Y.-S.; Yeh, J.-W. Effects of Substrate Bias on the Structure and Mechanical Properties of (Al1.5CrNb0.5Si0.5Ti)Nx Coatings. Thin Solid Film. 2012, 520, 6183–6188. [Google Scholar] [CrossRef]
- Liu, L.; Zhu, J.B.; Hou, C.; Li, J.C.; Jiang, Q. Dense and Smooth Amorphous Films of Multicomponent FeCoNiCuVZrAl High-Entropy Alloy Deposited by Direct Current Magnetron Sputtering. Mater. Des. (1980–2015) 2013, 46, 675–679. [Google Scholar] [CrossRef]
- El Garah, M.; Achache, S.; Michau, A.; Schuster, F.; Sanchette, F. Structural and Mechanical Properties of AlTiTaZr( N) Medium Entropy Films (MEF) Obtained by DC Magnetron Sputtering in Dynamic Mode. Surf. Coat. Technol. 2020, 396, 125941. [Google Scholar] [CrossRef]
- El Garah, M.; Achache, S.; Michau, A.; Schuster, F.; Sanchette, F. AlTiTaZr(-N) Medium-Entropy Films Deposited by Magnetron Sputtering with a Combinatorial Approach. J. Mater. Eng. Perform. 2021, 30, 4063–4071. [Google Scholar] [CrossRef]
- Yao, C.-Z.; Zhang, P.; Liu, M.; Li, G.-R.; Ye, J.-Q.; Liu, P.; Tong, Y.-X. Electrochemical Preparation and Magnetic Study of Bi–Fe–Co–Ni–Mn High Entropy Alloy. Electrochim. Acta 2008, 53, 8359–8365. [Google Scholar] [CrossRef]
- Yue, T.; Xie, H.; Lin, X.; Yang, H.; Meng, G. Microstructure of Laser Re-Melted AlCoCrCuFeNi High Entropy Alloy Coatings Produced by Plasma Spraying. Entropy 2013, 15, 2833–2845. [Google Scholar] [CrossRef]
- Alvi, S.; Jarzabek, D.M.; Kohan, M.G.; Hedman, D.; Jenczyk, P.; Natile, M.M.; Vomiero, A.; Akhtar, F. Synthesis and Mechanical Characterization of a CuMoTaWV High-Entropy Film by Magnetron Sputtering. ACS Appl. Mater. Interfaces 2020, 12, 21070–21079. [Google Scholar] [CrossRef]
- Radhika, N.; Sasikumar, J.; Arulmozhivarman, J. Tribo-Mechanical Behaviour of Ti-Based Particulate Reinforced As-Cast and Heat Treated A359 Composites. Silicon 2020, 12, 2769–2782. [Google Scholar] [CrossRef]
- Bachani, S.K.; Wang, C.-J.; Lou, B.-S.; Chang, L.-C.; Lee, J.-W. Microstructural Characterization, Mechanical Property and Corrosion Behavior of VNbMoTaWAl Refractory High Entropy Alloy Coatings: Effect of Al Content. Surf. Coat. Technol. 2020, 403, 126351. [Google Scholar] [CrossRef]
- Luo, H.; Luo, F.; Chen, Y.; Wang, J.; Liu, Q.; Li, F.; Zhou, X.; Guo, L. The Effect of Yttrium Addition on the Microstructure and Irradiation Hardening in V-4Cr-4Ti Alloy under Self-Ion Irradiation. Metals 2021, 11, 1024. [Google Scholar] [CrossRef]
- Chinnasamy, M.; Rathanasamy, R.; Pal, S.K.; Palaniappan, S.K. Effectiveness of Cryogenic Treatment on Cutting Tool Inserts: A Review. Int. J. Refract. Met. Hard Mater. 2022, 108, 105946. [Google Scholar] [CrossRef]
- Lu, S.; Li, X.; Liang, X.; He, J.; Shao, W.; Li, K.; Chen, J. Effect of Y Additions on the Oxidation Behavior of Vacuum Arc Melted Refractory High-Entropy Alloy AlMo0.5NbTa0.5TiZr at Elevated Temperatures. Vacuum 2022, 201, 111069. [Google Scholar] [CrossRef]
- Jiang, W.; Fan, Z.; Dai, Y.; Li, C. Effects of Rare Earth Elements Addition on Microstructures, Tensile Properties and Fractography of A357 Alloy. Mater. Sci. Eng. A 2014, 597, 237–244. [Google Scholar] [CrossRef]
- Tong, G.; Liu, H.; Liu, Y. Effect of Rare Earth Additions on Microstructure and Mechanical Properties of AZ91 Magnesium Alloys. Trans. Nonferrous Met. Soc. China 2010, 20, s336–s340. [Google Scholar] [CrossRef]
- Long, Y.; Che, J.; Wu, Z.; Lin, H.-T.; Zhang, F. High Entropy Alloy Borides Prepared by Powder Metallurgy Process and the Enhanced Fracture Toughness by Addition of Yttrium. Mater. Chem. Phys. 2021, 257, 123715. [Google Scholar] [CrossRef]
- Li, G.R.; Liu, M.; Wang, H.M.; Zhang, D.; Tang, F.; Wang, C.W.; Zhao, Y.T.; Chen, G.; Kai, X.Z. Effect of the Rare Earth Element Yttrium on the Structure and Properties of Boron-Containing High-Entropy Alloy. JOM 2020, 72, 2332–2339. [Google Scholar] [CrossRef]
- Sha, M.; Zhou, Y.; Wang, N.; Yu, D.; Wang, Z.; Ai, X.; Wang, H.; Shen, M.; Li, S. Precipitation Behavior of Yttrium-Rich Nano-Phases in AlCoCrFeNi2.1Yx High-Entropy Alloy. Mater. Res. Express 2020, 7, 126510. [Google Scholar] [CrossRef]
- Zhang, L.J.; Zhang, M.D.; Zhou, Z.; Fan, J.T.; Cui, P.; Yu, P.F.; Jing, Q.; Ma, M.Z.; Liaw, P.K.; Li, G.; et al. Effects of Rare-Earth Element, Y, Additions on the Microstructure and Mechanical Properties of CoCrFeNi High Entropy Alloy. Mater. Sci. Eng. A 2018, 725, 437–446. [Google Scholar] [CrossRef]
- Hong, X.; Hsueh, C.-H. Effects of Yttrium Addition on Microstructures and Mechanical Properties of CoCrNi Medium Entropy Alloy. Intermetallics 2022, 140, 107405. [Google Scholar] [CrossRef]
- Kauffmann, A.; Stüber, M.; Leiste, H.; Ulrich, S.; Schlabach, S.; Szabó, D.V.; Seils, S.; Gorr, B.; Chen, H.; Seifert, H.-J.; et al. Combinatorial Exploration of the High Entropy Alloy System Co-Cr-Fe-Mn-Ni. Surf. Coat. Technol. 2017, 325, 174–180. [Google Scholar] [CrossRef]
- Cemin, F.; Jimenez, M.J.M.; Leidens, L.M.; Figueroa, C.A.; Alvarez, F. A Thermodynamic Study on Phase Formation and Thermal Stability of AlSiTaTiZr High-Entropy Alloy Thin Films. J. Alloys Compd. 2020, 838, 155580. [Google Scholar] [CrossRef]
- Demchyna, R.O.; Chykhrij, S.I.; Kuz’ma, Y.B. Y–Cu–P System. J. Alloys Compd. 2002, 345, 170–174. [Google Scholar] [CrossRef]
- Spedding, F.H.; Daane, A.H.; Herrmann, K.W. The Crystal Structures and Lattice Parameters of High-Purity Scandium, Yttrium and the Rare Earth Metals. Acta Cryst. 1956, 9, 559–563. [Google Scholar] [CrossRef]
- Stadelmann, P. Image Analysis and Simulation Software in Transmission Electron Microscopy. Microsc. Microanal. 2003, 9, 60–61. [Google Scholar] [CrossRef]
- Miracle, D.B.; Senkov, O.N. A Critical Review of High Entropy Alloys and Related Concepts. Acta Mater. 2017, 122, 448–511. [Google Scholar] [CrossRef]
- Dalla Torre, F.H.; Dubach, A.; Schällibaum, J.; Löffler, J.F. Shear Striations and Deformation Kinetics in Highly Deformed Zr-Based Bulk Metallic Glasses. Acta Mater. 2008, 56, 4635–4646. [Google Scholar] [CrossRef]
- Achache, S.; Sanchette, F. Effect of Tantalum Addition on Properties of Cu–Zr–Based Thin Film Metallic Glasses (TFMGs). Coatings 2020, 10, 515. [Google Scholar] [CrossRef]
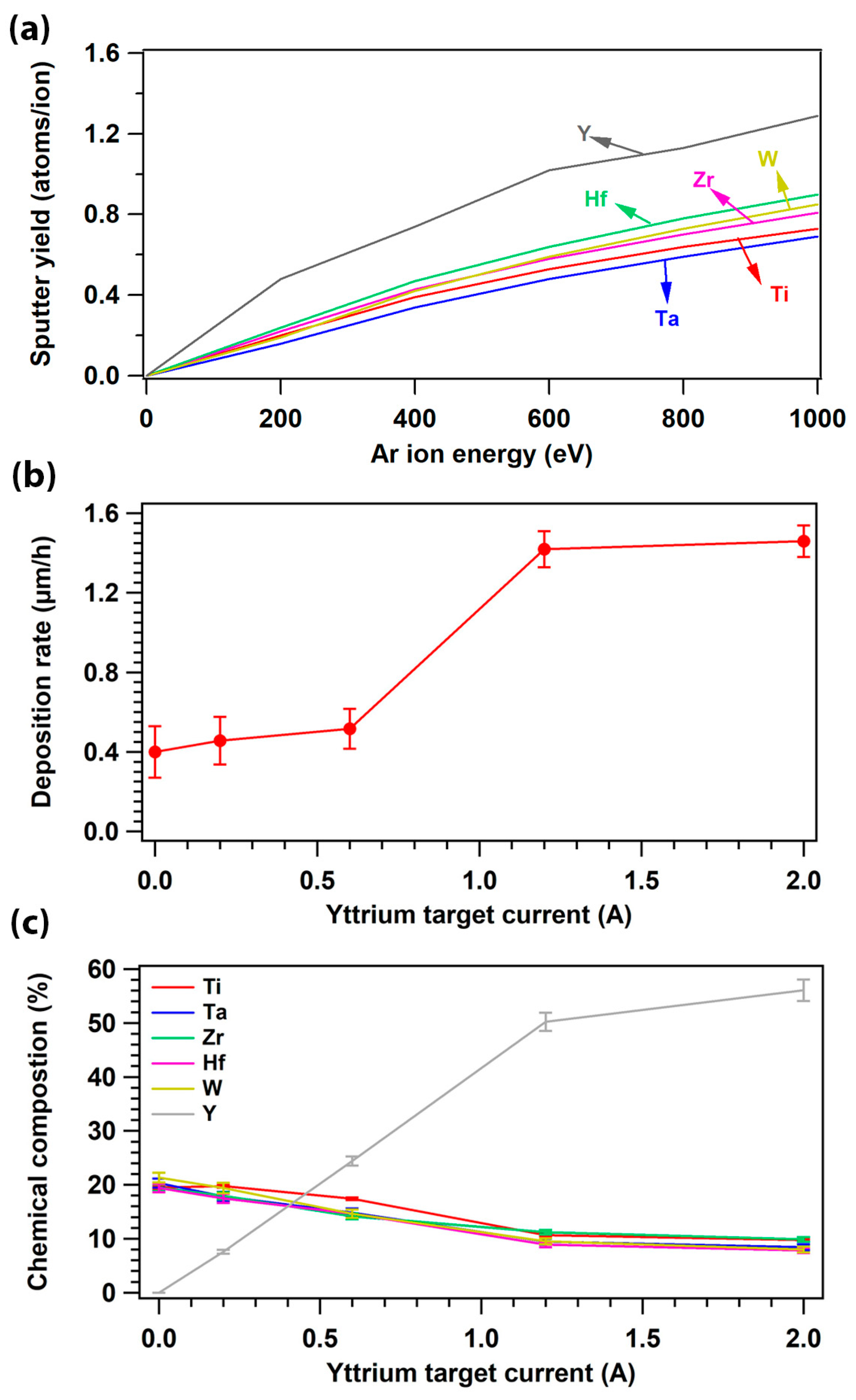
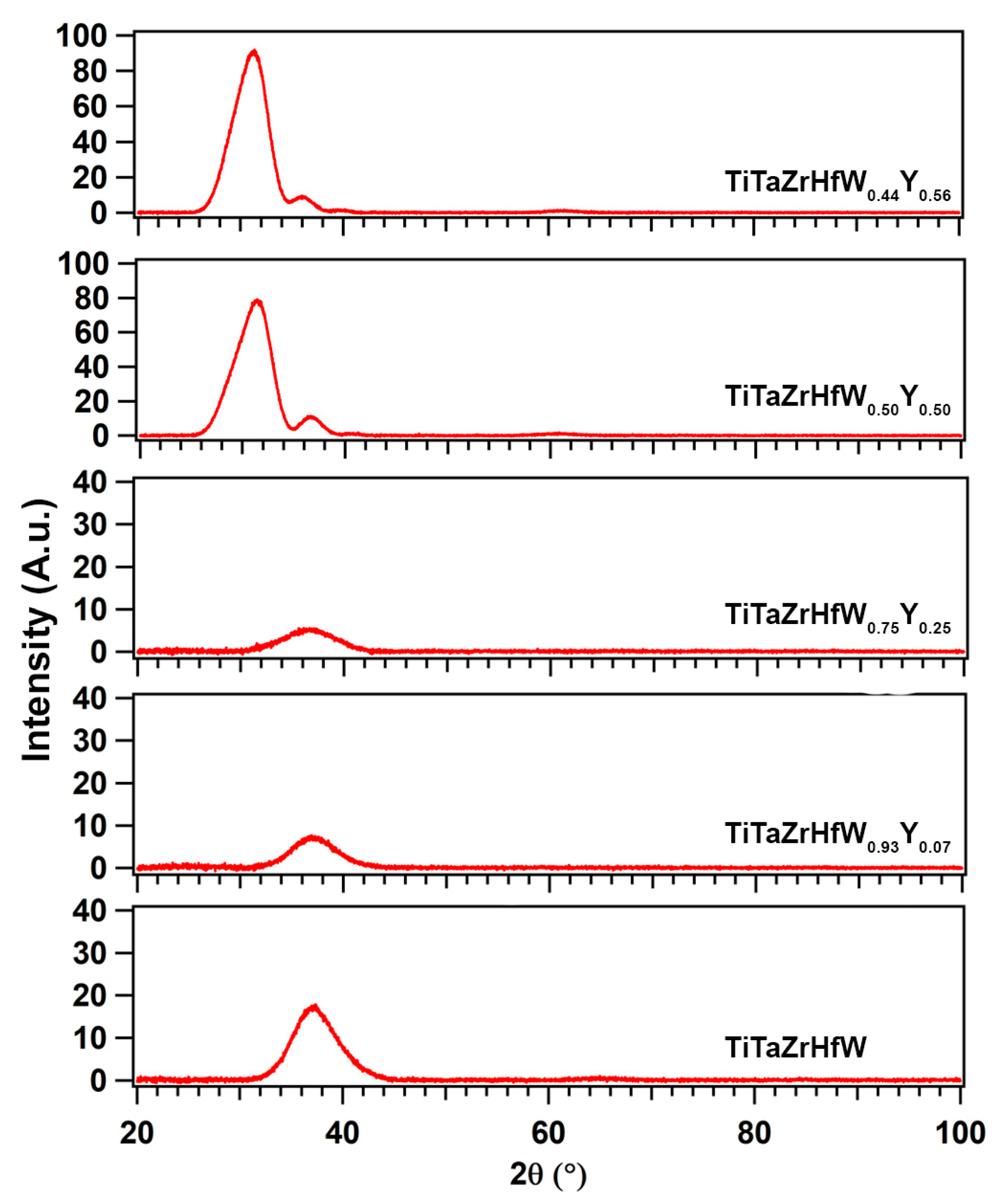
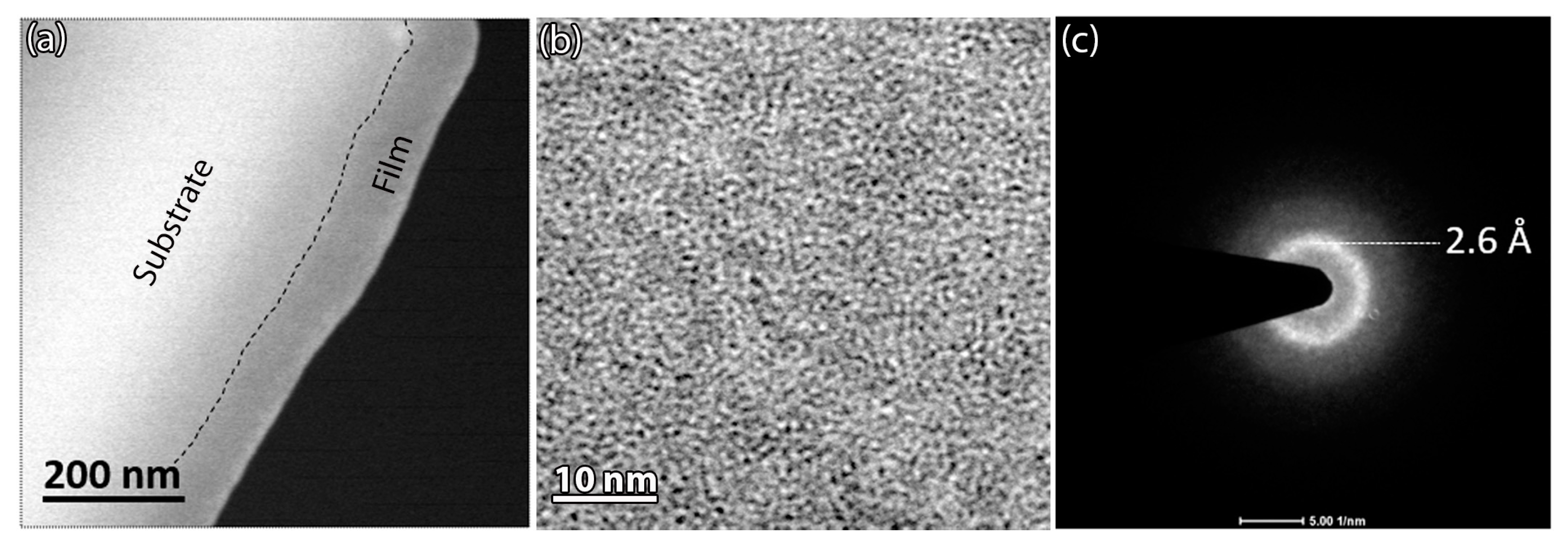

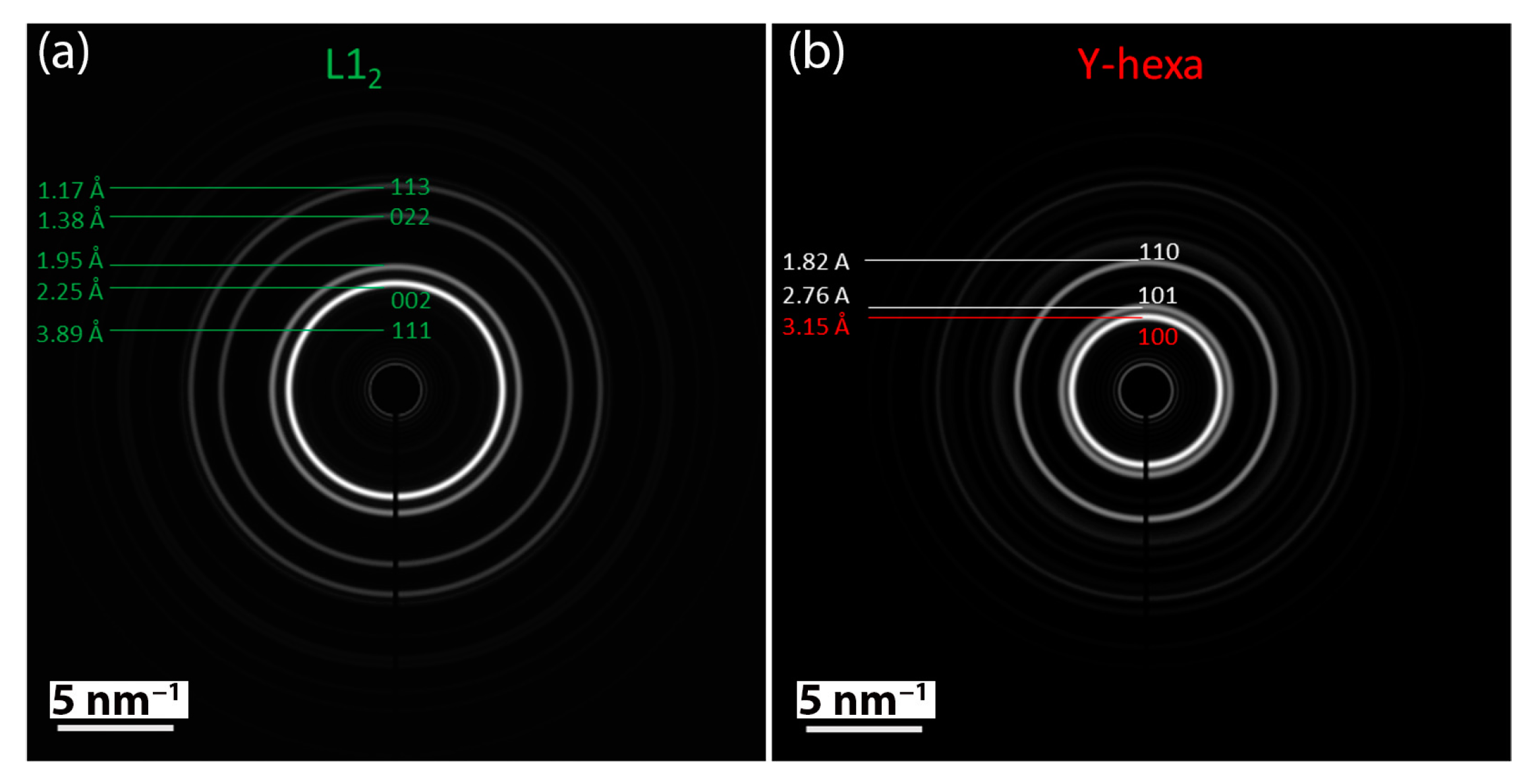
| Element (Melting Point, Atomic Size) | Ti | Ta | Zr | Hf | W | Y |
|---|---|---|---|---|---|---|
| Ti (1660 °C, 176 pm) | - | 1 | 0 | 0 | −6 | 15 |
| Ta (2850 °C, 200 pm) | - | - | 3 | 3 | −7 | 27 |
| Zr (1852 °C, 206 pm) | - | - | - | 0 | −9 | 9 |
| Hf (2200 °C, 208 pm) | - | - | - | - | −6 | 11 |
| W (3410 °C, 193 pm) | - | - | - | - | - | 24 |
| Y (1500 °C, 212 pm) | - | - | - | - | - | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
El Garah, M.; Patout, L.; Bouissil, A.; Charai, A.; Sanchette, F. The Effect of Yttrium Addition on Microstructure and Mechanical Properties of Refractory TiTaZrHfW High-Entropy Films. Coatings 2023, 13, 1380. https://doi.org/10.3390/coatings13081380
El Garah M, Patout L, Bouissil A, Charai A, Sanchette F. The Effect of Yttrium Addition on Microstructure and Mechanical Properties of Refractory TiTaZrHfW High-Entropy Films. Coatings. 2023; 13(8):1380. https://doi.org/10.3390/coatings13081380
Chicago/Turabian StyleEl Garah, Mohamed, Loïc Patout, Abdelhakim Bouissil, Ahmed Charai, and Frederic Sanchette. 2023. "The Effect of Yttrium Addition on Microstructure and Mechanical Properties of Refractory TiTaZrHfW High-Entropy Films" Coatings 13, no. 8: 1380. https://doi.org/10.3390/coatings13081380





