Morphological Evolution of La2NiO4 Coatings Synthesized by Reactive Magnetron Sputtering (RMS) at High Pressure as Cathode for Intermediate Temperature Solid Oxide Fuel Cell (IT-SOFC)
Abstract
1. Introduction
2. Experimental Procedure
2.1. Deposition Device
2.2. Characterization
3. Results and Discussion
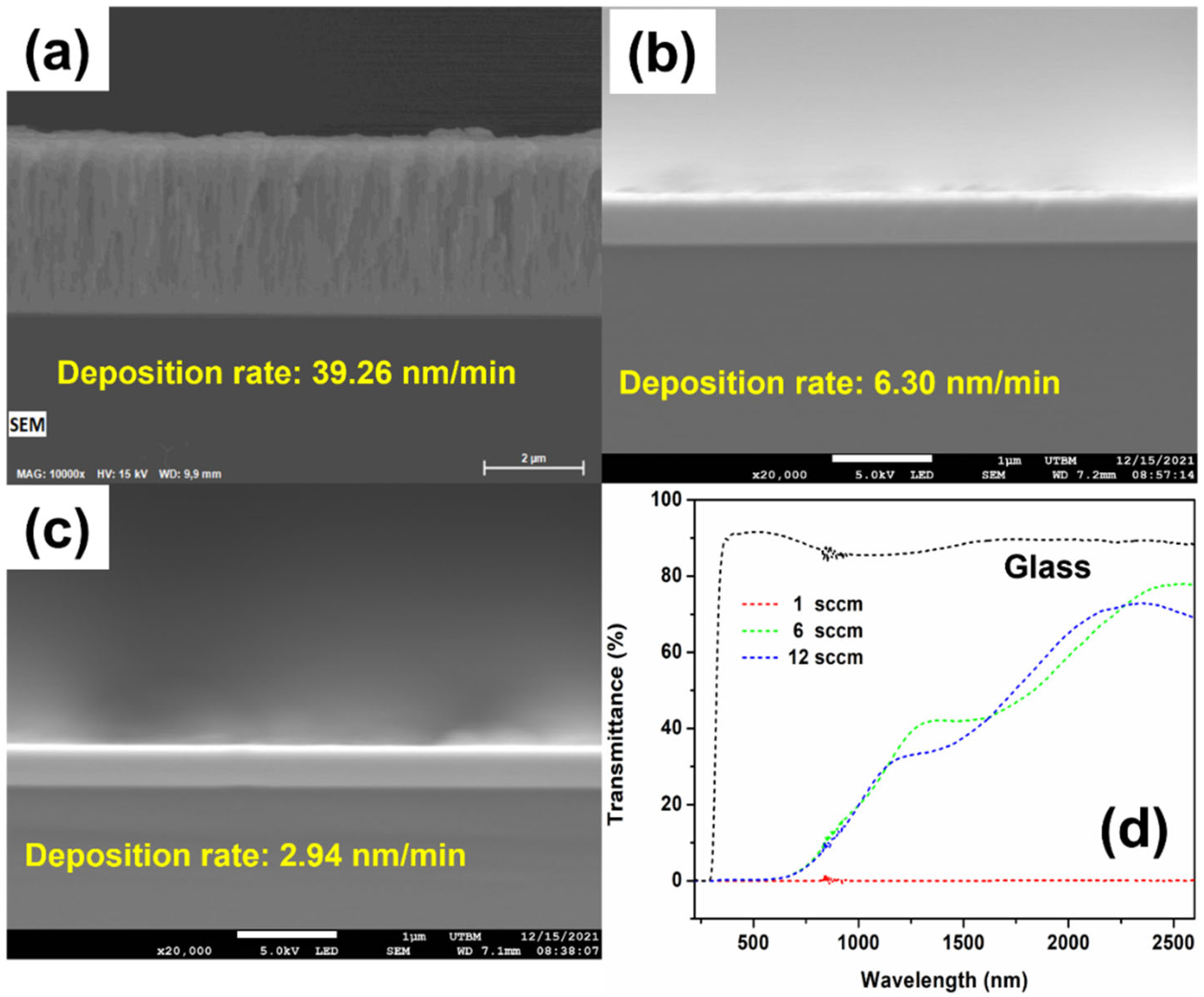
3.1. Deposition of La–Ni–O Coatings
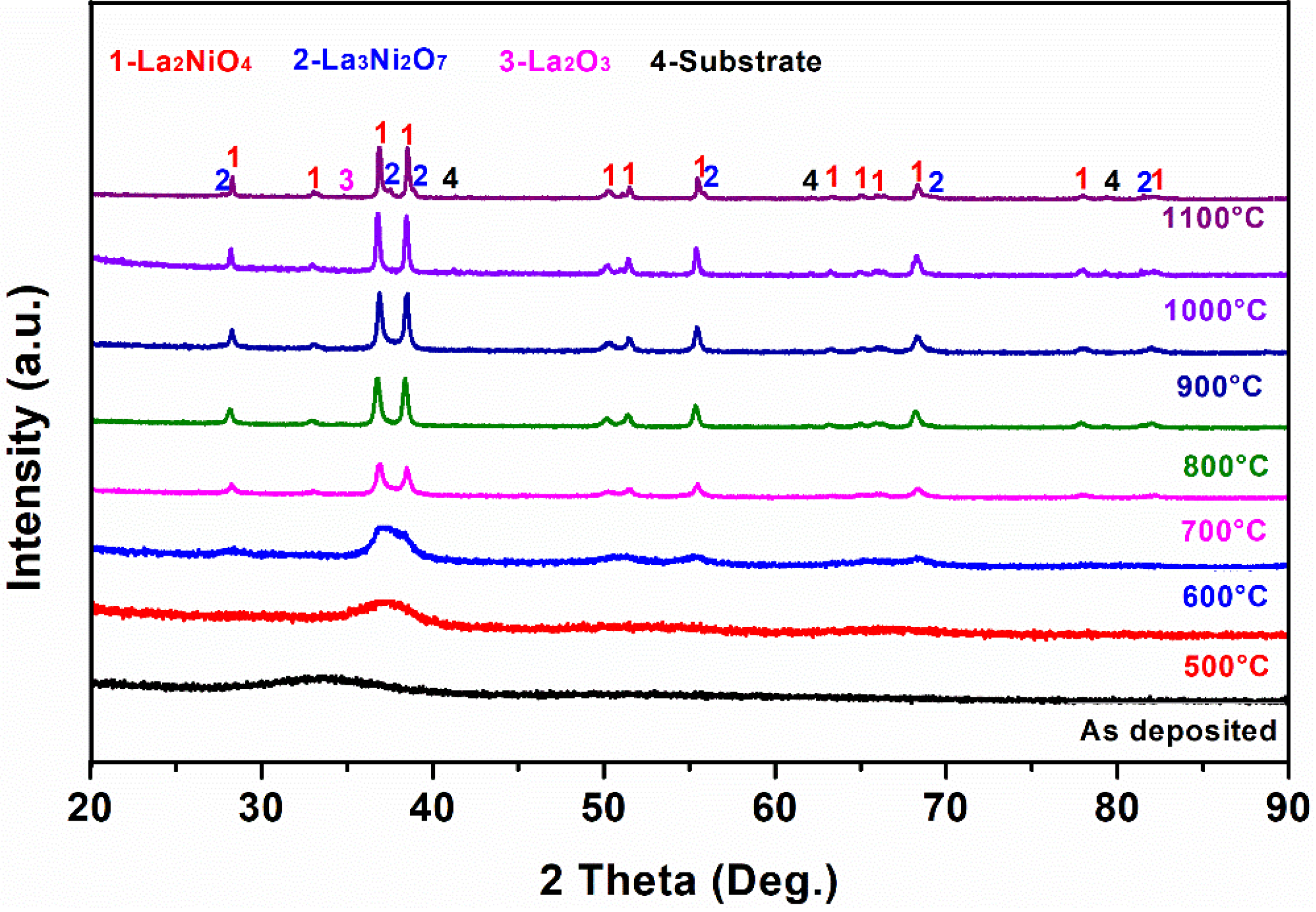
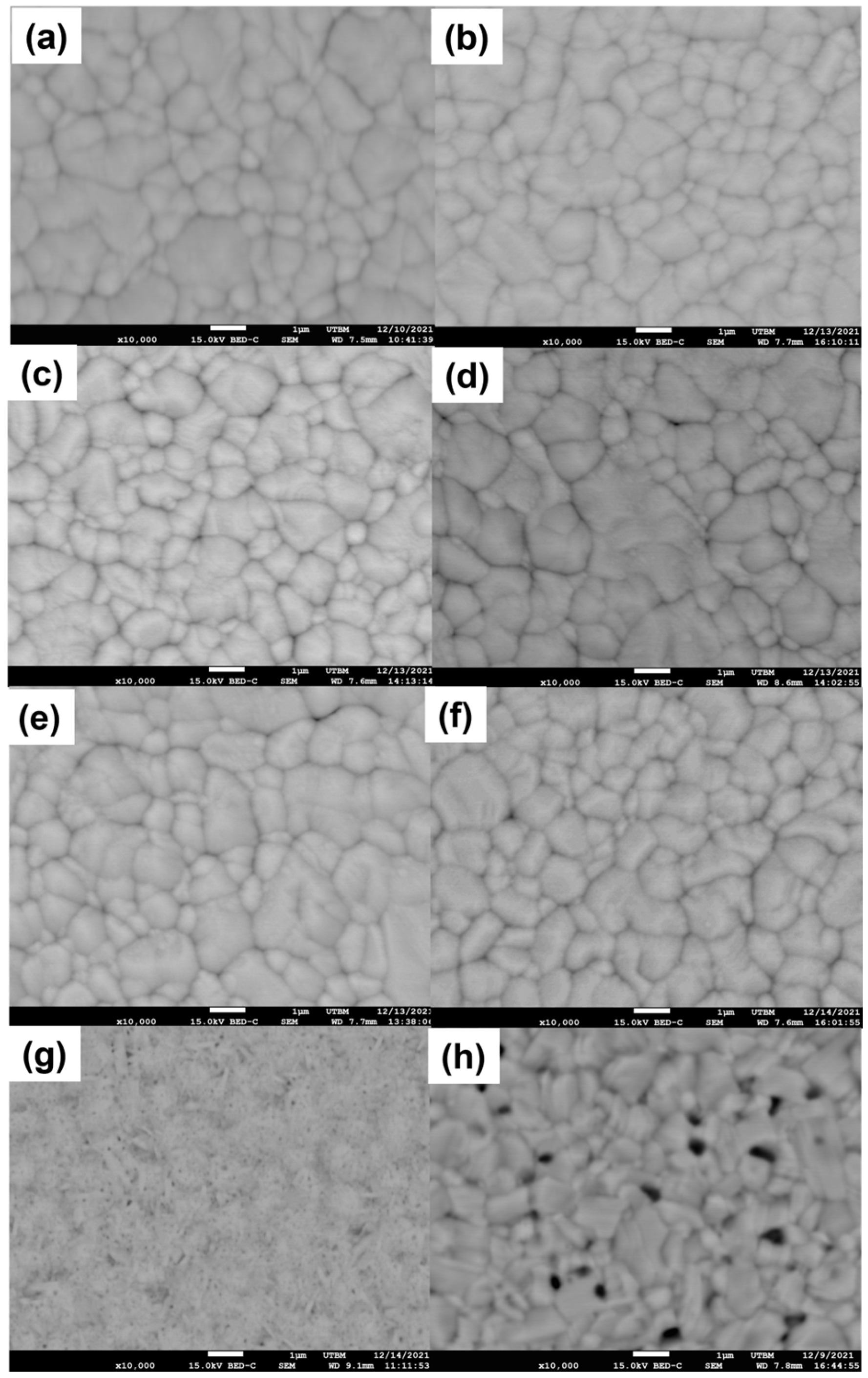
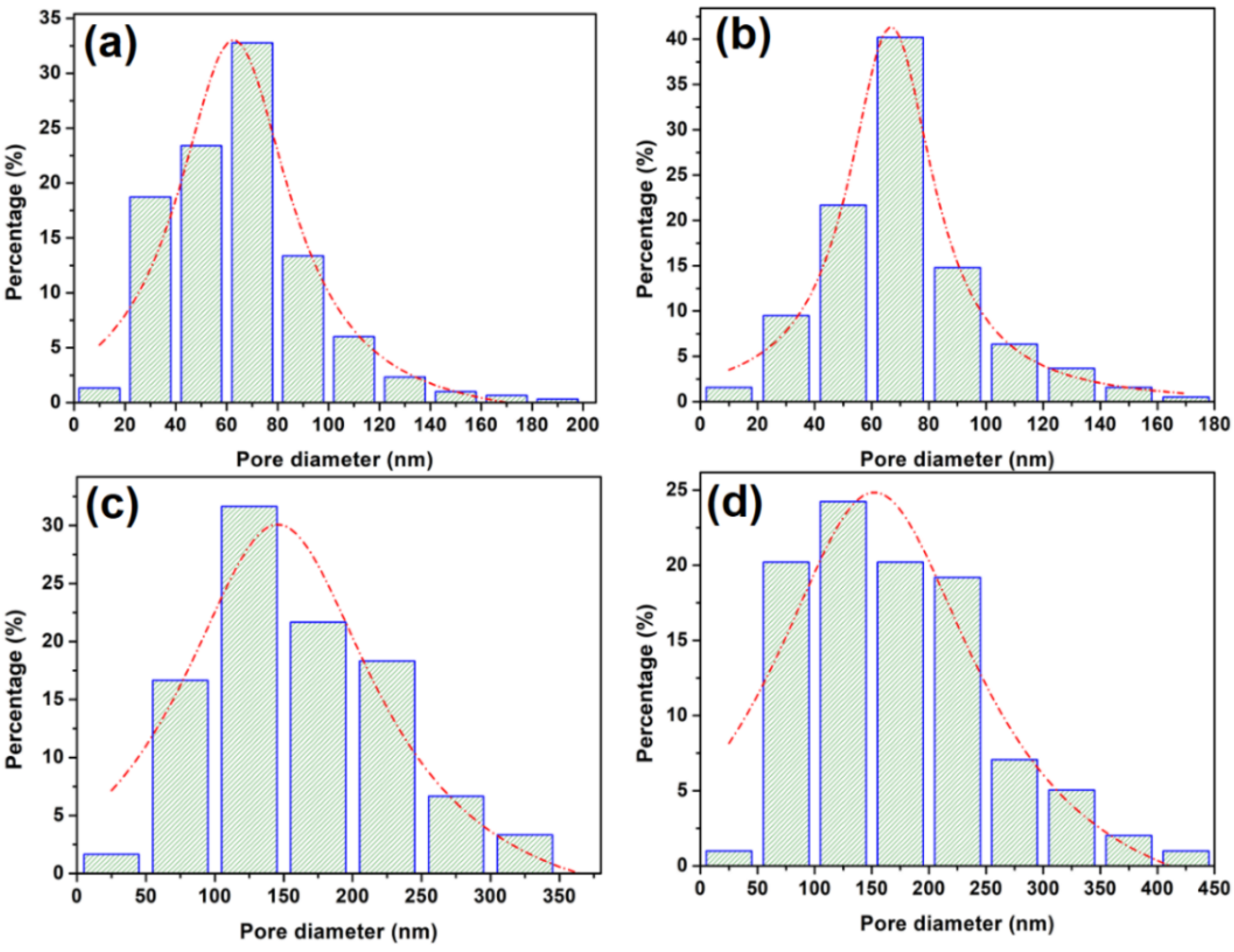
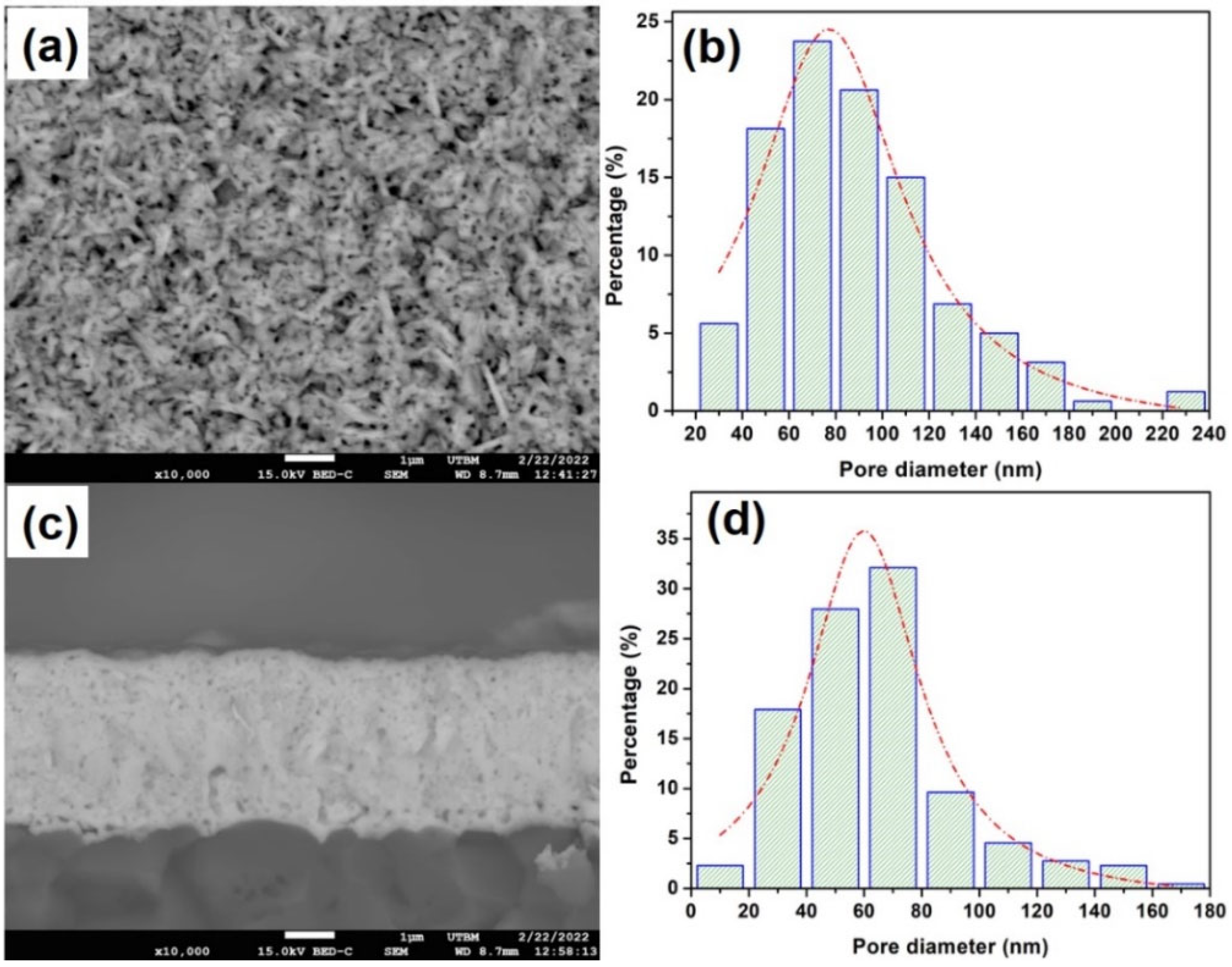
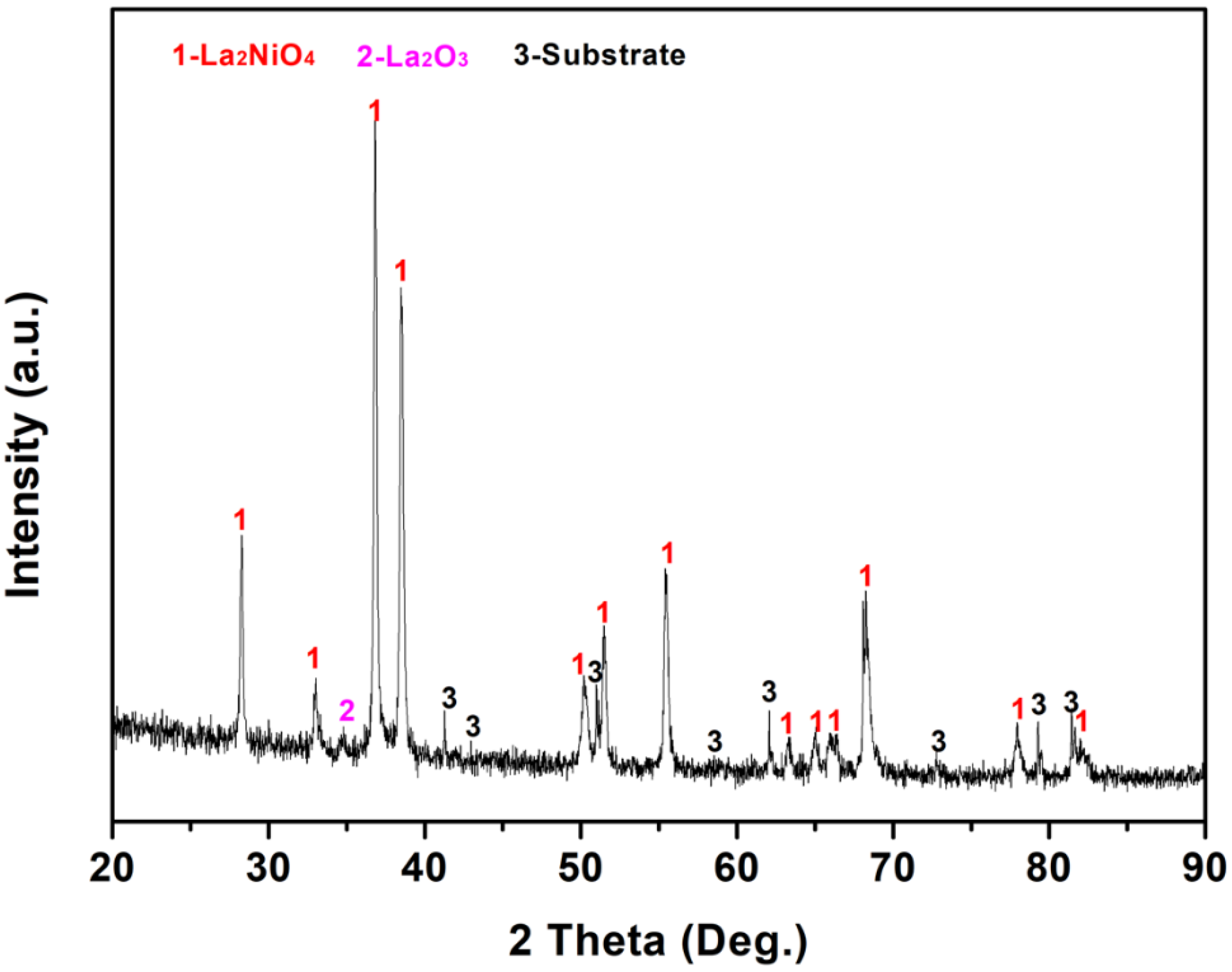
3.2. The Structure and Morphology Evolution of the La–Ni–O Coatings with the La/Ni Atomic Ratio of 2.04
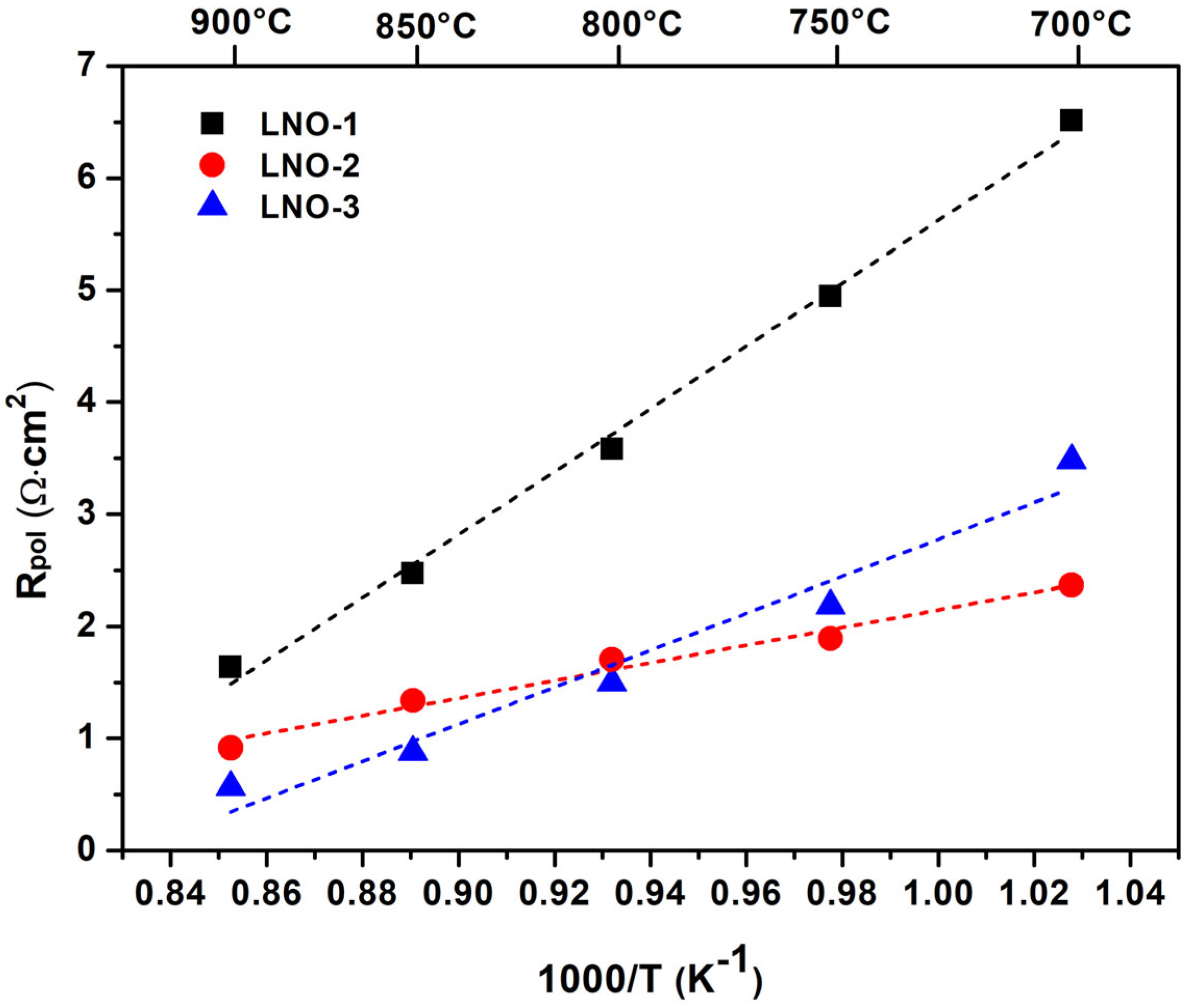
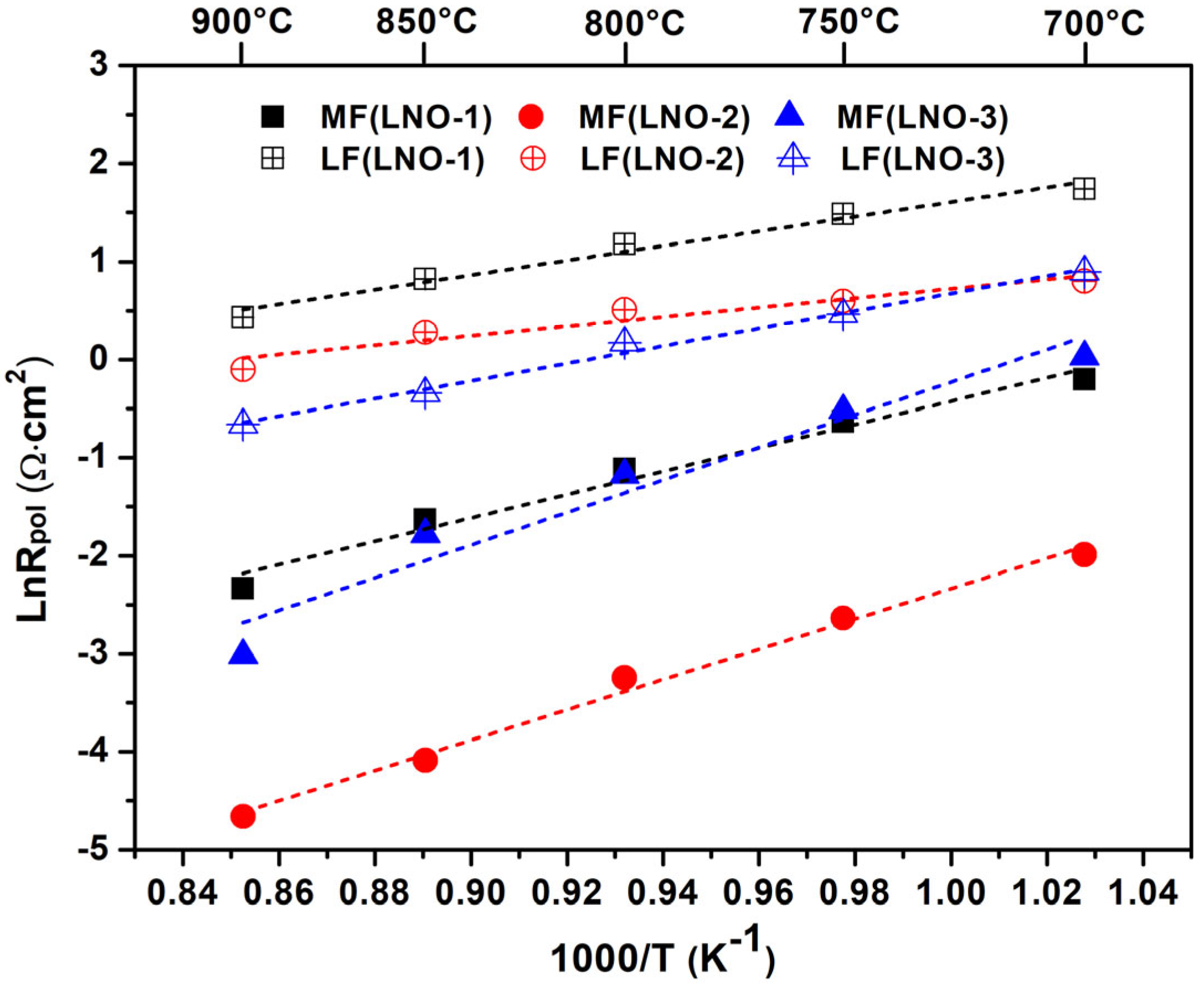
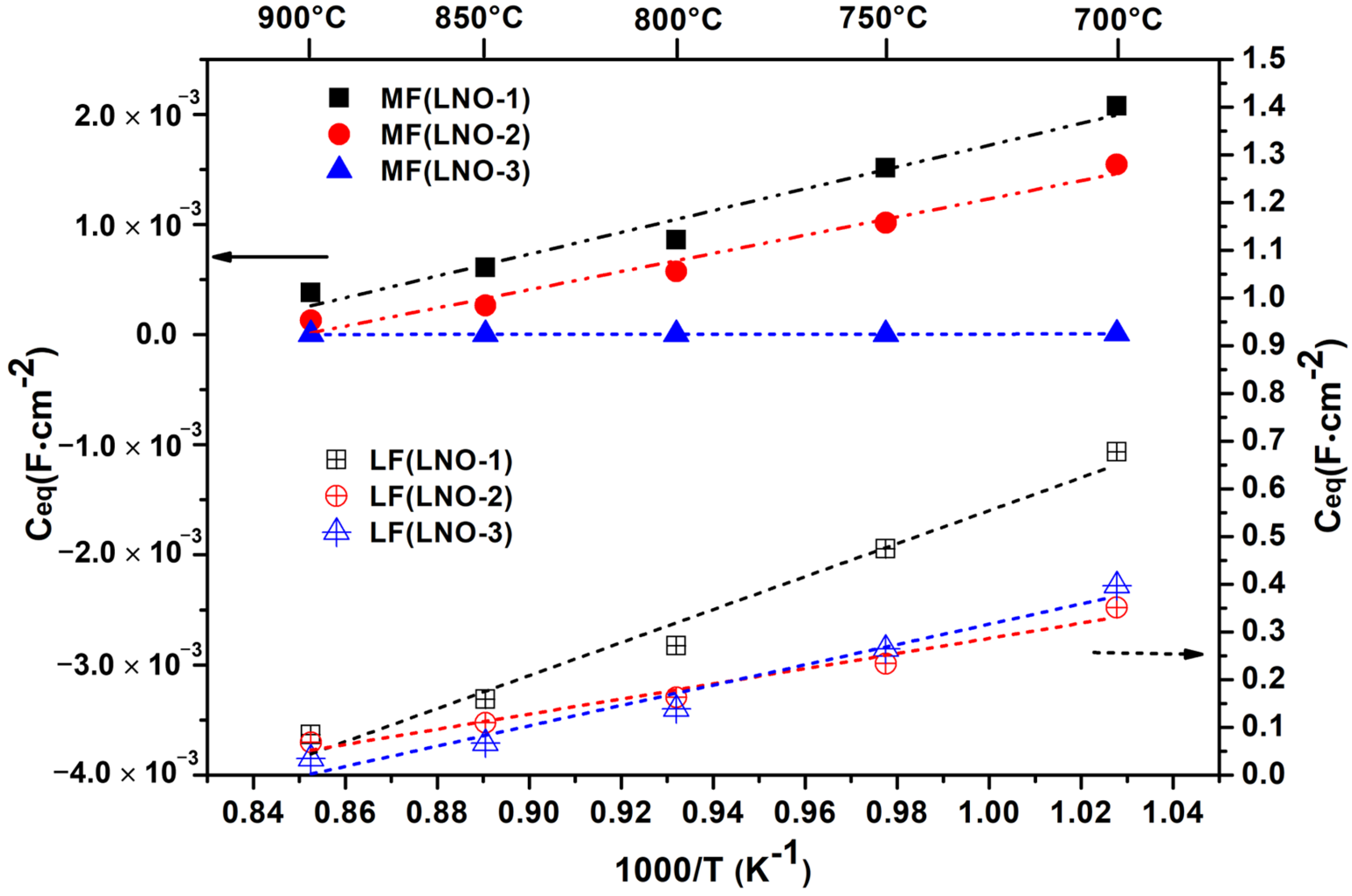
3.3. Electrical and Electrochemical Properties of the La2NiO4 Coatings
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Vohs, J.M.; Gorte, R.J. High-performance SOFC cathodes prepared by infiltration. Adv. Mater. 2009, 21, 943–956. [Google Scholar] [CrossRef]
- Yang, L.; Wang, S.; Blinn, K.; Liu, M.; Liu, Z.; Cheng, Z.; Liu, M. Enhaned sulfur and coking tolerance of a mixed ion conductor for SOFCs: BaZr0.1Ce0.7Y0.2–x YbxO3–δ. Science 2009, 326, 126–129. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Zhao, M.; Li, F.; Zhou, W.; Peterson, V.K.; Xu, X.; Shao, Z.; Gentle, I.; Zhu, Z. A niobium and tantalum co-doped perovskite cathode for solid oxide fuel cells operating below 500 °C. Nat. Commun. 2017, 8, 13990. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Choi, Y.; Yoo, S.; Ding, Y.; Yan, R.; Pei, K.; Qu, C.; Zhang, L.; Chang, I.; Zhao, B.; et al. A highly efficient multi-phase catalyst dramatically enhances the rate of oxygen reduction. Joule 2018, 2, 938–949. [Google Scholar] [CrossRef]
- Alenazey, F.; Alyousef, Y.; AlOtaibi, B.; Almutairi, G.; Minakshi, M.; Cheng, C.K.; Vo, D.V.N. Degradation behaviors of solid oxide fuel cell stacks in steady-state and cycling conditions. Energy Fuels 2020, 34, 14864–14873. [Google Scholar] [CrossRef]
- DiGiuseppe, G.; Sun, L. Electrochemical performance of a solid oxide fuel cell with an LSCF cathode under different oxygen concentrations. Int. J. Hydrogen Energy 2011, 36, 5076–5087. [Google Scholar] [CrossRef]
- Zhang, Y.; Chen, B.; Guan, D.; Xu, M.; Ran, R.; Ni, M.; Zhou, W.; O’hayre, R.; Shao, Z. Thermal-expansion offset for high-performance fuel cell cathodes. Nature 2021, 591, 246–251. [Google Scholar] [CrossRef]
- Xu, K.; Pei, K.; Zhao, B.; Zhao, Y.; Niu, Q.; Chen, Y. An oxygen reduction reaction active and durable SOFC cathode/electrolyte interface achieved via a cost-effective spray-coating. Int. J. Hydrogen Energy 2021, 46, 32242–32249. [Google Scholar] [CrossRef]
- Dailly, J.; Fourcade, S.; Largeteau, A.; Mauvy, F.; Grenier, J.C.; Marrony, M. Perovskite and A2MO4-type oxides as new cathode materials for protonic solid oxide fuel cells. Electrochim. Acta 2010, 55, 5847–5853. [Google Scholar] [CrossRef]
- Burriel, M.; Santiso, J.; Rossell, M.D.; Tendeloo, G.V.; Figueras, A.; Garcia, G. Enhancing total conductivity of La2NiO4+δ epitaxial thin films by reducing thickness. J. Phys. Chem. C 2008, 112, 10982–10987. [Google Scholar] [CrossRef]
- Flura, A.; Dru, S.; Nicollet, C.; Vibhu, V.; Fourcade, S.; Lebraud, E.; Rougier, A.; Bassat, J.-M.; Grenier, J.-C. Chemical and structural changes in Ln2NiO4+δ (Ln = La, Pr or Nd) lanthanide nickelates as a function of oxygen partial pressure at high temperature. J. Solid State Chem. 2015, 228, 189–198. [Google Scholar] [CrossRef]
- Fondard, J.; Billard, A.; Bertrand, G.; Briois, P. Ln2NiO4+δ (Ln = La, Pr, Nd) coatings deposited by reactive magnetron sputtering as cathode material for intermediate temperature solid oxide fuel cell. Vacuum 2018, 152, 97–108. [Google Scholar] [CrossRef]
- Wu, J.; Liu, X. Recent development of SOFC metallic interconnect. J. Mater. Sci. Technol. 2010, 26, 293–305. [Google Scholar] [CrossRef]
- Zhao, K.; Wang, Y.P.; Chen, M.; Xu, Q.; Kim, B.H.; Huang, D.P. Electrochemical evaluation of La2NiO4+δ as a cathode material for intermediate temperature solid oxide fuel cells. Int. J. Hydrogen Energy 2014, 39, 7120–7130. [Google Scholar] [CrossRef]
- Philippeau, B.; Mauvy, F.; Mazataud, C.; Fourcade, S.; Grenier, J.C. Comparative study of electrochemical properties of mixed conducting Ln2NiO4+δ (Ln = La, Pr and Nd) and La0.6Sr0.4Fe0.8Co0.2O3−δ as SOFC cathodes associated to Ce0.9Gd0.1O2−δ, La0.8Sr0.2Ga0.8Mg0.2O3−δ and La9Sr1Si6O26.5 electrolytes. Solid State Ion. 2013, 249, 17–25. [Google Scholar] [CrossRef]
- Yang, S.H.; Kim, K.H.; Yoon, H.H.; Kim, W.J.; Choi, H.W. Comparison of combustion and solid-state reaction methods for the fabrication of SOFC LSM cathodes. Mol. Cryst. Liq. Cryst. 2011, 539, 50–390. [Google Scholar] [CrossRef]
- Solovyev, A.; Burmistrov, I.N.; Rabotkin, S.; Shipilova, A.; Yalovenko, D.V.; Semenov, V.; Bredikhin, S.I. Electrochemical Characterization of Intermediate-Temperature Solid Oxide Fuel Cells with PVD-Coated Electrolyte. ECS Trans. 2021, 103, 105. [Google Scholar] [CrossRef]
- Gannon, P.; Deibert, M.; White, P.; Smith, R.; Chen, H.; Priyantha, W.; Gorokhovsky, V. Advanced PVD protective coatings for SOFC interconnects. Int. J. Hydrogen Energy 2008, 33, 3991–4000. [Google Scholar] [CrossRef]
- Jung, W.; Kim, J.J.; Tuller, H.L. Investigation of nanoporous platinum thin films fabricated by reactive sputtering: Application as micro-SOFC electrode. J. Power Source 2015, 275, 860–865. [Google Scholar] [CrossRef]
- Briois, P.; Perry, F.; Billard, A. Structural and electrical characterisation of lanthanum nickelate reactively sputter-deposited thin films. Thin Solid Films 2008, 516, 3282–3286. [Google Scholar] [CrossRef]
- Fondard, J.; Billard, A.; Bertrand, G.; Briois, P. Synthesis and characterization of La2NiO4+δ coatings deposited by reactive magnetron sputtering using plasma emission monitoring. Solid State Ion. 2014, 265, 73–79. [Google Scholar] [CrossRef]
- Anders, A.X. A structure zone diagram including plasma-based deposition and ion etching. Thin Solid Films 2010, 518, 4087–4090. [Google Scholar] [CrossRef]
- Amow, G.; Davidson, I.J.; Skinner, S.J. A comparative study of the Ruddlesden-Popper series, Lan+1NinO3n+1 (n = 1, 2 and 3), for solid-oxide fuel-cell cathode applications. Solid State Ion. 2006, 177, 1205–1210. [Google Scholar] [CrossRef]
- Mauvy, F.; Lalanne, C.; Bassat, J.M.; Grenier, J.C.; Zhao, H.; Huo, L.; Stevens, P. Electrode properties of Ln2NiO4+δ (Ln = La, Nd, Pr): AC impedance and DC polarization studies. J. Electrochem. Soc. 2006, 153, A1547. [Google Scholar] [CrossRef]
- Minakshi, M.; Mitchell, D.R.; Munnangi, A.R.; Barlow, A.J.; Fichtner, M. New insights into the electrochemistry of magnesium molybdate hierarchical architectures for high performance sodium devices. Nanoscale 2018, 10, 13277–13288. [Google Scholar] [CrossRef] [PubMed]
- Barmi, M.J.; Minakshi, M. Tuning the redox properties of the nanostructured CoMoO4 electrode: Effects of surfactant content and synthesis temperature. ChemPlusChem 2016, 81, 964–977. [Google Scholar] [CrossRef]
- Zhao, H.; Mauvy, F.; Lalanne, C.; Bassat, J.M.; Fourcade, S.; Grenier, J.C. New cathode materials for ITSOFC: Phase stability, oxygen exchange and cathode properties of La2−xNiO4+δ. Solid State Ion. 2008, 179, 2000–2005. [Google Scholar] [CrossRef]
- Escudero, M.J.; Aguadero, A.; Alonso, J.A.; Daza, L. A kinetic study of oxygen reduction reaction on La2NiO4 cathodes by means of impedance spectroscopy. J. Electroanal. Chem. 2007, 611, 107–116. [Google Scholar] [CrossRef]
- Benamira, M.; Ringuedé, A.; Cassir, M.; Horwat, D.; Lenormand, P.; Ansart, F.; Bassat, J.M.; Viricelle, J.P. Enhancing oxygen reduction reaction of YSZ/La2NiO4+δ using an ultrathin La2NiO4+δ interfacial layer. J. Alloys Compd. 2018, 746, 413–420. [Google Scholar] [CrossRef]
- Garbayo, I.; Esposito, V.; Sanna, S.; Morata, A.; Pla, D.; Fonseca, L.; Sabaté, N.; Tarancón, A. Porous La0.6Sr0.4CoO3−δ thin film cathodes for large area micro solid oxide fuel cell power generators. J. Power Source 2014, 248, 1042–1049. [Google Scholar] [CrossRef]
- Boukamp, B.A.; Hildenbrand, N.; Nammensma, P.; Blank, D.H. The impedance of thin dense oxide cathodes. Solid State Ion. 2011, 192, 404–408. [Google Scholar] [CrossRef]





| Parameters | La Target | Ni Target |
|---|---|---|
| Ar flow rate (sccm) | 200 | 200 |
| O2 flow rate (sccm) | 6 | 6 |
| Total pressure (Pa) | 2.4 | 2.4 |
| Drawing distance (mm) | 70 | 70 |
| Power (W) | 500 | 102 |
| Frequency (kHz) | 50 | 50 |
| Toff (μs) | 5 | 5 |
| Annealing Treatment | Average Grain Size (nm) |
|---|---|
| As deposited | 1.9 |
| 500 °C for 2 h | 3.7 |
| 600 °C for 2 h | 5.1 |
| 700 °C for 2 h | 27.0 |
| 800 °C for 2 h | 32.0 |
| 900 °C for 2 h | 36.2 |
| 1000 °C for 2 h | 42.9 |
| 1100 °C for 2 h | 71.2 |
| 1000 °C 2 h–750 °C 120 h | 47.6 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ye, X.; Luo, H.; Hou, M.; Bertrand, P.; Billard, A.; Briois, P. Morphological Evolution of La2NiO4 Coatings Synthesized by Reactive Magnetron Sputtering (RMS) at High Pressure as Cathode for Intermediate Temperature Solid Oxide Fuel Cell (IT-SOFC). Coatings 2023, 13, 1113. https://doi.org/10.3390/coatings13061113
Ye X, Luo H, Hou M, Bertrand P, Billard A, Briois P. Morphological Evolution of La2NiO4 Coatings Synthesized by Reactive Magnetron Sputtering (RMS) at High Pressure as Cathode for Intermediate Temperature Solid Oxide Fuel Cell (IT-SOFC). Coatings. 2023; 13(6):1113. https://doi.org/10.3390/coatings13061113
Chicago/Turabian StyleYe, Xiaolei, Huan Luo, Ming Hou, Pierre Bertrand, Alain Billard, and Pascal Briois. 2023. "Morphological Evolution of La2NiO4 Coatings Synthesized by Reactive Magnetron Sputtering (RMS) at High Pressure as Cathode for Intermediate Temperature Solid Oxide Fuel Cell (IT-SOFC)" Coatings 13, no. 6: 1113. https://doi.org/10.3390/coatings13061113
APA StyleYe, X., Luo, H., Hou, M., Bertrand, P., Billard, A., & Briois, P. (2023). Morphological Evolution of La2NiO4 Coatings Synthesized by Reactive Magnetron Sputtering (RMS) at High Pressure as Cathode for Intermediate Temperature Solid Oxide Fuel Cell (IT-SOFC). Coatings, 13(6), 1113. https://doi.org/10.3390/coatings13061113






