In Situ Growth of High-Performance ZnAl-Layered Double Hydroxides/Al2O3 Composite Coatings on Aluminum Alloy
Abstract
:1. Introduction
2. Materials and Methods
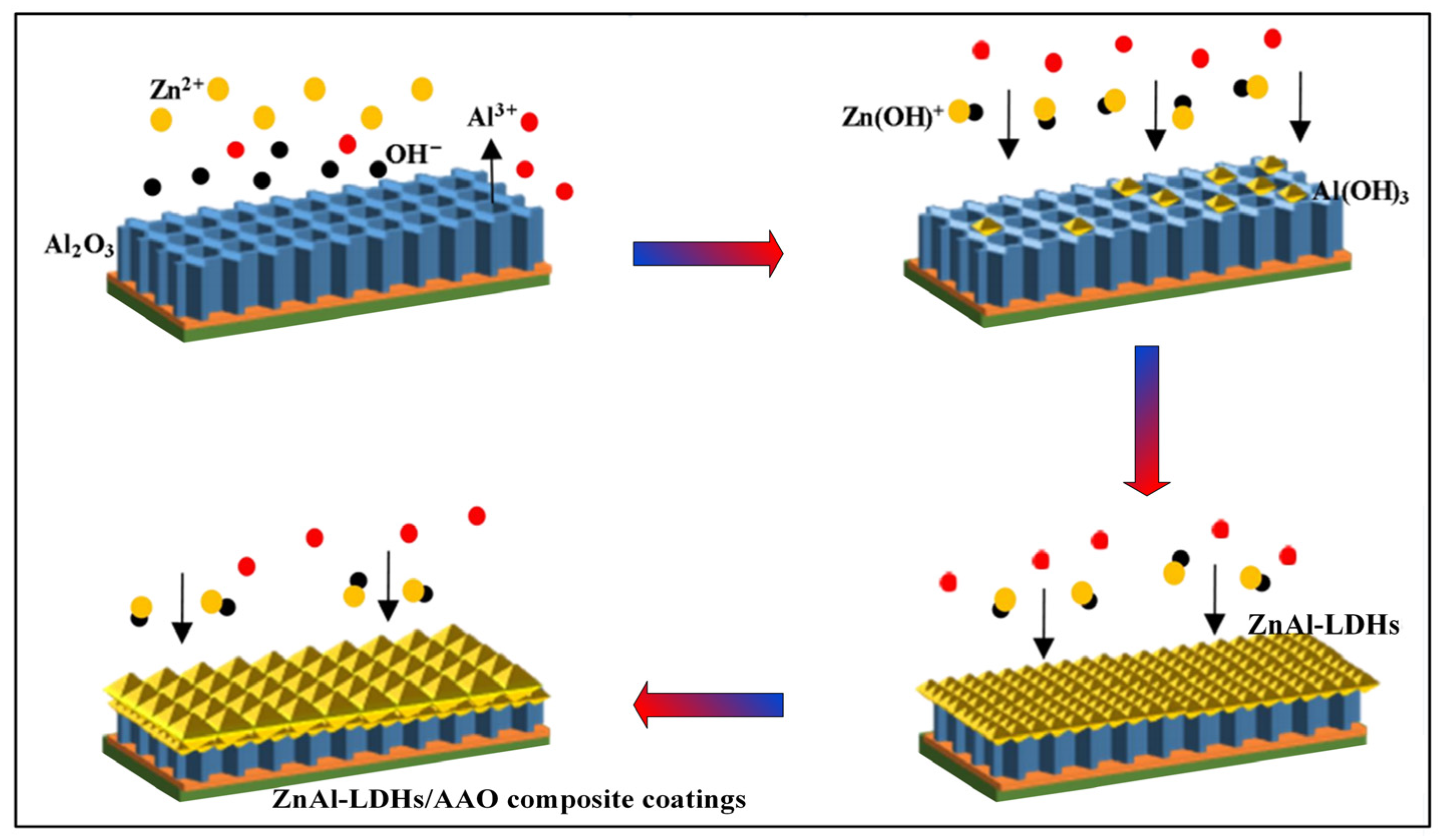
2.1. Preparation of ZnAl-LDHs/AAO Composite Coatings
2.2. Characterization and Tests
3. Results and Discussion
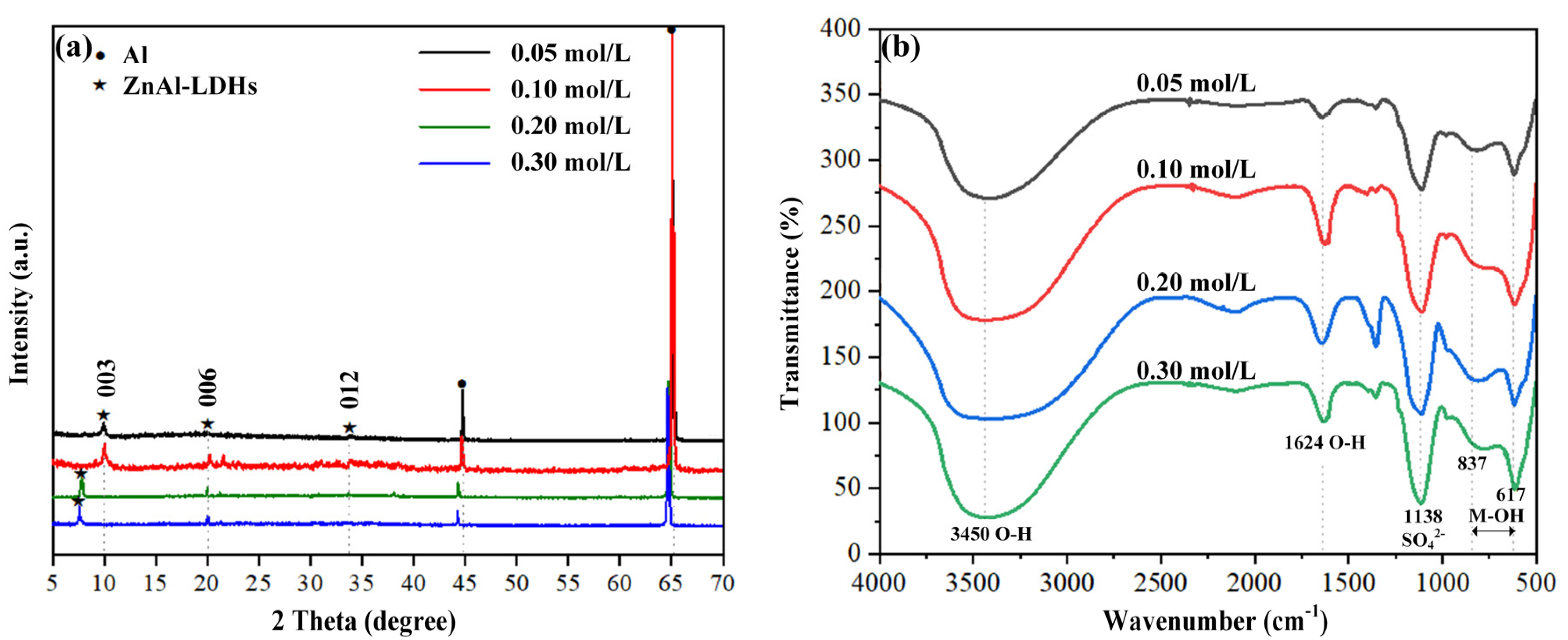
3.1. Chemical and Phase Composition
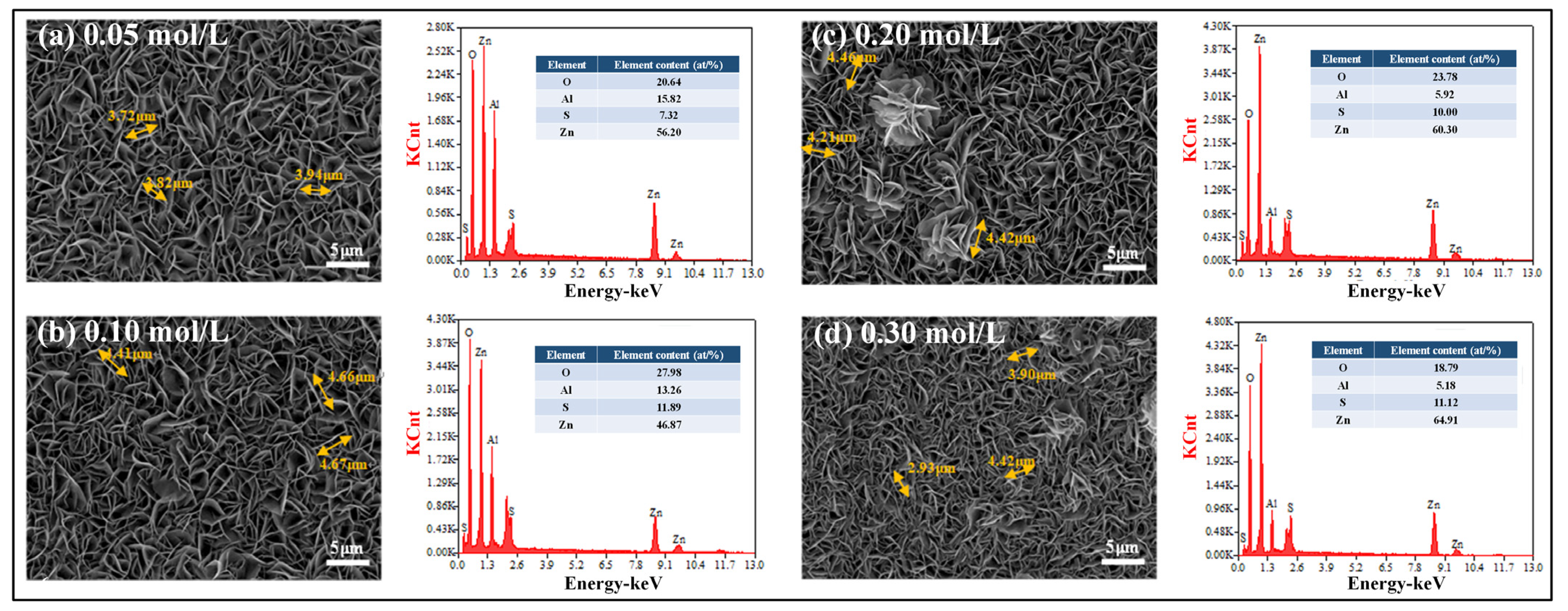
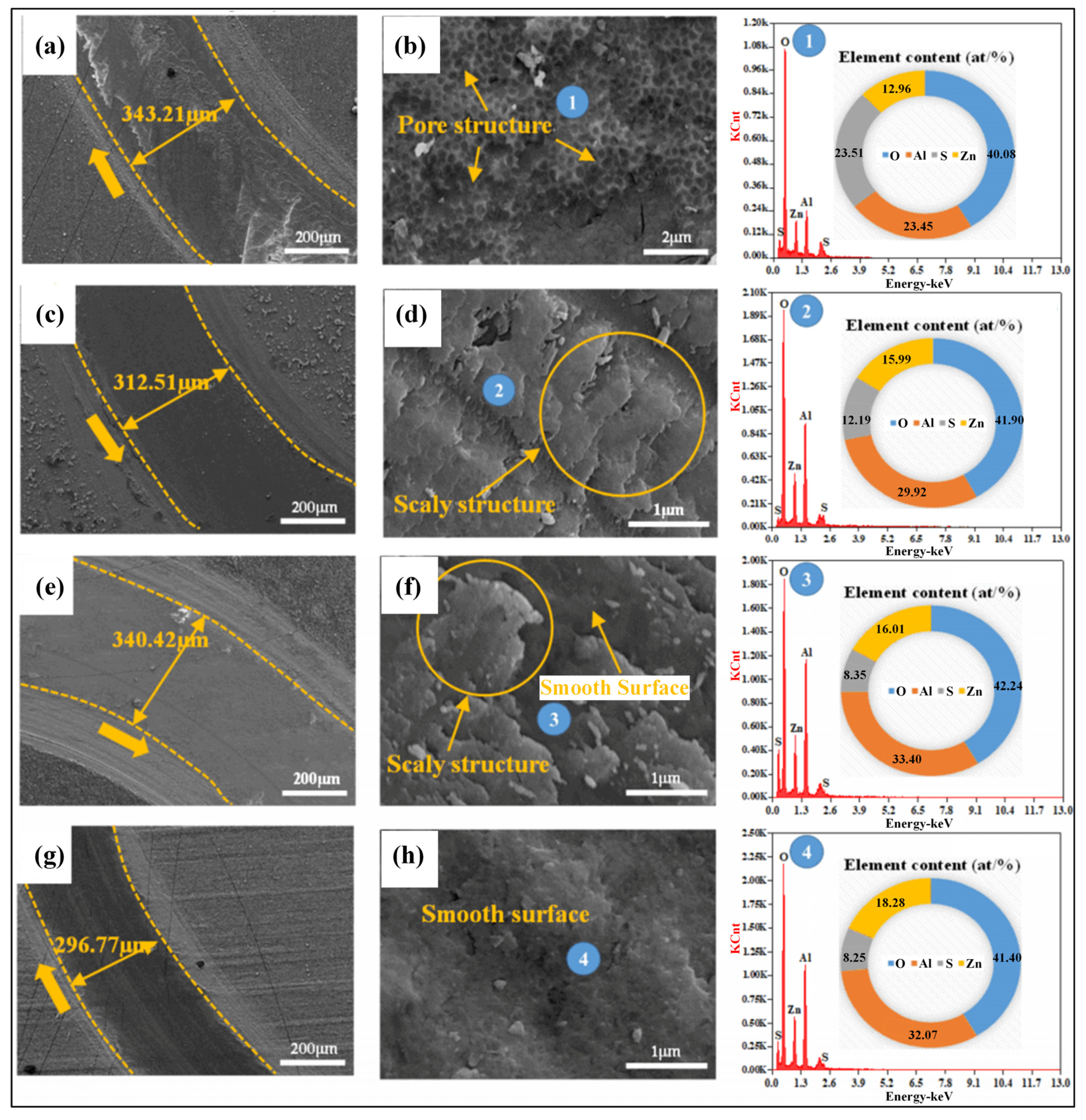
3.2. Surface and Cross-Sectional Morphology
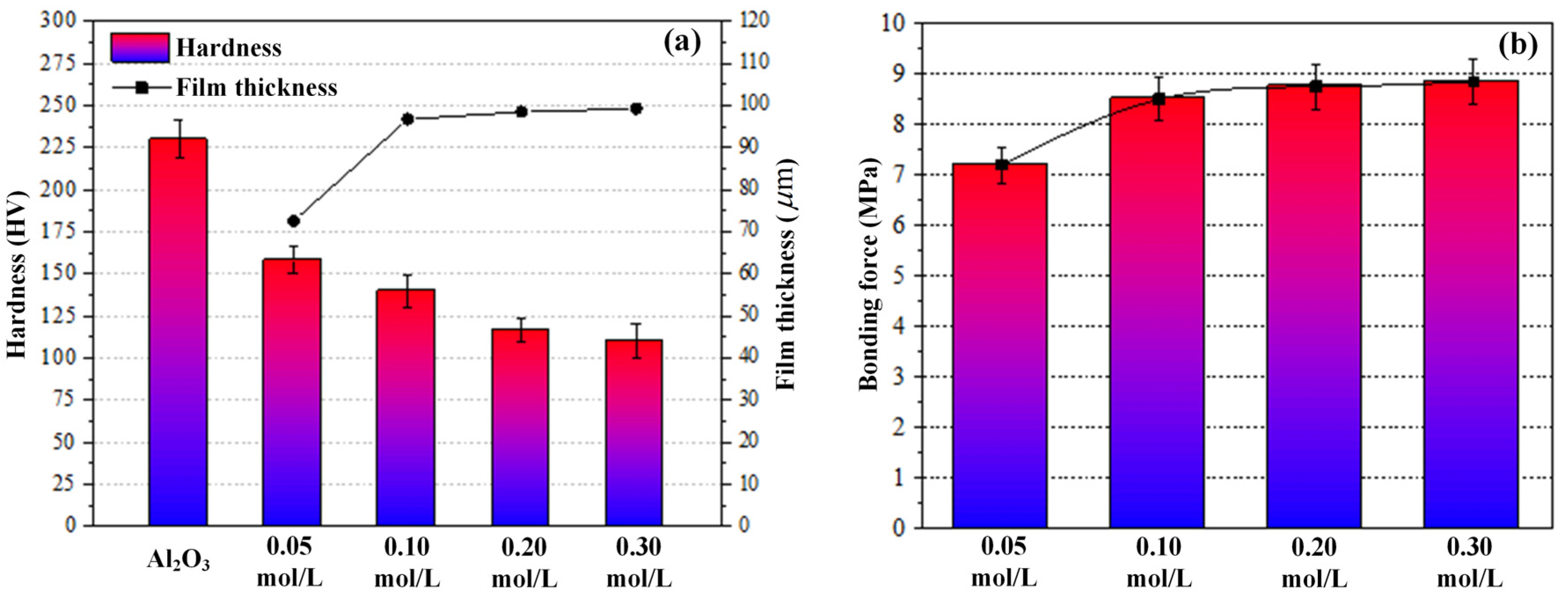
3.3. Hardness and Interfacial Bonding Strength
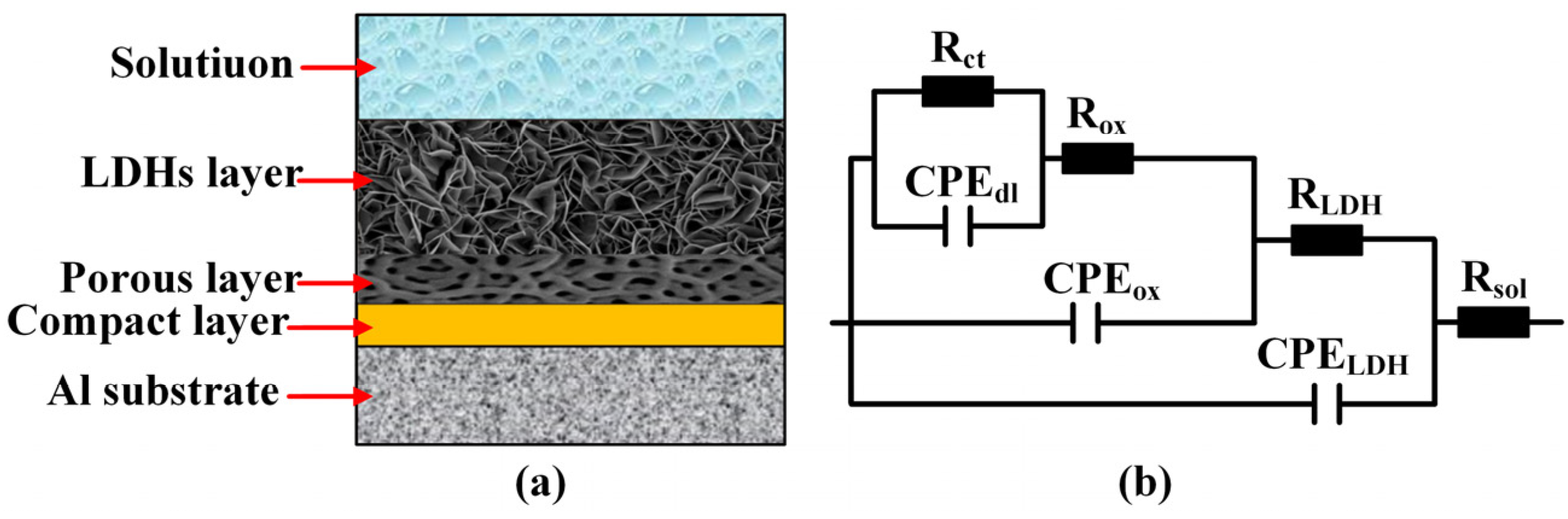
3.4. Corrosion Resistance of ZnAl-LDHs/AAO Composite Coatings
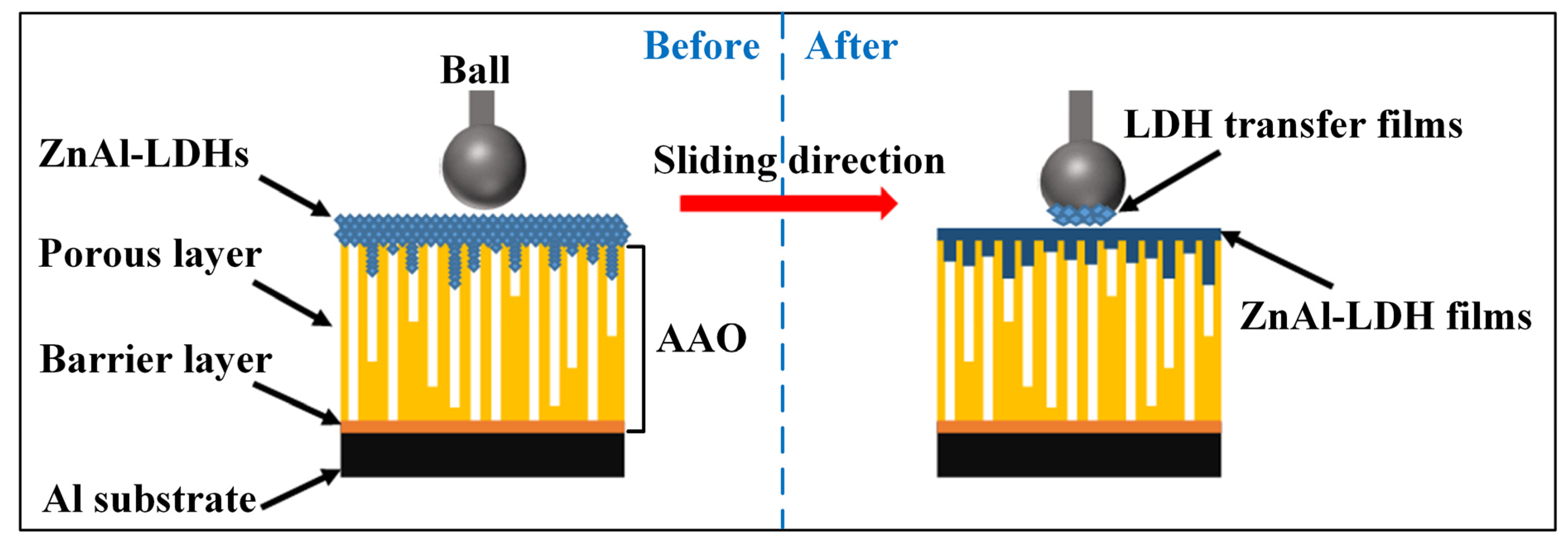
3.5. Tribological Behavior of ZnAl-LDHs/AAO Composite Coatings
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Ma, Z.; Feng, A.; Chen, D.; Shen, J. Recent advances in friction stir welding/processing of aluminum alloys: Microstructural avolution and mechanical properties. Crit. Rev. Solid State 2018, 43, 269–333. [Google Scholar] [CrossRef]
- Karthik, G.M.; Kim, H.S. Heterogeneous aspects of additive manufactured metallic parts: A review. Met. Mater. Int. 2021, 27, 1–39. [Google Scholar] [CrossRef]
- Guo, F.; Wu, S.; Liu, J.; Wu, Z.; Fu, S.; Ding, S. A time-domain stepwise fatigue assessment to bridge small-scale fracture mechanics with large-scale system dynamics for high-speed maglev lightweight bogies. Eng. Fract. Mech. 2021, 248, 107711. [Google Scholar] [CrossRef]
- Zhang, F.; Zhao, L.; Chen, H.; Xu, S.; Evans, D.; Duan, X. Corrosion resistance of superhydrophobic layered double hydroxide films on aluminum. Angew. Chem. Int. Ed. 2008, 47, 2466–2469. [Google Scholar] [CrossRef]
- Bai, B.N.P.; Ramasesh, B.S.; Surappa, M.K. Dry sliding wear of A356-Al-SiCp somposites. Wear 1992, 157, 295–304. [Google Scholar] [CrossRef]
- Li, S.; Yao, W.; Liu, J.; Yu, M.; Ma, K. Effect of SiC nanoparticle concentration on the properties of oxide films formed on Ti-10V-2Fe-3Al alloy. Vacuum 2016, 123, 1–7. [Google Scholar] [CrossRef]
- Li, S.; Yao, W.; Liu, J.; Yu, M.; Wu, L.; Ma, K. Study on anodic oxidation process and property of composite film formed on Ti-10V-2Fe-3Al alloy in SiC nanoparticle suspension. Surf. Coat. Technol. 2015, 277, 234–241. [Google Scholar] [CrossRef]
- Cui, L.; Gao, S.; Li, P.; Zeng, R.; Zhang, F.; Li, S.; Han, E. Corrosion resistance of a self-healing micro-arc oxidation/polymethyltrimethoxysilane composite coating on magnesium alloy AZ31. Corros. Sci. 2017, 118, 84–95. [Google Scholar] [CrossRef]
- Lu, X.; Blawert, C.; Tolnai, D.; Subroto, T.; Kainer, K.; Zhang, T.; Wang, F.; Zheludkevich, M. 3D reconstruction of plasma electrolytic oxidation coatings on Mg alloy via synchrotron radiation tomography. Corros. Sci. 2018, 139, 395–402. [Google Scholar] [CrossRef]
- Totaro, P.; Khusid, B. Multistep anodization of 7075-T6 aluminum alloy. Surf. Coat. Technol. 2021, 421, 127407. [Google Scholar] [CrossRef]
- Wu, L.; Ding, X.; Zheng, Z.; Ma, Y.; Atrens, A.; Chen, X.; Xie, Z.; Sun, D.; Pan, F. Fabrication and characterization of an actively protective Mg-Al LDHs/Al2O3 composite coating on magnesium alloy AZ31. Appl. Surf. Sci. 2019, 487, 558–568. [Google Scholar] [CrossRef]
- Wu, L.; Liu, L.; Zhan, Q.; Wen, C.; Hao, X.; Liu, J.; Chen, Y.; Atrens, A. Morphology, microstructure and tribological properties of anodic films formed on Ti10V2Fe3Al alloy in different electrolytes. Rare Met. 2021, 40, 2905–2916. [Google Scholar] [CrossRef]
- Zaraska, L.; Sulka, G.; Szeremeta, J.; Jaskula, M. Porous anodic alumina formed by anodization of aluminum alloy (AA1050) and high purity aluminum. Electrochim. Acta 2010, 55, 4377–4386. [Google Scholar] [CrossRef]
- Kikuchi, T.; Taniguchi, T.; Suzuki, R.; Natsui, S. Fabrication of a plasma electrolytic oxidation/anodic aluminum oxide multi-layer film via one-step anodizing aluminum in ammonium carbonate. Thin Solid Film. 2020, 697, 137799. [Google Scholar] [CrossRef]
- Bensalah, W.; Feki, M.; Wery, M.; Ayedi, H. Chemical dissolution resistance of anodic oxide layers formed on aluminum. Trans. Nonferrous Met. Soc. China 2011, 21, 1673–1679. [Google Scholar] [CrossRef]
- Montero-Moreno, J.M.; Sarret, M.; Muller, C. Influence of the aluminum surface on the final results of a two-step anodizing. Surf. Coat. Technol. 2007, 201, 6352–6357. [Google Scholar] [CrossRef]
- Ko, C.; Kuo, Y.; Chen, S.; Chen, S.; Guo, J.; Wang, Y. Formation of aluminum composite passive film on magnesium alloy by integrating sputtering and anodic aluminum oxidation processes. Thin Solid Films 2020, 709, 138151. [Google Scholar] [CrossRef]
- Sul, Y.; Johansson, C.; Jeong, Y.; Albrektsson, T. The electrochemical oxide growth behaviour on titanium in acid and alkaline electrolytes. Med. Eng. Phys. 2001, 23, 329–346. [Google Scholar] [CrossRef]
- Song, H.; Park, S.; Jeong, S.; Park, Y. Surface characteristics and bioactivity of oxide films formed by anodic spark oxidation on titanium in different electrolytes. J. Mater. Process. Technol. 2009, 209, 864–870. [Google Scholar] [CrossRef]
- Liu, L.; Wu, L.; Chen, X.; Sun, D.; Chen, Y.; Zhang, G.; Ding, X.; Pan, F. Enhanced protective coatings on Ti-10V-2Fe-3Al alloy through anodizing and post-sealing with layered double hydroxides. J. Mater. Sci. Technol. 2020, 37, 104–113. [Google Scholar] [CrossRef]
- Rahimi, S.; Khiabani, A.B.; Yarmand, B.; Kolahi, A. Comparison of corrosion and antibacterial properties of Al alloy treated by plasma electrolytic oxidation and anodizing methods. Mater. Today-Proc. 2018, 5, 15667–15676. [Google Scholar] [CrossRef]
- Zhang, J.; Song, B.; Wei, Q.; Bourell, D.; Shi, Y. A review of selective laser melting of aluminum alloys: Processing, microstructure, property and developing trends. J. Mater. Sci. Technol. 2019, 35, 270–284. [Google Scholar] [CrossRef]
- Feng, Y.; Williams, G.; Leroux, F.; Taviot-Gueho, C.; O’Hare, D. Selective anion-exchange properties of second-stage layered double hydroxide heterostructures. Chem. Mater. 2006, 18, 4312–4318. [Google Scholar] [CrossRef]
- Wu, L.; Zhang, G.; Tang, A.; Liu, Y.; Atrens, A.; Pan, F. Communication-fabrication of protective layered double hydroxide films by conversion of anodic films onmagnesium alloy. J. Electrochem. Soc. 2017, 164, 339–341. [Google Scholar] [CrossRef]
- Zhang, G.; Wu, L.; Tang, A.; Ma, Y.; Song, G.; Zheng, D.; Jiang, B.; Atrens, A.; Pan, F. Active corrosion protection by a smart coating based on a MgAl-layered double hydroxide on a cerium-modified plasma electrolytic oxidation coating on Mg alloy AZ31. Corros. Sci. 2018, 139, 370–382. [Google Scholar] [CrossRef]
- Wu, L.; Yang, D.; Zhang, G.; Zhang, Z.; Zhang, S.; Tang, A.; Pan, F. Fabricationand characterization of Mg-M layered double hydroxide films on anodized magnesium alloy AZ31. Appl. Surf. Sci. 2018, 431, 77–186. [Google Scholar] [CrossRef]
- Zhang, G.; Wu, L.; Tang, A.; Weng, B.; Atrens, A.; Ma, S.; Liu, L.; Pan, F. Sealing of anodized magnesium alloy AZ31 with MgAl layered double hydroxides layers. RSC Adv. 2018, 8, 2248–2259. [Google Scholar] [CrossRef]
- Wang, C.; Huang, Y.; Li, J.; Wang, M.; Du, X.; Chen, D. Preparation of superhydrophobic Li-Al-Ala LDH/SA film with enhanced corrosion resistance and mechanical stability on AZ91D Mg alloy. J. Mater. Sci. 2022, 57, 14780–14798. [Google Scholar] [CrossRef]
- Wang, H.; Liu, Y.; Liu, W.; Wang, R.; Wen, J.; Sheng, H.; Peng, J.; Erdemir, A.; Luo, J. Tribological behavior of NiAI-Layered double hydroxide nanoplatelets as oil-based lubricant additives. ACS Appl. Mater. Interfaces 2017, 9, 30891–30899. [Google Scholar] [CrossRef]
- Wang, H.; Liu, Y.; Liu, W.; Liu, Y.; Wang, K.; Li, J.; Ma, T.; Eryilmaz, O.; Shi, Y.; Erdemir, A.; et al. Superlubricity of polyalkylene glycol aqueous solutions enabled by ultrathin layered double hydroxide nanosheets. ACS Appl. Mater. Interfaces 2019, 11, 20249–20256. [Google Scholar] [CrossRef]
- Zhao, S.; Tsen, W.; Hu, F.; Zhong, F.; Liu, H.; Wen, S.; Zheng, G.; Qin, C.; Gong, C. Layered double hydroxide-coated silica nanospheres with 3D architecture-modified composite anion exchange membranes for fuel cell applications. J. Mater. Sci. 2020, 55, 2967–2983. [Google Scholar] [CrossRef]
- Zhang, Y.; Wuu, D.; Huang, J. Preparation of AgNWs@NiO-Co3O4 dopant material for an activated carbon thin-film electrode of pseudocapacitors. J. Mater. Sci. 2021, 56, 15229–15240. [Google Scholar]
- Zhang, Z.; Zhou, J.; Wei, H.; Dai, Y.; Li, S.; Shi, H.; Xu, G. Construction of hierarchical NiFe-LDH/FeCoS2/CFC composites as efficient bifunctional electrocatalysts for hydrogen and oxygen evolution reaction. J. Mater. Sci. 2022, 55, 16625–16640. [Google Scholar] [CrossRef]
- Qi, J.; Ruan, C.; Hu, R.; Sui, Y.; He, Y.; Meng, Q.; Wei, F.; Ren, Y.; Wei, W. Flexible wire-shaped symmetric supercapacitors with Zn-Co layered double hydroxide nanosheets grown on Ag-Coated cotton wire. J. Mater. Sci. 2022, 55, 16683–16696. [Google Scholar]
- Ding, P.; Li, Z.; Wang, Q.; Zhang, X.; Tang, S.; Song, N.; Shi, L. In situ growth of layered double hydroxide films under dynamic processes: Influence of metal cations. Mater. Lett. 2012, 77, 1–3. [Google Scholar] [CrossRef]
- Xue, L.; Lu, Z.; Cheng, Y.; Sun, X.; Lin, H.; Xiao, X.; Liu, X.; Zhuo, S. Three-dimensional layered double hydroxide membranes: Fabrication technique, growth mechanism, and enhanced photocatalytic activity. Chem. Commun. 2018, 54, 8494–8497. [Google Scholar] [CrossRef]
- Liu, J.; Shi, H.; Yu, M.; Du, R.; Rong, G.; Li, S. Effect of divalent metal ions on durability and anticorrosion performance of layered double hydroxides on anodized 2A12 aluminum alloy. Surf. Coat. Technol. 2019, 373, 56–64. [Google Scholar] [CrossRef]
- Shen, G.; Zhang, L.; Gu, Z.; Zheng, Z.; Liu, Y.; Tan, G.; Jie, X. Zinc aluminum-layered double hydroxide(LDH)-graphene oxide(GO) lubricating and corrosion-resistant composite coating on the surface of magnesium alloy. Surf. Coat. Technol. 2022, 437, 128354. [Google Scholar]
- Zhang, Y.; Wang, Y.; Li, C.; Zhou, B.; Cheng, K.; Wei, Y. Preparation and corrosion resistance of the ZnAl-LDHs film on 6061 Al alloy surface. Acta. Metall. Sin. 2018, 54, 1417–1427. [Google Scholar]
- Li, S.; Bhushan, B. Lubrication performance and mechanisms of Mg/Al-, Zn/Al-, and Zn/Mg/Al-layered double hydroxide nanoparticles as lubricant additives. Appl. Surf. Sci. 2016, 378, 308–319. [Google Scholar] [CrossRef]
- Wu, H.; Zhang, Y.; Long, S.; Zhang, L.; Jie, X. Tribological behavior of graphene anchored Mg-Al layered double hydroxide film on Mg alloy pre-sprayed Al coating. Appl. Surf. Sci. 2020, 530, 146536. [Google Scholar]
- Ying, L.; Xie, Y.; Fu, Z.; Shi, H.; Wang, G. Study on direct current electro-deposition of copper nanowires in anodic alumina membrane pores. Coatings 2019, 9, 378. [Google Scholar]
- Zhang, K.; Yu, S. Preparation of wear and corrosion resistant micro-arc oxidation coating on 7N01 aluminum alloy. Surf. Coat. Technol. 2020, 388, 125453. [Google Scholar] [CrossRef]
- Snihirova, D.; Lamaka, S.; Montemor, M. “SMART” protective ability of water based epoxy coatings loaded with CaCO3 microbeads impregnated with corrosion inhibitors applied on AA2024 substrates. Electrochim. Acta 2012, 83, 439–447. [Google Scholar] [CrossRef]
- Long, R.; Zhao, C.; Jin, Z.; Zhang, Y.; Pan, Z.; Sun, S.; Gao, W. Influence of groove dimensions on the tribological behavior of textured cylindrical roller thrust bearings under starved lubrication. Ind. Lubr. Tribol. 2021, 73, 971–979. [Google Scholar] [CrossRef]
- Zhao, C.; Long, R.; Zhang, Y.; Wang, Y.; Wang, Y. Influence of characteristic parameters on the tribological properties of vein-bionic textured cylindrical roller thrust bearings. Tribol. Int. 2022, 175, 107861. [Google Scholar] [CrossRef]
- Rosenkranz, A.; Stratmann, A.; Gachot, C.; Burghardt, G.; Jacobs, G.; Mucklich, F. Improved wear behavior of cylindrical roller thrust bearings by three-beam laser interference. Adv. Eng. Mater. 2016, 18, 854–862. [Google Scholar]
- Xie, H.; Cheng, Y.; Li, S.; Cao, J.; Cao, L. Wear and corrosion resistant coatings on surface of cast A356 aluminum alloy by plasma electrolytic oxidation in moderately concentrated aluminate electrolytes. Trans. Nonferrous Met. Soc. China 2017, 27, 336–351. [Google Scholar] [CrossRef]
- Martini, C.; Ceschini, L.; Tarterini, F.; Paillard, J.; Curran, J. PEO layers obtained from mixed aluminate-phosphate baths on Ti-6Al-4V: Dry sliding behaviour and influence of a PTFE topcoat. Wear 2010, 269, 747–756. [Google Scholar] [CrossRef]
- Soffritti, C.; Fortini, A.; Nastruzzi, A.; Sola, R.; Merlin, M.; Garagnani, G. Dry sliding behavior of an aluminum alloy after innovative hard anodizing treatments. Materials 2021, 14, 3281. [Google Scholar] [CrossRef]
- Kim, H.; Kim, D.; Ahn, H. Tribological properties of nanoporous anodic aluminum oxide film. Surf. Coat. Technol. 2010, 205, 1431–1437. [Google Scholar] [CrossRef]
- Meyer, E. Controlling friction atom by atom. Science 2015, 348, 1089. [Google Scholar] [CrossRef] [PubMed]




| Groups | Ecorr (V vs. SCE) | icorr (A/cm2) |
|---|---|---|
| AAO | −0.775 | 3.020 × 10−7 |
| 0.05 mol/L | −0.640 | 5.416 × 10−9 |
| 0.10 mol/L | −0.541 | 3.479 × 10−9 |
| 0.20 mol/L | −0.499 | 3.415 × 10−9 |
| 0.30 mol/L | −0.462 | 2.435 × 10−9 |
| Groups | Rsol (ohm) | CLDHs (F) | RLDHs (ohm) | Cox (F) | Rox (ohm) | Cdl (F) | Rct (ohm) | χ2 |
|---|---|---|---|---|---|---|---|---|
| AAO | 10.61 | - | - | 2.927 × 10−7 | 6.778 × 103 | 5.953 × 10−8 | 1.345 × 106 | 8.2 × 10−4 |
| 0.05 mol/L | 9.52 | 3.231 × 10−8 | 2.543 × 103 | 5.010 × 10−8 | 2.451 × 104 | 3.549 × 10−8 | 4.598 × 106 | 3.6 × 10−3 |
| 0.10 mol/L | 8.04 | 1.576 × 10−8 | 4.396 × 103 | 5.375 × 10−8 | 2.029 × 104 | 2.721 × 10−8 | 5.527 × 106 | 2.4 × 10−3 |
| 0.20 mol/L | 10.23 | 1.431 × 10−8 | 4.523 × 103 | 6.678 × 10−8 | 1.297 × 104 | 1.943 × 10−8 | 7.321 × 106 | 2.8 × 10−3 |
| 0.30 mol/L | 9.64 | 1.303 × 10−8 | 6.041 × 103 | 8.846 × 10−8 | 1.186 × 104 | 1.209 × 10−8 | 1.289 × 107 | 4.2 × 10−3 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ying, L.; Zhao, C.; Sun, Z.; Nie, C.; Liu, R.; Wang, Z.; Wu, Y. In Situ Growth of High-Performance ZnAl-Layered Double Hydroxides/Al2O3 Composite Coatings on Aluminum Alloy. Coatings 2023, 13, 1511. https://doi.org/10.3390/coatings13091511
Ying L, Zhao C, Sun Z, Nie C, Liu R, Wang Z, Wu Y. In Situ Growth of High-Performance ZnAl-Layered Double Hydroxides/Al2O3 Composite Coatings on Aluminum Alloy. Coatings. 2023; 13(9):1511. https://doi.org/10.3390/coatings13091511
Chicago/Turabian StyleYing, Lixia, Chao Zhao, Zexian Sun, Chongyang Nie, Ruxin Liu, Zhiyong Wang, and Yunlong Wu. 2023. "In Situ Growth of High-Performance ZnAl-Layered Double Hydroxides/Al2O3 Composite Coatings on Aluminum Alloy" Coatings 13, no. 9: 1511. https://doi.org/10.3390/coatings13091511




